Abstract
Background
Race is an important predictor of TKA outcomes in the United States; however, analyses of race can be confounded by socioeconomic factors, which can result in difficulty determining the root cause of disparate outcomes after TKA.
Questions/purposes
We asked: (1) Are race and socioeconomic factors at the individual level associated with patient-reported pain and function 2 years after TKA? (2) What is the interaction between race and community poverty and patient-reported pain and function 2 years after TKA?
Methods
We identified all patients undergoing TKA enrolled in a hospital-based registry between 2007 and 2011 who provided 2-year outcomes and lived in New York, Connecticut, or New Jersey. Of patients approached to participate in the registry, more than 82% consented and provided baseline data, and of these patients, 72% provided 2-year data. Proportions of patients with complete followup at 2 years were lower among blacks (57%) than whites (74%), among patients with Medicaid insurance (51%) compared with patients without Medicaid insurance (72%), and among patients without a college education (67%) compared with those with a college education (71%). Our final study cohort consisted of 4035 patients, 3841 (95%) of whom were white and 194 (5%) of whom were black. Using geocoding, we linked individual-level registry data to US census tracts data through patient addresses. We constructed a multivariate linear mixed-effect model in multilevel frameworks to assess the interaction between race and census tract poverty on WOMAC pain and function scores 2 years after TKA. We defined a clinically important effect as 10 points on the WOMAC (which is scaled from 1 to 100 points, with higher scores being better).
Results
Race, education, patient expectations, and baseline WOMAC scores are all associated with 2-year WOMAC pain and function; however, the effect sizes were small, and below the threshold of clinical importance. Whites and blacks from census tracts with less than 10% poverty have similar levels of pain and function 2 years after TKA (WOMAC pain, 1.01 ± 1.59 points lower for blacks than for whites, p = 0.53; WOMAC function, 2.32 ± 1.56 lower for blacks than for whites, p = 0.14). WOMAC pain and function scores 2 years after TKA worsen with increasing levels of community poverty, but do so to a greater extent among blacks than whites. Disparities in pain and function between blacks and whites are evident only in the poorest communities; decreasing in a linear fashion as poverty increases. In census tracts with greater than 40% poverty, blacks score 6 ± 3 points lower (worse) than whites for WOMAC pain (p = 0.03) and 7 ± 3 points lower than whites for WOMAC function (p = 0.01).
Conclusions
Blacks and whites living in communities with little poverty have similar patient-reported TKA outcomes, whereas in communities with high levels of poverty, there are important racial disparities. Efforts to improve TKA outcomes among blacks will need to address individual- and community-level socioeconomic factors.
Level of Evidence
Level III, therapeutic study.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-016-4919-8) contains supplementary material, which is available to authorized users.
Introduction
In orthopaedics, racial disparities in the use of TKA and in outcomes such as revision and mortality have remained constant for decades [1]. Blacks in the United States experience more short-term complications and present for TKA later than whites (with more preoperative pain and worse function), and have worse long-term outcomes as measured by pain, function, and patient satisfaction [5, 13, 23, 24]. However, because blacks experience poverty disproportionately [6] and because poverty also is associated with poor TKA outcomes [8, 21], the root of the problem remains obscure.
US census tracts are small geographic areas containing approximately 4000 individuals, designed to be homogeneous with respect to population characteristics, economic status, and living conditions [25, 26]. There are 73,057 US census tracts, of which 7762 are in New York, New Jersey, and Connecticut. Census tract-level data are sensitive to gradients in health across communities [15] and capture variables that are missed at the individual level [18]. Resources linked to better health outcomes are less prevalent in neighborhoods where 20% or more of the population lives below the poverty level [7].
In the United States there are racial disparities in multiple health outcomes including the management and outcomes of cancer, diabetes, and life expectancy [7]. There also are persistent disparities in the use and outcomes of arthroplasty [23].
We assessed the association of race and socioeconomic factors on patient-reported outcomes after TKA, and analyzed the interaction between race and community poverty on those associations. To do this we linked patient-level data from a single-institution TKA registry to census tract data using geocodable addresses. Using a multivariate linear mixed effect model we assessed the interaction between race and census tract poverty level and WOMAC pain and function 2 years after TKA.
Materials and Methods
This retrospective study was conducted at a large-volume orthopaedic hospital that performs more than 4000 TKAs yearly, using a longitudinally maintained hospital institutional review board-approved TKA registry. All patients undergoing TKA between May 1, 2007, and February 25, 2011, were approached to participate in the registry, and registry data were collected at baseline and 2 years after TKA.
Of patients approached to participate in the registry, more than 82% consented to be in the registry and provided data. We included patients who provided baseline and 2-year data, had identified race, were 18 years or older, and had a geocodable address in New York, New Jersey, or Connecticut. We focused on the three states closest to our hospital to ensure a representative group of patients (not skewed toward patients traveling for boutique care). Patients were excluded if they had ICD-9 codes for fracture or were undergoing revision or bilateral TKAs [9]. We excluded patients with a contralateral TKA within 2 years of the index surgery so that the 2-year outcome data would reflect the index surgery. We excluded Asians, Pacific Islanders, and Native Americans owing to the marked socioeconomic differences between these groups and blacks and whites in the United States, and because of their small numbers. We used hospital administrative data to characterize the race of patients who did not self-report race, and used weighted Cohen’s kappa statistics to examine the agreement between administrative data with self-reported data on race.
The registry included 10,594 patients who had primary TKAs, of whom 9232 (82%) completed baseline questionnaires, and of those who returned a baseline survey, 72% returned a 2-year survey. Five thousand thirty-one patients were 18 years or older, provided a valid US address at the time of surgery, had no contralateral surgery or revision surgery during 2 years of followup, and completed a 2-year postoperative questionnaire. Five hundred thirty patients were excluded because they lived outside New York, New Jersey, or Connecticut and 276 were excluded because they did not have geocodable addresses. Our final study cohort included 4035 patients.
Those who declined to participate in the registry were older (mean age, 70 versus 67 years) and more likely female (67% versus 63%) with more comorbidities (one or more Elixhauser comorbidities, 38% versus 31%). Proportionally more blacks (7.4% versus 6.5%) and Hispanics (6% versus 3%) declined to participate in the registry compared with whites (all p values < 0.001).
Rates of followup at 2 years were lower among blacks (57%) than whites (74%), among those with Medicaid insurance (51%) compared with those without Medicaid insurance (72%), and among those without a college education (67%) compared with those with a college education (71%). When we compared the baseline characteristics of patients included in our sample with characteristics of patients lost to followup at 2 years, there were no differences in WOMAC pain (54 versus 55; p = 0.40) or function (53 versus 52; p = 0.53) among either blacks or whites. Because we did not geocode the patients without 2 years of followup, we could not compare area-based measures such as census tract poverty level in our sample with data from patients lost to followup. Fewer nonwhites (7% versus 12%; p = 0.02) and fewer Hispanic patients (0.8% versus 4%; p = 0.01) lacked geocodable addresses. More Hispanic patients did not self-report race (14% versus 4%; p = 0.02). The agreement between administrative and self-reported race was high, with a weighted Kappa of 0.72 (95% CI, 0.72–0.72), indicating substantial agreement.
After obtaining consent, we collected individual-level self-reported and administrative data at baseline, and self-reported data 2 years after TKA. Individual-level data were analyzed in univariate and multivariate models to determine predictors of TKA outcome at the individual level. We then linked the individual-level data to area-based census tract measures through geocoded addresses, constructed multivariable linear mixed-effect models, and analyzed the interaction between race and the percent of the community below the poverty level, and the association with patient-reported pain and function after TKA. The institutional ethical review board approved this study.
Baseline data collected on all registry patients included age, sex, BMI, ethnicity (non-Hispanic or Hispanic), race, insurance status (Medicare, Medicaid, or other), and education (some college or above, or no college). Patient-reported measures collected included preoperative Hospital for Special Surgery (HSS) Expectations score [20], baseline and 2-year Knee Osteoarthritis Outcomes (KOOS) pain score, and KOOS function score from which the WOMAC was derived [4]. Administrative data included Charlson-Deyo comorbidities [10], patient address, and race (if available) when not self-identified by the patient. The agreement between self-reported race and race as recorded administratively was evaluated using Cohen’s kappa statistics. Cohen’s Kappa ranges from 0 to 1, where larger numbers mean better agreement. Baseline patient expectations were measured using the HSS Total Knee Replacement Expectations Survey [20], a validated instrument that assesses patients’ preoperative expectations in areas specific to TKA, including pain relief and resumption of activities such as sports and recreation. The survey uses a 1 to 100 scale with a higher score indicating higher expectations for the outcome of surgery. A difference of 7 is considered the minimum clinically important difference (MCID) [19, 20].
We derived WOMAC pain and function scores from the KOOS questionnaire [4]. The WOMAC is a lower extremity-specific scale that has been validated for TKA; we used a 0 to 100 scale with a higher score indicating better status. The MCID for the WOMAC is considered to be a difference of greater than 10 points [3, 27].
We used geocoding of individual patients’ addresses to link registry patients to specific census tracts. We obtained census tract-level variables from the American Community Survey/US Census using the Geographic Information Systems [16]. We screened various census tract variables for use as an area-based socioeconomic measure, including the percent of the population living below the poverty level (census tract poverty), percent living alone, percent receiving Medicaid, percent older than 65 years, Gini coefficient (representing income distribution and commonly used as a measure of inequality) [1], percent single mothers, percent with insurance, median house income, percent black, and percent Hispanic.
To assess for exclusion bias in our study cohort, we compared the administrative data of those who declined to participate in the registry with those who consented to participate. We also compared the baseline characteristics of those with and without geocodable addresses and those with and without 2-year WOMAC pain and function scores. As we did not geocode patients without 2 years of followup, we have no census tract area-based measure to use in a comparison of patients in our sample with patients lost to followup.
The primary study outcome was the association of race and socioeconomic factors on patient-reported outcomes after TKA, to permit analysis of the interaction between race and community poverty on those associations. The secondary outcomes were comparisons between baseline characteristics of black and white patients at the time of TKA and differences in outcomes for black and white patients at 2-years after TKA.
Statistical Analysis
Baseline patient characteristics were summarized for all patients using descriptive statistics. Baseline characteristics of white and black patients, patients with and without self-reported race, patients with and without WOMAC pain and function scores at 2 years, and patients with and without geocodable addresses were compared using a t-test or Wilcoxon rank sum test for continuous variables and chi-square or Fisher’s exact test for categorical variables. Census tract-level variables were summarized for all census tracts using descriptive statistics. Spearman’s correlation coefficient was calculated to quantify the correlation between census tract-level variables.
A multivariate linear mixed-effect model with random intercept for each census tract was first performed by including only patient-level variables as predictors of WOMAC pain and function 2 years after TKA. We explored age, sex, BMI, race, ethnicity, education, Charlson-Deyo comorbidities, insurance status, HSS Expectations score, and baseline WOMAC pain and function for associations with WOMAC pain and function at 2 years in univariate analysis, and we included any terms identified at a probability less than 0.05 in our model to try to account for potentially confounding variables. We also included Charlson-Deyo comorbidities in our model because it was a variable of interest. The model then was expanded to include census tract-level variables mentioned above, one at a time. If a census tract-level variable was statistically significant in the model, the interaction between race and this variable was assessed. “Census tract poverty” was the area-based measure we used in our final model because it has been shown to be sensitive to gradients in health [6, 16, 17].
Our final sample included 4035 patients, of whom 3841 (95%) were white and 194 (5%) were black (Table 1). Compared with whites, blacks were younger, more likely to be female, with higher BMI and more comorbidities. Blacks had lower educational attainment and were more likely to be insured by Medicaid. The mean WOMAC pain scores at baseline and at 2 years were 6 points lower (worse) for blacks than for whites, and the mean WOMAC function scores at baseline and at 2 years were 7 points lower for blacks than for whites. However, blacks and whites experienced similar levels of improvement from baseline to 2 years. The change in WOMAC pain was 33 ± 20 for blacks versus 34 ± 22 for whites, (p = 0.75) and the change in WOMAC function was 30 ± 21 for blacks versus 32 ± 20 for whites, (p = 0.49). There was no difference in the mean HSS Expectations score. More blacks than whites lived in neighborhoods with 20% or greater of the population below the poverty level (38% versus 4%; p < 0.0001) (Table 1).
Table 1.
Characteristics of the cohort
| Characteristic | Total | White | Black | p value |
|---|---|---|---|---|
| Number of patients | 4035 (100%) | 3841 (95%) | 194 (5%) | |
| Age at surgery (years), mean (SD) | 67 (9.6) | 673 (9.5) | 65 (11) | 0.007 |
| Female | 2482 (62%) | 2331 (61%) | 151 (78%) | < 0.001 |
| BMI (kg/m2) | 30 (5.9) | 30 (5.8) | 33 (6.7) | < 0.001 |
| Hispanic | 84 (2%) | 78 (2%) | 6 (3%) | 0.30 |
| One or more comorbidities | 1135 (28%) | 1050 (27%) | 85 (44%) | < 0.001 |
| College or above | 2438 (61%) | 2352 (62%) | 86 (45%) | < 0.001 |
| Insurance payer | < 0.001 | |||
| Medicaid | 60 (2%) | 29 (0.8%) | 31 (16%) | |
| Medicare | 2535 (63%) | 2437 (63%) | 98 (50%) | |
| Other insurance | 1440 (36%) | 1375 (36%) | 65 (34%) | |
| ASA class | 0.04 | |||
| Missing | 2 | 2 | 0 | |
| I–II | 3211 (80%) | 3068 (80%) | 143 (74%) | |
| III–IV | 822 (20%) | 771 (20%) | 51 (26%) | |
| Hospital for Special Surgery Expectations score, mean (SD) | 78 (18) | 79 (18) | 76 (18) | 0.09 |
| WOMAC pain at baseline, mean (SD) | 54 (18) | 55 (18) | 49 (20) | < 0.001 |
| WOMAC pain at 2 years, mean (SD) | 87 (16) | 88 (16) | 82 (22) | 0.005 |
| Delta WOMAC pain, mean (SD) | 33 (20) | 33 (20) | 34 (22) | 0.75 |
| WOMAC function at baseline, mean (SD) | 53 (18) | 54 (18) | 47 (19) | < 0.001 |
| WOMAC function at 2 years, mean (SD) | 85 (17) | 85 (16) | 78 (22) | < 0.001 |
| Delta WOMAC function at 2 years, mean (SD) | 32 (20) | 32 (20) | 30 (21) | 0.49 |
| Census tract poverty level | < 0.001 | |||
| < 10% | 3226 (80%) | 3154 (82%) | 72 (37%) | |
| 10%–20% | 601 (15%) | 552 (14%) | 49 (25%) | |
| 20%–30% | 119 (3%) | 82 (2%) | 37 (19%) | |
| 30%–40% | 57 (1%) | 35 (0.9%) | 22 (11%) | |
| > 40% | 32 (0.8%) | 18 (0.5%) | 14 (7%) |
Patients in the registry came from a total of 1998 census tracts in New York, New Jersey, and Connecticut (of a total of 7762 census tracts in these three states) (Fig. 1). Of these census tracts, 1111 (56%) were linked to only one registry patient address, 753 (38%) were linked to two to four patient addresses, and the remaining (7%) were linked to five to 20 patient addresses (Appendix 1. Supplemental materials are available with the online version of CORR ®.).
Fig. 1.
A map of New York, New Jersey, and Connecticut shows the distribution of addresses of the study cohort in 1998 census tracts.
Ninety-five percent of included census tracts were categorized as urban. The median census tract population was 3% black (range, 0%–99%) and 9% Hispanic (range, 0%–85%). Median census tract family income was USD 86,176 (range, USD 9502–USD 250,000) (Appendix 2. Supplemental materials are available with the online version of CORR ®.). There were strong correlations at the census tract level between census tract poverty and the percentage with Medicaid insurance coverage (rho = 0.72; p < 0.001), the percentage black (rho = 0.41; p < 0.001), and the percentage Hispanic (rho = 0.48; p < 0.001), and a negative correlation with median household income (rho = −0.72; p < 0.001) (Appendix 3. Supplemental materials are available with the online version of CORR ®.). We chose census tract poverty as the area-based measure for all census tract-based analyses because it has been shown to perform as well as complex composite measures and it is sensitive to gradients in health [4, 21]. Five percent of the registry patients lived in census tract poverty level of 20% or greater. Seven percent of black registry patients and 0.5% of white registry patients lived in census tract poverty level of 40% or more of the population under the poverty level (Table 1).
Results
After controlling for potentially relevant confounding variables such as sex, race, BMI, education, insurance, baseline HSS Expectations score, and baseline WOMAC pain and function, we found that black race, having no college education, lower HSS Expectations score, and worse baseline WOMAC pain or function scores were associated with worse WOMAC pain and function scores 2 years after TKA, but the magnitude of the effects were small, and did not achieve the MCID (Table 2). For example, 2-year estimates of WOMAC pain were only 3.6 points lower for blacks than for whites, and the 2-year estimate of WOMAC function was only 4.5 points lower for blacks than for whites. A 7-point difference in baseline HSS Expectations score (the MCID) was associated with only a 0.7-point difference in WOMAC pain and a 0.8-point difference in WOMAC function at 2 years. A 10-point difference in baseline WOMAC pain score was associated with an increase of only 2 points in WOMAC pain at 2 years and an increase of 3 points in WOMAC function at 2 years. Age, sex, comorbidities, and insurance status were not associated with WOMAC pain or function at 2 years. Although more blacks than whites were insured with Medicaid, insurance status was not associated with outcomes.
Table 2.
Multivariate analysis of predictors of pain and function at 2 years
| Individual level variable | WOMAC pain at 2 years | WOMAC function at 2 years | ||
|---|---|---|---|---|
| Estimate (standard error) | p value | Estimate (standard error) | p value | |
| Age at surgery | 0.08 (0.05) | 0.10 | −0.07 (0.05) | 0.16 |
| Sex (female versus male) | −0.74 (0.60) | 0.22 | −0.24 (0.60) | 0.69 |
| BMI | 0.00 (0.05) | 0.95 | −0.05 (0.05) | 0.33 |
| Race (black versus white) | −3.55 (1.42) | 0.01 | −4.46 (1.40) | 0.002 |
| Ethnicity (Hispanic versus non-Hispanic) | −1.55 (1.97) | 0.43 | −1.01 (1.99) | 0.61 |
| Education (no college versus college or above) | −3.43 (0.62) | < 0.001 | −1.95 (0.62) | 0.002 |
| Charlson-Deyo comorbidities (≥ 1 versus 0) | −0.91 (0.66) | 0.17 | −1.26 (0.66) | 0.05 |
| Insurance (Medicare versus Medicaid) | −0.03 (2.84) | 0.99 | 0.81 (2.81) | 0.77 |
| Insurance (other versus Medicaid) | 0.87 (2.82) | 0.76 | 1.31 (2.79) | 0.64 |
| Hospital for Special Surgery Expectations score | 0.10 (0.02) | < 0.001 | 0.12 (0.02) | < 0.001 |
| WOMAC pain at baseline | 0.23 (0.02) | < 0.001 | − | − |
| WOMAC function at baseline | − | − | 0.31 (0.02) | < 0.001 |
When we incorporated census tract poverty level into our models, we found that higher census tract poverty level, having no college education, lower baseline HSS Expectations score, and worse baseline WOMAC pain scores were all associated with worse WOMAC pain scores at 2 years (Table 3); higher census tract poverty level, greater age, black race, having no college education, lower baseline HSS Expectations score, and worse baseline WOMAC function scores all were associated with worse WOMAC function scores at 2 years. However, none of these associations achieved the MCID in this outcome at 2 years. When we added an interaction term between race and census tract poverty to our models, we found a strong relationship between the two variables (Table 4). Specifically, progressively higher levels of census tract poverty were associated with a stepwise worsening of WOMAC pain and function at 2 years in all patients, but the effect was much more pronounced among blacks than whites. For example, with the other variables in the model held constant (WOMAC pain = 54; age = 67; BMI = 30; Expectations score = 78; sex = female; and comorbidities = 0), in neighborhoods with little poverty (10% of the census tract population below the poverty level, or “census tract poverty 10%”), the estimated difference in WOMAC pain scores between white and black patients was minimal (1 ± 2 points; p = 0.53). However, in communities with census tract poverty of 40%, the estimated difference was much greater (6 ± 3 points; p = 0.03). Similarly, estimated WOMAC function scores at 2 years were only 2 ± 2 lower in blacks than whites in communities with census tract poverty less than 10% (p = 0.14), but 7 ± 4 lower in communities with census tract poverty greater than 40% (p = 0.01).
Table 3.
Effect of adding census tract variables to individual-level data
| Individual level variable | WOMAC pain at 2 years | WOMAC function at 2 years | ||||||
|---|---|---|---|---|---|---|---|---|
| Individual-level data only | Census tract and individual-level data | Individual-level data only | Census tract and individual-level data | |||||
| Estimate (standard error) | p value | Estimate (standard error) | p value | Estimate (standard error) | p value | Estimate (standard error) | p value | |
| Age at surgery | 0.05 (0.03) | 0.13 | 0.09 (0.05) | 0.08 | −0.08 (0.03) | 0.009 | −0.08 (0.03) | 0.009 |
| Sex (female versus male) | −0.66 (0.60) | 0.27 | −0.63 (0.60) | 0.30 | −0.17 (0.60) | 0.78 | −0.15 (0.60) | 0.80 |
| BMI | 0.00 (0.05) | 0.95 | 0.01 (0.05) | 0.92 | −0.05 (0.05) | 0.33 | −0.05 (0.05) | 0.33 |
| Race (black versus white) | −2.00 (1.47) | 0.17 | −2.20 (1.48) | 0.14 | −3.15 (1.45) | 0.03 | −3.25 (1.45) | 0.03 |
| Education (no college versus college or above) | −2.67 (0.84) | 0.002 | −3.35 (0.62) | < 0.001 | −1.27 (0.84) | 0.13 | −1.89 (0.62) | 0.002 |
| Charlson-Deyo comorbidities (≥ 1 versus 0) | −0.85 (0.66) | 0.19 | −0.87 (0.66) | 0.18 | −1.20 (0.65) | 0.07 | −1.21 (0.66) | 0.07 |
| Hospital for Special Surgery Expectations score | 0.10 (0.02) | < 0.001 | 0.10 (0.02) | < 0.001 | 0.12 (0.02) | < 0.001 | 0.12 (0.02) | < 0.001 |
| WOMAC pain at baseline | 0.23 (0.02) | < 0.001 | 0.23 (0.02) | < 0.001 | – | – | – | – |
| WOMAC function at baseline | – | – | – | – | 0.31 (0.02) | < 0.001 | 0.31 (0.02) | < 0.001 |
| Percent below poverty level | – | – | −0.14 (0.04) | 0.001 | − | – | −0.12 (0.04) | 0.005 |
Table 4.
WOMAC pain and function 2 years after TKA: interaction between race and census-tract poverty level
| Census tract poverty level* | Race | WOMAC pain at 2 years | WOMAC function at 2 years | ||||
|---|---|---|---|---|---|---|---|
| Estimate (standard error) | Estimated difference (standard error) | p value | Estimate (standard error) | Estimated difference (standard error) | p value | ||
| 10% | Black White |
87 (2) 88 (2) |
−1 (2) | 0.53 | 83 (2) 85 (2) |
−2 (2) | 0.14 |
| 20% | Black White |
85 (2) 87 (2) |
−3 (2) | 0.07 | 81 (2) 85 (2) |
−4 (2) | 0.01 |
| 30% | Black White |
82 (2) 86 (2) |
−5 (2) | 0.03 | 78 (2) 84 (2) |
−6 (2) | 0.008 |
| 40% | Black White |
79 (3) 85 (2) |
−6 (3) | 0.03 | 76 (3) 83 (2) |
−7 (4) | 0.01 |
*Percentage of population in census tract below poverty level; estimation based on linear mixed-effect model using the following assumptions: WOMAC pain = 54; age = 67; BMI = 30 kg/m2; expectation score = 78; sex = female; comorbidities = 0.
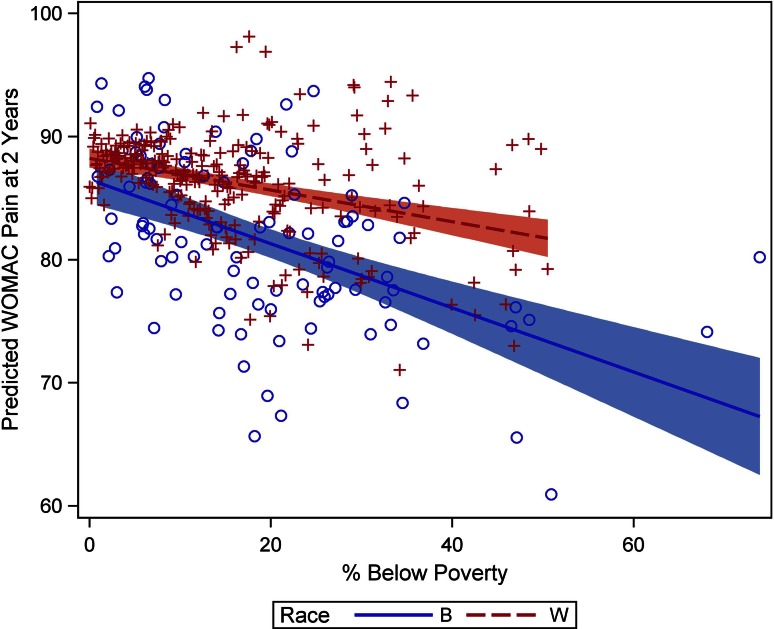
Looked at in another way, comparing blacks in communities with census tract poverty of 40% with blacks in communities with census tract poverty of 10%, WOMAC pain scores at 2 years were predicted to be 8 points lower (87 versus 79) and decrease in a linear fashion as community poverty increases, whereas whites in these two types of communities had a difference in predicted WOMAC pain scores of only 3 points (88 versus 85). Similarly, WOMAC function scores were predicted to be 7 points lower (83 versus 76) for blacks in communities with census tract poverty of 40% compared with those with census tract poverty of 10%, but only 2 points lower (85 versus 83) for whites. The relationship between 2-year WOMAC pain outcome (Fig. 2) and WOMAC function outcome (Fig. 3) and census tract poverty for blacks and for whites is shown in the scatterplots.
Fig. 2.
The scatterplot shows the relationship of 2-year WOMAC pain scores with census tract poverty for blacks and whites.
Fig. 3.
The scatterplot shows the relationship of 2-year WOMAC function scores with census tract poverty for blacks and whites.
Discussion
Racial disparities in use and outcomes of arthroplasty have persisted for decades [5, 13, 23, 24]. The extent to which this is attributable to socioeconomic differences has been difficult to discern, as poverty also is associated with poor pain and function after TKA [2]. By linking individual-level registry data that includes self-reported outcomes and individual level socioeconomic variables to area-based census tract poverty levels, we were able to analyze the interaction between race and community poverty and patient-reported outcomes after TKA. We found that although blacks have worse pain and function 2 years after TKA than whites, the difference is strongly associated with census tract poverty. Although predicted differences in WOMAC pain and function scores between blacks and whites were only 1 to 2 points among patients living in wealthy communities (census tracts poverty of 10%), differences between the races were 6 to 7 points in communities with high census tract poverty. Among blacks, 2-year WOMAC pain and function scores were predicted to be 7 to 8 points lower in those living in impoverished communities compared with those living in wealthy communities. Our study suggests not only that there is an important relationship between community poverty and patient-reported outcomes after TKA, but also that poverty had a disproportionately strong association with TKA outcomes among black patients when compared with white patients.
Our study was limited by a small number of patients living in poor communities, and a small number of blacks relative to the size of the total cohort. However, to our knowledge, this is the largest TKA cohort to date in which the association of race on patient-reported outcomes after TKA was analyzed. A systematic review of predictors of patient-reported outcomes among those who had TKAs found only seven studies that included race as an independent variable. The number of blacks in the individual studies ranged from only 22 to 101, and blacks comprised only 11% of pooled cases [11]. In addition, 95% of our cohort was categorized as urban, limiting the generalizability to rural or suburban areas.
Second, our study included only patients undergoing TKA with 2-year registry followup, thus possibly selecting for patients with better outcomes [14]. Although we found little difference in the baseline WOMAC pain and function of patients with and without 2-year followup data, we did find demographic differences. There were more blacks, more Medicaid insured, and more without any college education among the patients without 2-year surveys. At the individual level, our data indicated that being black and having less education were risk factors for poor WOMAC pain and function scores, suggesting that if anything, the findings of this study are likely to have underestimated the differences in WOMAC pain and function 2 years after TKA between blacks and whites associated with increasing community level poverty. We also excluded patients without identifiable race, but this number (n = 23) was small. In addition, patients without geocodable addresses were excluded, but fewer black and Hispanic patients than whites were excluded on this basis. At a teaching hospital such as ours, trainees more often perform surgery on Medicaid patients, but Medicaid insurance was not associated with poorer outcomes in our analysis. At the individual level, blacks undergoing TKA had worse preoperative pain and function, suggesting a delay seeking surgical treatment, and had worse outcomes at 2 years, although these differences were small and not clinically important. While we showed that race and multiple other factors including education, HSS Expectations score, and baseline pain or function scores were associated with WOMAC pain and function 2 years after TKA, the effect sizes were small.
A recent systematic review found that of 33 articles analyzing health disparities in orthopaedic patients, only three controlled for income-related and insurance-related factors in their statistical analysis [22]. A study performed in a predominantly white cohort showed that individuals of lower socioeconomic status present for arthroplasty later and have worse preoperative pain and function [15]. A British study showed that black patients have higher rates of infection- and noninfection-related complications after arthroplasty [13], but key differences in healthcare access between countries limit the generalizability of these findings to an American population. Individuals with lower socioeconomic status are more likely to have surgery performed in low-volume hospitals where outcomes may be poorer and the likelihood of complications is higher [12, 24]. Although socioeconomic status may have a greater effect on TKA outcomes than race, in our study, Medicaid insurance, which is a proxy for individual poverty, was not an independent predictor of poor outcome [23].
We showed that although blacks have worse WOMAC pain and function 2 years after TKA than whites, the difference is strongly associated with census tract poverty level. Blacks also had poorer baseline pain and functional status than whites, but this was controlled for in our model. In our model, disparities in 2-year WOMAC pain and function scores between blacks and whites are minimal when patients live in wealthy communities but are present in communities with high census tract poverty levels. Our study suggests an important association of community poverty on patient-reported outcomes after TKA, and that poverty has a disproportionately strong association with TKA outcomes among black patients than among white patients Our study is consistent with other studies [2, 7, 8, 21], showing a strong interaction between community poverty and race on health outcomes. For example, in a study analyzing the interaction of neighborhood poverty and race as they affect mortality, the effect of increasing poverty was greater for blacks than for whites. Blacks from communities with census tract poverty greater than 20% had predicted mortality rates three times higher than blacks from wealthy communities, whereas the mortality rate for whites was only 50% higher in poor areas [6]. Among blacks, community level poverty appears to be a better socioeconomic predictor of health outcomes than individual-level variables such as insurance status [3, 21].
Our study confirms the important association of socioeconomic factors, in particular community poverty level, and disparities in patient-reported outcomes after TKA in the United States. The results of our study will help institutions with surgeons performing joint arthroplasties to identify patients at the greatest risk of poor outcomes. Race and patient address are collected in all hospital administrative databases, and can be used to identify a subpopulation that can be targeted for interventions to improve their outcomes. In addition, in an era of bundled payments, recognition that blacks in high census tract poverty communities are a patient subset at high risk for poor outcomes could allow for more accurate risk adjustment by payers such as Medicare and Medicaid. More accurate risk adjustment could serve to encourage hospitals to accept these patients. Our study showed that increasing community-level poverty has a disproportionately strong association with TKA outcomes among black patients than among white patients, and also may contribute to lower use of orthopaedic surgery among patients living in impoverished communities.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Footnotes
This work was funded by the Clinical and Translational Science Center at Weill Cornell (UL1TR000457-06) (SMG), the Agency for Healthcare Research and Quality Grant U18 HS016075 (SMG), and the Block Family Foundation (SMG).
One of the authors certifies that he (MPF), or a member of his immediate family, has or may receive payments or benefits, during the study period, in an amount of USD 100,001–USD 1,000,000, from Zimmer Biomet (Warsaw, IN, USA), and an amount of USD 10,000–USD 100,000 from Lima (Arlington, TX, USA).
One of the authors certifies that he (MLP), or a member of his immediate family, has or may receive payments or benefits, during the study period, an amount of USD 10,000–USD 100,000 from Zimmer Biomet (Warsaw, IN, USA) related to this work.
One or more of the authors certify that he (JTN), or a member of his immediate family, has or may receive payments or benefits, during the study period, an amount of less than USD 10,000 from the National Center for Advancing Translational Sciences (Bethesda, MD, USA) related to this work.
One or more of the authors certify that she (Y-YL), or a member of her immediate family, has or may receive payments or benefits, during the study period, an amount of USD 10,000–USD 100,000 from the National Center for Advancing Translational Sciences ((Bethesda, MD, USA) related to this work.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
All work was performed at the Departments of Medicine and Orthopaedics at the Hospital for Special Surgery (New York, NY, USA).
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-016-4919-8) contains supplementary material, which is available to authorized users.
A comment to this article is available at http://dx.doi.org/10.1007/s11999-016-4947-4.
References
- 1.Alonge O, Peters DH. Utility and limitations of measures of health inequities: a theoretical perspective. Glob Health Action. 2015;8:27591. doi: 10.3402/gha.v8.27591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrack RL, Ruh EL, Chen J, Lombardi AV, Jr, Berend KR, Parvizi J, Della Valle CJ, Hamilton WG, Nunley RM. Impact of socioeconomic factors on outcome of total knee arthroplasty. Clin Orthop Relat Res. 2014;472:86–97. doi: 10.1007/s11999-013-3002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellamy N. Instruments to assess osteoarthritis: current status and future needs. Ann Rheum Dis. 1995;54:692–693. doi: 10.1136/ard.54.9.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellamy N. Outcome measurement in osteoarthritis clinical trials. J Rheumatol Suppl. 1995;43:49–51. [PubMed] [Google Scholar]
- 5.Cai X, Cram P, Vaughan-Sarrazin M. Are African American patients more likely to receive a total knee arthroplasty in a low-quality hospital? Clin Orthop Relat Res. 2012;470:1185–1193. doi: 10.1007/s11999-011-2032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Racial disparities in total knee replacement among Medicare enrollees–United States, 2000–2006. MMWR Morb Mortal Wkly Rep. 2009;58:133–138. [PubMed] [Google Scholar]
- 7.Chen JT, Rehkopf DH, Waterman PD, Subramanian SV, Coull BA, Cohen B, Ostrem M, Krieger N. Mapping and measuring social disparities in premature mortality: the impact of census tract poverty within and across Boston neighborhoods, 1999–2001. J Urban Health. 2006;83:1063–1084. doi: 10.1007/s11524-006-9089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clement ND, Jenkins PJ, MacDonald D, Nie YX, Patton JT, Breusch SJ, Howie CR, Biant LC. Socioeconomic status affects the Oxford knee score and short-form 12 score following total knee replacement. Bone Joint J. 2013;95:52–58. doi: 10.2106/JBJS.M.00029. [DOI] [PubMed] [Google Scholar]
- 9.Daneshvar P, Forster AJ, Dervin GF. Accuracy of administrative coding in identifying hip and knee primary replacements and revisions. J Eval Clin Pract. 2012;18:555–559. doi: 10.1111/j.1365-2753.2010.01622.x. [DOI] [PubMed] [Google Scholar]
- 10.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 11.Do DP. The dynamics of income and neighborhood context for population health: do long-term measures of socioeconomic status explain more of the black/white health disparity than single-point-in-time measures? Soc Sci Med. 2009;68:1368–1375. doi: 10.1016/j.socscimed.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman SM, McHugh K, Parks M, Figgie MP, Lee YY, Fields K, Smethurst R, Bass A. racial disparities in pain and function after total knee arthroplasty in the United States: a systematic literature review. J Rheumatol. 2016;43:765–770. doi: 10.3899/jrheum.150950. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim SA, Stone RA, Han X, Cohen P, Fine MJ, Henderson WG, Khuri SF, Kwoh CK. Racial/ethnic differences in surgical outcomes in veterans following knee or hip arthroplasty. Arthritis Rheum. 2005;52:3143–3151. doi: 10.1002/art.21304. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Lonner JH, Nelson CL, Lotke PA. Response bias: effect on outcomes evaluation by mail surveys after total knee arthroplasty. J Bone Joint Surg Am. 2004;86:15–21. doi: 10.1302/0301-620X.86B7.15255. [DOI] [PubMed] [Google Scholar]
- 15.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures the Public Health Disparities Geocoding Project. Am J Public Health. 2003;93:1655–1671. doi: 10.2105/AJPH.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95:312–323. doi: 10.2105/AJPH.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter? The Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156:471–482. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 18.Krieger N, Waterman P, Chen JT, Soobader MJ, Subramanian SV, Carson R. Zip code caveat: bias due to spatiotemporal mismatches between zip codes and US census-defined geographic areas: the Public Health Disparities Geocoding Project. Am J Public Health. 2002;92:1100–1102. doi: 10.2105/AJPH.92.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mancuso CA, Salvati EA, Johanson NA, Peterson MG, Charlson ME. Patients’ expectations and satisfaction with total hip arthroplasty. J Arthroplasty. 1997;12:387–396. doi: 10.1016/S0883-5403(97)90194-7. [DOI] [PubMed] [Google Scholar]
- 20.Mancuso CA, Sculco TP, Wickiewicz TL, Jones EC, Robbins L, Warren RF, Williams-Russo P. Patients’ expectations of knee surgery. J Bone Joint Surg Am. 2001;83:1005–1012. doi: 10.1302/0301-620X.83B7.12105. [DOI] [PubMed] [Google Scholar]
- 21.Neuburger J, Hutchings A, Black N, van der Meulen JH. Socioeconomic differences in patient-reported outcomes after a hip or knee replacement in the English National Health Service. J Public Health (Oxf). 2013;35:115–124. doi: 10.1093/pubmed/fds048. [DOI] [PubMed] [Google Scholar]
- 22.Schoenfeld AJ, Tipirneni R, Nelson JH, Carpenter JE, Iwashyna TJ. The influence of race and ethnicity on complications and mortality after orthopedic surgery: a systematic review of the literature. Med Care. 2014;52:842–851. doi: 10.1097/MLR.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 23.Singh JA, Lu X, Rosenthal GE, Ibrahim S, Cram P. Racial disparities in knee and hip total joint arthroplasty: an 18-year analysis of national Medicare data. Ann Rheum Dis. 2014;73:2107–2115. doi: 10.1136/annrheumdis-2013-203494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.SooHoo NF, Zingmond DS, Ko CY. Disparities in the utilization of high-volume hospitals for total knee replacement. J Natl Med Assoc. 2008;100:559–564. doi: 10.1016/S0027-9684(15)31303-1. [DOI] [PubMed] [Google Scholar]
- 25.Subramanian SV, Chen JT, Rehkopf DH, Waterman PD, Krieger N. Racial disparities in context: a multilevel analysis of neighborhood variations in poverty and excess mortality among black populations in Massachusetts. Am J Public Health. 2005;95:260–265. doi: 10.2105/AJPH.2003.034132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramanian SV, Chen JT, Rehkopf DH, Waterman PD, Krieger N. Comparing individual- and area-based socioeconomic measures for the surveillance of health disparities: a multilevel analysis of Massachusetts births, 1989–1991. Am J Epidemiol. 2006;164:823–834. doi: 10.1093/aje/kwj313. [DOI] [PubMed] [Google Scholar]
- 27.Wells G, Anderson J, Beaton D, Bellamy N, Boers M, Bombardier C, Breedveld F, Carr A, Cranney A, Dougados M, Felson D, Kirwan J, Schiff M, Shea B, Simon L, Smolen J, Strand V, Tugwell P, van Riel P, Welch VA. Minimal clinically important difference module: summary, recommendations, and research agenda. J Rheumatol. 2001;28:452–454. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.