Abstract
Objective
The role of vitamin D deficiency in coronary artery disease (CAD) progression is uncertain. Chronic inflammation in epicardial adipose tissue (EAT) has been implicated in the pathogenesis of CAD. However, the molecular mechanism underlying vitamin D deficiency-enhanced inflammation in the EAT of diseased coronary arteries remains unknown. We examined a mechanistic link between 1,25-dihydroxyvitamin D (VD3)-mediated suppression of NF-κB transporter, karyopherin alpha 4 (KPNA4) expression and NF-κB activation in preadipocytes. Furthermore, we determined whether vitamin D deficiency accelerates CAD progression by increasing KPNA4 and nuclear NF-κB levels in EAT.
Approach and Results
Nuclear protein levels were detected by immunofluorescence and Western blot. Exogenous KPNA4 was transported into cells by a transfection approach and constituted lentiviral vector. Swine were administered vitamin D-deficient or vitamin D-sufficient hypercholesterolemic diet. After one year, the histopathology of coronary arteries and nuclear protein expression of EAT were assessed. VD3 inhibited NF-κB activation and reduced KPNA4 levels through increased VDR expression. Exogenous KPNA4 rescued VD3-dependent suppression of NF-κB nuclear translocation and activation. Vitamin D deficiency caused extensive CAD progression and advanced atherosclerotic plaques, which are linked to increased KPNA4 and nuclear NF-κB levels in the EAT.
Conclusions
VD3 attenuates NF-κB activation by targeting KPNA4. Vitamin D deficiency accelerates CAD progression at least in part through enhanced chronic inflammation of EAT by upregulation of KPNA4, which enhances NF-κB activation. These novel findings provide mechanistic evidence that vitamin D supplementation could be beneficial for the prevention and treatment of CAD.
Keywords: Atherosclerosis, Vitamin D, KPNA4, NF-κB, Epicardial Adipose Tissue, Coronary Artery Disease
Introduction
Coronary artery disease (CAD) and its complication, myocardial infarction arising from atherosclerosis, are the most common life-threatening cardiovascular disorders. Vascular endothelial dysfunction, increased inflammatory cytokine release, immune cell infiltrates, and subsequent stimulation of vascular smooth muscle cell proliferation are the pathophysiological basis of atherosclerosis. The inflammatory signals are classically considered to originate from blood–borne immune cells 1. However, accumulated data from the past decade indicate that pathogenic inflammatory adipokines from epicardial adipose tissue (EAT) contribute to the development and progression of CAD 2–4. The majority of clinical data have demonstrated that EAT volume is a strong predictor of coronary atherosclerosis and directly associated with the severity of atherosclerotic lesions 5–7. EAT is located between the outer wall of the myocardium and the visceral layer of pericardium. Almost all coronary atherosclerotic lesions occur in the segments of coronary arteries that are encased in EAT. In contrast, the arterial segments associated with the myocardial bridge, which is absent of EAT, have limited or no atherosclerotic plaques 8, 9. Furthermore, the excision of dysfunctional EAT slows the progression of porcine coronary atherosclerosis and transplantation of inflammatory perivascular adipose tissue accelerates the progression of atherosclerosis 10–12. These studies provide sufficient evidence that the inflammation-induced dysregulation of EAT plays an important role in promoting the progression of CAD. Compared with other adipose deposits, EAT displays a unique phenotype and is able to produce local inflammatory cytokines to influence the progression of CAD 13. These cytokines predominately consist of nuclear factor kappa B (NF-κB) transcription factor and the products of its target genes, including monocyte chemoattractant protein-1, interleukin (IL)-6, IL-8, IL-1β, and tumor necrosis factor (TNF)-α. Therefore, it is important to distinguish EAT from the pericardial fat, which consists of EAT and paracardial fat. The latter is located between the parietal and visceral pericardium 14, 15.
Activation of NF-κB has been implicated in physiological immunity and pathological inflammation in many different tissues and cells 16–18. The NF-κB family consists of five members: RelA (p65), NF-κB1 (p105/p50), NF-κB2 (p100), RelB and c-Rel, which share an approximately 300 amino acid long N-terminal Rel homology domain. In the resting state, the inhibitor of κB protein (IκB), IκBα, binds to NF-κB dimer through a robust nuclear export sequence. This binding masks the nuclear localization signals (NLSs) of p65 to keep the dimer in the cytoplasm. Upon activation either by canonical or non-canonical pathways, IκBα is phosphorylated which leads to rapid, signal-induced polyubiquitination and degradation by the proteasome. The liberating NF-κB complex with unmasked NLSs binds to karyopherin alpha 4 (KPNA4) (also known importin α3), a nuclear membrane protein that functions as a part of the shuttling of protein nuclear translocation 19. KPNA4- importin β complex recognizes and binds to NLSs in the NF-κB dimer, p65/p50. The reaction docks KPNA4- importin β- NF-κB complex to the cytoplasmic side of the nuclear pore complex, leading to the translocation of NF-κB dimer into the nucleus. It has been shown that activation of the NF-κB complex is facilitated predominantly by KPNA4-mediated nuclear translocation 20. The nuclear NF-κB dimer acts as a transcription factor that binds to both promoter and enhancer elements in its target genes to regulate both transcriptional activities and chromatin remodeling.
Vitamin D exerts anti-inflammatory activity and has a cardiovascular protective function 21–23, in addition to its regulation of calcium and phosphorus metabolism. Vitamin D is synthesized in the skin by ultraviolet light exposure or from diet and hydroxylated in the liver to generate 25-hydroxyvitamin D (25(OH)D), the plasma vitamin D marker. The latter is hydroxylated by 1α-hydroxylase in the proximal renal tubule or local tissues to generate VD3, a biologically active form of vitamin D. VD3 as a nuclear hormone regulates gene transcription and exerts its biological effects through binding to the vitamin D receptor (VDR). Recent human studies have shown that vitamin D deficiency is an independent risk factor associated with CAD 24–26. However, whether vitamin D deficiency directly causes the progression of CAD or vice versa remains unclear. It is possible that patients with progressive CAD reduce outdoor activity or vitamin D intake due to low appetite, leading to vitamin D deficiency. It has been shown that vitamin D signaling inhibits NF-κB activation in many kinds of cells including adipocytes 27–31. However, the exact molecular mechanism remains to be elucidated. Vitamin D deficiency increases the expression of the NF-κB target gene, monocyte chemoattractant protein-1, TNF-α, and IL-6 in swine EAT 32, and CAD patients with vitamin D deficiency have elevated inflammatory cytokines in the EAT 33. These results indicate that vitamin D signaling negatively regulates NF-κB activation in EAT. However, the molecular mechanism underlying vitamin D deficiency-enhanced NF-κB activation in EAT is largely unknown. Here we examined VD3-mediated suppression of KPNA4 expression and reduction of NF-κB activation, and a mechanistic link between the two proteins in swine epicardial preadipocytes. Furthermore, we determined whether vitamin D deficiency accelerates CAD progression by increasing nuclear NF-κB and KPNA4 levels in the EAT of swine fed an atherogenic diet.
Material and methods
Materials and Methods are available in the online-only Data.
Results
VD3 inhibits NF-κB activation
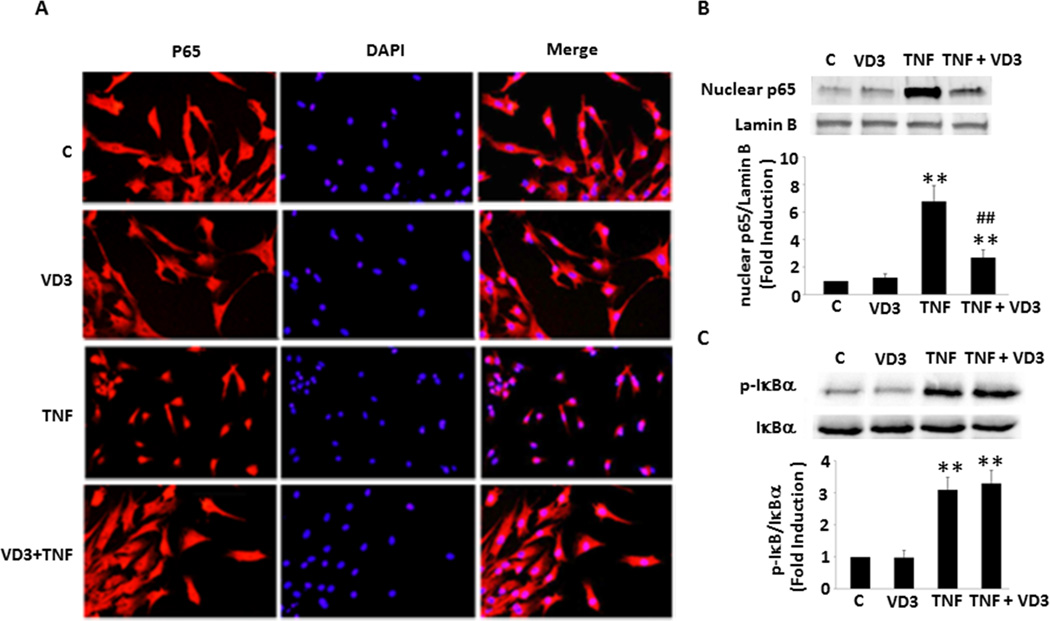
To determine the molecular mechanism by which VD3 inhibits NF-κB activation, we established a primary culture of preadipocytes from adult swine EAT. After the treatment with 10−8 mol/L VD3 (the same concentration used in all experiments in this study) for 24 hours and TNF-α (20 ng/ml, an optimum dose was used in this study, see Suppl. Figure IA) for 25 minutes, the cells were fixed for immunofluorescent assay. As shown in Figure 1A, VD3 alone had no effect on the intracellular location of p65, a major NF-κB unit, compared with control. TNF-α stimulated p65 nuclear translocation from cytoplasm, showing that almost all p65 were located in the nuclei. Treatment with VD3 prevented TNF-α stimulated p65 translocation from cytoplasm to nuclei. To confirm and quantify VD3-dependent suppression of NF-κB activation, the preadipocytes were treated as described above and the nuclear extract was prepared for Western blot analysis. As shown in Figure 1B, treatment with TNF-α resulted in a 4−7-fold increase in the levels of nuclear p65. Preincubation of VD3 had no effect on basal nuclear p65 levels, but significantly reduced TNF-α induced nuclear p65 by 65%. The nuclear translocation of p65 is essential and the critical step for NF-κB activation. These results provide convincing evidence that TNF-α stimulates NF-κB activation through cytoplasmic to nuclear translocation in the preadipocytes, which is blocked by VD3. In addition to its suppressive effect on TNF-α-stimulated activation of NF-κB, VD3 also inhibited another pro-inflammatory stimulus, IL-1β-induced NF-κB nuclear translocation (Suppl. Figure IB).
Figure 1.
VD3-dependent suppression of TNF-α-induced p65 nuclear translocation not related to IκBα in the swine epicardial preadipocytes. (A) VD3 had no effect on basal p65 localization, but prevented TNF-α-induced nuclear translocation, as determined by immunofluorescent assay. (B) Western blot confirmed immunofluorescence results shown in panel A. (C) VD3 had no effect on IκBα expression and TNF-α-induced IκBα phosphorylation. **p<0.01 vs. individual control, ##p<0.01 vs. TNF-α alone (n=3). C: control; TNF: TNF-α.
To determine whether IκB plays a potential role in VD3-mediated inhibition of NF-κB activation, we treated the preadipocytes with VD3 or/and TNF-α for different time periods. As shown in Figure 1C, treatment with TNF-α led to 4-fold increase in IκBα phosphorylation. However, VD3 had no significant effect on TNF-α-stimulated IκBα phosphorylation, suggesting that the exact molecular mechanisms remain unclear and another potential target may be responsible for the VD3 effect.
VD3 suppresses KPNA4 expression through increased VDR expression
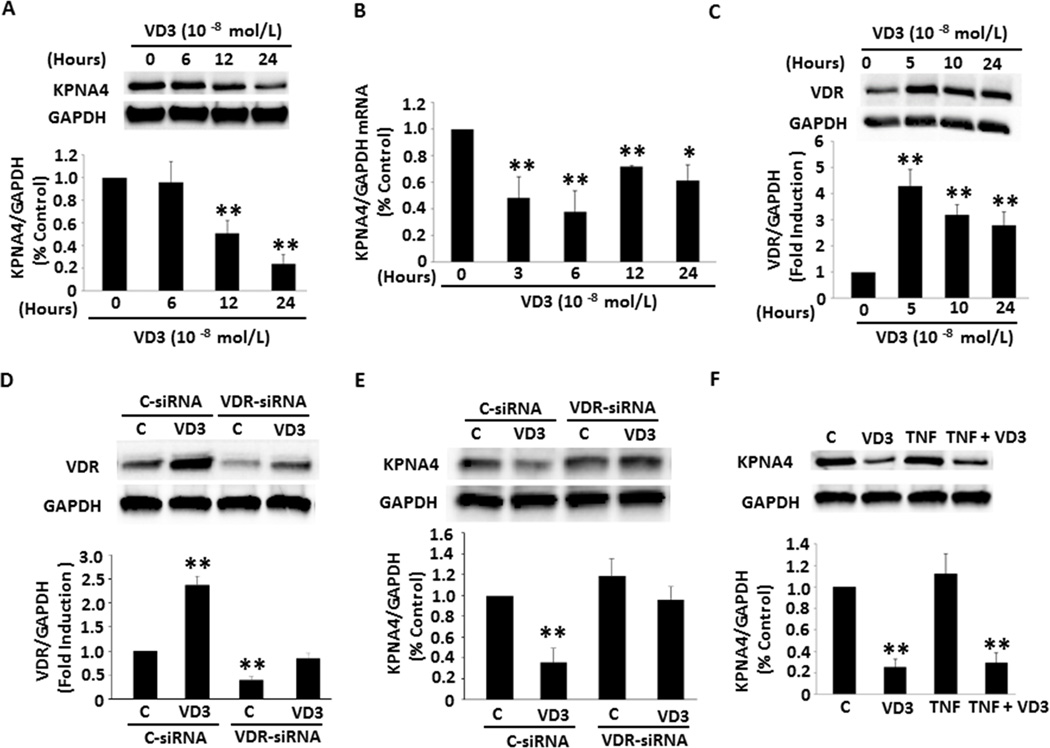
To explore the molecular mechanism regarding the suppressive role of VD3 in NF-κB activation, we focused on the nuclear membrane transporter KPNA4, a major shuttle responsible for the transportation of activated NF-κB from cytoplasm to nucleus. As demonstrated in Figure 2A, VD3 inhibited KPNA4 mRNA expression starting at 3-hour treatment through 24 hours of VD3 exposure. The maximum inhibitory effect of VD3 was ~ 70% after 6-hour incubation. The reduction of mRNA levels was accompanied with decreased KPNA4 protein expression. Treating cells with VD3 for 12 and 24 hours led to a 50% and 75% reduction of KPNA4 translation, respectively (Figure 2B). These results suggest that VD3 inhibits NF-κB activation possibly through suppression of KPNA4 transcription and translation.
Figure 2.
Effect of VD3 and TNF-α on KPNA4 signature and the role of VD3 in VDR expression. (A and B) VD3 reduced KPNA4 protein and mRNA levels. (C) VD3 increased VDR expression. (D and E) VDR siRNA significantly reduced VDR expression and VD3-induced VDR levels, and knockdown of VDR by the specific siRNA eliminated VD3 effect on KPNA4. (F) TNF-α did not affect either KPNA4 expression or VD3-mediated suppression of KPNA4. The experiments were repeated three to five times. **p<0.01, *p<0.05 vs. individual no treatment or control groups.
To examine whether VD3 amplifies VDR expression, we incubated the preadipocytes with VD3 at different time periods. As shown in Figure 2C, exposure to VD3 for 5 hours resulted in a 4-fold increase in VDR expression. VDR levels decreased with time, but maintained significantly high level compared with control throughout 24-hour treatment. To determine whether the increased VDR levels contributes to the effect of VD3 on the suppression of KPNA4 expression, we knocked down VDR expression by the specific siRNA. As expected in Figure 2D, VDR siRNA effectively blocked VDR expression and significantly reduced VD3-induced VDR levels compared with those in control siRNA. Knockdown of VDR disrupted the suppressive role of VD3 in KPNA4 expression (Figure 2E), suggesting that VD3-mediated inhibition of KPNA4 expression is VDR-dependent. VD3 can be synthesized and degraded locally in many different cells by 1-α-hydroxylase and 24-hydroxylase, respectively. To determine whether these two enzymes express in the preadipocytes, which are regulated by VD3, the cellular extracts prepared as above were detected with antibodies against these two enzymes. The expression of both enzymes was significantly lower than that of VDR. VD3 had no significant effect on 1-α-hydroxylase and 24-hydroxylase expression (Suppl. Figure IIA and B).
To explore whether prohibitin plays a potential role in the VD3-mediated anti-inflammatory process, we treated preadipocytes with VD3 or TNF-α for different time periods. Neither VD3 nor TNF-α had a significant effect on prohibitin mRNA or protein levels (Suppl. Figure IIC and D). These results ruled out the participation of prohibitin in the anti-inflammatory role of VD3 in the preadipocytes. To detect whether TNF-α exerts any effect on KPNA4 expression, we incubated the cells with TNF-α for 6, 12, and 24 hours. Western blot data showed no significant effect of TNF-α on KPNA4 protein levels (Suppl. Figure IIIA). To examine whether TNF-α rescues VD3/VDR-induced reduction of KPNA4 expression, we treated the cells with TNF-α in presence or absence of VD3. TNF-α had no significant effect on VDR expression (Suppl. Figure IIIB). As shown in Figure 2F, VD3 alone significantly decreased KPNA4 expression and the effect was not markedly changed by the combined treatment of VD3 and TNF-α. These results indicate that TNF-α–dependent activation of NF-κB is not implicated in KPNA4 expression, but VD3 prevents the translocation of activated NF-κB from cytoplasm to nuclei, which is likely due to suppression of KPNA4 expression.
VD3 reduces NF-κB activation through targeting KPNA4
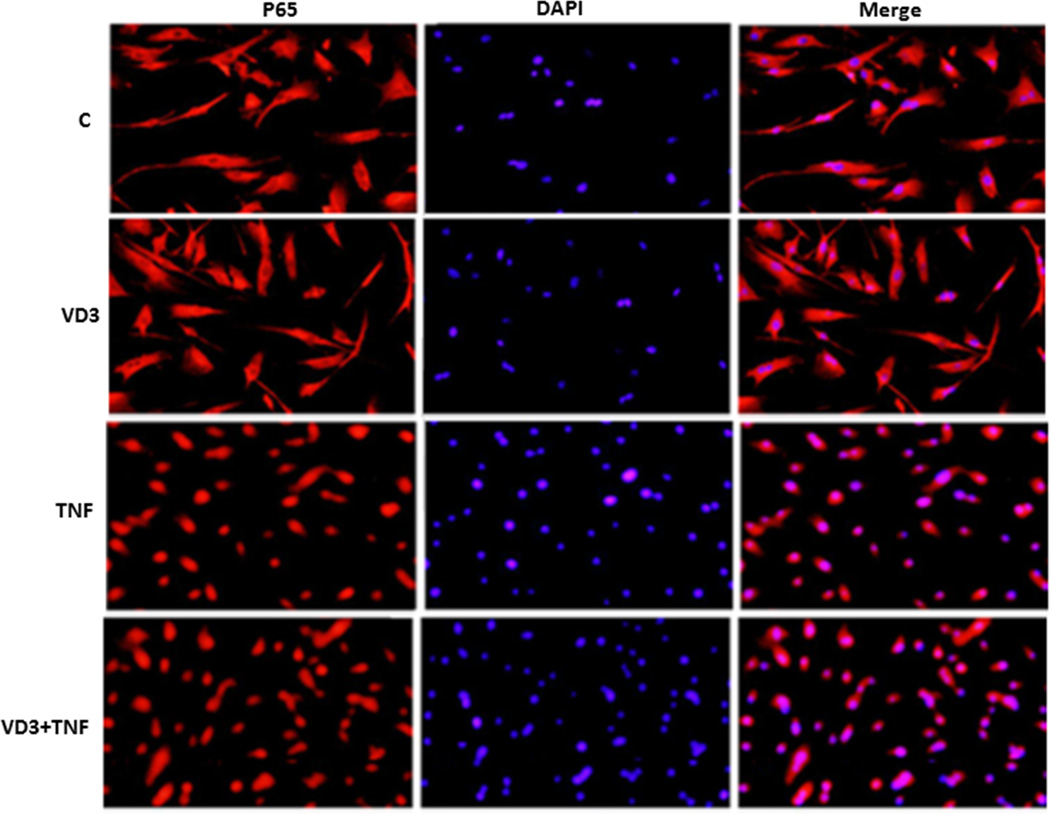
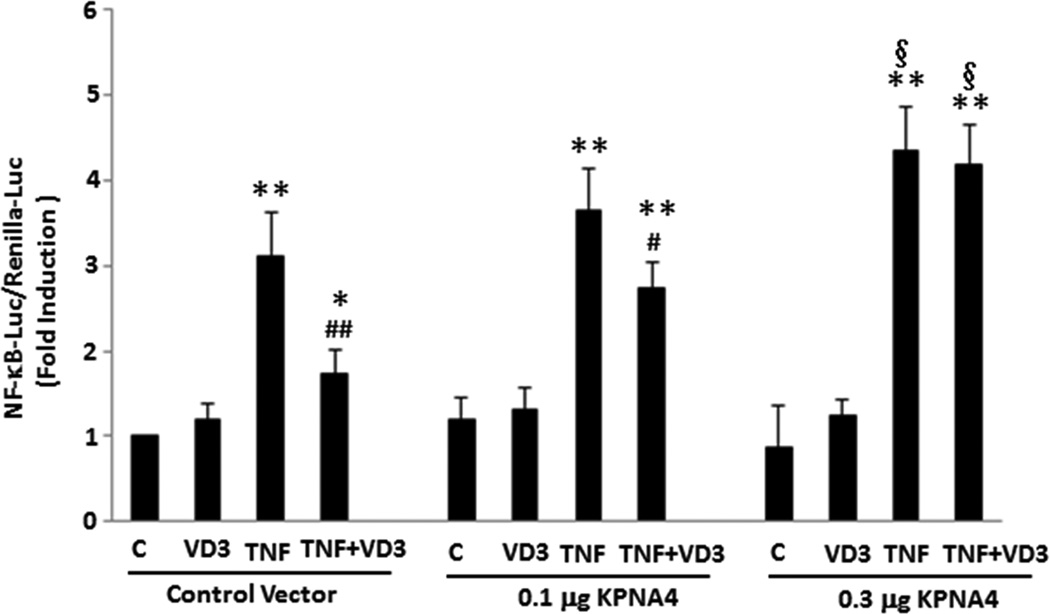
To determine a mechanistic link between vitamin D-dependent reduction of KPNA4 and suppression of NF-κB activation, we used two independent gain-of-function approaches to elucidate this question. First, we made KPNA4-lentivirus-GFP expression vector to generate the viruses, and then used the viruses to infect the preadipocytes. KPNA4-positive cells were selected by puromycin and exogenous KPNA4 expression was confirmed by the detection of GFP expression (Suppl. Figure IV). We then cultured KNPA4 positive preadipocytes, and pretreated them with VD3 for 24 hours and TNF-α for 25 minutes. Cells were then fixed for immunofluorescent assay. As shown in Figure 3, VD3 alone had no effect on the cellular distribution of p65, and TNF-α promoted p65 nuclear accumulation in KPNA4-positive cells, which were similar in wild-type cells. However, in KPNA4-positive cells, VD3 failed to block TNF-α-induced p65 nuclear translocation, suggesting that rescuing vitamin D-mediated suppression of KNPA4 levels by increased KPNA4 expression restores the ability of TNF-α-mediated NF-κB nuclear translocation in the presence of VD3. Second, to examine whether increased KPNA4 expression participates in the effect of VD3 on NF-κB function, we transfected CMV6 promoter-driven KPNA4 expression vector with NF-κB -luciferase/renilla-luciferase into preadiopocytes, then treated cells with VD3 for 24 hours and TNF-α for the final 8 hours. As shown in Figure 4, treatment with TNF-α led to a 3-fold increase in NF-κB luciferase activity normalized to renilla-luciferase activity (p < 0.01). This was significantly reduced by ~ 50% with VD3 treatment (p < 0.01), indicating that VD3 is able to block TNF-α-mediated NF-κB activation. Co-transfection with 0.1 µg of KPNA4 expression vector modestly decreased VD3-dependent inhibition of NF-κB activation by approximately 25% (p < 0.05). Increased KPNA4 expression by co-transfection with 0.3 µg of the vector led to complete recovery of vitamin D-mediated inhibition, suggesting that KPNA4 dose-dependently rescues VD3-mediated suppression of NF-κB activity. Of note, transfection of 0.3 µg of KPNA4 significantly increased TNFα-induced NF-κB luciferase activity compared with that of control vector (p < 0.05), suggesting that increased KPNA4 levels would promote available NF-κB from cytoplasm to nuclei. Taken together, these results provide a mechanistic link between VD3-mediated suppression of KPNA4 expression and NF-κB activation, and suggest that KPNA4 is a major target responsible for vitamin D-mediated suppression of NF-κB activation in the preadipocytes.
Figure 3.
VD3 failed to prevent p65 nuclear translocation induced by TNF-α in the cells with overexpression of KPNA4. The experiments were repeated three times and the representative immunofluorescent images are shown.
Figure 4.
Forced expression of KPNA4 significantly rescued VD3-dependent inhibition of NF-κB activity. The cells were transfected with NF-κB-Luc/Renilla-Luc in the presence of different doses of CMV6-KPNA4 expression vector or control vector. After 24 hours of transfection, the cells were treated with VD3 for 24 hours and TNF-α for the final 8 hours. **p<0.01, *p<0.05 vs. individual control groups. ##p<0.01, #p<0.05 vs. individual TNF-α alone groups, § p<0.05 vs. TNF-α with the control vector (n=4–6).
Vitamin D deficiency exacerbates the progression of swine CAD linked to increased KPNA4 and nuclear p65 levels in the EAT
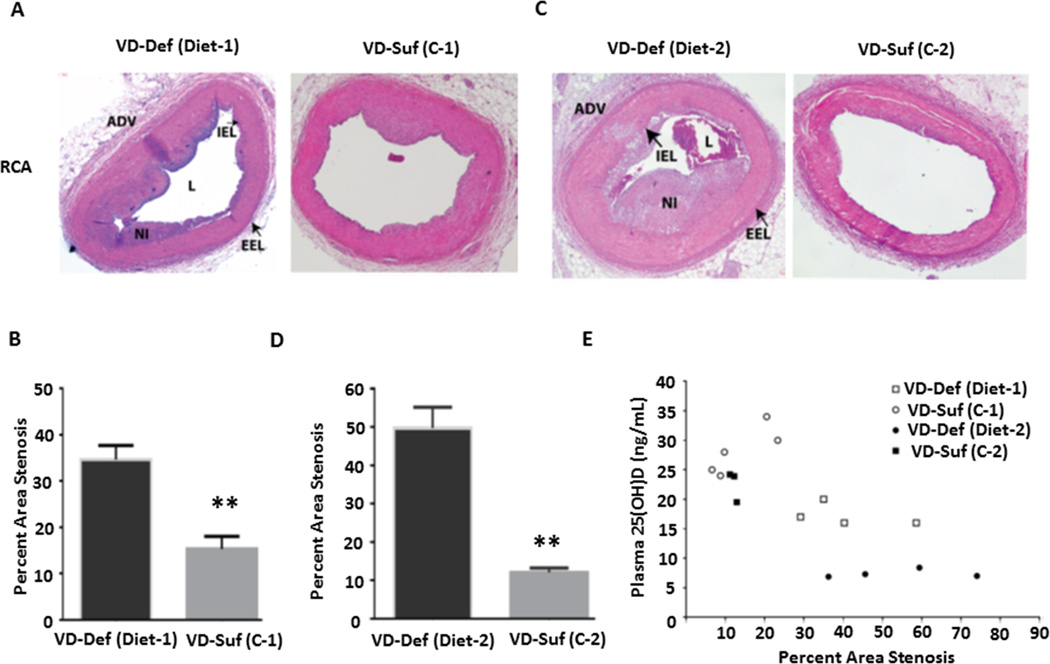
To determine a causal relationship between vitamin D deficiency and CAD progression, we fed swine with vitamin D-deficient or vitamin D-sufficient hypercholesterolemic diets for one year. In one group of animals, vitamin D-deficient hypercholesterolemic diet (Diet-1) contained about 500 IU/day of vitamin D and the sufficient control diet (C-1) contained 1,500 IU/day of vitamin D. In another group, vitamin D-deficient hypercholesterolemic diet (Diet-2) contained undetectable vitamin D levels, and the control diet (C-2) contained 1,300 IU/day of vitamin D. Just prior to euthanization, the plasma 25(OH)D levels were 17 ± 1.8 ng/ml in the vitamin D-deficient Diet-1 group vs. 28 ± 4.0 ng/ml in the vitamin D-sufficient C-1 group compared with 7.2 ± 0.7 ng/ml in the Diet-2 group vs. 23 ± 5.3 ng/ml in the C-2 group. Their age, body weight, and plasma cholesterol levels at the time of euthanization were similar between the vitamin D-deficient and control groups (Suppl. Table 1). As shown in Figure 5, administration of the vitamin D-sufficient hypercholesterolemic diets for one year led to the development of atherosclerotic plaques, which were similar between C-1 and C-2 groups (~15% vs. ~12% stenosis). The novel results here are that vitamin D deficiency accelerated CAD progression on either Diet-1 or Diet-2 versus the control diet (~35% vs. ~15% stenosis on Diet-1, p<0.01; ~50% vs. ~12% stenosis on Diet-2, p<0.01), and that as shown in Figure 5E, the severity of vitamin D deficiency appears to be causally related to the extent of CAD progression (~35% stenosis on Diet-1 in Figure 5C with 25(OH)D levels of ~17 ng/ml vs. ~ 50% stenosis on Diet-2 in Figure 5D with 25(OH)D levels at ~7 ng/ml). Furthermore, the atherosclerotic plaques were more advanced in swine fed Diet-2 (Figure 5C) compared with the plaques in swine fed Diet-1 (Figure 5A).
Figure 5.
Vitamin D deficiency accelerated the progression of CAD induced by hypercholesterolemic diets. The representative images of HE staining from swine right coronary arteries (RCA) are shown and the pooled data in the graph display the quantification of stenosis area in the RCAs from the swine fed on vitamin D-deficient Diet-1(VD-Def, n=4) and sufficient control (VD-Suf, C-1, n=5) diet (A and B) for one year, and vitamin D-deficient Diet-2 (n=4) and sufficient control (C-2) diet (n=3) (C and D) for one year. The relationship between plasma 25(OH)D level versus % stenosis in each swine is shown in (E). **p<0.01 vs. individual control group. ADV: adventitia, IEL: internal elastic lamina, L: lumen, EEL: external elastic lamina, NI: neointima.
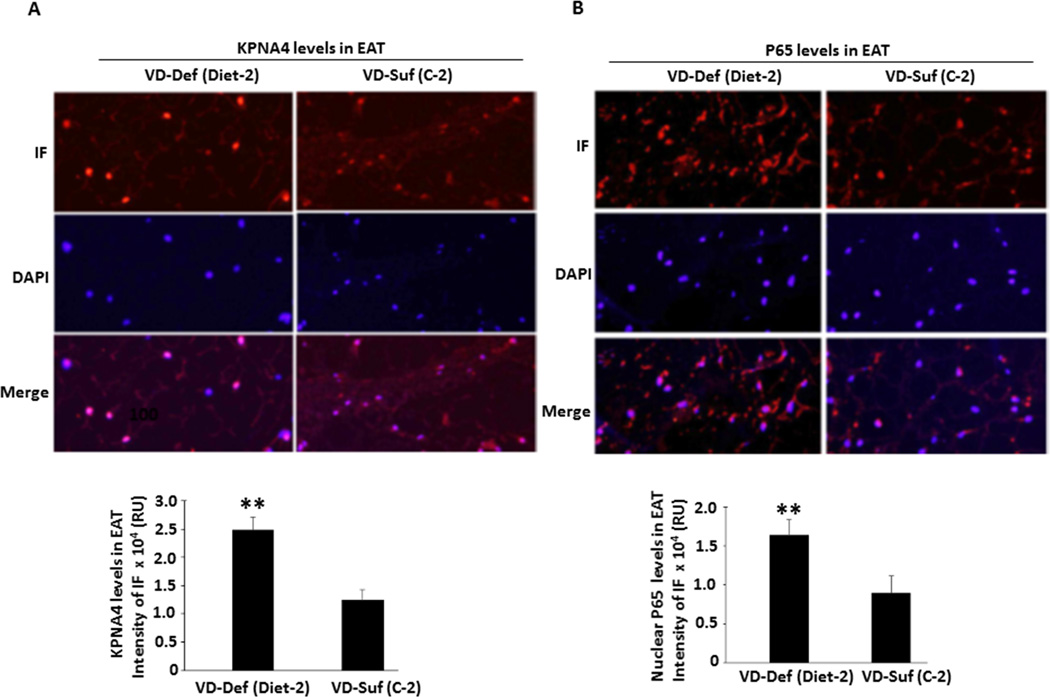
To examine whether vitamin D deficiency-dependent exacerbation of CAD progression is linked to an increase in KPNA4 and nuclear NF-κB levels in EAT, the EAT of the right coronary arteries as shown in Suppl. Figure V from the swine on Diet-2 or C-2 diet was isolated and embedded for quantitative immunofluorescent assay. The administration of vitamin D-deficient Diet-2 led to a more than 2-fold increase in KPNA4 expression (p < 0.01) and nuclear NF-κBp65 levels (p < 0.01) in the EAT compared with the levels in the EAT of vitamin D-sufficient swine (Figure 6). These results suggest that vitamin D deficiency-induced increase in KPNA4 expression promotes the transport of more activated NF-κB complexes into the nuclei, leading to NF-κB activation and an inflammatory reaction in EAT, contributing to the progression of CAD.
Figure 6.
Quantitative immunofluorescence (IF) showing increased nuclear p65 and KPNA4 levels in the EAT of swine fed on vitamin D deficient hypercholesterolemic Diet-2 vs. sufficient hypercholesterolemic diet (C-2). The experiments were performed using the sections from right coronary arteries and the EAT of three different swine from each group. KPNA4 (A) and nuclear p65 (B) levels were determined by the overlap of the staining using the specific antibodies and DAPI. The representative immunofluorescent images are shown and the graphs display the intensity of immunofluorescence measured in relative units (RU) in approximately 150 nuclei in 5–6 random fields from three animals per group. **p<0.01 vs. individual control group.
Discussion
The major findings in this study are: 1) VD3 inhibits NF-κB activation and reduces KPNA4 levels through increased VDR expression in preadipocytes from swine EAT; 2) exogenous KPNA4 rescues VD3-dependent suppression of NF-kB activation; and 3) vitamin D deficiency exacerbates the progression of swine coronary artery disease linked to increased KPNA4 and nuclear p65 levels in the EAT.
VD3 has been shown to exert anti-inflammatory activities in adipose tissue 34. Activation of NF-κB signaling plays a central role in an inflammatory reaction 17. Several studies have demonstrated that VD3 inhibits inflammation and cytokine release through suppression of NF-κB activation 27–31. However, the precise molecular mechanism remains largely unknown. In this study, we established the primary preadipocyte culture from adult swine EAT to elucidate the inhibitory effect of VD3 on TNF-α-stimulated inflammatory reaction. It has been shown that human preadipocytes produce significantly higher inflammatory adipokines than mature adipocytes 35. We found that TNF-α rapidly stimulated NF-κB translocation from cytoplasm to nuclei and drove the transcription of the target gene containing κB -response elements, and the effects were significantly blocked by VD3. Furthermore, VD3 also inhibited IL-1β-induced NF-κB activation. These findings provide strong evidence that VD3 is able to prevent the activation of the NF-κB signaling pathway in preadipocytes. Some 29, 30, but not all 31 studies have shown that VD3 reduces NF-κB activation via targeting IκBα expression or phosphorylation, a key step in NF-κB activation that releases the NF-κB complex, leading to its nuclear translocation. In our culture system, we confirmed that TNF-α stimulated phosphorylation of IκBα. However, we did not see a significant effect of VD3 on the either expression or TNF-α -stimulated phosphorylation of IκBα. While the inconsistent results might be due to different cell types or different cultured conditions, our results rule out that IκBα is a critical target responsible for VD3-dependent suppression of NF-κB activation in the preadipocytes.
Previous studies have shown that activated NF-κB is transported into nuclei predominantly by KPNA4, 18, 20 and VD3 is able to inhibit KPNA4 expression in bronchial smooth muscle cells 36. However, it remains to be elucidated whether VD3-mediated inhibition of KPNA4 is responsible for its reduction of NF-κB activity. We demonstrated that VD3 exerted its maximum inhibitory effect on KPNA4 transcription and translation after 6-hour and 24-hour incubation, respectively. VD3 induced VDR expression with a maximal effect at 5 hours and the induction was still significant, but gradually decreased with the time of treatment. The effect is temporally orchestrated with its inhibitory role in KPNA4 transcription. Furthermore, the suppressive role of VD3 in KNPA4 expression disappeared by knockdown of VDR, suggesting that VD3 inhibits KPNA4 expression through amplification of VDR levels. Preadipocytes also expressed low levels of 1α-hydoxylase and 24-hydroxylase. However, VD3 had no significant effect on their expression. These results indicate that VD3 gradually decreases its inhibitory effect on KPNA4 mRNA levels predominately associated with attenuated VDR induction rather than increase in its degradation or decrease in local VD3 synthesis.
Since KPNA4 is responsible for NF-κB transportation from cytoplasm to nuclei, a critical step for NF-κB activation, VD3-mediated suppression of KPNA4 transcription leading to impaired KPNA4 protein levels may be associated with attenuated NF-κB activation in the preadipocytes. To determine a causal link between VD3-dependent inhibition of KPNA4 expression and NF-κB activation, we performed two independent gain-of-function experiments. Our key finding in the present study is that increases in KPNA4 levels by two different exogenous expression vectors provide multiple KPNA4 shuttles for activated NF-κB transportation from cytoplasm to nuclei. This process rescues VD3-dependent suppression of TNF-α-induced NF-κB nuclear translocation and transcriptional activity. The novel finding provides a mechanistic explanation that VD3 inhibits NF-κB activation through suppression of KPNA4 expression, reducing the reserved nuclear transporter number and thus leading to the retention of accumulated free NF-κB dimer in the cytoplasm.
It remains unclear whether VD3 directly or indirectly inhibits KPNA4 transcription. Prohibitin has been shown to inhibit KPNA4 expression in intestinal epithelial cells,37 and VD3 increases prohibitin levels in bronchial smooth muscle cells 38. These studies suggest that vitamin D may suppress KPNA4 expression through upregulation of prohibitin expression in the preadipocytes. However, our data revealed no effect of VD3 on prohibitin mRNA and protein levels, suggesting that prohibitin does not mediate VD3-dependent suppression of KPNA4 expression in the swine preadipocytes. Whether VD3 negatively controls NF-κB activity through a direct binding to KPNA4 promoter needs further investigation.
Human observational study has shown that vitamin D deficiency is associated with the extent of CAD 26. However, data from the observational studies cannot answer the critical question whether vitamin D deficiency leads to CAD progression or vice versa. In addition, there are many confounders that affect the conclusion from observational studies, including participants with different ages, sexes, races, outdoor activity, vitamin D intake, obesity, histories of renal failure, stroke, hypertension, and other chronic diseases. In contrast, the anatomy and physiological function of coronary arteries in swine are similar to human and the confounding factors mentioned above can be eliminated using the swine model. We administered two groups of swine with hypercholesterolemic diets containing deficient or sufficient vitamin D for one year. All other conditions were kept the same for each swine. Our results provide a direct causal relationship between vitamin D deficiency and CAD progression. One year later, we repeated the above experiment using a severely vitamin D-deficient diet. Combining the data from the different vitamin D diets, the findings in this study show that the severity of vitamin D deficiency seemed to be causally related to CAD progression and advanced stage atherosclerotic plaques in swine fed hypercholesterolemic diets.
Although a mechanistic link between dysfunction of EAT and CAD has not been completely established, increased EAT volume resulting in the dysregulation of inflammatory adipokine release contributes to the development and progression of CAD 3, 4, 39. Studies have shown that the administration of inflammatory mediators outside the arterial adventitia leads to immune cell invasion, increases in intimal thickness, and arterial remodeling 40, 41, and increased coronary vasa vasorum neovascularization precedes endothelial dysfunction in swine fed a high-cholesterol diet 42, 43. These data suggest that local adipokines from EAT could diffuse into interstitial fluid across the adventitia into the vascular wall by paracrine factors or be directly transported into downstream coronary arteries by vasocrine factors. This hypothesis supports a notion of “outside to inside signaling of atherosclerosis”, in which the inflammatory milieu in EAT promotes the development and progression of CAD. Recently, McKenney et al. 11 clearly showed in atherosclerotic swine that EAT excision attenuated the progression of CAD, but their observation did not have a clear mechanistic link. Here we provide critical data on EAT signaling mechanisms underlying CAD. Vitamin D deficiency increases nuclear translocation of freely available cytoplasmic NF-κB complexes generated by an atherogenic diet through increased KPNA4 expression, leading to enhanced NF-κB activation in EAT, which contributes to CAD progression.
Supplementary Material
Highlights.
VD3 inhibits NF-κB activation and reduces KPNA4 levels in preadipocytes of swine EAT.
VD3 exerts its effect through increased expression of VDR in preadipocytes.
Exogenous KPNA4 rescues vitamin D3-dependent suppression of nuclear translocation and action of NF-κB.
Vitamin D deficiency exacerbates the progression of swine CAD linked to increased KPNA4 and nuclear p65 levels in the EAT.
Acknowledgments
The authors thank their colleagues in the laboratory in handling and monitoring swine at various stages of the project.
Sources of funding
This work was supported by research grants R01 HL116042 and R01 HL120659 to DK Agrawal from the National Heart, Lung and Blood Institute, NIH, USA, and by the State of Nebraska LB692 Clinical and Translational Research Grant to S Chen. The National Center for Research Resources provided support for the Creighton University Animal Resource Facility (G20RR024001).
Abbreviation list
- EAT
Epicardial adipose tissue
- CAD
Coronary artery disease
- VD3
1,25-dihydroxyvitamin D
- 25(OH)D
25-hydroxyvitamin D
- VDR
Vitamin D receptor
- KPNA4
Karyopherin alpha 4
- TNF-α
Tumor necrosis factor α
- NF-κB
Nuclear factor kappa B
- IκB
The inhibitor of κB protein
- IL
Interleukin
Footnotes
Disclosures
The authors have no conflict of interest to declare.
References
- 1.Weber C, Noels H. Atherosclerosis: Current pathogenesis and therapeutic options. Nature medicine. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 2.Iacobellis G, Malavazos AE, Corsi MM. Epicardial fat: From the biomolecular aspects to the clinical practice. The international journal of biochemistry & cell biology. 2011;43:1651–1654. doi: 10.1016/j.biocel.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O'Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 4.Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214:3–10. doi: 10.1016/j.atherosclerosis.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 5.Kim SH, Chung JH, Kwon BJ, Song SW, Choi WS. The associations of epicardial adipose tissue with coronary artery disease and coronary atherosclerosis. International heart journal. 2014;55:197–203. doi: 10.1536/ihj.13-303. [DOI] [PubMed] [Google Scholar]
- 6.Okada K, Ohshima S, Isobe S, Harada K, Hirashiki A, Funahashi H, Arai K, Hayashi D, Hayashi M, Ishii H, Murohara T. Epicardial fat volume correlates with severity of coronary artery disease in nonobese patients. Journal of cardiovascular medicine. 2014;15:384–390. doi: 10.2459/JCM.0b013e32836094da. [DOI] [PubMed] [Google Scholar]
- 7.Shimabukuro M, Hirata Y, Tabata M, Dagvasumberel M, Sato H, Kurobe H, Fukuda D, Soeki T, Kitagawa T, Takanashi S, Sata M. Epicardial adipose tissue volume and adipocytokine imbalance are strongly linked to human coronary atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1077–1084. doi: 10.1161/ATVBAHA.112.300829. [DOI] [PubMed] [Google Scholar]
- 8.Lee SS, Wu TL. The role of the mural coronary artery in prevention of coronary atherosclerosis. Archives of pathology. 1972;93:32–35. [PubMed] [Google Scholar]
- 9.Ishii T, Asuwa N, Masuda S, Ishikawa Y. The effects of a myocardial bridge on coronary atherosclerosis and ischaemia. The Journal of pathology. 1998;185:4–9. doi: 10.1002/(SICI)1096-9896(199805)185:1<4::AID-PATH50>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Manka D, Chatterjee TK, Stoll LL, Basford JE, Konaniah ES, Srinivasan R, Bogdanov VY, Tang Y, Blomkalns AL, Hui DY, Weintraub NL. Transplanted perivascular adipose tissue accelerates injury-induced neointimal hyperplasia: Role of monocyte chemoattractant protein-1. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1723–1730. doi: 10.1161/ATVBAHA.114.303983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKenney ML, Schultz KA, Boyd JH, Byrd JP, Alloosh M, Teague SD, Arce-Esquivel AA, Fain JN, Laughlin MH, Sacks HS, Sturek M. Epicardial adipose excision slows the progression of porcine coronary atherosclerosis. J Cardiothorac Surg. 2014;9:2. doi: 10.1186/1749-8090-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohman MK, Luo W, Wang H, Guo C, Abdallah W, Russo HM, Eitzman DT. Perivascular visceral adipose tissue induces atherosclerosis in apolipoprotein e deficient mice. Atherosclerosis. 2011;219:33–39. doi: 10.1016/j.atherosclerosis.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owen MK, Noblet JN, Sassoon DJ, Conteh AM, Goodwill AG, Tune JD. Perivascular adipose tissue and coronary vascular disease. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1643–1649. doi: 10.1161/ATVBAHA.114.303033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacks HS, Fain JN. Human epicardial adipose tissue: A review. Am Heart J. 2007;153:907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Bertaso AG, Bertol D, Duncan BB, Foppa M. Epicardial fat: Definition, measurements and systematic review of main outcomes. Arq Bras Cardiol. 2013;101:e18–e28. doi: 10.5935/abc.20130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayden MS, Ghosh S. Regulation of nf-kappab by tnf family cytokines. Seminars in immunology. 2014;26:253–266. doi: 10.1016/j.smim.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Killeen MJ, Linder M, Pontoniere P, Crea R. Nf-kappabeta signaling and chronic inflammatory diseases: Exploring the potential of natural products to drive new therapeutic opportunities. Drug discovery today. 2014;19:373–378. doi: 10.1016/j.drudis.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, Hunninghake GM, Vera MP, Registry M, Blackwell TS, Baron RM, Feinberg MW. Microrna-181b regulates nf-kappab-mediated vascular inflammation. The Journal of clinical investigation. 2012;122:1973–1990. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aggarwal A, Agrawal DK. Importins and exportins regulating allergic immune responses. Mediators Inflamm. 2014;2014:476357. doi: 10.1155/2014/476357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fagerlund R, Kinnunen L, Kohler M, Julkunen I, Melen K. Nf-{kappa}b is transported into the nucleus by importin {alpha}3 and importin {alpha}4. The Journal of biological chemistry. 2005;280:15942–15951. doi: 10.1074/jbc.M500814200. [DOI] [PubMed] [Google Scholar]
- 21.Arnson Y, Itzhaky D, Mosseri M, Barak V, Tzur B, Agmon-Levin N, Amital H. Vitamin d inflammatory cytokines and coronary events: A comprehensive review. Clinical reviews in allergy & immunology. 2013;45:236–247. doi: 10.1007/s12016-013-8356-0. [DOI] [PubMed] [Google Scholar]
- 22.Chen S, Law CS, Grigsby CL, Olsen K, Gardner DG. A role for the cell cycle phosphatase cdc25a in vitamin d-dependent inhibition of adult rat vascular smooth muscle cell proliferation. The Journal of steroid biochemistry and molecular biology. 2010;122:326–332. doi: 10.1016/j.jsbmb.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, Yeghiazarians Y, Gardner DG. Cardiomyocyte-specific deletion of the vitamin d receptor gene results in cardiac hypertrophy. Circulation. 2011;124:1838–1847. doi: 10.1161/CIRCULATIONAHA.111.032680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai H, Detrick B, Fishman EK, Gerstenblith G, Brinker JA, Hollis BW, Bartlett J, Cofrancesco J, Jr, Tong W, Tai H, Chen S, Bhatia S, Lai S. Vitamin d deficiency is associated with the development of subclinical coronary artery disease in african americans with hiv infection: A preliminary study. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2012;60:801–807. doi: 10.231/JIM.0b013e318250bf99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seker T, Gur M, Yuksel Kalkan G, Kuloglu O, Yildiz Koyunsever N, Yildiray Sahin D, Turkoglu C, Akyol S, Elbasan Z, Harbalioglu H, Cayli M. Serum 25-hydroxyvitamin d level and extent and complexity of coronary artery disease. Journal of clinical laboratory analysis. 2014;28:52–58. doi: 10.1002/jcla.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verdoia M, Schaffer A, Sartori C, Barbieri L, Cassetti E, Marino P, Galasso G, De Luca G. Vitamin d deficiency is independently associated with the extent of coronary artery disease. Eur J Clin Invest. 2014;44:634–642. doi: 10.1111/eci.12281. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Zhang J, Ge X, Du J, Deb DK, Li YC. Vitamin d receptor inhibits nuclear factor kappab activation by interacting with ikappab kinase beta protein. The Journal of biological chemistry. 2013;288:19450–19458. doi: 10.1074/jbc.M113.467670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deb DK, Chen Y, Zhang Z, Zhang Y, Szeto FL, Wong KE, Kong J, Li YC. 1,25-dihydroxyvitamin d3 suppresses high glucose-induced angiotensinogen expression in kidney cells by blocking the nf-{kappa}b pathway. American journal of physiology. Renal physiology. 2009;296:F1212–F1218. doi: 10.1152/ajprenal.00002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding C, Wilding JP, Bing C. 1,25-dihydroxyvitamin d3 protects against macrophage-induced activation of nfkappab and mapk signalling and chemokine release in human adipocytes. PloS one. 2013;8:e61707. doi: 10.1371/journal.pone.0061707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mutt SJ, Karhu T, Lehtonen S, Lehenkari P, Carlberg C, Saarnio J, Sebert S, Hypponen E, Jarvelin MR, Herzig KH. Inhibition of cytokine secretion from adipocytes by 1,25-dihydroxyvitamin d(3) via the nf-kappab pathway. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:4400–4407. doi: 10.1096/fj.12-210880. [DOI] [PubMed] [Google Scholar]
- 31.Tan X, Wen X, Liu Y. Paricalcitol inhibits renal inflammation by promoting vitamin d receptor-mediated sequestration of nf-kappab signaling. Journal of the American Society of Nephrology : JASN. 2008;19:1741–1752. doi: 10.1681/ASN.2007060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta GK, Agrawal T, DelCore MG, Mohiuddin SM, Agrawal DK. Vitamin d deficiency induces cardiac hypertrophy and inflammation in epicardial adipose tissue in hypercholesterolemic swine. Experimental and molecular pathology. 2012;93:82–90. doi: 10.1016/j.yexmp.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dozio E, Briganti S, Vianello E, Dogliotti G, Barassi A, Malavazos AE, Ermetici F, Morricone L, Sigruener A, Schmitz G, Romanelli MM. Epicardial adipose tissue inflammation is related to vitamin d deficiency in patients affected by coronary artery disease. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2015;25:267–273. doi: 10.1016/j.numecd.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Ding C, Gao D, Wilding J, Trayhurn P, Bing C. Vitamin d signalling in adipose tissue. The British journal of nutrition. 2012;108:1915–1923. doi: 10.1017/S0007114512003285. [DOI] [PubMed] [Google Scholar]
- 35.Gao D, Trayhurn P, Bing C. 1,25-dihydroxyvitamin d3 inhibits the cytokine-induced secretion of mcp-1 and reduces monocyte recruitment by human preadipocytes. International journal of obesity. 2013;37:357–365. doi: 10.1038/ijo.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agrawal T, Gupta GK, Agrawal DK. Calcitriol decreases expression of importin alpha3 and attenuates rela translocation in human bronchial smooth muscle cells. Journal of clinical immunology. 2012;32:1093–1103. doi: 10.1007/s10875-012-9696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theiss AL, Jenkins AK, Okoro NI, Klapproth JM, Merlin D, Sitaraman SV. Prohibitin inhibits tumor necrosis factor alpha-induced nuclear factor-kappa b nuclear translocation via the novel mechanism of decreasing importin alpha3 expression. Molecular biology of the cell. 2009;20:4412–4423. doi: 10.1091/mbc.E09-05-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agrawal T, Gupta GK, Agrawal DK. Vitamin d deficiency decreases the expression of vdr and prohibitin in the lungs of mice with allergic airway inflammation. Experimental and molecular pathology. 2012;93:74–81. doi: 10.1016/j.yexmp.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y, Cheng X, Hong K, Huang C, Wan L. How to interpret epicardial adipose tissue as a cause of coronary artery disease: A meta-analysis. Coronary artery disease. 2012;23:227–233. doi: 10.1097/MCA.0b013e328351ab2c. [DOI] [PubMed] [Google Scholar]
- 40.Shimokawa H, Ito A, Fukumoto Y, Kadokami T, Nakaike R, Sakata M, Takayanagi T, Egashira K, Takeshita A. Chronic treatment with interleukin-1 beta induces coronary intimal lesions and vasospastic responses in pigs in vivo. The role of platelet-derived growth factor. The Journal of clinical investigation. 1996;97:769–776. doi: 10.1172/JCI118476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyata K, Shimokawa H, Kandabashi T, Higo T, Morishige K, Eto Y, Egashira K, Kaibuchi K, Takeshita A. Rho-kinase is involved in macrophage-mediated formation of coronary vascular lesions in pigs in vivo. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:2351–2358. doi: 10.1161/01.atv.20.11.2351. [DOI] [PubMed] [Google Scholar]
- 42.Herrmann J, Lerman LO, Rodriguez-Porcel M, Holmes DR, Jr, Richardson DM, Ritman EL, Lerman A. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovascular research. 2001;51:762–766. doi: 10.1016/s0008-6363(01)00347-9. [DOI] [PubMed] [Google Scholar]
- 43.Kwon HM, Sangiorgi G, Ritman EL, McKenna C, Holmes DR, Jr, Schwartz RS, Lerman A. Enhanced coronary vasa vasorum neovascularization in experimental hypercholesterolemia. The Journal of clinical investigation. 1998;101:1551–1556. doi: 10.1172/JCI1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.