Abstract
Background
Sleep and depression are comorbid problems that contribute to the development of chronic medical conditions (CMC) over time. Although racial and gender differences in the bidirectional associations between sleep, depression, and CMC are known, very limited information exists on heterogeneity of the residual effects of sleep problems over depressive symptoms on CMC across race by gender groups.
Aim
Using a life-course perspective, the present study compared race by gender groups for residual effects of restless sleep over depressive symptoms on CMC.
Methods
We used data from waves 1 (year 1986), 4 (year 2001), and 5 (year 2011) of the Americans’ Changing Lives Study (ACL). The study followed 294 White men, 108 Black men, 490 White women, and 237 Black women for 25 years. Restless sleep, depressive symptoms (Center for Epidemiological Studies-Depression Scale [CES-D]) and number of chronic medical conditions (hypertension, diabetes, chronic lung disease, heart disease, stroke, cancer, and arthritis) were measured in 1986, 2001, and 2011. We employed multi-group cross-lagged modeling, with chronic medical conditions as the outcome, and race by gender as the groups.
Results
Major group differences were found in the residual effect of restless sleep on CMC over depressive symptoms across race by gender groups. Restless sleep in 2001 predicted CMC 10 years later in 2011 among Black women (Standardized Adjusted B=.135, P<.05) and White men (Standardized Adjusted =0.145, P<.01) and White women (Standardized Adjusted B=.171, P<.001) but not Black men (Standardized Adjusted B = .001, P > 0.05).
Conclusion
Race by gender heterogeneity in the residual effect of restless sleep over depressive symptoms on CMC over 25 years suggests that comorbid poor sleep and depressive symptoms differently contribute to development of multi-morbidity among subpopulations based on the intersection of race and gender. Thus, interventions that try to prevent comorbid sleep problems and depression as a strategy to prevent medical conditions may benefit from tailoring based on the intersection of race and gender.
Keywords: Additive Effect, Residual Effects, Intersectionality, Ethnic Groups, Sleep Quality, Depressive Symptoms, Chronic Medical Conditions
Background
Sleep problems (1–3) and depressive symptoms (4) are comorbid mental health problems and both contribute to development of chronic medical conditions (CMC) across populations. Race and gender may, however, alter how poor sleep (5) and depression (6,7) increase the risk of CMC at the community level. For instance, the link between poor sleep and diabetes is stronger for Whites than Blacks (5), and baseline depressive symptoms increase the risk of CMC over 25 years among Whites but not Blacks (6).
Both cross-sectional (8,9) and longitudinal (10,11) studies have documented a link between poor sleep and CMC. Reviews have shown that sleep problems tend to co-occur with a variety of CMC including cardiovascular, pulmonary, gastrointestinal, and infectious diseases (8). In a community sample of 772 individuals, those with insomnia were significantly more likely to report a CMC (9). Among community dwelling adults aged 55 to 84, 41% of those with 4 or more CMC, 22% of those with 1 to 3 conditions, and only 10% of those with no CMC reported fair or poor sleep quality. Similarly, 69% of individuals with 4 or more CMC, 52% of those with one to 3 CMC, and 36% of those with no CMC reported sleep problems (12).
Furthermore, the effects of race on sleep (5,13,14) and depression (15,16) also depend on gender. While cross-sectional studies have suggested that Black men report less nighttime awakenings but more daytime sleepiness than Black women, White men, and White women (17,18), longitudinal research has documented a higher risk of sleep disturbance over time among Black women compared to other race by gender groups (19,20). The link between depression and medical conditions also appears to depend on race and gender (21–25). For instance, major depressive disorder (MDD) is associated with higher risk of obesity among Black women, but lower risk of obesity among Black men (22). When the additive effects of anxiety and depression on obesity are considered, among Black women, lifetime generalized anxiety disorder (GAD), but not MDD, was associated with high body mass index (BMI) (24). In another study, among Whites but not Blacks, baseline depressive symptoms predicted an increase in CMC over 25 years of follow-up (6). In a nationally representative sample, lifetime MDD was associated with at least one CMC among Blacks, but not Whites (26). Because of these findings, Griffith and colleagues (27) suggested that the residual effects of sleep problems over depressive symptoms on medical conditions should be compared in race and gender groups simultaneously to better understand how the intersection of race and gender shapes these complex links across diverse populations.
There are several reasons to compare race by gender groups for the residual effects of poor sleep over depression on CMC. First and foremost, despite the well-known comorbidity between sleep and depression (28,29), very little is known about how race and gender modify the additive effects of sleep problems and depression on CMC over time. Due to the racial and gender differences in the distribution and pattern of comorbidities between sleep problems (5,13,14), depressive symptoms (30–37), and CMC (26,30,31), the residual effect of poor sleep (38) over depression (6,26) on CMC may also be race and gender dependent (6,26). Other support for this argument comes from studies suggesting that race, gender, and their intersection change the correlates of depression (6,22,24,25,26), as well as residual effects of other risk factors while depression is controlled (39–42). For instance, according to the Black-White health paradox (43,44), despite higher prevalence of CMC (30,31), Blacks are less likely to be depressed than Whites (15,16). Interestingly, the results of this literature are mixed (26,45,46), and some studies have even reported a stronger link between depression and CMC among Blacks than Whites (7,45,47). Finally, there is a need for long-term, longitudinal studies that examine potential mechanisms behind health disparities and begin to sort out potential causal ordering. Most of the literature on the moderating effects of race on the contribution of depression (6,7,26,45) and sleep (5) on CMC has used cross-sectional designs or only short-term follow-up (6,7,26,45), limiting causal inference.
In this study we applied a life-course perspective to better understand race by gender differences in the residual effects of sleep problems over depressive symptoms on CMC. Based on this approach, understanding the subsequent health of individuals requires information on cumulative and additive exposures to multiple risk factors over the course of life (48–50). This is particularly appropriate for studying racial and ethnic health disparities in the U.S. where Blacks experience higher levels of accumulation of exposures at different life stages that collectively contribute to disparities in racial differences in morbidity and mortality (50). According to this framework, CMC are the final product of a number of underlying processes that operate additively and multiplicatively across the lifespan even if CMC develops late in life (48). The major emphasis is on the relevance of previous and cumulative exposures that collectively explain subsequent distributions of the disease in the population. In this study, we hypothesize that poor sleep and depressive symptoms in mid-life will have long-term health effects that may be detectable decades later. Given prior research, we expect these effects to differ based on the intersection of race and gender.
Methods
Design and setting
Data were from wave 1 (1986), wave 4 (2001), and wave 5 (2011) of the Americans’ Changing Lives Study (ACL), a nationally-representative longitudinal study of the United States (U.S.) population conducted at the University of Michigan. Details of the study design, sampling, and data collection methods are published elsewhere (51,52).
Participants and sampling
The ACL used a stratified multistage probability sampling strategy, with oversampling of African Americans and those who are age 60 and older. In 1986, the study enrolled 3,617 community-dwelling adults age 25 or older who lived in the continental U.S. Wave 1 included 70% of sampled households and 68% of sampled individuals. Waves 4 and 5 included 74% and 81% of survivors in 2001/02, and 2011/12, respectively. The current analysis is limited to 1,129 individuals who were followed for 25 years, composed of White men (n=294), Black men (n=108), White women (n=490), and Black women (n=237). Data were collected via face-to-face interviews in the first wave, while waves 4 to 5 were conducted via either face-to-face or telephone interviews. In waves 4 and 5, for a small number of cases, when participants were unavailable for a given wave, data were collected from a proxy interviewee.
Measures
Baseline data were collected on demographic characteristics, socioeconomic status, restless sleep, depressive symptoms, and number of CMC. Sleep problems and depressive symptoms were measured at wave 1 and wave 4. Number of CMC was measured at wave 1, wave 4, and wave 5.
Socio-demographics
Demographic variables included age (continuous measure), gender (dichotomous variable with male as the reference group), and race (Black and White, with White as the reference group). Education (less than 12 years of education, high school degree or some college [reference group], and college degree or higher) was used to measure socioeconomic status. Groups were based on the intersection of gender and race.
Depressive Symptoms
Depressive symptoms were measured with a brief version of the Center for Epidemiological Studies-Depression scale (CES-D) which included 10-items (53). Items measured the extent to which in the past week respondents felt depressed, happy, lonely, sad, that everything was an effort, that people were unfriendly, that they did not feel like eating, that people dislike them, that they could not get going, and that they enjoyed life. The sleep item was removed from the depression scale and used to indicate sleep problems (below). Item responses were 1 (“hardly ever”) to 3 (“most of the time”). Positively worded items were reverse-coded, and a total score was computed across the 10 items (54–56), resulting in a continuous measure of depressive symptoms for baseline and follow-up, ranging from 10 to 30. Higher scores indicated greater severity of depressive symptoms. This abbreviated CES-D has acceptable reliability and a similar factor structure to the original version (57).
Number of Chronic Medical Conditions (CMC)
Number of CMC was measured based on self-reported data. Participants were asked whether a health care provider had ever told them they had any of the following seven CMC: hypertension, diabetes, chronic lung disease, heart disease, stroke, cancer, and arthritis. Participants were also asked if they were currently taking medication for these conditions. Based on dichotomous responses, a summed score was calculated, ranging from 0 to 7, where a higher score indicated more CMC. Thus the count of CMC at each time point (e.g. CMC 2011) was the number of all CMC a patient had at the time of interview (e.g. 2011). A detailed description of the measurement of CMC is provided in House and colleagues (52).
Restless Sleep
We used the following item to measure sleep problems: “During the past week, my sleep was restless.” Responses ranged from 1 (“hardly ever”) to 3 (“most of the time”). The same single item measure has been previously used to measure restless sleep in the absence (58,59) or presence (60–62) of other domains of sleep quality. Burgard and Ailshire in 2009 (59) and Leggett and colleagues in 2015 (58) used the same single item measure to study sleep disturbance. Self-reported restless sleep is shown to be associated with adverse sleep symptoms, sleep burden, and high risk obstructive sleep apnea (61). Restless sleep, as an important aspect of sleep quality, is now receiving increased research attention (59–61,63). Due to the longitudinal design of the study, we were able to model changes in restless sleep from baseline to follow up.
Statistical Analysis
All statistical analyses were conducted in SPSS 20.0 (IBM Corp, Armonk, NY). For univariate analyses, we reported means or frequencies (%) when appropriate. For bivariate associations, we used the Pearson correlation test, ANOVA, and Tukey test for post-hoc comparisons. For multivariate analysis, we used multi-group cross-lagged models, where groups were defined based on the intersection of race and gender.
Cross-lagged models are effective statistical methods to detect sequential effects over long periods of follow-up in the presence of repeated observations (64, 65). This approach allows for the stability of each construct over time (autoregressive paths) and also estimation of lagged effects between the independent and dependent variables over time. In all models, age, age-squared, and education were covariates. Depressive symptoms and sleep were independent variables, and number of CMC was the dependent variable. The cross-lagged model adjusts for number of CMC at each time point, so the outcome is increase in CMC compared to the earlier wave. Groups were defined based on the intersection of race and gender (6,66). Multi-group models yielded separate estimates for each group (67). Considering age square as a confounder in addition to age is an accepted practice for long term studies which focus on trajectory of health outcomes over a long period of time (68–74). This is because particularly for long follow-up periods, influence of age on health outcomes is not linear, and adding age square to the model can capture the non-linear effect (j shape or the reverse j shape) of age on health outcome.
To conduct these analyses, we used AMOS (Analysis of Moment Structures), a module in SPSS. AMOS uses Full Information Maximum Likelihood (FIML) to handle missing data (75,76). The adequacy of model fit was assessed by examining the comparative fit index (CFI), Chi-square to degree of freedom ratio (CMIN/DF), which reflects minimum discrepancy divided by its degrees of freedom, and the root mean square error of approximation (RMSEA). A CMIN/DF of less than 4, a CFI above .90, and a RMSEA value of .06 or less are indicators of good fit of the model (77,78). Considering the large sample in the present study, and the over-sensitivity of the Chi-square measure to sample size, significant Chi-square was not considered as an indicator of fit (79). A significance level of p < 0.05 was considered as statistically significant. Adjusted path coefficients with 95% confidence intervals (CI) are reported.
Results
The current analysis included 1,129 individuals who were followed for 25 years and completed surveys in wave 1, wave 4, and wave 5 of the ACL. Participants were White men (n=294), Black men (n=108), White women (n=490), and Black women (n=237).
Table 1 provides descriptive statistics for baseline age, education, CES-D, sleep problems, and CMC by race and gender at baseline and follow-up. The majority of participants (64%) were female with a mean age of 41 (SD = 11) years at baseline in 1986. Although most participants did not have any CMC at baseline, most of them had developed at least one CMC during the 25 years of follow-up. An ANOVA showed significant differences in age, age-squared, education, CES-D, sleep problems, and CMC by race and gender at baseline and follow-up. Post-hoc comparisons are shown in Table 2. Depressive symptoms were lowest among White men, with significant differences from Black men, White men and White women. The only significant difference on restless sleep was between Black women and White men (Table 2).
Table 1.
Descriptive statistics and ANOVA comparing race and gender groups
| White Male | Black Male | White Female | Black Female | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Min-max | Mean(SD) | n | Min-max | Mean(SD) | n | Min-max | Mean(SD) | n | Min-max | Mean(SD) | P | |
| Age | 294 | 25.00–70.00 | 41.27(11.56) | 108 | 25.00–68.00 | 40.39(10.34) | 490 | 25.00–75.00 | 44.59(13.11) | 237 | 24.00–71.00 | 41.47(11.87) | <.001 |
| Education | 294 | 1.00–17.00 | 13.50(2.73) | 107 | 3.00–17.00 | 12.18(2.79) | 488 | 0.00–17.00 | 12.82(2.53) | 237 | 2.00–17.00 | 11.84(2.56) | <.001 |
| CESD-10 1986 | 294 | 10.00–224.00 | 12.75(2.80) | 108 | 10.00–23.00 | 14.51(3.52) | 490 | 9.00–29.00 | 13.86(3.82) | 237 | 9.00–29.00 | 15.61(4.21) | <.001 |
| CESD-10 2001 | 256 | 8.00–24.00 | 12.11(2.61) | 74 | 9.00–23.00 | 13.20(3.14) | 435 | 9.00–25.00 | 12.82(3.26) | 159 | 10.00–28.00 | 13.91(3.66) | <.001 |
| Sleep 1986 | 292 | 1.00–3.00 | 1.60(0.65) | 108 | 1.00–3.00 | 1.61(0.65) | 490 | 1.00–3.00 | 1.69(0.72) | 236 | 1.00–3.00 | 1.78(0.72) | 0.023 |
| Sleep 2001 | 256 | 1.00–3.00 | 1.55(0.64) | 74 | 1.00–3.00 | 1.58(0.72) | 435 | 1.00–3.00 | 1.65(0.71) | 159 | 1.00–3.00 | 1.75(0.72) | 0.034 |
| CMC 1986 | 294 | 0.00–4.00 | 0.39(0.66) | 108 | 0.00–4.00 | 0.53(0.81) | 490 | 0.00–4.00 | 0.57(0.80) | 237 | 0.00–5.00 | 0.72(0.88) | <.001 |
| CMC 2001 | 256 | 0.00–4.00 | 0.93(0.95) | 75 | 0.00–4.00 | 1.00(0.93) | 436 | 0.00–5.00 | 1.05(0.93) | 159 | 0.00–5.00 | 1.33(1.11) | <.001 |
| CMC 2011 | 294 | 0.00–5.00 | 1.29(1.02) | 108 | 0.00–5.00 | 1.53(1.06) | 490 | 0.00–5.00 | 1.45(1.03) | 237 | 0.00–4.00 | 1.71(0.98) | <.001 |
Note CMC: Chronic Medical Conditions, CESD: Center for Epidemiological Studies-Depression
Table 2.
Post-hoc analysis for comparison of study variables by race and gender
| Mean difference (SE) |
Mean difference (SE) | Mean difference (SE) | Mean difference (SE) | Mean difference (SE) | ||
|---|---|---|---|---|---|---|
| Age | Education | CESD-10 1986 | Sleep 1986 | CMC 1986 | ||
| White Male | Black Male | 0.88(1.37) | 1.32(0.30)* | −1.76(0.41)* | −0.01(0.08) | −0.14(0.09) |
| White Female | −3.32(0.90)* | .68(0.19)* | −1.11(0.27)* | −0.09(0.05) | −.18(0.06)* | |
| Black Female | −0.20(1.07) | 1.67(0.23)* | −2.86(0.32)* | −.177(0.06)* | −.33(0.07)* | |
| Black Male | White Male | −0.88(1.37) | −1.32(0.30)* | 1.76(0.41)* | 0.01(0.08) | 0.14(0.09) |
| White Female | −4.21(1.30)* | −0.64(0.28) | 0.65(0.39) | −0.08(0.07) | −0.04(0.08) | |
| Black Female | −1.08(1.42) | 0.34(0.30) | −1.10(0.42)* | −0.17(0.08) | −0.19(0.09) | |
| White Female | White Male | 3.32*(0.90)* | −.68(0.19)* | 1.11(0.27)* | 0.09(0.05) | .18(0.06)* |
| Black Male | 4.21*(1.30)* | 0.64(0.28) | −0.65(0.39) | 0.08(0.07) | 0.04(0.08) | |
| Black Female | 3.13*(0.97)* | .98(0.21)* | −1.75(0.29)* | −0.09(0.06) | −0.15(0.06) | |
| Black Female | White Male | 0.20(1.07) | −1.67(0.23)* | 2.86(0.32)* | .177(0.06)* | .33(0.07)* |
| Black Male | 1.08(1.42) | −0.34(0.30) | 1.10(0.42)* | 0.17(0.08) | 0.19(0.09) | |
| White Female | −3.13(0.97)* | −.98(0.21)* | 1.75(0.29)* | 0.09(0.06) | 0.15(0.06) | |
p<0.05;
p<0.01
; p<0.001;
Table 3 shows correlations between age, education, depressive symptoms, restless sleep, and CMC for each group. Among all groups, restless sleep and depressive symptoms were correlated; however, the depressive symptoms - CMC and also restless sleep - CMC associations varied based on race and gender.
Table 3.
Bivariate correlations by race and gender
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| White Male | ||||||
| 1 Age | 1 | .992** | −.196** | −.076 | −.031 | .342** |
| 2 Age squared | 1 | −.206** | −.086 | −.036 | .335** | |
| 3 Education | 1 | −.145* | −.046 | −.182** | ||
| 4 CESD-10 1986 | 1 | .354** | .146* | |||
| 5 Sleep 1986 | 1 | .243** | ||||
| 6 CMC 1986 | 1 | |||||
| Black Male | ||||||
| 1 Age | 1 | .991** | −.262** | −.208* | −.084 | .483** |
| 2 Age squared | 1 | −.279** | −.192* | −.078 | .495** | |
| 3 Education | 1 | −.091 | −.147 | −.171 | ||
| 4 CESD-10 1986 | 1 | .537** | .091 | |||
| 5 Sleep 1986 | 1 | .126 | ||||
| 6 CMC 1986 | 1 | |||||
| White Female | ||||||
| 1 Age | 1 | .992** | −.139** | −.257** | −.024 | .344** |
| 2 Age squared | 1 | −.131** | −.249** | −.016 | .351** | |
| 3 Education | 1 | −.106* | −.112* | −.218** | ||
| 4 CESD-10 1986 | 1 | .492** | .048 | |||
| 5 Sleep 1986 | 1 | .171** | ||||
| 6 CMC 1986 | 1 | |||||
| Black Female | ||||||
| 1 Age | 1 | .991** | −.350** | −.202** | −.058 | .509** |
| 2 Age squared | 1 | −.354** | −.195** | −.054 | .511** | |
| 3 Education | 1 | −.080 | −.035 | −.397** | ||
| 4 CESD-10 1986 | 1 | .508** | −.015 | |||
| 5 Sleep 1986 | 1 | .097 | ||||
| 6 CMC 1986 | 1 |
Note CMC: Chronic Medical Conditions, CESD; Center for Epidemiological Studies-Depression
p<0.05;
p<0.01
; p<0.001;
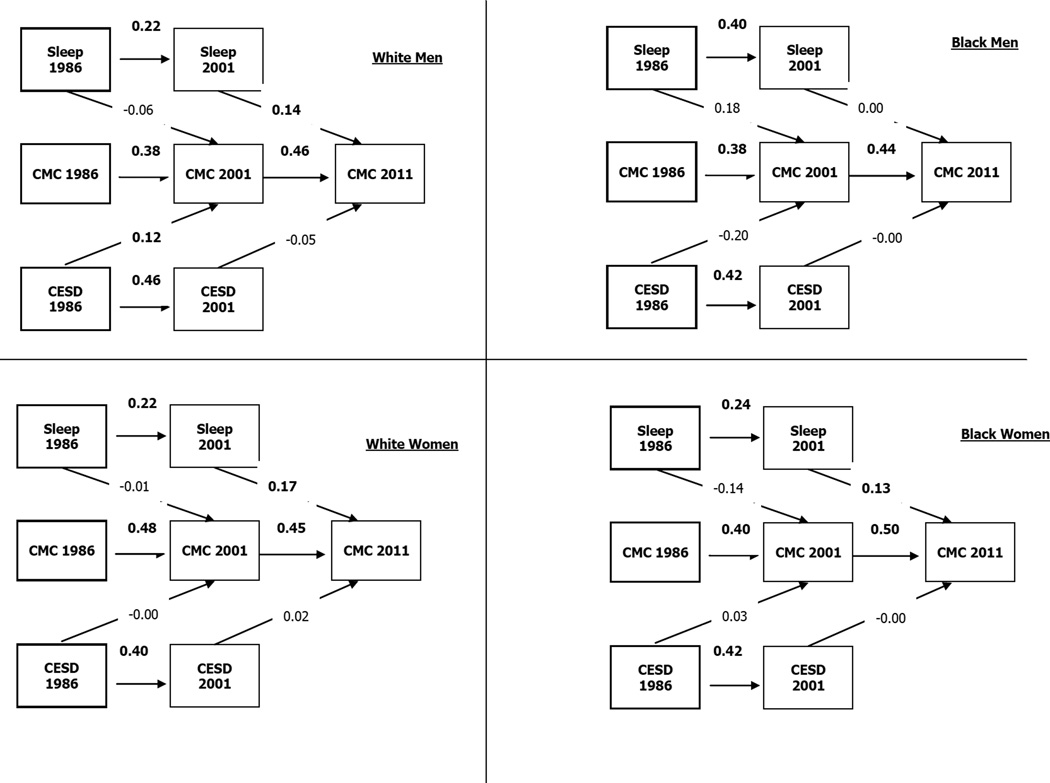
The fit of the multi-group cross-lagged model was good (Chi-square = 322.474, p = <0.001, CFI = .960, ×2/df = 4.243, RMSEA = .054, 90% CI =.048– .060). Restless sleep in 2001 predicted CMC in 2011 among all race by gender groups except among Black men. Among White men only, baseline depressive symptoms at 1986 predicted CMC in 2001. Among White women, age and age-squared were predictive of CMC in 2011 (Table 4, Figure 1-a to 1-d).
Table 4.
Summary of the cross-lagged model by race and gender
| Estimate (SE) | Estimate (SE) | Estimate (SE) | Estimate (SE) | ||
|---|---|---|---|---|---|
| White Male | Black Male | White Female | Black Female | ||
| Lagged | |||||
| Sleep 1986 | → CMC 2001 | −.061(.091) | .178(.171) | −.015(.063) | −.142(.126) |
| CESD-10 1986 | → CMC 2001 | .120(.021)* | −.196(.032) | −.005(.012) | .034(.021) |
| Sleep 2001 | → CMC 2011 | .145(.085) ** | .001(.150) | .171(.060)*** | .135(.089) * |
| CESD-10 2001 | → CMC 2011 | −.053(.021) | −.003(.034) | .023(.013) | −.002(.017) |
| Autoregressive paths | |||||
| Sleep 1986 | → Sleep 2001 | .225(.060)*** | .403(.119) *** | .221(.046) *** | .239(.077)** |
| CMC 1986 | → CMC 2001 | .384(.085) *** | .383(.117) *** | .477(.049)*** | .397(.089)*** |
| CESD-10 1986 | → CESD-10 2001 | .462(.052)*** | .418(.095)*** | .402(.038)*** | .416(.063)*** |
| CMC 2001 | → CMC 2011 | .457(.056)*** | .442(.115)*** | .449(.046)*** | .504(.055)*** |
| Covariates | |||||
| Age | → CMC 2011 | .442(.036) | −.037(.070) | .639(.025)* | .594(.035) |
| Age squared | → CMC 2011 | −.402(.000) | .045(.001) | −.583(.000) | −.643(.000) |
| Education | → CMC 2011 | −.062(.020) | −.006(.036) | −.009(.016) | −.086(.023) |
p<0.05;
p<0.01
; p<0.001;
CMC; Chronic Medical Conditions, CESD; Depression
Figure 1.
Summary of cross-lagged model by race and gender
Chi-square = 322.474, P = <0.001, CFI = .960, ×2/df = 4.243, RMSEA = .054 (90% CI =.048–.060)
Discussion
According to the findings, the intersection of race and gender modifies the residual effects of restless sleep over depressive symptoms on CMC over a 25-year period in the US. Specifically, Black men were the only group where their restless sleep in 2001 did not predict their CMC in 2011, above and beyond the effect of depressive symptoms. Thus Black men may be less vulnerable to the residual effects of restless sleep on development of CMC compared to Black women, White men, and White women, above and beyond the effect of depressive symptoms.
At baseline, poorer sleep was reported among Black compared to White men. Using data from the 2005 National Health Interview Survey (NHIS), Nunes and colleagues found that compared to Whites, Blacks less frequently report sleeping at least 7 hours and are more likely to experience short sleep duration (80). In 2011 Pigeon and colleagues found higher prevalence of sleep disturbance among Blacks compared to Whites (81). Our bivariate associations (Table 3) show a lower correlation between restless sleep and depressive symptoms (CES-D) at baseline for White men (r =.35) than Black men and Black women (r between .51 and.54) or White women (r =.49). This finding suggests that White men may not associate sleep problems with other depressive symptoms as much as other groups. That is sleep problems may be a less salient symptom in depressive episodes for White men.
The residual effect of restless sleep over depressive symptoms on CMC which was present in all other race by gender groups was absent among Black men. Based on the Black-White health paradox (6,37,43), Blacks are believed to be more resilient to the effect of mental health on physical health. According to Jackson’s hypothesis, the weaker effects of psychological stressors on mental health and CMC of Black men compared to White men may be due to engagement of Black men in negative health behaviors such as smoking or drinking to self-medicate their stressful conditions (82–84). Future research should investigate whether unhealthy coping mechanisms among Black men as hypothesized by Jackson and colleagues, explains their weakened association between mental and physical health problems, a phenomenon called the Black-White health paradox (83,84). Specifically, future research should examine if risk behaviors differently attenuate the effect of restless sleep and depression on CMC among Black men than other groups.
Our finding is not in line with the literature which suggests more severe consequences of psychological problems (such as depression and sleep) for Blacks than Whites (16,26,85). Lower access to and trust in the mental health care system and higher stigma for mental health problems among Blacks than Whites are all expected to worsen the consequences of any mental health problem among Blacks (16,26,86). If they seek care, Blacks are more likely to receive mental health treatment in primary care settings than Whites, which is known to be associated with lower quality of mental health treatments (26,87). Compared to Whites, Blacks endorse more negative beliefs regarding pharmaceutical treatment of psychological problems (16), are more likely to prefer non-pharmacologic approaches (e.g., counseling and prayer), as they more commonly believe that such medications are addictive (88). Blacks are also believed to less frequently receive a prescription for sleep problems (89). Given this background we might have expected a stronger effect of sleep on CMC for Blacks than Whites, which was not found in the current study.
Depression and poor sleep are both associated with altered inflammation and immune function (90–94). Thus, chronic inflammation and oxidative stress may be the main pathophysiological mechanism behind the effect of depression and sleep on CMC. Significant evidence has shown dysregulation of homeostatic buffering mechanisms regulating oxidation and inflammation that exist in healthy individuals in the presence of depression, sleep disorder, and CMC (95). Thus, changes in inflammation, oxidative stress and immune function may be the mechanisms that at least in part explain the effect of depression and poor sleep on CMC over time (96–100). It is still not clear if peripheral and central inflammation fully mediate the comorbidities between poor sleep, depression, and CMC (101).
The prevention, as well as identification and treatment of sleep disturbance should be considered a core element of CMC prevention strategies and also health disparity reduction programs. The absence of a relationship between restless sleep and CMC in the first follow-up period (i.e., during late mid-life) and the consistency of the restless sleep - CMC associations (except for Black men) in the second follow-up period (i.e., during older adulthood) suggests that it may be especially important to address the problem of restless sleep among older adults compared to adults at mid-life.
Unfortunately, sleep disturbance is particularly under recognized and treated among minorities. Although there are few studies on early detection and screening of poor sleep within communities, building on strategies to screen for other conditions (e.g., depressive symptoms) in community-based settings, development of similar tools to aid in the detection of sleep problems is a reasonable goal (102,103). There may be an opportunity for health coaches or lay community workers to screen, detect, and treat sleep problems in community and primary care settings, particularly for Black women. Effective interventions that improve sleep quality (104,107) may reduce the burden of CMC. Such programs in community and primary health-care settings may benefit from tailoring based on the race of the participant. Zozula et al. (108) argued that race should be considered in conducting an education program for ongoing consultation to primary care providers. The authors conducted a study to provide an educational intervention on sleep disorders for professionals at a community health clinic with high rates of minority patients. The educational intervention resulted in a four-fold increase in referrals for sleep disorder assessment (108).
The results of the current study should be interpreted in the light of four main limitations. First and foremost, restless sleep was measured using a single item with a score ranging from 1 to 3. A single sleep item may not be an adequate substitute for a multi-item measure of overall sleep disturbance (59). Future research should test the replicability of our results using multi-item measures and also other aspects of sleep quality such as sleep efficiency, latency, or disorder which may provide different results. We operationalized our predictor as increase in restless sleep from baseline to follow-up which should not be interpreted as deterioration in sleep quality over time, or chronicity of sleep problems. Such a conclusion requires more detailed information and long-term measurement of sleep problems over a long period of time, which may better reflect chronicity of sleep problems during the follow-up period. Second, measurement of CMC was limited to self-reported data with only a limited number of CMC assessed. We also did not analyze type of CMC, and sleep is known to have different effects on various medical conditions. Third, we studied symptoms of depression, not clinical depression. Fourth, due to the long duration of follow-up (25 years), bias due to mortality selection, particularly among Blacks and those with high baseline CMC cannot be ruled out. The study has strengths as well. As we used a nationally representative study with over 25-years of follow-up, our findings are generalizable to the U.S. population as a whole. In addition, the current study is one of very few studies with long-term follow-up on race differences on the additive effects of sleep and depression on the development of CMC.
To conclude, our findings suggest a differential residual effect of restless sleep over depressive symptoms on subsequent CMC 25 years based on the intersection of race and gender. Restless sleep in 2001 predicted CMC 10 years later in 2011 among Black women and White men and women but not Black men. This finding suggests that restless sleep and depressive symptoms are differently important as risk factors for development of CMC across diverse populations based on race and gender.
Acknowledgments
Funding
The Americans' Changing Lives (ACL) study was supported by Grant # AG018418 from the National Institute on Aging (DHHS/NIH), and per the NIH Public Access Policy requires that peer-reviewed research publications generated with NIH support are made available to the public through PubMed Central. NIH is not responsible for the data collection or analyses represented in this article. The ACL study was conducted by the Institute of Social Research, University of Michigan. Dr. Leggett and Dr. Pepin were supported by a National Institute of Mental Health T32 MH073553 postdoctoral fellowship. Shervin Assari is supported by the Heinz C. Prechter Bipolar Research Fund and the Richard Tam Foundation at the University of Michigan Comprehensive Depression Center.
Footnotes
Authors’ contribution:
The study was designed and drafted by Assari. Data analysis was conducted by Assari. Sonnega, Leggett, and Pepin contributed to the draft and revision. All authors approved the last version.
Conflict of Interest
Assari, Sonnega, Leggett, and Pepin declare that they have no conflicts of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all participants included in the study.
Animal Studies
No animal studies were carried out by the authors for this article.
References
- 1.Touma C, Pannain S. Does lack of sleep cause diabetes? Cleve Clin J Med. 2011;78:549–558. doi: 10.3949/ccjm.78a.10165. [DOI] [PubMed] [Google Scholar]
- 2.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71:1027–1036. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 3.Sands-Lincoln M, Grandner M, Whinnery J, Keenan BT, Jackson N, Gurubhagavatula I. The association between obstructive sleep apnea and hypertension by race/ethnicity in a nationally representative sample. J Clin Hypertens (Greenwich) 2013 Aug;15(8):593–599. doi: 10.1111/jch.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patten SB, Williams JV, Lavorato DH, Modgill G, Jetté N, Eliasziw M. Major depression as a risk factor for chronic disease incidence: longitudinal analyses in a general population cohort. Gen Hosp Psychiatry. 2008;30(5):407–413. doi: 10.1016/j.genhosppsych.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Jackson CL, Redline S, Kawachi I, Hu FB. Association between sleep duration and diabetes in black and white adults. Diabetes Care. 2013;36(11):3557–3565. doi: 10.2337/dc13-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assari S, Burgard SA, Zivin K. Long Term Reciprocal Associations between Depression and Chronic Medical Conditions; Longitudinal Support for Black-White Health Paradox. J Racial Ethn Health Disparities. 2015;2(2):1–10. 133. doi: 10.1007/s40615-015-0116-9. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez HM, Tarraf W. Comorbid cardiovascular disease and major depression among ethnic and racial groups in the United States. Int Psychogeriatr. 2013;25(5):833–841. doi: 10.1017/S1041610212002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parish JM. Sleep-related problems in common medical conditions. Chest. 2009;135(2):563–572. doi: 10.1378/chest.08-0934. [DOI] [PubMed] [Google Scholar]
- 9.Taylor DJ, Mallory LJ, Lichstein KL, Durrence H, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30(2):213. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 10.Foley DJ, Monjan A, Simonsick EM, Wallace RB, Blazer DG. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999;22(Suppl 2):S366–S372. [PubMed] [Google Scholar]
- 11.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Reviews. 2004;8(2):119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 12.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Williams LL. Factors associated with sleep disruption among community-dwelling older adults in the health and retirement study. University of Alabama at Birmingham; 2009. [Google Scholar]
- 14.Budhrani PH. Race/Ethnicity, Subjective and Objective Sleep Quality, Physical and Psychological Symptoms in Breast Cancer Survivors. 2013 [Google Scholar]
- 15.Matheson FI, Smith KL, Fazli GS, Moineddin R, Dunn JR, Glazier RH. Physical health and gender as risk factors for usage of services for mental illness. J Epidemiol Community Health. 2014;68(10):971–978. doi: 10.1136/jech-2014-203844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams DR, González HM, Neighbors H, Nesse R, Abelson JM, Sweetman J, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean Blacks, and Non-Hispanic Whites: results from the National Survey of American Life. Arch Gen Psychiatr. 2007;64(3):305–315. doi: 10.1001/archpsyc.64.3.305. [DOI] [PubMed] [Google Scholar]
- 17.Whitney CW, Enright PL, Newman AB, Bonekat W, Foley D, Quan SF. Correlates of daytime sleepiness in 4,578 elderly persons: the Cardiovascular Health Study. Sleep. 1998;21:27–36. doi: 10.1093/sleep/21.1.27. [DOI] [PubMed] [Google Scholar]
- 18.Newman AB, Enright PL, Manolio TA, Haponik EF, Wahl PW Cardiovascular Health Research Study Group. Sleep disturbance, psychosocial correlates, and cardiovascular disease in 5,201 older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 1997;45:1–7. doi: 10.1111/j.1532-5415.1997.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 19.Foley DJ, Monjan A, Simonsick EM, Wallace RB, Blazer DG. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999;22(Suppl 2):S366–S372. [PubMed] [Google Scholar]
- 20.Blazer DG, Hays JC, Foley DJ. Sleep compaints in older adults: Racial comparison. J Gerontol A Biol Sci Med Sci. 1995;50:M280–M284. doi: 10.1093/gerona/50a.5.m280. [DOI] [PubMed] [Google Scholar]
- 21.Assari S. Psychosocial Correlates of Body Mass Index in the United States: Intersection of Race, Gender and Age. Iranian Journal of Psychiatry and Behavioral Sciences. 2015 doi: 10.17795/ijpbs-3458. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Assari S. Association between obesity and depression among American Blacks: Role of Ethnicity and Gender. 2014;1(1):36–44. [Google Scholar]
- 23.Jackson JS, Knight KM, Rafferty JA. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am J Public Health. 2010;100(5):933–939. doi: 10.2105/AJPH.2008.143446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assari S. Additive Effects of Anxiety and Depression on Body Mass Index among Blacks: Role of Ethnicity and Gender. Int Cardiovasc Res J. 2014;8(2):44–51. [PMC free article] [PubMed] [Google Scholar]
- 25.Assari S, Caldwell CH. Gender and Ethnic Differences in the Association between Obesity and Depression among Black Adolescents. J Racial Ethn Health Disparities. 2015;2(3) doi: 10.1007/s40615-015-0096-9. [DOI] [PubMed] [Google Scholar]
- 26.Watkins DC, Johnson-Lawrence V, Assari S. Race and Ethnic Group Differences in Comorbid Major Depressive Disorder, Generalized Anxiety Disorder, and Chronic Medical Conditions. J Racial Ethn Health Disparities. 2015 doi: 10.1007/s40615-015-0085-z. 10.1007/s40615-015-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffith DM, Ellis KR, Allen JO. An intersectional approach to social determinants of stress for African American men: men's and women's perspectives. Am J Mens Health. 2013;7(4 Suppl):19S–30S. doi: 10.1177/1557988313480227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendlewicz J. Sleep disturbances: core symptoms of major depressive disorder rather than associated or comorbid disorders. World J Biol Psychiatry. 2009;10(4):269–275. doi: 10.3109/15622970802503086. [DOI] [PubMed] [Google Scholar]
- 29.Staner L. Comorbidity of insomnia and depression. Sleep Med Rev. 2010;14(1):35–46. doi: 10.1016/j.smrv.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Cabassa LJ, Humensky J, Druss B, Lewis-Fernández R, Gomes AP, Wang S, et al. Do race, ethnicity, and psychiatric diagnoses matter in the prevalence of multiple chronic medical conditions? Med Care. 2013;51(6):540–547. doi: 10.1097/MLR.0b013e31828dbb19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson-Lawrence VD, Griffith DM, Watkins DC. The effects of race, ethnicity and mood/anxiety disorders on the chronic physical health conditions of men from a national sample. Am J Men’s Health. 2013;7(4S):58S–67S. doi: 10.1177/1557988313484960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Assari S. Race and ethnic differences in associations between cardiovascular diseases, anxiety, and depression in the United States. Int J Travel Med Glob Health. 2014;2(3):103–109. [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor RJ, Nguyen AW, Sinkewicz M, Joe S, Chatters LM. Comorbid mood and anxiety disorders, suicidal behavior, and substance abuse among Black Caribbeans in the U.S.A. J Af Am Stud. 2012;17(4):409–425. [Google Scholar]
- 34.Alegría M, Mulvaney-Day N, Woo M, Torres M, Gao S, Oddo V. Correlates of twelve-month mental health service use among Latinos: results from the National Latino and Asian American Study (NLAAS) Am J Public Health. 2007;97(1):76–83. doi: 10.2105/AJPH.2006.087197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.González HM, Tarraf W, Whitfield KE, Vega WA. The epidemiology of major depression and ethnicity in the United States. J Psychol Res. 2010;44(15):1043–1051. doi: 10.1016/j.jpsychires.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joe S, Baser RE, Breeden G, Neighbors H, Jackson JS. Prevalence of and risk factors for lifetime suicide attempts among Blacks in the United States. J Am Med Assoc. 2006;296(17):2112–2123. doi: 10.1001/jama.296.17.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watkins DC, Hudson DL, Caldwell CH, Siefert K, Jackson JS. Discrimination, mastery, and depressive symptoms among African American men. Res Soc Work Pract. 2011;21(3):269–277. doi: 10.1177/1049731510385470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson CL, Redline S, Emmons KM. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health. 2015;18(36):417–440. doi: 10.1146/annurev-publhealth-031914-122838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Assari S, Dejman M, Neighbors HW. Ethnic Differences in Separate and Additive Effects of Anxiety and Depression on Self-rated Mental Health Among Blacks. J Racial and Ethnic Health Disparities. 2015:1–8. doi: 10.1007/s40615-015-0154-3. [DOI] [PubMed] [Google Scholar]
- 40.Assari S. Additive Effects of Anxiety and Depression on Body Mass Index among Blacks: Role of Ethnicity and Gender. Int Cardiovasc Res J. 2014;8(2):44–51. [PMC free article] [PubMed] [Google Scholar]
- 41.Lankarani MM, Lankarani RM3. Ethnicity Modifies the Additive Effects of Anxiety and Drug Use Disorders on Suicidal Ideation among Black Adults in the United States. Int J Prev Med. 2013;4(11):1251–1257. [PMC free article] [PubMed] [Google Scholar]
- 42.Assari S. Separate and Combined Effects of Anxiety, Depression and Problem Drinking on Subjective Health among Black, Hispanic and Non-Hispanic White Men. Int J Prev Med. 2014;5(3):269–279. [PMC free article] [PubMed] [Google Scholar]
- 43.Keyes CLM. The Black–White paradox in health: Flourishing in the face of social inequality and discrimination. Journal of personality. 2009;77(6):1677–1706. doi: 10.1111/j.1467-6494.2009.00597.x. [DOI] [PubMed] [Google Scholar]
- 44.Barnes DM, Keyes KM, Bates LM. Racial differences in depression in the United States: how do subgroup analyses inform a paradox? Social psychiatry and psychiatric epidemiology. 2013;48(12):1941–1949. doi: 10.1007/s00127-013-0718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Assari S. Chronic Medical Conditions and Major Depressive Disorder: Differential Role of Positive Religious Coping among African Americans, Caribbean Blacks and Non-Hispanic Whites. Int J Prev Med. 2014;5(4):405–413. [PMC free article] [PubMed] [Google Scholar]
- 46.Assari S. Chronic medical conditions and major depressive disorder: differential role of positive religious coping among African Americans, Caribbean Blacks and Non-Hispanic Whites. Int J Prev Med. 2014;5(4):405–413. [PMC free article] [PubMed] [Google Scholar]
- 47.Hankerson SH, Fenton MC, Geier T, Keyes KM, Weissman MM, Hasin DS. Racial differences in symptoms, comorbidity, and treatment for major depressive disorder among Black and White adults. J Nat Med Assoc. 2011;103(7):576–584. doi: 10.1016/s0027-9684(15)30383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuh D, Ben-Shlomo Y, editors. A life course approach to chronic disease epidemiology. Oxford: Oxford University Press; 1997. pp. 101–120. [Google Scholar]
- 49.Lumey LH. Reproductive outcomes in women prenatally exposed to undernutrition: a review of findings from the Dutch famine birth cohort. Proc Nutr Soc. 1998;57(1):129–135. doi: 10.1079/pns19980019. [DOI] [PubMed] [Google Scholar]
- 50.Davey Smith G, Hart C, Blane D, Gillis C, Hawthorne V. Lifetime socioeconomic position and mortality: prospective observational study. BMJ. 1997;314:547–552. doi: 10.1136/bmj.314.7080.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.House JS, et al. The Social Stratification of Aging and Health. Journal of Health and Social Behavior. 1994;35(3):213–234. [PubMed] [Google Scholar]
- 52.House JS, Kessler RC, Herzog AR. Age, Socioeconomic Status, and Health. Milbank Quarterly. 1990;68(3):383–411. [PubMed] [Google Scholar]
- 53.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. ApplPsychol Measure. 1977;1:385–401. [Google Scholar]
- 54.Amtmann D, Kim J, Chung H, Bamer AM, Askew RL, Wu S, Cook KF, Johnson KL. Comparing CESD-10, PHQ-9, and PROMIS depression instruments in individuals with multiple sclerosis. Rehabil Psychol. 2014;59(2):220–229. doi: 10.1037/a0035919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang W, O'Brien N, Forrest JI, Salters KA, Patterson TL, Montaner JS, Hogg RS, Lima VD. Validating a shortened depression scale (10 item CES-D) among HIV-positive people in British Columbia, Canada. PLoS One. 2012;7(7):e40793. doi: 10.1371/journal.pone.0040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 57.Burgard SA, Elliott MR, Zivin K, House JS. Working conditions and depressive symptoms: a prospective study of US adults. J Occup Environ Med. 2013;55(9):1007–1014. doi: 10.1097/JOM.0b013e3182a299af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leggett A, Burgard S, Zivin K. The Impact of Sleep Disturbance on the Association Between Stressful Life Events and Depressive Symptoms. J Gerontol B Psychol Sci Soc Sci. 2015 Sep 1; doi: 10.1093/geronb/gbv072. pii: gbv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burgard SA, Ailshire JA. Putting work to bed: stressful experiences on the job and sleep quality. J Health Soc Behav. 2009 Dec;50(4):476–492. doi: 10.1177/002214650905000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwartz J, Bottorff JL, Richardson CG. Secondhand smoke exposure, restless sleep, and sleep duration in adolescents. Sleep Disord. 2014;2014:374732. doi: 10.1155/2014/374732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fülöp T, Hickson DA, Wyatt SB, Bhagat R, Rack M, Gowdy O, Jr, Flessner MF, Taylor HA. Sleep-disordered breathing symptoms among African-Americans in the Jackson Heart Study. Sleep Med. 2012 Sep;13(8):1039–1049. doi: 10.1016/j.sleep.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kutner NG, Bliwise DL, Zhang R. Linking race and well-being within a biopsychosocial framework: variation in subjective sleep quality in two racially diverse older adult samples. J Health Soc Behav. 2004 Mar;45(1):99–113. doi: 10.1177/002214650404500107. [DOI] [PubMed] [Google Scholar]
- 63.Devins GM, Edworthy SM, Paul LC, Mandin H, Seland TP, Klein G, Costello CG, Shapiro CM. Restless sleep, illness intrusiveness, and depressive symptoms in three chronic illness conditions: rheumatoid arthritis, end-stage renal disease, and multiple sclerosis. J Psychosom Res. 1993;37(2):163–170. doi: 10.1016/0022-3999(93)90083-r. [DOI] [PubMed] [Google Scholar]
- 64.You S, Hong S, Kim EJ, Kim J. Multivariate autoregressive cross-lagged modeling of the reciprocal longitudinal relationship between perceived control and academic achievement. Psychol Rep. 2008 Jun;102(3):873–883. doi: 10.2466/pr0.102.3.873-883. [DOI] [PubMed] [Google Scholar]
- 65.Oud JHL. Continuous time modeling of reciprocal relationships in the cross-lagged panel design. In: Boker SM, Wenger MJ, editors. Data analytic techniques for dynamic systems in the social and behavioral sciences. Mahwah, NJ: Erlbaum; 2007. pp. 87–129. [Google Scholar]
- 66.Kline RB. Principles and practice of structural equation modeling. 3rd. New York: Guilford Press; 2010. [Google Scholar]
- 67.Mayer A, Nagengast B, Fletcher J, Steyer R. Analyzing average and conditional effects with multigroup multilevel structural equation models. Front Psychol. 2014;5:304. doi: 10.3389/fpsyg.2014.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kobayashi KM, Prus SG. Examining the gender, ethnicity, and age dimensions of the healthy immigrant effect: factors in the development of equitable health policy. Int J Equity Health. 2012 Feb 16;11:8. doi: 10.1186/1475-9276-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wahrendorf M, Blane D. Does labour market disadvantage help to explain why childhood circumstances are related to quality of life at older ages? Results from SHARE. Aging Ment Health. 2015 Jul;19(7):584–594. doi: 10.1080/13607863.2014.938604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, Coresh J, Knopman D, Power MC, Rawlings A, Sharrett AR, Wruck LM, Mosley TH. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014 Oct;71(10):1218–1227. doi: 10.1001/jamaneurol.2014.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang N, Bécares L, Chandola T. Does the timing of parental migration matter for child growth? A life course study on left-behind children in rural China. BMC Public Health. 2015 Sep 25;15(1):966. doi: 10.1186/s12889-015-2296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Minelli L, Pigini C, Chiavarini M, Bartolucci F. Employment status and perceived health condition: longitudinal data from Italy. BMC Public Health. 2014 Sep 12;14:946. doi: 10.1186/1471-2458-14-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roalf DR, Gur RE, Ruparel K, Calkins ME, Satterthwaite TD, Bilker WB, Hakonarson H, Harris LJ, Gur RC. Within-individual variability in neurocognitive performance: age- and sex-related differences in children and youths from ages 8 to 21. Neuropsychology. 2014 Jul;28(4):506–518. doi: 10.1037/neu0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cuypers K, Kvaløy K, Bratberg G, Midthjell K, Holmen J, Holmen TL. Being Normal Weight but Feeling Overweight in Adolescence May Affect Weight Development into Young Adulthood-An 11-Year Followup: The HUNT Study, Norway. J Obes. 2012;2012:601872. doi: 10.1155/2012/601872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Allison PD. Sage University Papers Series on Quantitative Applications in the Social Sciences, 07–136. Thousand Oaks, CA: Sage; 2002. Missing data. [Google Scholar]
- 76.Arbuckle AJ. Amos 20 user's guide. Crawfordville, FL: Amos Development; 2012. [Google Scholar]
- 77.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 78.Lei M, Lomax RG. The effect of varying degrees of nonormality in structural equation modeling. Structural Equation Modeling. 2005;12:1–27. [Google Scholar]
- 79.Lindwall M, Larsman P, Hagger MS. The reciprocal relationship between physical activity and depression in older European adults: a prospective cross-lagged panel design using SHARE data. Health Psychol. 2011;30(4):453–462. doi: 10.1037/a0023268. [DOI] [PubMed] [Google Scholar]
- 80.Nunes J, Jean-Louis G, Zizi F, Casimir GJ, von Gizycki H, Brown CD, McFarlane SI. Sleep duration among black and white Americans: results of the National Health Interview Survey. J Natl Med Assoc. 2008;100(3):317–322. doi: 10.1016/s0027-9684(15)31244-x. [DOI] [PubMed] [Google Scholar]
- 81.Pigeon WR, Heffner K, Duberstein P, Fiscella K, Moynihan J, Chapman BP. Elevated sleep disturbance among blacks in an urban family medicine practice. J Am Board Fam Med. 2011;24(2):161–168. doi: 10.3122/jabfm.2011.02.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mezuk B, Rafferty JA, Kershaw KN, Hudson D, Abdou CM, Lee H, et al. Reconsidering the role of social disadvantage in physical and mental health: stressful life events, health behaviors, race, and depression. Am J Epidemiol. 2010;172(11):1238–1249. doi: 10.1093/aje/kwq283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mezuk B, Abdou CM, Hudson D, Kershaw KN, Rafferty JA, Lee H, Jackson JS. "White Box" Epidemiology and the Social Neuroscience of Health Behaviors: The Environmental Affordances Model. Soc Ment Health. 2013;3(2) doi: 10.1177/2156869313480892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jackson JS, Knight KM. Social structures, aging, and self-regulation in the elderly. New York: Springer; 2006. Race and self-regulatory health behaviors: the role of the stress response and the HPA axis in physical and mental health disparities; pp. 189–207. [Google Scholar]
- 85.Gonzalez HM, Tarraf W. Comorbid cardiovascular disease and major depression among ethnic and racial groups in the United States. Int Psychogeriatr. 2013;25(5):833–841. doi: 10.1017/S1041610212002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Uebelacker LA, Strong D, Weinstock LM, Miller IW. Use of item response theory to understand differential functioning of DSM-IV major depression symptoms by race, ethnicity and gender. Psychol Med. 2009;39(4):591–601. doi: 10.1017/S0033291708003875. [DOI] [PubMed] [Google Scholar]
- 87.Agyemang AA, Mezuk B, Perrin P, Rybarczyk B. Quality of depression treatment in Black Americans with major depression and comorbid medical illness. Gen Hosp Psychiatry. 2014;36(4):431–436. doi: 10.1016/j.genhosppsych.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Givens JL, Katz IR, Bellamy S, Holmes WC. Stigma and the acceptability of depression treatments among African Americans and Whites. J Gen Intern Med. 2007;22(9):1292–1297. doi: 10.1007/s11606-007-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.CDC. Prescription Sleep Aid Use Among Adults: United States, 2005–2010. http://www.cdc.gov/nchs/data/databriefs/db127.htm#aid. [PubMed]
- 90.Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011;24(6):519–525. doi: 10.1097/YCO.0b013e32834b9db6. [DOI] [PubMed] [Google Scholar]
- 91.Berk M, Williams LJ, Jacka FN, O'Neil A, Pasco JA, Moylan S, Allen NB, Stuart AL, Hayley AC, Byrne ML, Maes M. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013 Sep 12;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.lavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014 May;140(3):774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13(6):467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dinan TG. Inflammatory markers in depression. Curr Opin Psychiatry. 2009;22(1):32–36. doi: 10.1097/YCO.0b013e328315a561. [DOI] [PubMed] [Google Scholar]
- 95.Rawdin BJ, Mellon SH, Dhabhar FS, Epel ES, Puterman E, Su Y, Burke HM, Reus VI, Rosser R, Hamilton SP, Nelson JC, Wolkowitz OM. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav Immun. 2013;31:143–152. doi: 10.1016/j.bbi.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martin-Subero M, Anderson G, Kanchanatawan B, Berk M, Maes M. Comorbidity between depression and inflammatory bowel disease explained by immune-inflammatory, oxidative, and nitrosative stress; tryptophan catabolite; and gut-brain pathways. CNS Spectr. 2015 Aug;26:1–15. doi: 10.1017/S1092852915000449. [DOI] [PubMed] [Google Scholar]
- 97.Jo WK, Zhang Y, Emrich HM, Dietrich DE. Glia in the cytokine-mediated onset of depression: fine tuning the immune response. Front Cell Neurosci. 2015 Jul 10;9:268. doi: 10.3389/fncel.2015.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bakunina N, Pariante CM, Zunszain PA. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology. 2015 Jan 10; doi: 10.1111/imm.12443. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ogłodek E, Szota A, Just M, Moś D, Araszkiewicz A. The role of the neuroendocrine and immune systems in the pathogenesis of depression. Pharmacol Rep. 2014;66(5):776–781. doi: 10.1016/j.pharep.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 100.Kallaur AP, Lopes J, Oliveira SR, Simão AN, Reiche EM, de Almeida ER, Morimoto HK, de Pereira WL, Alfieri DF, Borelli SD, Kaimen-Maciel DR, Maes M. Immune-Inflammatory and Oxidative and Nitrosative Stress Biomarkers of Depression Symptoms in Subjects with Multiple Sclerosis: Increased Peripheral Inflammation but Less Acute Neuroinflammation. Mol Neurobiol. 2015 Sep 24; doi: 10.1007/s12035-015-9443-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 101.Kobrosly R, van Wijngaarden E. Associations between immunologic, inflammatory, and oxidative stress markers with severity of depressive symptoms: an analysis of the 2005–2006 National Health and Nutrition Examination Survey. Neurotoxicology. 2010;31(1):126–133. doi: 10.1016/j.neuro.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 102.Shochat T, Hadas N, Kerkhofs M, Herchuelz A, Penzel T, Peter JH, Lavie P. The SleepStrip: an apnoea screener for the early detection of sleep apnoea syndrome. Eur Respir J. 2002;19(1):121–126. doi: 10.1183/09031936.02.00227302. [DOI] [PubMed] [Google Scholar]
- 103.Maurer JT. Early diagnosis of sleep related breathing disorders. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2008 Doc;7 Epub 2010 Oct 7. [PMC free article] [PubMed] [Google Scholar]
- 104.Germain A, Moul DE, Franzen PL, Miewald JM, Reynolds CF, 3rd, Monk TH, Buysse DJ. Effects of a brief behavioral treatment for late-life insomnia: preliminary findings. J Clin Sleep Med. 2006;2(4):403–406. [PubMed] [Google Scholar]
- 105.Montgomery P, Dennis J. Cognitive behavioural interventions for sleep problems in adults aged 60+ Cochrane Database Syst Rev. 2002;(2):Cd003161. doi: 10.1002/14651858.CD003161. [DOI] [PubMed] [Google Scholar]
- 106.Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151(8):1172–1180. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- 107.Murtagh DR, Greenwood KM. Identifying effective psychological treatments for insomnia: a meta-analysis. J Consult Clin Psychol. 1995;63(1):79–89. doi: 10.1037//0022-006x.63.1.79. [DOI] [PubMed] [Google Scholar]
- 108.Zozula R, Rosen RC, Jahn EG, Engel SH. Recognition of sleep disorders in a community-based setting following an educational intervention. Sleep Med. 2005;6(1):55–61. doi: 10.1016/j.sleep.2004.09.004. [DOI] [PubMed] [Google Scholar]