Abstract
Background
Individuals with osteoarthritis (OA) of the lower limb find normal locomotion tiring compared with individuals without OA, possibly because OA of any lower limb joint changes limb mechanics and may disrupt transfer of potential and kinetic energy of the center of mass during walking, resulting in increased locomotor costs. Although recovery has been explored in asymptomatic individuals and in some patient populations, the effect of changes in these gait parameters on center of mass movements and mechanical work in patients with OA in specific joints has not been well examined. The results can be used to inform clinical interventions and rehabilitation that focus on improving energy recovery.
Questions/purposes
We hypothesized that (1) individuals with end-stage lower extremity OA would exhibit a decrease in walking velocity compared with asymptomatic individuals and that the joint affected with OA would differntially influence walking velocity, (2) individuals with end-stage lower extremity OA would show decreased energy recovery compared with asymptomatic individuals and that individuals with end-stage hip and ankle OA would have greater reductions in recovery than would individuals with end-stage knee OA owing to restrictions in hip and ankle motion, and (3) that differences in the amplitude and congruity of the center of mass would explain the differences in energy recovery that are observed in each population.
Methods
Ground reaction forces at a range of self-selected walking speeds were collected from individuals with end-stage radiographic hip OA (n = 27; 14 males, 13 females; average age, 55.6 years; range, 41–70 years), knee OA (n = 20; seven males, 13 females; average age, 61.7 years; range, 49–74 years), ankle OA (n = 30; 14 males, 16 females; average age, 57 years; range, 45–70 years), and asymptomatic individuals (n = 13; eight males, five females; average age, 49.8 years; range, 41–67 years). Participants were all patients with end-stage OA who were scheduled to have joint replacement surgery within 4 weeks of testing. All patients were identified by the orthopaedic surgeon as having end-stage radiographic disease and to be a candidate for joint replacement surgery. Patients were excluded if they had pain at any other lower extremity joint, previous joint replacement surgery, or needed to use an assistive device for ambulation. Patients were enrolled if they met the study inclusion criteria. Our study was comparative and cohorts could be compared with each other, however, the asymptomatic group served to verify our methods and provided a recovery standard with which we could compare our patients. Potential and kinetic energy relationships (% congruity) and energy exchange (% recovery) were calculated. Linear regressions were used to examine the effect of congruity and amplitude of energy fluctuations and walking velocity on % recovery. Analysis of covariance was used to compare energy recovery between groups.
Results
The results of this study support our hypothesis that individuals with OA walk at a slower velocity than asymptomatic individuals (1.4 ± 0.2 m/second, 1.2–1.5 m/second) and that the joint affected by OA also affects walking velocity (p < 0.0001). The cohort with ankle OA (0.9 ± 0.2 m/second, 0.77–0.94 m/second) walked at a slower speed relative to the cohort with hip OA (1.1 ± 0.2 m/second, 0.96–1.1 m/second; p = 0.002). However, when comparing the cohorts with ankle and knee OA (0.9 ± 0.2 m/second, 0.77–0.94 m/second) there was no difference in walking speed (p = 0.16) and the same was true when comparing the cohorts with knee and hip OA (p = 0.14). Differences in energy recovery existed when comparing the OA cohorts with the asymptomatic cohort and when examining differences between the OA cohorts. After adjusting for walking speeds these results showed that asymptomatic individuals (65% ± 3%, 63%–67%) had greater recovery than individuals with hip OA (54% ± 10%, 50%–58%; p = 0.014) and ankle OA (47% ± 13%, 40%–52%; p = 0.002) but were not different compared with individuals with knee OA (57% ± 10%, 53%–62%; p = 0.762). When speed was accounted for, 80% of the variation in recovery not attributable to speed was explained by congruity with only 10% being explained by amplitude.
Conclusions
OA in the hip, knee, or ankle reduces effective exchange of potential and kinetic energy, potentially increasing the muscular work required to control movements of the center of mass.
Clinical Relevance
The fatigue and limited physical activity reported in patients with lower extremity OA could be associated with increased mechanical work of the center of mass. Focused gait retraining potentially could improve walking mechanics and decrease fatigue in these patients.
Introduction
Lower limb osteoarthritis (OA), affecting the knee, hip, and ankle, is a debilitating disease that affects millions of people older than 45 years in the United States [8, 16, 26]. People affected by OA report general pain and fatigue after daily activities and walk at reduced speeds relative to asymptomatic adults [3, 24–27, 29], Fatigue related to hip and knee OA also is associated with higher cardiac and ventilatory costs of gait [3, 29]. In addition, the exchange of potential energy and kinetic energy which is associated with changes in the timing and magnitude of center of mass energy fluctuations, and which in turn can be influenced by walking speed, joint motion, and footfall timing [4–7, 14, 15], appears to be reduced in patients with mild to moderate knee OA perhaps leading to higher levels of muscular work [9, 27].
The center of mass during normal walking on a relatively stiff leg follows the cycle of an inverted pendulum allowing stored gravitational potential energy of the center of mass at midstance to be converted to kinetic energy to further drive the center of mass forward and upward [4–7, 13–15]. The efficiency of energy exchange (% recovery) between potential energy and kinetic energy can be as much as 70% during preferred-speed walking [4–7]. When energy exchange is efficient, it can reduce the amount of muscular effort needed to accelerate and decelerate the center of mass [4–7, 11, 12, 14, 15, 19]. Energy exchange is affected by age and disease [2, 10, 13, 18, 27, 28]. However, the effect of OA in all three major lower extremity joints on the mechanical work of the center of mass is poorly understood. Although calculation of energy recovery is a long-standing technique that has been applied to studies of nonhuman animals and asymptomatic humans [4–7, 15], only a few studies have examined gait in subjects older than 45 years and gait as affected by disorders [10, 13, 18], and those who have examined OA have examined the effect of knee OA on recovery [11, 27]. There is little information regarding the effect of hip and ankle OA on recovery, although it can be predicted from models of energy recovery [4–7, 27].
Percentage recovery can be affected by fluctuations in the relative magnitude of potential and kinetic energy and the phase relationships (% congruity; the amount of time during the stride when the potential and kinetic energy curves change in the same direction) (Fig. 1) [1, 4–7, 14, 15, 27]. Magnitude and congruity of potential energy and kinetic energy fluctuations can be influenced by mechanical factors such as changes in stride length and restrictions in hip, knee, and ankle motion, which are common to individuals with lower extremity OA [25].
Fig. 1.
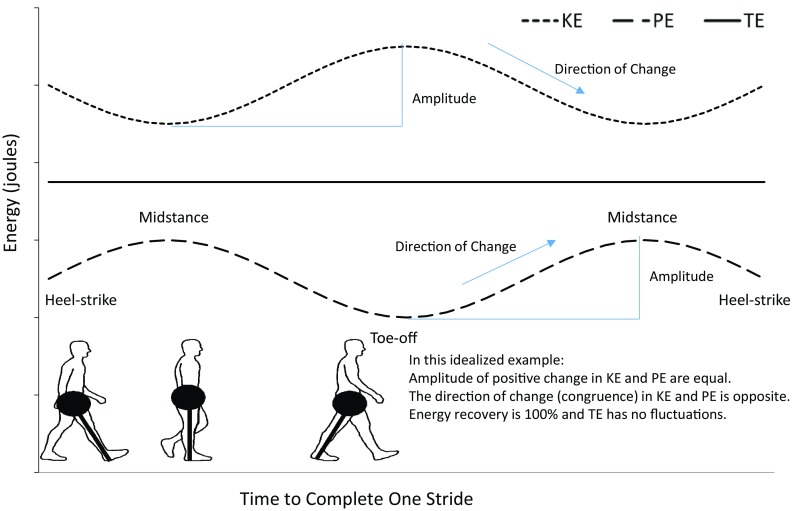
The association of potential energy (PE), kinetic energy (KE), and total energy (TE) of the center of mass in a theoretical model of 100% recovery is presented. When the positive fluctuations of potential energy and kinetic energy are of the same amplitude and moving in opposite directions at all times during the stride (100% out of phase calculated as 0% congruity), it leads to a pattern in which total energy does not fluctuate. In this example, recovery (as calculated following the methods described in the text) would be 100%. The points in the gait cycle are indicated on a schematic of the pendular model of human walking in the lower left with the mass of the body concentrated at one end. Disruption in amplitude or congruity (and shape) of potential energy and kinetic energy can lead to reduced recoveries.
The goals of this study were to (1) examine the effect of end-stage lower extremity OA (hip, knee, or ankle) on walking speed in comparison to an asymptomatic cohort, (2) examine patterns of energy exchange between patients with lower extremity OA in specific joints and in comparison to an asymptomatic cohort, and (3) examine the aspects of center of mass movements that drive energy recovery in each population. We hypothesized that individuals with lower extremity OA would exhibit a decrease in velocity compared with an asymptomatic cohort and that walking speed would be different between the OA cohorts based on the joint affected. In addition, we hypothesized that the OA cohorts would have decreased energy recovery during walking compared with an asymptomatic cohort and that individuals with hip and ankle OA would have a greater reduction in recovery compared with individuals with knee OA owing to restrictions in hip and ankle motion previously reported in these populations [25]. Finally, we hypothesized that congruity in the movement of the center of mass would explain the differences in energy recovery when comparing these study cohorts.
Materials and Methods
Ninety individuals were recruited from the orthopaedic clinic during a course of 18 months, 30 with diagnosed ankle OA, 27 with diagnosed hip OA, 20 with diagnosed knee OA, and 13 asymptomatic individuals with an age range for the entire sample between 41 and 71 years old (Table 1). All patients with OA were diagnosed by board-certified orthopaedic surgeons (MB, DA, SW, JD, JN, ME) as having unilateral OA in a single joint and the absence of pain in all other lower extremity joints. Establishing comparable levels of OA severity across joints is challenging. Kellgren-Lawrence grades can be determined for patients with knee OA, but currently, no established OA grading systems have been validated and accepted for the ankle and hip. Therefore, to provide a consistent recruitment strategy, we included only individuals with end-stage, symptomatic OA who were scheduled for a total joint arthroplasty within 4 weeks of testing. Individuals were excluded if they were unable to ambulate without the use of an assistive device, had pain in more than one lower extremity joint on either limb, or had prior lower extremity joint replacement surgery.
Table 1.
Demographic comparisons between the four study groups
| Demographic | Asymptomatic group (n = 13) |
Hip OA group (n = 27) |
Knee OA group (n = 20) |
Ankle OA group (n = 30) |
p value |
|---|---|---|---|---|---|
| Age (years) | 49.8 ± 7.4 41–67 |
55.6 ± 6.2 41–70 |
61.7 ± 6.5 49–74 |
57 ± 5.4 45–70 |
< 0.0001* ^ |
| Mass (kg) | 70.3 ± 9.3 56–87 |
85.3 ± 20.4 59–127 |
88.4 ± 21.7 53–125 |
88.9 ± 19.8 58–134 |
0.030 + |
| Height (m) | 1.67 ± 0.19 (1.07–1.85) |
1.73 ± 0.09 (1.58–1.93) |
1.69 ± 0.09 (1.58–1.83) |
1.69 ± 0.10 (1.53–1.85) |
0.402 |
| BMI (kg/m2) | 26.2 ± 8.6 (21.3–54.1) |
28.3 ± 5.4 (20.4–43.7) |
30.7 ± 6.6 (21.3–46.0) |
30.8 ± 5.2 (21.3–40.1) |
0.084 |
| Sex | M = 8 (62%) F = 5 (38%) |
M = 14 (52%) F = 13 (48%) |
M = 7 (35%) F = 13 (65%) |
M = 14 (47%) F = 16 (53%) |
OA = osteoarthritis; mean ± SD (range) for age, mass, height, and BMI; *difference between asymptomatic and all OA groups; +difference between asymptomatic and ankle OA cohorts; ^difference between knee OA and hip OA cohorts.
Although this was a comparative study across end-stage, symptomatic OA populations and an asymptomatic group may be unnecessary, we included this group to verify our methods as this was a relatively new approach and used data from multiple force plates [27]. Considerable data exist examining energy recovery in asymptomatic young people [4–7, 18] and an older population [18], and we wanted to ensure that our methods yielded results consistent with these studies before we explored energy recovery in patients with OA. In addition, the asymptomatic cohort was included to obtain a relative measure of the effect of OA in any joint and to be able to provide a reference recovery value for comparison. The asymptomatic cohort was a sample of convenience based on available individuals who were selected to reduce, to the extent possible, differences in age and gender in the OA cohorts. Asymptomatic individuals had to be pain-free with no history of lower extremity joint surgery and no clinical diagnosis of lower extremity OA. Before this study all participants signed informed consent as part of a larger study of gait patterns that had been approved by the medical center’s institutional review board.
Although individuals in the asymptomatic cohort were on average younger (p = 0.035, < 0.0001, and 0.002, respectively, for hip, knee, and ankle OA cohorts) compared with any individuals in the OA cohort in this study, their age range overlapped that of individuals in all of the OA cohorts (Table 1). There were also differences in body mass (p = 0.024) between the asymptomatic individuals and the ankle OA cohort, but no difference in weight existed between OA cohorts and no differences existed in BMI between any cohorts including asymptomatic individuals (Table 1). Moreover, the range of body mass of the asymptomatic individuals overlapped the range of body masses of all the OA cohorts.
An eight-camera motion analysis system sampling at 120 Hz (Motion Analysis Corporation, Santa Rosa, CA, USA) was used in conjunction with four force plates embedded in the walkway sampling at 1200 Hz (AMTI, Watertown, MA, USA) to collect ground reaction forces during self-selected speed level walking. Each participant was asked to wear form-fitting shorts and a shirt and to walk barefoot during testing to control for changes in the ground reaction forces associated with variations in footwear. A modified Helen-Hayes marker set was used for testing [29], but only the sacral marker was used for this analysis to calculate walking velocity. Seven walking trials were collected along a 10-m walkway at self-selected speeds. For a walking trial to be accepted and used for analysis, the individuals had to contact three isolated force plates and maintain a constant speed throughout the trial. Participants were asked to walk at a comfortable speed that was similar to the speed they would walk when grocery shopping.
Outcome Measures
The outcomes were calculated and assessed in a fashion similar to assessment in an earlier study [22] and are briefly summarized here. Potential energy and kinetic energy fluctuations were calculated from all three components of ground reaction force for complete strides using multiple force plates. Integration of the acceleration data provided velocity, whereas double integration provided the displacement of the center of mass, which were used to calculate the kinetic energy of the center of mass in all three planes and the potential energy of the center of mass in the vertical plane. As much as was possible with a population with a confirmed disorder, only steps that could be considered steady state were used based on inclusion criteria described. The percent of center of mass energy recovery (% R) was calculated according to the formula [1, 5–7, 9, 10, 27] (KE = kinetic energy, PE = potential energy, TE = total energy):
| 1 |
Percentage congruity (% C), the percent of time throughout the stride in which potential energy and kinetic energy changed in the same direction, is a measure of phase relationships between the energy curves with high values indicating in-phase curves, and also was calculated following the equation for congruity described by Ahn et al. [1].
| 2 |
Amplitude differences between kinetic energy and potential energy oscillations were calculated by determining the amplitude differences (kinetic energy–potential energy) between the oscillation peaks throughout the stride and then the differences were averaged.
Statistical Analysis
The participant mean across all trials for each variable was determined and used for statistical analysis. Parametric Pearson correlation analyses were performed to determine the associations between center of mass percent recovery and congruity, energy oscillation amplitude differences, and velocity. Regression lines and R2 values were calculated for the variables of interest which included velocity, recovery, congruity, and amplitude. Center of mass percent recovery was averaged across 0.25-m/second increments of velocity from 0.5 to 2.0 m/second to graphically compare individuals across speed pools. Independent t-tests were run to compare the mean recoveries for the symptomatic and asymptomatic individuals at the self-selected walking speed. An alpha level of 0.05 was used to indicate statistically significant differences between groups. All statistical analyses were performed using JMP®, Version Pro 10.0.0 (SAS Institute Inc, Cary, NC, USA).
Results
The results of this study support our hypothesis that individuals with OA walk at a slower velocity than asymptomatic individuals and that the joint affected by OA also affects walking velocity. All of the OA cohorts walked at a slower speed than the asymptomatic population (1.38 ± 0.22 m/second; 95% CI, 1.25–1.5; p < 0.0001), with individuals with hip OA (1.05 ± 0.22 m/second; 95% CI, 0.97–1.3), or knee OA walking the fastest (0.95 ± 0.20 m/second; 95% CI, 0.85–1.05) of the OA cohorts, while the ankle OA cohort walked the slowest (0.86 ± 0.23 m/second; 95% CI, 0.78–0.94). The ankle OA cohort walked at a lower speed relative to the hip OA cohort (p = 0.002). However, when comparing the ankle OA cohort with the knee OA cohort there was no difference in walking speed (p = 0.16) and the same was true when comparing the knee OA and hip OA cohorts (p = 0.14) (Table 2). Each of the OA cohorts achieved a maximum walking speed (ankle OA= 1.36 m/second; knee OA= 1.29 m/second; hip OA=1.39 m/second) at or near the average walking speed of the asymptomatic cohort (mean asymptomatic velocity = 1.38 m/second) (Table 2).
Table 2.
Differences in walking speed between the study groups
| Variable | Asymptomatic group (n = 13) | Hip OA group (n = 27) | Knee OA group (n = 20) | Ankle OA group (n = 30) | p value |
|---|---|---|---|---|---|
| Velocity (m/second) | 1.4 ± 0.2 (1.2–1.5) | 1.1 ± 0.2 (0.96–1.1) | 0.9 ± 0.2 (0.85–0.94) | 0.9 ± 0.2 (0.77–0.94) | < 0.0001*+ |
| % Recovery | 65.0 ± 3.0 (63–67) | 54.0 ± 10.0 (50–58) | 57 ± 10.0 (53–62) | 47.0 ± 13.0 (40–52) | < 0.0001# ^ ♦ |
| % Congruity | 10 ± 3.5 (7.8–12) | 17.6 ± 10.0 (13.5–22) | 16.6 ± 8.5 (11.8–21) | 26.9 ± 10.9 (22.8–31.1) | < 0.0001*♦ + |
OA = osteoarthritis; mean ± SD (95% CI) for velocity, energy recovery, and % congruity in the sample of asymptomatic individuals and those with isolated osteoarthritis of the hip, knee, and ankle; *difference between asymptomatic and all OA groups; +difference between ankle OA and hip OA cohorts; #difference between asymptomatic and hip and ankle OA cohorts; ^difference between knee and hip OA cohorts; ♦difference between knee and ankle OA cohorts.
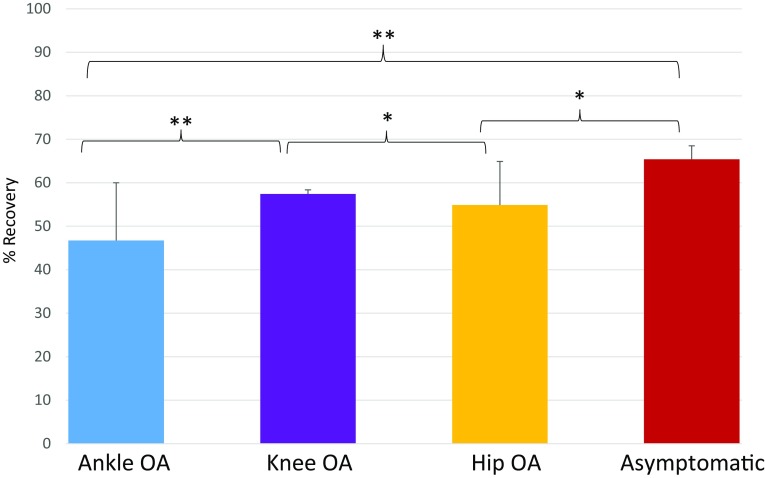
Differences in energy recovery existed when comparing the OA cohorts with the asymptomatic cohort and when examining differences between the OA cohorts. The mean values for percentage recovery during self-selected walking speed was 54% ± 10% for the cohort with hip OA, 57% ± 10% for the knee OA cohort, 47% ± 13% for the ankle OA cohort, and 65% ± 3% for the asymptomatic cohort (Fig. 2). After adjusting for walking speeds these results showed that asymptomatic individuals had greater values of recovery than individuals with hip OA (p = 0.014) and ankle OA (p = 0.002) but not greater values than individuals with knee OA (p = 0.762). In addition, the knee OA cohort showed greater values of recovery than the hip OA cohort (p = 0.033). The knee OA cohort also showed higher recovery compared with the ankle OA cohort (p = 0.005) (Fig. 2). Thus overall, individuals with knee OA had the least loss of recovery compared with other OA groups, whereas individuals with hip OA and ankle OA had similarly low values of recovery.
Fig. 2.
The mean and 1 SD for % recovery are shown for individuals with isolated ankle, knee, or hip osteoarthritis (OA) and a cohort of asymptomatic individuals. *p < 0.05; **p < 0.001.
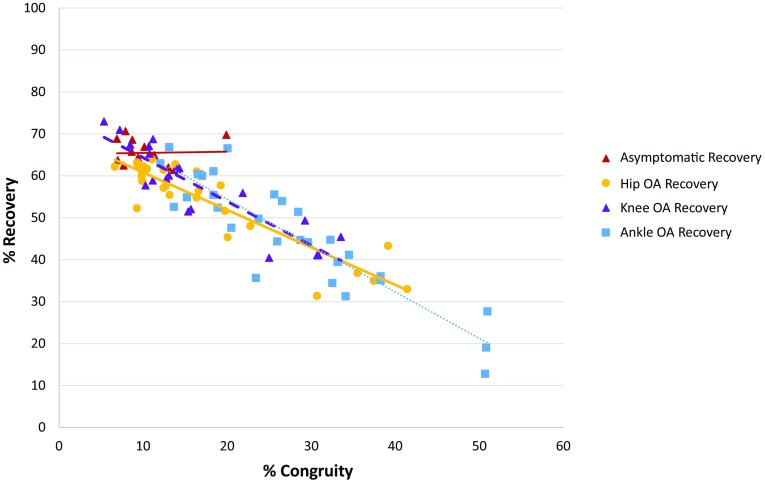
The results of our study indicate that congruity in center of mass movement is associated with energy recovery in the OA cohorts, but not for the asymptomatic cohort. However, oscillation amplitude does not appear to be associated with changes in energy recovery for any of the study cohorts. A strong negative relationship exists between center of mass percent recovery and congruity (Fig. 3) for all OA groups, with an R2 of 0.82 (95% CI, 0.66–0.73) for the hip OA cohort, an R2 of 0.8 (95% CI, 0.7–0.79) for the knee OA cohort, and an R2 of 0.82 (95% CI, 0.7–0.82) for the ankle OA cohort, all of which were significant at a probability less than 0.0001. However, no relationship existed between recovery and oscillation amplitude difference for the three OA groups and the asymptomatic cohort. The lack of a relationship between recovery and congruity in the asymptomatic cohort is likely the result of the narrow speed range in this group. When speed was accounted for, 80% of the variation in recovery not attributable to speed was explained by congruity, with only 10% being explained by amplitude.
Fig. 3.
The values and linear regression lines for % recovery versus % congruity for individuals with isolated ankle, knee, or hip osteoarthritis (OA) and a cohort of asymptomatic individuals are shown.
Discussion
Individuals with OA of the lower limb experience pain and fatigue during normal activities, but little is known about the source of this fatigue. One possibility is that fatigue is associated with higher levels of muscular work necessary to move the center of mass owing to a decrease in the efficiency of exchanging potential energy and kinetic energy in these individuals [5–7, 9, 10, 14, 15, 18, 19, 27]. This effect has been shown for patients with knee OA [9, 27], but to our knowledge, no study has examined the degree to which ankle and hip OA influence walking speed or energy recovery and determined which center of mass parameter influences energy recovery in these OA cohorts. The goal of this project was to determine the effect of isolated lower limb joint OA on energy recovery. We found that OA in any joint (hip, knee, ankle) reduces walking velocity, energy recovery, and that lower energy recovery is influenced by velocity and an increase in congruity of the potential and kinetic energy curves. In addition, the results of this study showed that the loss of recovery is greatest in individuals with hip and ankle OA, which could be associated with increased locomotor cost in these two OA cohorts.
This study had numerous limitations. The participants walked across multiple force plates rather than a single large surface, which required a more complicated analysis, but a previous study [27] and the asymptomatic individuals in that study and in the current study provide results similar to those in other studies [4–7, 13] and appear to provide valid measures of recovery in asymptomatic individuals, indicating that this method can be applied to this population. Using this method would allow for this type of analysis to be completed in many standard gait laboratories using multiple force plates rather than a single long force plate. We examined patients with advanced OA to be able to compare patients with disease at the hip, knee, and ankle. Although the patient selection allowed for comparison across groups, the results are applicable only to patients in the late stages of OA. In patients with end-stage disease there can be differences in limb alignment and the use of pain-relief medications. Both of these factors were not considered in the current study, but could affect walking mechanics and therefore energy recovery. All individuals in the OA cohort were mild or moderately overweight and represented an older age group compared with the asymptomatic cohort, but there were no differences in age or body mass across OA cohorts, who were the main focus of this analysis.
Individuals with OA in this study walked with slower velocities than asymptomatic individuals in this study and in other studies [4–7, 13]. Individuals with ankle OA walked the slowest of all the cohorts, reflecting the deep disability associated with end-stage ankle OA [25, 26]. The walking speed in our cohort with ankle OA is similar to those reported for patients with end-stage hip [22, 25], knee [25, 27], and ankle OA [17, 20, 21, 23, 25].
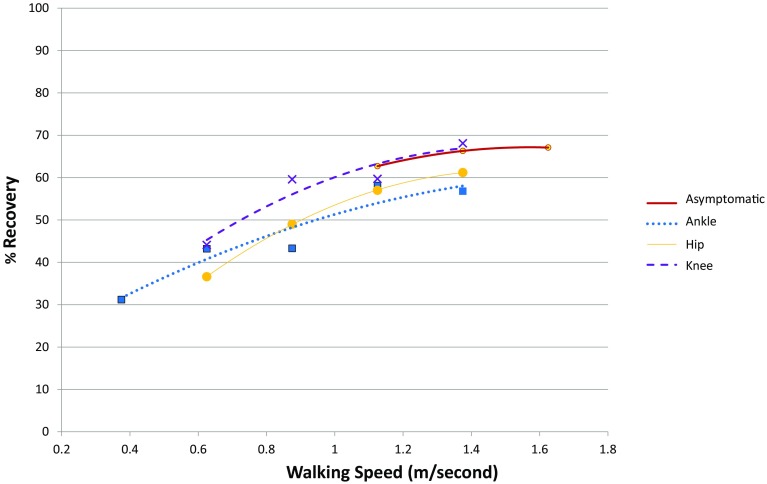
The presence of OA in any joint reduced energy recovery. The lower recovery values seen for this population at a self-selected speed could be the result of a slower walking velocity in individuals with OA compared with asymptomatic individuals. However, individuals with OA showed the same parabolic relationships between speed and recovery seen in asymptomatic individuals and often overlapped much of the speed range of the asymptomatic cohort (Fig. 4). When these individuals increased their walking speed to a range that was consistent with asymptomatic individuals, the recovery values increased (Fig. 4). Individuals with knee OA were able to achieve recovery levels as high as those of the asymptomatic cohort, although on average recovery in the knee OA group was significantly lower than the asymptomatic cohort. A larger study of knee OA and recovery incorporating faster speeds showed that individuals with knee OA had lower recovery values compared with asymptomatic individuals at multiple speeds [27]. In contrast, the individuals in the current study with hip or ankle OA did not ever achieve recovery values equivalent to those of the asymptomatic cohort even at the same speeds. This suggests that joint mechanics have a potentially strong effect on energy recovery regardless of gait velocity.
Fig. 4.
The relationship between % recovery and speed (m/second) for individuals with isolated ankle, knee, or hip osteoarthritis (OA) and a cohort of asymptomatic individuals is shown. Points indicate the average recovery for 0.25-ms−1 increments of velocity and the lines indicate the second-order polynomial regression lines for each group to indicate the overall pattern of change with speed increments.
Although the presence of knee OA disrupts energy exchange, its effect was mild relative to the effect of hip and ankle OA, reflecting high levels of disability [25, 26]. It has been shown that the presence of hip or ankle OA has a strong effect on the pattern of the vertical force during walking that is in turn reflected in the center of mass motion [25].
Changes in the congruity and the amplitude of the center of mass curves can alter energy recovery. The results of our study indicate that energy recovery is influenced more by changes in congruity than by changes in the amplitude of the center of mass. One way in which joint mechanics appears to play a role is in changing the congruity (phase relationships) of the curve, which is evidenced by the strong and consistent negative relationship in the whole sample and in specific cohorts when assessing recovery and congruity. For example, hip OA may disrupt energy exchange because of the reduced ROM, especially in hip extension during terminal stance [25]. The reduction in hip ROM may limit the rise and fall of the center of mass as a result, reducing the ability to store gravitational potential energy, which enhances the congruity of the energy curves by shifting the timing of pushoff relative to the timing of the peak potential energy. For similar reasons, ankle OA has an even-more-profound influence on center of mass motion as reflected in previously reported flat-topped, low-magnitude vertical force curves [25]. The reduced plantar flexion during the last third of stance phase [25] will influence profoundly the phase relationships between potential energy and kinetic energy and enhance congruity. With a reduction in plantar flexion recovery is reduced through a delayed pushoff which allows kinetic energy to be high as the center of mass rises.
Before this study, although energy recovery had been used to examine the effect of aging [18], patients with different disorders [2, 10] and knee OA [9, 27], the effects of hip and ankle OA have not been explored in this context. One of the goals for patient care and treatment for individuals with OA is to establish gait patterns that allow normal activities to be conducted with minimal pain and fatigue. Returning energy recovery to values closer to those in unaffected populations may reduce muscular effort during walking and reduce pain and fatigue. To achieve this, it is imperative to restore normal walking mechanics, ideally achieving a full range of plantar flexion and hip extension. The data presented here support the prediction that people affected by OA in any lower extremity joint (hip, knee, or ankle) consistently exhibit gait patterns that yield low levels of energy exchange, and this is most pronounced among patients with ankle and hip OA. Therefore, future research will need to examine the effect of various surgical and nonsurgical interventions on energy recovery. An understanding of how total joint arthroplasty and the subsequent recovery process affect measures of energy exchange could be instrumental in determining the need for changes to current postoperative physical therapy programs with the ultimate goal of restoring normal walking mechanics and increasing postoperative physical activity after surgery. In addition, future work will need to focus on understanding the kinematic changes that are driving recovery in affected populations and develop appropriate interventions for normalizing lower extremity movement patterns and improving energy recovery.
Acknowledgments
We thank Michael Bolognesi MD; David Attarian MD; Samuel Wellman MD; James Nunley MD; James DeOrio MD; and Mark Easley MD (all from the Department of Orthopaedic Surgery, Duke University Medical Center, Durham, NC, USA) for assistance in recruiting the patients with OA who were tested in this study.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Duke University, Durham, NC, USA.
A comment to this article is available at http://dx.doi.org/10.1007/s11999-016-4965-2.
References
- 1.Ahn AN, Furrow E, Biewener AA. Walking and running in the red-legged running frog, Kassina maculata. J Exp Biol. 2004;207:399–410. doi: 10.1242/jeb.00761. [DOI] [PubMed] [Google Scholar]
- 2.Bennett BC, Abel MF, Wolovick A, Franklin T, Allaire PE, Kerrigan DC. Center of mass movement and energy transfer during walking in children with cerebral palsy. Arch Phys Med Rehabil. 2005;86:2189–2194. doi: 10.1016/j.apmr.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Bernardi M, Macaluso A, Sproviero E, Castellano V, Coratella D, Felici F, Rodio A, Piacentini MF, Marchetti M, Ditunno JF., Jr Cost of walking and locomotor impairment. J Electromyogr Kinesiol. 1999;9:149–157. doi: 10.1016/S1050-6411(98)00046-7. [DOI] [PubMed] [Google Scholar]
- 4.Cavagna GA, Heglund NC, Taylor CR. Mechanical work in terrestrial locomotion: two basic mechanisms for minimizing energy expenditure. Am J Physiol. 1977;233:R243–261. doi: 10.1152/ajpregu.1977.233.5.R243. [DOI] [PubMed] [Google Scholar]
- 5.Cavagna GA, Kaneko M. Mechanical work and efficiency in level walking and running. J Physiol. 1977;268:467–481. doi: 10.1113/jphysiol.1977.sp011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavagna GA, Tesio L, Fuchimoto T, Heglund NC. Ergometric evaluation of pathological gait. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:607–613. doi: 10.1152/jappl.1983.55.2.606. [DOI] [PubMed] [Google Scholar]
- 7.Cavagna GA, Thys H, Zamboni A. The sources of external work in level walking and running. J Physiol. 1976;262:639–657. doi: 10.1113/jphysiol.1976.sp011613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Osteoarthritis. Available at: http://www.cdc.gov/arthritis/basics/osteoarthritis.htm. Accessed November 10, 2015.
- 9.Detrembleur C, De Nayer J, van den Hecke A. Celecoxib improves the efficiency of the locomotor mechanism in patients with knee osteoarthritis: a randomised, placebo, double-blind and cross-over trial. Osteoarthritis Cartilage. 2005;13:206–210. doi: 10.1016/j.joca.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Detrembleur C, Dierick F, Stoquart G, Chantraine F, Lejeune T. Energy cost, mechanical work, and efficiency of hemiparetic walking. Gait Posture. 2003;18:47–55. doi: 10.1016/S0966-6362(02)00193-5. [DOI] [PubMed] [Google Scholar]
- 11.Duff-Raffaele M, Kerrigan DC, Corcoran PJ, Saini M. The proportional work of lifting the center of mass during walking. Am J Phys Med Rehabil. 1996;75:375–379. doi: 10.1097/00002060-199609000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Grabowski A, Farley CT, Kram R. Independent metabolic costs of supporting body weight and accelerating body mass during walking. J Appl Physiol. 1985;2005(98):579–583. doi: 10.1152/japplphysiol.00734.2004. [DOI] [PubMed] [Google Scholar]
- 13.Iida H, Yamamuro T. Kinetic analysis of the center of gravity of the human body in normal and pathological gaits. J Biomech. 1987;20:987–995. doi: 10.1016/0021-9290(87)90328-9. [DOI] [PubMed] [Google Scholar]
- 14.Kuo AD. The six determinants of gait and the inverted pendulum analogy: a dynamic walking perspective. Hum Mov Sci. 2007;26:617–656. doi: 10.1016/j.humov.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Kuo AD, Donelan JM, Ruina A. Energetic consequences of walking like an inverted pendulum: step-to-step transitions. Exerc Sport Sci Rev. 2005;33:88–97. doi: 10.1097/00003677-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F. National Arthritis Data W. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis JS, Jr, Adams SB, Jr, Queen RM, DeOrio JK, Nunley JA, Easley ME. Outcomes after total ankle replacement in association with ipsilateral hindfoot arthrodesis. Foot Ankle Int. 2014;35:535–542. doi: 10.1177/1071100714528495. [DOI] [PubMed] [Google Scholar]
- 18.Mian OS, Thom JM, Ardigo LP, Narici MV, Minetti AE. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiol (Oxf). 2006;186:127–139. doi: 10.1111/j.1748-1716.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- 19.Neptune RR, Zajac FE, Kautz SA. Muscle mechanical work requirements during normal walking: the energetic cost of raising the body’s center-of-mass is significant. J Biomech. 2004;37:817–825. doi: 10.1016/j.jbiomech.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Queen RM, Adams SB, Jr, Viens NA, Friend JK, Easley ME, Deorio JK, Nunley JA. Differences in outcomes following total ankle replacement in patients with neutral alignment compared with tibiotalar joint malalignment. J Bone Joint Surg Am. 2013;95:1927–1934. doi: 10.2106/JBJS.L.00404. [DOI] [PubMed] [Google Scholar]
- 21.Queen RM, De Biassio JC, Butler RJ, DeOrio JK, Easley ME, Nunley JAJ. Leonard Goldner Award 2011: changes in pain, function, and gait mechanics two years following total ankle arthroplasty performed with two modern fixed-bearing prostheses. Foot Ankle Int. 2012;33:535–542. doi: 10.3113/FAI.2012.0535. [DOI] [PubMed] [Google Scholar]
- 22.Queen RM, Schaeffer JF, Butler RJ, Berasi CC, Kelley SS, Attarian DE, Bolognesi MP. Does surgical approach during total hip arthroplasty alter gait recovery during the first year following surgery? J Arthroplasty. 2013;28:1639–1643. doi: 10.1016/j.arth.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Queen RM, Sparling TL, Butler RJ, Adams SB, Jr, DeOrio JK, Easley ME, Nunley JA. Patient-reported outcomes, function, and gait mechanics after fixed and mobile-bearing total ankle replacement. J Bone Joint Surg Am. 2014;96:987–993. doi: 10.2106/JBJS.M.00971. [DOI] [PubMed] [Google Scholar]
- 24.Schepens SL, Kratz AL, Murphy SL. Fatigability in osteoarthritis: effects of an activity bout on subsequent symptoms and activity. J Gerontol A Biol Sci Med Sci. 2012;67:1114–1120. doi: 10.1093/gerona/gls076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitt D, Vap A, Queen RM. Effect of end-stage hip, knee, and ankle osteoarthritis on walking mechanics. Gait Posture. 2015;42:373–379. doi: 10.1016/j.gaitpost.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Segal AD, Shofer J, Hahn ME, Orendurff MS, Ledoux WR, Sangeorzan BJ. Functional limitations associated with end-stage ankle arthritis. J Bone Joint Surg Am. 2012;94:777–783. doi: 10.2106/JBJS.K.01177. [DOI] [PubMed] [Google Scholar]
- 27.Sparling TL, Schmitt D, Miller CE, Guilak F, Somers TJ, Keefe FJ, Queen RM. Energy recovery in individuals with knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:747–755. doi: 10.1016/j.joca.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tesio L, Civaschi P, Tessari L. Motion of the center of gravity of the body in clinical evaluation of gait. Am J Phys Med. 1985;64:57–70. [PubMed] [Google Scholar]
- 29.Waters RL, Perry J, Conaty P, Lunsford B, O’Meara P. The energy cost of walking with arthritis of the hip and knee. Clin Orthop Relat Res. 1987;214:278–284. [PubMed] [Google Scholar]