Abstract
Mitochondrial dysfunction results in high levels of oxidative stress and mitochondrial damage, leading to disruption of endothelial homeostasis. Recent discoveries have clarified several pathways whereby mitochondrial dysregulation contributes to endothelial dysfunction and vascular disease burden. One such pathway centers around PGC-1α, a transcriptional coactivator linked to mitochondrial biogenesis and antioxidant defense, among other functions. Although primarily investigated for its therapeutic potential in obesity and skeletal muscle differentiation, the ability of PGC-1α to alter a multitude of cellular functions has sparked interest in its role in the vasculature. Within this context, recent studies demonstrate that PGC-1α plays a key role in endothelial cell and smooth muscle cell regulation through effects on oxidative stress, apoptosis, inflammation, and cell proliferation. The ability of PGC-1α to impact these parameters is relevant to vascular disease progression, particularly in relation to atherosclerosis. Upregulation of PGC-1α can prevent the development of, and even encourage regression of, atherosclerotic lesions. Therefore, PGC-1α is poised to serve as a promising target in vascular disease. This review details recent findings related to PGC-1α in vascular regulation, regulation of PGC-1α itself, the role of PGC-1α in atherosclerosis, and therapies that target this key protein.
Keywords: vasculature, PGC-1α, endothelium, cardiovascular disease
Introduction
The Vascular Endothelium
Endothelial cells form a continuous single-layer sheet (endothelium) lining the inside of blood vessels. The endothelium is essential in modulating vascular tone in part by production of vasoactive substances that act on surrounding smooth muscle cells to constrict or dilate vessels. In healthy human vessels exposed to physiological laminar flow, nitric oxide (NO) is released from endothelial cells and serves to relax vessels and maintain endothelial homeostasis. In contrast, in vessels affected by vascular pathologies such as atherosclerosis, heart failure, and hypertension, NO bioavailability is diminished as rising levels of reactive oxygen species (ROS) react with NO or disrupt NO production, leading to impaired endothelium-dependent dilation.1–5 This resulting endothelial dysfunction is a key component of cardiovascular disease progression through impaired vasodilatory responses and an increasingly inflammatory environment.6 Since endothelial dysfunction carries prognostic significance,7 extensive effort has been devoted to prevent or reverse this phenomenon. However, despite years of intense study and development of targeted therapies, cardiovascular diseases remain highly prevalent. A deeper understanding of involved pathways, as well as identification of new therapeutic targets, is thus warranted.
Although combatting vascular ROS production is a promising avenue to reduce vascular disease burden, clinical trials targeting global ROS production have been largely negative;8, 9 thus alternative strategies are needed. Several cellular mechanisms contributing to vascular endothelial dysfunction have been characterized, including endoplasmic reticulum stress,10, 11 high glucose levels,12 decreased formation of endothelial progenitor cells,13 and overproduction of superoxide by NADPH oxidases.14 In addition, mitochondrial dysfunction appears to participate in the development of endothelial dysfunction, largely through excessive production of ROS by the mitochondrial respiratory chain.15, 16 As a result, antioxidants targeting the mitochondria are being considered for clinical testing, including MitoQ and MitoVit-E.17, 18 Instead of simply targeting a single molecule in a single pathway, one alternative approach is to identify elements that act through broad regulatory mechanisms and confer systemic protection. PGC-1α (peroxisome proliferator activated receptor gamma coactivator-1 alpha), a nuclear protein that regulates an array of endothelial and smooth muscle cell processes, has emerged as a promising therapeutic candidate.19 This review considers the role of PGC-1α from a vascular biology perspective and will cover mechanisms whereby PGC-1α contributes to maintenance of endothelial and smooth muscle cell homeostasis, the regulation of PGC-1α itself, and strategies to boost levels of PGC-1α in the vasculature.
The Many Roles of PGC-1α
PGC-1α is a transcriptional coactivator that recruits nuclear receptors or transcription factors to regulate transcription of downstream genes in both the nucleus and the mitochondria. Instead of binding directly to nuclear or mitochondrial DNA, PGC-1α localizes in complexes containing several other proteins. The presence of multiple binding domains that enable interactions with proteins, coactivators, and RNA20 to orchestrate DNA binding factors and chromatin conformation helps to orient PGC-1α at the nexus of transcriptional control. Activation of PGC-1α and resulting recruitment of this transcription machinery is elicited by cellular homeostatic cues, such as changes in metabolic status, hormone levels,21 and redox balance, which correspondingly activate messenger pathways, including the cellular energy sensor AMP activated protein kinase (AMPK)22, calcium, and cAMP.23
First discovered in 1998 for its role in adaptive thermogenesis,24 PGC-1α-related data have amassed rapidly, demonstrating largely beneficial or protective effects in aging, obesity, diabetes, neurodegeneration, and exercise-induced changes in skeletal muscle. PGC-1α controls thermogenesis in brown fat, as well as white-to-brown adipocyte differentiation.24, 25 In skeletal muscle, PGC-1α increases insulin sensitivity and regulates fiber type switching.26, 27 Increases in PGC-1α can also prevent muscle atrophy.28 Repression of PGC-1α is implicated in the neurodegenerative process of Parkinson’s disease.29 These broad functional effects of PGC-1α are due, in part, to its wide distribution throughout the tissues of the body, and to its ability to regulate an array of signaling pathways and transcription control elements, such as PPARγ,24 CREB-binding protein,30 nuclear respiratory factor-1 (NRF-1),31 forkhead box O1 (FOXO1),32 thyroid hormone receptor,24 estrogen receptor,33 and more. These downstream regulatory pathways are nicely reviewed by Finck and Kelly.34 Given the existence of PGC-1α at the core of several cellular- and organ-level homeostatic mechanisms, burgeoning interest in PGC-1α’s role in vascular biology has resulted in the generation of many exciting reports that position this protein as a key player in vascular regulation.
PGC-1α and Vascular Biology
Regulation of PGC-1α in the Vasculature
The vasculature acts to match blood flow to the metabolic energy requirements of tissue. When considering the large variety of metabolic signaling pathways that converge on PGC-1α, it is evident that vascular mechanisms must be in place to control levels and activity of PGC-1α itself in order to establish balance between blood flow and energy expenditure. Indeed, within blood vessels, PGC-1α is responsive to a variety of inputs. Importantly, PGC-1α is increased during mechanical stimulation of the endothelium by shear stress in a SIRT1-dependent manner.35 PGC-1α levels are also regulated by endothelial vasoactive substances, such as NO and hydrogen peroxide (H2O2). PGC-1α acts downstream of NO, and its regulation by this key vasodilator is time-dependent. Short-term treatment (<12h) of endothelial cells with NO donors acts to decrease protein levels of PGC-1α, whereas long-term treatment (>24h) has the opposite effect. This relationship between short-term NO treatment and reduction in levels of PGC-1α was shown to be dependent on FOXO3a levels, and overexpression of FOXO3a prevents an NO-mediated decrease in PGC-1α.36 Interestingly, PGC-1α levels correlate with production of hydrogen peroxide, another vasodilatory substance. Although not demonstrated in endothelial cells, H2O2 is required for the exercise-induced increase in PGC-1α in skeletal muscle.37 It remains unknown whether H2O2 is necessary in this same fashion in the vasculature. That PGC-1α is the target of, and can be increased by, two vasodilatory factors – especially ones that are canonically viewed as antagonistic – warrants further investigation of the functional vascular effects exerted by NO and H2O2 via downstream effects on PGC-1α. Further characterization is needed of mechanisms influencing PGC-1α levels or activation, including exploring connections with other vasoactive substances like prostacyclin or thromboxane, and clarifying the mechanosensitive release of PGC-1α through cell surface receptors.
Post-translational modification is a major mechanism through which PGC-1α is regulated. Serine 570 phosphorylation and subsequent lysine acetylation in response to angiotensin II (Ang II) stimulation leads to inactivation of PGC-1α in vascular smooth muscle cells. This acetylation impairs PGC-1α-FOXO1 interactions, resulting in depressed antioxidant defense.38 It is important to note that Ang II is implicated in hypertension and atherosclerosis, providing a potential link between Ang II-mediated PGC-1α acetylation and cardiovascular diseases.39 Acetylation of PGC-1α is a bidirectional event. Silent mating type information regulation 2 homologs (Sirtuins) are histone deacetylases that have been linked to longevity and cardiovascular protection.40 Sirtuin 1 (SIRT1) is a NAD-dependent histone deacetylase that increases expression of PGC-1α and promotes formation of a complex between the transcription factor FOXO3a and PGC-1α to improve antioxidant defense. Moreover, SIRT1 overexpression reduces PGC-1α acetylation in the presence of oxidative stress in cultured endothelial cells.41 This two-way regulation of PGC-1α via acetylation and deacetylation events exposes its importance as a homeostatic control mechanism. Micro RNAs (miRNA), small, non-coding molecules typically associated with repression of gene translation, act as another means of vascular regulation of PGC-1α. In endothelial cells, miR-19b, miR-221, and miR-222 have been shown to decrease PGC-1α expression and promote endothelial dysfunction.42 Application of the pro-inflammatory cytokines TNFα and IFNγ resulted in mobilization of these miRNAs and subsequent repression of PGC-1α, establishing a direct relationship between PGC-1α and endothelial inflammation, which we discuss in further detail below.
PGC-1α in the Vascular Endothelium
The endothelium is responsible for regulating blood flow, largely through agonist and shear mediated mechanisms. As stated above, shear stress on the vascular endothelium releases vasodilatory NO, and stimulates production of PGC-1α.35 NO and PGC-1α are known to independently combat ROS production thereby limiting endothelial dysfunction.4, 43–45 NO, for instance, can directly scavenge superoxide or target the mitochondrial electron transport chain (ETC) to reduce levels of ROS.46, 47 Likewise, PGC-1α combats excessive levels of oxidative stress by enhancing transcription of antioxidant genes, including manganese superoxide dismutase (MnSOD), responsible for converting superoxide in the mitochondrial matrix to hydrogen peroxide; catalase, responsible for decomposing hydrogen peroxide; and glutathione peroxidase, also responsible for diminishing hydrogen peroxide levels.44 Borniquel et al demonstrated that NO and PGC-1α can operate together to limit ROS levels, but the relationship is complex. Short-term NO treatment downregulates PGC-1α to reduce mitochondrial antioxidant defense genes expression, whereas long-term treatment boosts antioxidant defense through increases in PGC-1α. This time-dependent regulation of antioxidant mechanisms through PGC-1α may help to explain the known pro- and anti-oxidant properties of NO.48 An increase in antioxidant genes is only one mechanism whereby PGC-1α stifles ROS production. ROS are also produced when mitochondrial membrane potential is hyperpolarized, leading to reduced exchange of ADP for ATP. Won et al revealed that PGC-1α can restore physiological membrane potential and dampen excessive ROS production by increasing ATP/ADP translocase activity.49
PGC-1α also exerts control over angiogenesis and associated endothelial cell migration. Angiogenesis is known to require eNOS-derived NO, as well as endothelial ROS.50 Previous studies in skeletal muscle tissue demonstrate the critical role of PGC-1α in raising capillary density.51, 52 However, the picture in isolated endothelial cells somewhat contrasts with the proangiogenic profile of PGC-1α in skeletal muscle. Borniquel et al discovered that PGC-1α overexpression limited NO-mediated endothelial cell migration, and treatment with NO donors decreased PGC-1α mRNA expression.53 These data suggest that the antioxidant properties of PGC-1α in endothelial cells are antithetical to the ROS-requiring, NO-mediated proangiogenic program. In a later study, Sawada et al reported an in vivo, endothelial-specific anti-angiogenic role of PGC-1α. Mice overexpressing PGC-1α in the vascular endothelium displayed blunted re-endothelialisation after carotid injury. Conversely, endothelial cells isolated from PGC-1α−/− mice displayed high capacity for migration with a concurrent repression of anti-angiogenic gene expression.54 Despite this described anti-migratory role of endothelial PGC-1α, a recent study showed that, although endothelial cells from PGC-1α−/− mice migrate faster than those from PGC-1α+/+ mice, this migration is aberrant. Cells lacking PGC-1α do not adhere as strongly within the extracellular matrix. In addition, the cell spreading that was observed lacked proper directionality, suggesting that PGC-1α is actually required for conserved angiogenesis, in contrast to the results above.55 A different report illustrated the critical role of PGC-1α in driving retinal angiogenesis via vascular endothelial growth factor.56 Although the exact mechanism requires additional clarification, PGC-1α’s participation in both pro- and anti-angiogenic responses places it at the central switch terminal for regulating vascular growth and architecture.
PGC-1α in Vascular Smooth Muscle Cells
Vascular homeostasis is not limited to contributions of the endothelial cell layer. Surrounding the endothelium are vascular smooth muscle cells (VSMCs), the ultimate effectors of vasomotion. VSMCs and endothelial cells display a dynamic interrelationship, and diffusion of endothelial substances57 or direct electrochemical connections58, 59 can elicit responses in nearby VSMCs. Of note, PGC-1α is expressed in both the vascular endothelium and VSMCs.60 Smooth muscle-specific functions of PGC-1α have been largely characterized in relation to VSMC proliferation and senescence, both of which result from excessive ROS production. In light of PGC-1α’s robust antioxidant-boosting effects in endothelial cells, it is not surprising that PGC-1α can indirectly limit ROS production in VSMCs, 60 and conversely, loss of PGC-1α in this tissue results in a reduction in the antioxidant program.61 PGC-1α overexpression can reduce ROS-mediated VSMC migration.62 Moreover, adenoviral-, palmitate-, or 17β-estradiol-mediated overexpression of PGC-1α can halt ROS-mediated VSMC proliferation, even in response to an exogenous stressor like oleic acid or elevated glucose.63–65 In addition, preventing Ang II-mediated inactivation of PGC-1α ameliorates VSMC senescence and hypertrophy.38 It appears that these anti-senescent effects in VSMCs are enabled by the interaction between PGC-1α and two longevity-associated factors, telomerase reverse transcriptase (TERT) and FOXO1.66–70 The ability of PGC-1α to enhance antioxidant gene transcription and attenuate ROS production in intimal and medial layers and to bolster anti-proliferative and anti-aging pathways emphasizes its potential to serve as a therapeutic target for vascular disease.
PGC-1α and Vascular Disease
Inflammation
Inflammation is intimately involved in the pathogenesis of several cardiovascular disease processes, including atherosclerosis, hypertension, and heart failure.71–73 A host of factors participate in the inflammatory response. Within the vasculature, ROS are inextricably linked to development of a pro-inflammatory phenotype, and we have already discussed PGC-1α’s powerful antioxidant properties. Another central pathway involves tumor necrosis factor-α (TNF-α), a pro-inflammatory signaling molecules that increases expression of downstream adhesion molecules, allowing for attachment of inflammatory response cells at sites of damage. TNF-α also increases cellular ROS levels. PGC-1α is able to mitigate TNF-induced production of mitochondrial and intracellular ROS, as well as expression of adhesion molecules throughout the vascular wall.60 In this same study, PGC-1α overexpression reduced activity of NF-κB, a major effector of pro-inflammatory pathways. A similar effect of PGC1α on NF-κB activity was observed in muscle cells.74 Oxidized low-density lipoprotein (oxLDL) is known to promote inflammation,75 and PGC-1α blocks oxLDL movement into cells, thereby halting the progression of inflammation.76 Confirmation of this anti-inflammatory effect of PGC-1α in human subjects is needed.
Atherosclerosis
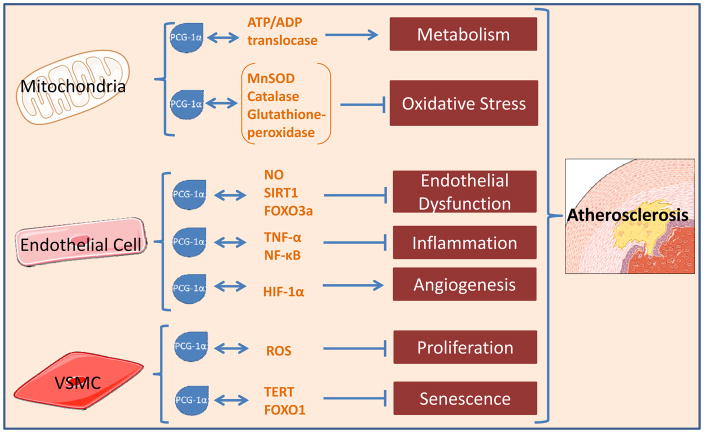
That PGC-1α broadly affects diverse functions in different vascular compartments, confirms its role as a master controller of vascular homeostasis. Of particular relevance to cardiovascular disease is its powerful induction of anti-inflammatory signals and upregulation of antioxidant proteins. Nowhere are these properties more relevant than in atherosclerosis. Once considered to be primarily a disease of lipid storage, atherosclerosis is now fundamentally considered an inflammatory process.71, 77 PGC-1α is poised to reduce inflammation and, as a result, lessen atherosclerotic disease burden (Figure 1). On a broad, population-based scale, one case-control study found a higher frequency of the loss-of-function Gly482Ser polymorphism in the gene encoding for PGC-1α in coronary artery disease (CAD) patients versus control patients.78 Further evidence for PGC-1α’s involvement in cardiovascular disease is the decreased protein levels, albeit increased mRNA expression, of PGC-1α in vessels from patients with atherosclerosis relative to vessels from healthy subjects.42 When examining human atherosclerotic lesions directly, PGC-1α expression is less in symptomatic versus asymptomatic plaques.76
Fig. 1.
Mechanisms through which PGC-1α influences the vasculature and regulates atherosclerotic disease progression. Manganese superoxide dismutase, MnSOD; nitric oxide, NO; sirtuin 1, SIRT 1; forkhead box O3, FOXO3a; tumor necrosis factor-alpha, TNFα; nuclear factor kappa-light-chain-enhancer of activated B cells, NF-κB; Hypoxia-inducible factor 1-alpha, HIF-1α; reactive oxygen species, ROS; telomerase reverse transcriptase, TERT; forkhead box O3, FOXO3a; vascular smooth muscle cell, VSMC.
Aside from these correlative links between lower levels of PGC-1α and presence of atherosclerosis, mechanistic studies validate PGC-1α’s athero-protective role. In the initial stages of atherosclerosis, bone marrow-derived inflammatory monocytes, invade the arterial wall, where they differentiate into macrophages. These macrophages ingest lipids and take up residence in the vascular endothelium as foam cells, forming an essential component of the atherosclerotic plaque, conferring plaque weakness and propensity for rupture. PGC-1α is found in the human macrophages inhabiting such plaques, and PGC-1α overexpression via conjugated linoleic acid (CLA) treatment can inhibit foam cell development by preventing oxidized lipid uptake into macrophages.76 Adopting an inverse approach, the same investigative team reported excess oxidized lipid uptake in PGC-1α−/− mice relative to wild-type controls. In contrast, results from a previous study suggest that PGC-1α deficiency did not contribute to enhanced atherosclerosis.79 Despite expression of increased levels of inflammatory markers in PGC-1α−/− mice bred on an ApoE−/− background, no difference in atherosclerosis burden was observed compared to wild type.79 This study showed that inflammation may be necessary but is not sufficient for promoting atherosclerosis in this model.
Xiong et al proposed that that an age-dependent effect resulted in the differences between these two studies, stating that the athero-protective role PGC-1α is only relevant in older mice.67 In a well-designed experiment using atheroprone LDLR−/− mice, administration of wild-type or PGC-1α −/− macrophages was performed along with a high-fat diet. Mice lacking macrophage PGC-1α developed larger atherosclerotic lesions than those with intact PGC-1α. These investigators confirmed the proposed age-dependent anti-atherosclerotic effects of PGC-1α by demonstrating that, indeed, young (6-month-old) PGC-1α −/−ApoE−/− mice fed a high-fat diet do not develop significant atherosclerosis, whereas a clear increase in lesion development in PGC-1α −/−ApoE−/− mice over PGC-1α+/+ ApoE−/− control mice was observed at 18 months.67 Evaluation of the athero-preventive role of PGC-1α in humans is a logical next step.
Therapeutic Strategies to Increase PGC-1α
Traditional therapeutics for atherosclerosis include lifestyle changes, pharmacological therapy, and percutaneous as well as surgical intervention. Our most effective preventive strategies (exercise and diet) are inexpensive, but difficult to implement. Pharmacological approaches are typically associated with greater compliance, but are often costly and address only one “risk factor” pathway. A promising advantage of targeting PGC-1α is the ability to address multiple pathways (inflammation, endothelial dysfunction, oxidative stress) simultaneously in a single pharmacological approach. Interest in the ability to manipulate master regulators, such as PGC-1α and miRNAs, as a means of disease treatment is on the rise. It is important to proceed with caution: such master regulators may not serve as the best therapeutic candidates due to their ability to influence many processes, thus generating concerns about non-specificity and unintended off-target effects on non-pathogenic pathways. Despite this concern, PGC-1α still appears to be a promising candidate in atherosclerosis in light of the discussed data. In general, an improvement in disease parameters appears to center around upregulation of PGC-1α levels or activity. Fortunately, several such strategies, distinct from current guideline-directed treatments, exist.
Calorie restriction and exercise, both known to harbor enormous potential as anti-aging programs, are able to increase levels of PGC-1α and improve organismal health,80–86 likely due to cellular energy depletion, which is known to activate PGC-1α.23 Importantly, the PGC-1α-inducing effect of exercise is limited by use of global antioxidants in untrained and trained individuals,87, 88 providing a potential explanation for the inability of indiscriminate antioxidant use to ameliorate adverse cardiovascular event occurrence.8 However, natural antioxidants, such as resveratrol, appear to activate PGC-1α through a reduction in overall PGC-1α acetylation.89 Lipoic acid (ALA), an endogenous antioxidant and mitochondrial cofactor known to be anti-atherosclerotic,90 increases PGC-1α to counter cardiovascular disease development.67 ZLN005 is a novel transcriptional activator of PGC-1α with vasculoprotective effects in a diabetic mouse model.91 Statins can also upregulate PGC-1α in the retinal vasculature to boost antioxidant defense.92 The effect of statins on PGC-1α is tissue-specific, producing PGC-1α upregulation in the heart, along with a concomitant increase in antioxidant defense, but PGC-1α downregulation in the skeletal muscle.93 These examples provide new direction for novel potential therapeutic adjuncts in the treatment of atherosclerosis. Most of these treatments are already known to be beneficial in humans, but evaluation of the protective mechanism via regulation of PGC-1α instead of traditional pathways would be worthwhile. It must be considered that these treatments are non-specific, making it difficult to predict off-target effects of using these therapeutics. Systemic approaches may also pose an issue due to the ubiquitous expression of PGC-1α in tissues such as liver, heart, adipose, and muscle. However, since activation or upregulation of PGC-1α is beneficial in relation to several disease processes,20, 94 this concern may not present a major obstacle and may actually be advantageous in aging patients or subjects with multiple comorbidities.
Several other caveats must be mentioned in the discussion of PGC-1α’s therapeutic potential. For example, forced PGC-1α overexpression has also been associated with detrimental off-target effects. One study reported that excessive overexpression of PGC-1α produced reversible cardiomyopathy in mice as a result of robust mitochondrial biogenesis in cardiac tissue;95 in contrast, modest (two-fold) elevations in cardiac PGC-1α expression do not allow for preservation of cardiac function.96 Others have reported a pro-tumorigenic effect of PGC-1α,97 likely as a result of improvements in cell survival, although a recent study suggests that PGC-1α may actually suppress tumor formation.98 Therefore, caution must be used when attempting to overexpress PGC-1α.
Future Directions
Future studies should continue to characterize PGC-1α’s therapeutic potential. One lingering issue is the identification of a physiologic range of PGC-1alpha upregulation in cardiovascular system in light of the previously mentioned reports of dose-dependent cardiotoxic effects. Furthermore, much work needs to be done to elucidate how to most effectively target PGC-1α, either through regulation of expression or posttranslational modification. It is currently difficult to speculate as to the most effective method of promoting PGC-1α’s protective effects, although most studies report a beneficial effect of boosted expression.
While investigators should continue to identify the molecular routes through which PGC-1α acts in the vasculature, it will also be important to integrate PGC-1α signaling with other signaling pathways, as was done by Xiong et al in characterizing the interaction between PGC-1α and telomerase.67 When performing these investigations, it will be critical to not only explore these relationships within the endothelial and smooth muscle cell layers, but also to extend this work into the adventitial layer, an increasingly recognized contributor to vascular disease.99 Similarly, we must strive to connect PGC-1α with other outcome variables in vascular disease, such as endothelial permeability, vascular stiffness and calcification, and the venous circulation. Another intriguing potential connection to explore is that between PGC-1α and the other pathways producing endothelial dysfunction, such as endothelial progenitor cell deficiency, high glucose, and endoplasmic reticulum stress. Doing so will provide further support for translating these findings to human subjects.
Summary and Conclusion
To effectively combat atherosclerosis, we must understand both the fundamental mechanisms contributing to the disease and options to combat disease development or induce disease regression. When considering the data demonstrating PGC-1α’s ability to act as a master regulator of cell function and counteract oxidative stress, lesion development, endothelial dysfunction, VSMC proliferation, and inflammation, this protein emerges as an attractive therapeutic candidate for atherosclerosis. Its broad localization, regulation, and functional significance highlight its potential. As interest in PGC-1α’s role in the vasculature continues to rise, further clarification of the involved pathways may lead to an improved ability to manipulate this pathway. Understanding PGC-1α will help us to better understand the atherosclerotic process, as well as the manner in which endothelial function is altered by disease from a novel angle.
Supplementary Material
Highlights.
PGC-1α reduces oxidative stress and inflammation in the vascular endothelium and vascular smooth muscle cells.
Upregulation of PGC-1α combats atherosclerosis and may serve as an adjunct therapeutic target in cardiovascular disease.
Several therapeutic strategies exist to upregulate PGC-1α.
Acknowledgments
The authors thank Dr. Alison Kriegel and Mark Paterson for critical review of this manuscript.
Source of funding
This work was funded by the NIH NHLBI Grant 4R01HL113612-04 (DDG)
Glossary
- Ang II
angiotensin II
- CAD
coronary artery disease
- eNOS
endothelial nitric oxide synthase
- FOXO1
forkhead box O1
- H2O2
hydrogen peroxide
- LDLR
low density lipoprotein receptor
- NO
nitric oxide
- PGC-1α
Peroxisome proliferator receptor gamma coactivator 1-alpha
- ROS
reactive oxygen species
- SIRT1
sirtuin 1
- TNFα
tumor necrosis factor alpha
- VSMCs
vascular smooth muscle cells
Footnotes
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 2.Kubo SH, Rector TS, Bank AJ, Williams RE, Heifetz SM. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation. 1991;84:1589–1596. doi: 10.1161/01.cir.84.4.1589. [DOI] [PubMed] [Google Scholar]
- 3.Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 4.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 5.Tomasian D, Keaney JF, Vita JA. Antioxidants and the bioactivity of endothelium-derived nitric oxide. Cardiovasc Res. 2000;47:426–435. doi: 10.1016/s0008-6363(00)00103-6. [DOI] [PubMed] [Google Scholar]
- 6.Galle J, Quaschning T, Seibold S, Wanner C. Endothelial dysfunction and inflammation: What is the link? Kidney Int Suppl. 2003:S45–49. doi: 10.1046/j.1523-1755.63.s84.12.x. [DOI] [PubMed] [Google Scholar]
- 7.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin e supplementation and cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 9.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta carotene and vitamin a on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 10.Galan M, Kassan M, Kadowitz PJ, Trebak M, Belmadani S, Matrougui K. Mechanism of endoplasmic reticulum stress-induced vascular endothelial dysfunction. Biochim Biophys Acta. 2014;1843:1063–1075. doi: 10.1016/j.bbamcr.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenna S, Han R, Trojanowska M. Endoplasmic reticulum stress and endothelial dysfunction. IUBMB Life. 2014;66:530–537. doi: 10.1002/iub.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cosentino F, Hishikawa K, Katusic ZS, Luscher TF. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation. 1997;96:25–28. doi: 10.1161/01.cir.96.1.25. [DOI] [PubMed] [Google Scholar]
- 13.Werner N, Wassmann S, Ahlers P, Schiegl T, Kosiol S, Link A, et al. Endothelial progenitor cells correlate with endothelial function in patients with coronary artery disease. Basic Res Cardiol. 2007;102:565–571. doi: 10.1007/s00395-007-0680-1. [DOI] [PubMed] [Google Scholar]
- 14.Ray R, Shah AM. Nadph oxidase and endothelial cell function. Clin Sci (Lond) 2005;109:217–226. doi: 10.1042/CS20050067. [DOI] [PubMed] [Google Scholar]
- 15.Kluge MA, Fetterman JL, Vita JA. Mitochondria and endothelial function. Circ Res. 2013;112:1171–1188. doi: 10.1161/CIRCRESAHA.111.300233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson SM, Duchen MR. Endothelial mitochondria: Contributing to vascular function and disease. Circ Res. 2007;100:1128–1141. doi: 10.1161/01.RES.0000261970.18328.1d. [DOI] [PubMed] [Google Scholar]
- 17.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cocheme HM, et al. Mitochondria-targeted antioxidant mitoq10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 18.Dhanasekaran A, Kotamraju S, Kalivendi SV, Matsunaga T, Shang T, Keszler A, et al. Supplementation of endothelial cells with mitochondria-targeted antioxidants inhibit peroxide-induced mitochondrial iron uptake, oxidative damage, and apoptosis. J Biol Chem. 2004;279:37575–37587. doi: 10.1074/jbc.M404003200. [DOI] [PubMed] [Google Scholar]
- 19.Bayeva M, Gheorghiade M, Ardehali H. Mitochondria as a therapeutic target in heart failure. J Am Coll Cardiol. 2013;61:599–610. doi: 10.1016/j.jacc.2012.08.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones AW, Yao Z, Vicencio JM, Karkucinska-Wieckowska A, Szabadkai G. Pgc-1 family coactivators and cell fate: Roles in cancer, neurodegeneration, cardiovascular disease and retrograde mitochondria-nucleus signalling. Mitochondrion. 2012;12:86–99. doi: 10.1016/j.mito.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Goldenthal MJ, Weiss HR, Marin-Garcia J. Bioenergetic remodeling of heart mitochondria by thyroid hormone. Mol Cell Biochem. 2004;265:97–106. doi: 10.1023/b:mcbi.0000044321.17680.a2. [DOI] [PubMed] [Google Scholar]
- 22.Canto C, Auwerx J. Pgc-1alpha, sirt1 and ampk, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: The central role of pgc-1alpha. Cardiovasc Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 24.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 25.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A pgc1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, et al. Transcriptional co-activator pgc-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 27.Benton CR, Holloway GP, Han XX, Yoshida Y, Snook LA, Lally J, et al. Increased levels of peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (pgc-1alpha) improve lipid utilisation, insulin signalling and glucose transport in skeletal muscle of lean and insulin-resistant obese zucker rats. Diabetologia. 2010;53:2008–2019. doi: 10.1007/s00125-010-1773-1. [DOI] [PubMed] [Google Scholar]
- 28.Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, et al. Pgc-1alpha protects skeletal muscle from atrophy by suppressing foxo3 action and atrophy-specific gene transcription. Proc Natl Acad Sci U S A. 2006;103:16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, et al. Paris (znf746) repression of pgc-1alpha contributes to neurodegeneration in parkinson’s disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O’Malley B, et al. Activation of ppargamma coactivator-1 through transcription factor docking. Science. 1999;286:1368–1371. doi: 10.1126/science.286.5443.1368. [DOI] [PubMed] [Google Scholar]
- 31.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator pgc-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 32.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, et al. Insulin-regulated hepatic gluconeogenesis through foxo1-pgc-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 33.Huss JM, Kopp RP, Kelly DP. Peroxisome proliferator-activated receptor coactivator-1alpha (pgc-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within pgc-1alpha. J Biol Chem. 2002;277:40265–40274. doi: 10.1074/jbc.M206324200. [DOI] [PubMed] [Google Scholar]
- 34.Finck BN, Kelly DP. Pgc-1 coactivators: Inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, sirt1, and vascular homeostasis. Proc Natl Acad Sci U S A. 2010;107:10268–10273. doi: 10.1073/pnas.1003833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borniquel S, Valle I, Cadenas S, Lamas S, Monsalve M. Nitric oxide regulates mitochondrial oxidative stress protection via the transcriptional coactivator pgc-1alpha. FASEB J. 2006;20:1889–1891. doi: 10.1096/fj.05-5189fje. [DOI] [PubMed] [Google Scholar]
- 37.Silveira LR, Pilegaard H, Kusuhara K, Curi R, Hellsten Y. The contraction induced increase in gene expression of peroxisome proliferator-activated receptor (ppar)-gamma coactivator 1alpha (pgc-1alpha), mitochondrial uncoupling protein 3 (ucp3) and hexokinase ii (hkii) in primary rat skeletal muscle cells is dependent on reactive oxygen species. Biochim Biophys Acta. 2006;1763:969–976. doi: 10.1016/j.bbamcr.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Xiong S, Salazar G, San Martin A, Ahmad M, Patrushev N, Hilenski L, et al. Pgc-1 alpha serine 570 phosphorylation and gcn5-mediated acetylation by angiotensin ii drive catalase down-regulation and vascular hypertrophy. J Biol Chem. 2010;285:2474–2487. doi: 10.1074/jbc.M109.065235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berk BC, Haendeler J, Sottile J. Angiotensin ii, atherosclerosis, and aortic aneurysms. J Clin Invest. 2000;105:1525–1526. doi: 10.1172/JCI9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winnik S, Auwerx J, Sinclair DA, Matter CM. Protective effects of sirtuins in cardiovascular diseases: From bench to bedside. Eur Heart J. 2015;36:3404–3412. doi: 10.1093/eurheartj/ehv290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olmos Y, Sanchez-Gomez FJ, Wild B, Garcia-Quintans N, Cabezudo S, Lamas S, et al. Sirt1 regulation of antioxidant genes is dependent on the formation of a foxo3a/pgc-1alpha complex. Antioxid Redox Signal. 2013;19:1507–1521. doi: 10.1089/ars.2012.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xue Y, Wei Z, Ding H, Wang Q, Zhou Z, Zheng S, et al. Microrna-19b/221/222 induces endothelial cell dysfunction via suppression of pgc-1alpha in the progression of atherosclerosis. Atherosclerosis. 2015;241:671–681. doi: 10.1016/j.atherosclerosis.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 43.Cannon RO., 3rd Role of nitric oxide in cardiovascular disease: Focus on the endothelium. Clin Chem. 1998;44:1809–1819. [PubMed] [Google Scholar]
- 44.Valle I, Alvarez-Barrientos A, Arza E, Lamas S, Monsalve M. Pgc-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc Res. 2005;66:562–573. doi: 10.1016/j.cardiores.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 45.Olmos Y, Valle I, Borniquel S, Tierrez A, Soria E, Lamas S, et al. Mutual dependence of foxo3a and pgc-1alpha in the induction of oxidative stress genes. J Biol Chem. 2009;284:14476–14484. doi: 10.1074/jbc.M807397200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guzik TJ, West NE, Pillai R, Taggart DP, Channon KM. Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. Hypertension. 2002;39:1088–1094. doi: 10.1161/01.hyp.0000018041.48432.b5. [DOI] [PubMed] [Google Scholar]
- 47.Dahm CC, Moore K, Murphy MP. Persistent s-nitrosation of complex i and other mitochondrial membrane proteins by s-nitrosothiols but not nitric oxide or peroxynitrite: Implications for the interaction of nitric oxide with mitochondria. J Biol Chem. 2006;281:10056–10065. doi: 10.1074/jbc.M512203200. [DOI] [PubMed] [Google Scholar]
- 48.Patel RP, Levonen A, Crawford JH, Darley-Usmar VM. Mechanisms of the pro- and anti-oxidant actions of nitric oxide in atherosclerosis. Cardiovasc Res. 2000;47:465–474. doi: 10.1016/s0008-6363(00)00086-9. [DOI] [PubMed] [Google Scholar]
- 49.Won JC, Park JY, Kim YM, Koh EH, Seol S, Jeon BH, et al. Peroxisome proliferator-activated receptor-gamma coactivator 1-alpha overexpression prevents endothelial apoptosis by increasing atp/adp translocase activity. Arterioscler Thromb Vasc Biol. 2010;30:290–297. doi: 10.1161/ATVBAHA.109.198721. [DOI] [PubMed] [Google Scholar]
- 50.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 51.Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, et al. The transcriptional coactivator pgc-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci U S A. 2009;106:21401–21406. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, et al. Hif-independent regulation of vegf and angiogenesis by the transcriptional coactivator pgc-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 53.Borniquel S, Garcia-Quintans N, Valle I, Olmos Y, Wild B, Martinez-Granero F, et al. Inactivation of foxo3a and subsequent downregulation of pgc-1 alpha mediate nitric oxide-induced endothelial cell migration. Mol Cell Biol. 2010;30:4035–4044. doi: 10.1128/MCB.00175-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sawada N, Jiang A, Takizawa F, Safdar A, Manika A, Tesmenitsky Y, et al. Endothelial pgc-1alpha mediates vascular dysfunction in diabetes. Cell Metab. 2014;19:246–258. doi: 10.1016/j.cmet.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garcia-Quintans N, Prieto I, Sanchez-Ramos C, Luque A, Arza E, Olmos Y, et al. Regulation of endothelial dynamics by pgc-1alpha relies on ros control of vegf-a signaling. Free Radic Biol Med. 2016;93:41–51. doi: 10.1016/j.freeradbiomed.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 56.Ueta T, Inoue T, Yuda K, Furukawa T, Yanagi Y, Tamaki Y. Intense physiological light upregulates vascular endothelial growth factor and enhances choroidal neovascularization via peroxisome proliferator-activated receptor gamma coactivator-1alpha in mice. Arterioscler Thromb Vasc Biol. 2012;32:1366–1371. doi: 10.1161/ATVBAHA.112.248021. [DOI] [PubMed] [Google Scholar]
- 57.Lilly B. We have contact: Endothelial cell-smooth muscle cell interactions. Physiology (Bethesda) 2014;29:234–241. doi: 10.1152/physiol.00047.2013. [DOI] [PubMed] [Google Scholar]
- 58.Billaud M, Lohman AW, Johnstone SR, Biwer LA, Mutchler S, Isakson BE. Regulation of cellular communication by signaling microdomains in the blood vessel wall. Pharmacol Rev. 2014;66:513–569. doi: 10.1124/pr.112.007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: Role in vasomotor control. Circ Res. 2000;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- 60.Kim HJ, Park KG, Yoo EK, Kim YH, Kim YN, Kim HS, et al. Effects of pgc-1alpha on tnf-alpha-induced mcp-1 and vcam-1 expression and nf-kappab activation in human aortic smooth muscle and endothelial cells. Antioxid Redox Signal. 2007;9:301–307. doi: 10.1089/ars.2006.1456. [DOI] [PubMed] [Google Scholar]
- 61.Xiong S, Salazar G, Patrushev N, Ma M, Forouzandeh F, Hilenski L, et al. Peroxisome proliferator-activated receptor gamma coactivator-1alpha is a central negative regulator of vascular senescence. Arterioscler Thromb Vasc Biol. 2013;33:988–998. doi: 10.1161/ATVBAHA.112.301019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qu A, Jiang C, Xu M, Zhang Y, Zhu Y, Xu Q, et al. Pgc-1alpha attenuates neointimal formation via inhibition of vascular smooth muscle cell migration in the injured rat carotid artery. Am J Physiol Cell Physiol. 2009;297:C645–653. doi: 10.1152/ajpcell.00469.2008. [DOI] [PubMed] [Google Scholar]
- 63.Jiang X, Zhang Y, Hou D, Zhu L, Xu W, Ding L, et al. 17beta-estradiol inhibits oleic acid-induced rat vsmc proliferation and migration by restoring pgc-1alpha expression. Mol Cell Endocrinol. 2010;315:74–80. doi: 10.1016/j.mce.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 64.Zhu L, Sun G, Zhang H, Zhang Y, Chen X, Jiang X, et al. Pgc-1alpha is a key regulator of glucose-induced proliferation and migration in vascular smooth muscle cells. PLoS One. 2009;4:e4182. doi: 10.1371/journal.pone.0004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y, Liu C, Zhu L, Jiang X, Chen X, Qi X, et al. Pgc-1alpha inhibits oleic acid induced proliferation and migration of rat vascular smooth muscle cells. PLoS One. 2007;2:e1137. doi: 10.1371/journal.pone.0001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiong S, Salazar G, Patrushev N, Alexander RW. Foxo1 mediates an autofeedback loop regulating sirt1 expression. J Biol Chem. 2011;286:5289–5299. doi: 10.1074/jbc.M110.163667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiong S, Patrushev N, Forouzandeh F, Hilenski L, Alexander RW. Pgc-1alpha modulates telomere function and DNA damage in protecting against aging-related chronic diseases. Cell Rep. 2015;12:1391–1399. doi: 10.1016/j.celrep.2015.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Armanios M. Telomeres and age-related disease: How telomere biology informs clinical paradigms. J Clin Invest. 2013;123:996–1002. doi: 10.1172/JCI66370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 72.Touyz RM. Molecular and cellular mechanisms in vascular injury in hypertension: Role of angiotensin ii. Curr Opin Nephrol Hypertens. 2005;14:125–131. doi: 10.1097/00041552-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 73.Anker SD, von Haehling S. Inflammatory mediators in chronic heart failure: An overview. Heart. 2004;90:464–470. doi: 10.1136/hrt.2002.007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eisele PS, Salatino S, Sobek J, Hottiger MO, Handschin C. The peroxisome proliferator-activated receptor gamma coactivator 1alpha/beta (pgc-1) coactivators repress the transcriptional activity of nf-kappab in skeletal muscle cells. J Biol Chem. 2013;288:2246–2260. doi: 10.1074/jbc.M112.375253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kiyan Y, Tkachuk S, Hilfiker-Kleiner D, Haller H, Fuhrman B, Dumler I. Oxldl induces inflammatory responses in vascular smooth muscle cells via urokinase receptor association with cd36 and tlr4. J Mol Cell Cardiol. 2014;66:72–82. doi: 10.1016/j.yjmcc.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 76.McCarthy C, Lieggi NT, Barry D, Mooney D, de Gaetano M, James WG, et al. Macrophage ppar gamma co-activator-1 alpha participates in repressing foam cell formation and atherosclerosis in response to conjugated linoleic acid. EMBO Mol Med. 2013;5:1443–1457. doi: 10.1002/emmm.201302587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shapiro MD, Fazio S. From lipids to inflammation: New approaches to reducing atherosclerotic risk. Circ Res. 2016;118:732–749. doi: 10.1161/CIRCRESAHA.115.306471. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y, Xu W, Li X, Tang Y, Xie P, Ji Y, et al. Association between ppargc1a gene polymorphisms and coronary artery disease in a chinese population. Clin Exp Pharmacol Physiol. 2008;35:1172–1177. doi: 10.1111/j.1440-1681.2008.04988.x. [DOI] [PubMed] [Google Scholar]
- 79.Stein S, Lohmann C, Handschin C, Stenfeldt E, Boren J, Luscher TF, et al. Apoe−/− pgc-1alpha−/− mice display reduced il-18 levels and do not develop enhanced atherosclerosis. PLoS One. 2010;5:e13539. doi: 10.1371/journal.pone.0013539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anderson RM, Barger JL, Edwards MG, Braun KH, O’Connor CE, Prolla TA, et al. Dynamic regulation of pgc-1alpha localization and turnover implicates mitochondrial adaptation in calorie restriction and the stress response. Aging Cell. 2008;7:101–111. doi: 10.1111/j.1474-9726.2007.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of pgc-1alpha and sirt1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 82.Ranhotra HS. Long-term caloric restriction up-regulates ppar gamma co-activator 1 alpha (pgc-1alpha) expression in mice. Indian J Biochem Biophys. 2010;47:272–277. [PubMed] [Google Scholar]
- 83.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, et al. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Corton JC, Brown-Borg HM. Peroxisome proliferator-activated receptor gamma coactivator 1 in caloric restriction and other models of longevity. J Gerontol A Biol Sci Med Sci. 2005;60:1494–1509. doi: 10.1093/gerona/60.12.1494. [DOI] [PubMed] [Google Scholar]
- 85.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, et al. Adaptations of skeletal muscle to exercise: Rapid increase in the transcriptional coactivator pgc-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 86.Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA. Exercise increases mitochondrial pgc-1alpha content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J Biol Chem. 2011;286:10605–10617. doi: 10.1074/jbc.M110.211466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, et al. Oral administration of vitamin c decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 89.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating sirt1 and pgc-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 90.Zhang WJ, Bird KE, McMillen TS, LeBoeuf RC, Hagen TM, Frei B. Dietary alpha-lipoic acid supplementation inhibits atherosclerotic lesion development in apolipoprotein e-deficient and apolipoprotein e/low-density lipoprotein receptor-deficient mice. Circulation. 2008;117:421–428. doi: 10.1161/CIRCULATIONAHA.107.725275. [DOI] [PubMed] [Google Scholar]
- 91.Zhang LN, Zhou HY, Fu YY, Li YY, Wu F, Gu M, et al. Novel small-molecule pgc-1alpha transcriptional regulator with beneficial effects on diabetic db/db mice. Diabetes. 2013;62:1297–1307. doi: 10.2337/db12-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zheng Z, Chen H, Wang H, Ke B, Zheng B, Li Q, et al. Improvement of retinal vascular injury in diabetic rats by statins is associated with the inhibition of mitochondrial reactive oxygen species pathway mediated by peroxisome proliferator-activated receptor gamma coactivator 1alpha. Diabetes. 2010;59:2315–2325. doi: 10.2337/db10-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bouitbir J, Charles AL, Echaniz-Laguna A, Kindo M, Daussin F, Auwerx J, et al. Opposite effects of statins on mitochondria of cardiac and skeletal muscles: A ‘mitohormesis’ mechanism involving reactive oxygen species and pgc-1. Eur Heart J. 2012;33:1397–1407. doi: 10.1093/eurheartj/ehr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dillon LM, Rebelo AP, Moraes CT. The role of pgc-1 coactivators in aging skeletal muscle and heart. IUBMB Life. 2012;64:231–241. doi: 10.1002/iub.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Russell LK, Mansfield CM, Lehman JJ, Kovacs A, Courtois M, Saffitz JE, et al. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res. 2004;94:525–533. doi: 10.1161/01.RES.0000117088.36577.EB. [DOI] [PubMed] [Google Scholar]
- 96.Liang H, Balas B, Tantiwong P, Dube J, Goodpaster BH, O’Doherty RM, et al. Whole body overexpression of pgc-1alpha has opposite effects on hepatic and muscle insulin sensitivity. Am J Physiol Endocrinol Metab. 2009;296:E945–954. doi: 10.1152/ajpendo.90292.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhalla K, Hwang BJ, Dewi RE, Ou L, Twaddel W, Fang HB, et al. Pgc1alpha promotes tumor growth by inducing gene expression programs supporting lipogenesis. Cancer Res. 2011;71:6888–6898. doi: 10.1158/0008-5472.CAN-11-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Torrano V, Valcarcel-Jimenez L, Cortazar AR, Liu X, Urosevic J, Castillo-Martin M, et al. The metabolic co-regulator pgc1alpha suppresses prostate cancer metastasis. Nat Cell Biol. 2016;18:645–656. doi: 10.1038/ncb3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meijles DN, Pagano PJ. Nox and inflammation in the vascular adventitia. Hypertension. 2016;67:14–19. doi: 10.1161/HYPERTENSIONAHA.115.03622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.