Abstract
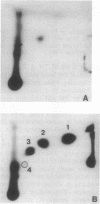
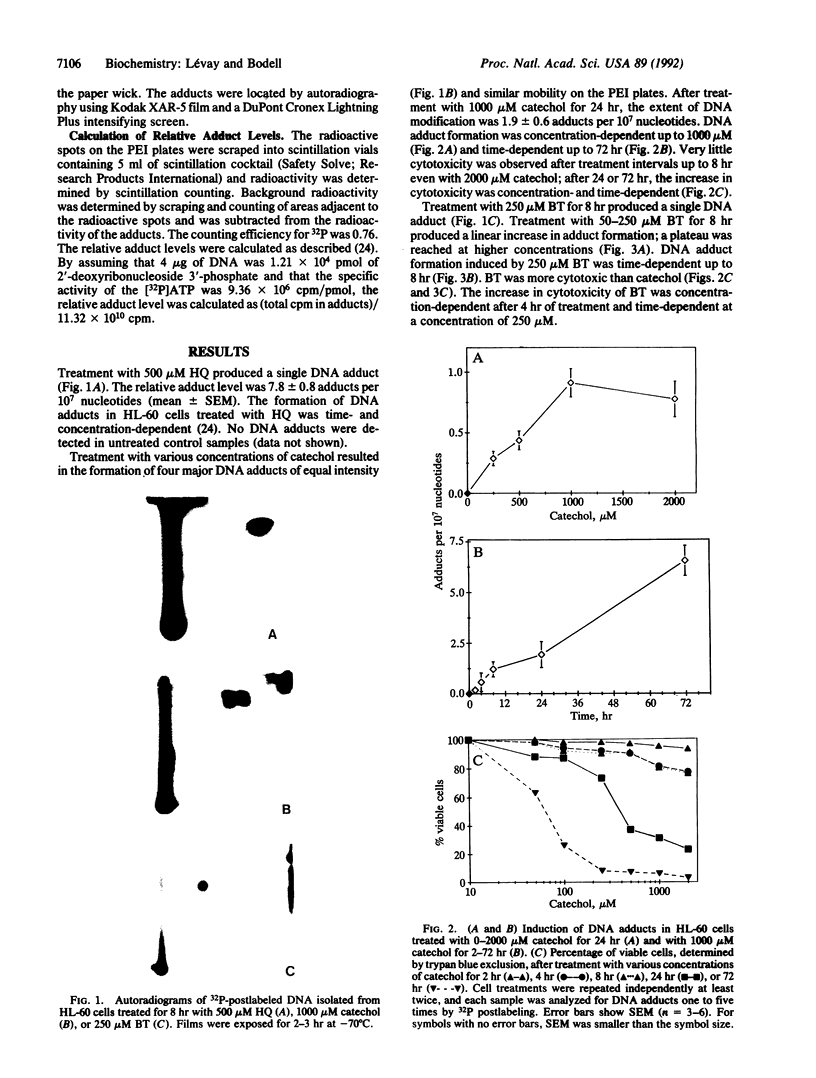
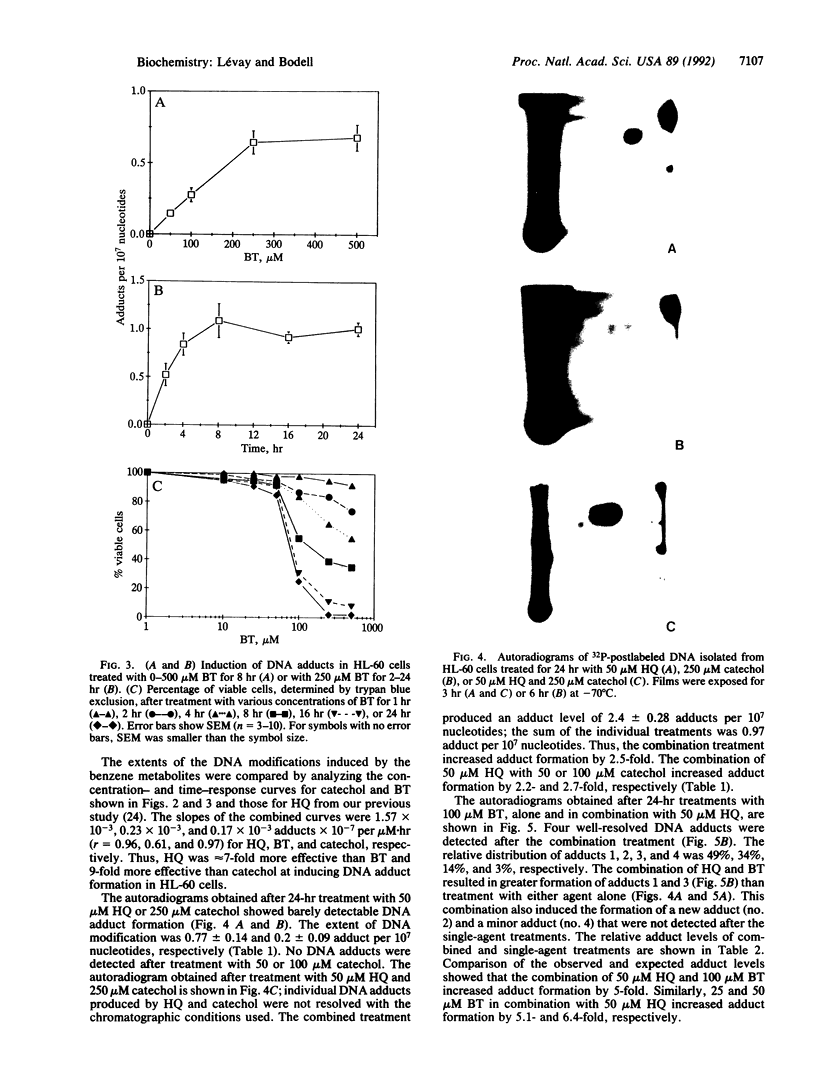
Using P1 nuclease enhanced 32P postlabeling, we investigated DNA adduct formation in HL-60 promyelocytic leukemia cells treated with the benzene metabolites hydroquinone, catechol, and 1,2,4-benzenetriol. Comparison of the slopes of the dose-response curves showed that hydroquinone was 7-9 times more effective than 1,2,4,-benzenetriol and catechol at inducing DNA adducts. Comparison of hydroquinone with catechol showed a good correlation between adduct formation and cytotoxicity. Similar comparisons of hydroquinone and 1,2,4,-benzenetriol suggest that cellular processes in addition to DNA adduct formation contributed to cytotoxicity. In cells treated with the combination of hydroquinone and either catechol or 1,2,4,-benzenetriol, DNA adduct formation was 3-6 times greater than the sum of adduct formation produced by single-agent treatments. Treatment with hydroquinone and 1,2,4,-benzenetriol produced DNA adducts not detected after treatment with either metabolite alone. The synergistic interaction of benzene metabolites in the production of DNA adducts may play an important role in the genotoxic effects of benzene in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews L. S., Lee E. W., Witmer C. M., Kocsis J. J., Snyder R. Effects of toluene on the metabolism, disposition and hemopoietic toxicity of [3H]benzene. Biochem Pharmacol. 1977 Feb 15;26(4):293–300. doi: 10.1016/0006-2952(77)90180-0. [DOI] [PubMed] [Google Scholar]
- Arfellini G., Grilli S., Colacci A., Mazzullo M., Prodi G. In vivo and in vitro binding of benzene to nucleic acids and proteins of various rat and mouse organs. Cancer Lett. 1985 Sep 15;28(2):159–168. doi: 10.1016/0304-3835(85)90071-0. [DOI] [PubMed] [Google Scholar]
- Barale R., Marrazzini A., Betti C., Vangelisti V., Loprieno N., Barrai I. Genotoxicity of two metabolites of benzene: phenol and hydroquinone show strong synergistic effects in vivo. Mutat Res. 1990 May;244(1):15–20. doi: 10.1016/0165-7992(90)90101-o. [DOI] [PubMed] [Google Scholar]
- Billings R. E. Mechanisms of catechol formation from aromatic compounds in isolated rat hepatocytes. Drug Metab Dispos. 1985 May-Jun;13(3):287–290. [PubMed] [Google Scholar]
- Bodell W. J., Banerjee M. R. Reduced DNA repair in mouse satellite DNA after treatment with methylmethanesulfonate, and N-methyl-N-nitrosourea. Nucleic Acids Res. 1976 Jul;3(7):1689–1701. doi: 10.1093/nar/3.7.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H. S., Freedman M. L., Goldstein B. D. The problem of benzene in our environment: clinical and molecular considerations. Am J Med Sci. 1978 Mar-Apr;275(2):124–136. doi: 10.1097/00000441-197803000-00001. [DOI] [PubMed] [Google Scholar]
- Eastmond D. A., Smith M. T., Irons R. D. An interaction of benzene metabolites reproduces the myelotoxicity observed with benzene exposure. Toxicol Appl Pharmacol. 1987 Oct;91(1):85–95. doi: 10.1016/0041-008x(87)90196-7. [DOI] [PubMed] [Google Scholar]
- Eastmond D. A., Smith M. T., Ruzo L. O., Ross D. Metabolic activation of phenol by human myeloperoxidase and horseradish peroxidase. Mol Pharmacol. 1986 Dec;30(6):674–679. [PubMed] [Google Scholar]
- Gill D. P., Ahmed A. E. Covalent binding of [14C]benzene to cellular organelles and bone marrow nucleic acids. Biochem Pharmacol. 1981 May 15;30(10):1127–1131. doi: 10.1016/0006-2952(81)90452-4. [DOI] [PubMed] [Google Scholar]
- Goldstein B. D. Benzene toxicity: a critical evaluation: hematotoxicity in humans. J Toxicol Environ Health Suppl. 1977;2:69–105. [PubMed] [Google Scholar]
- Greenlee W. F., Gross E. A., Irons R. D. Relationship between benzene toxicity and the disposition of 14C-labelled benzene metabolites in the rat. Chem Biol Interact. 1981 Jan;33(2-3):285–299. doi: 10.1016/0009-2797(81)90047-8. [DOI] [PubMed] [Google Scholar]
- Guy R. L., Dimitriadis E. A., Hu P. D., Cooper K. R., Snyder R. Interactive inhibition of erythroid 59Fe utilization by benzene metabolites in female mice. Chem Biol Interact. 1990;74(1-2):55–62. doi: 10.1016/0009-2797(90)90058-u. [DOI] [PubMed] [Google Scholar]
- Huff J. E., Haseman J. K., DeMarini D. M., Eustis S., Maronpot R. R., Peters A. C., Persing R. L., Chrisp C. E., Jacobs A. C. Multiple-site carcinogenicity of benzene in Fischer 344 rats and B6C3F1 mice. Environ Health Perspect. 1989 Jul;82:125–163. doi: 10.1289/ehp.8982125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalf G. F. Recent advances in the metabolism and toxicity of benzene. Crit Rev Toxicol. 1987;18(2):141–159. doi: 10.3109/10408448709089859. [DOI] [PubMed] [Google Scholar]
- Kawanishi S., Inoue S., Kawanishi M. Human DNA damage induced by 1,2,4-benzenetriol, a benzene metabolite. Cancer Res. 1989 Jan 1;49(1):164–168. [PubMed] [Google Scholar]
- Kuo C. F., Fridovich I. Stimulation of the activity of horseradish peroxidase by nitrogenous compounds. J Biol Chem. 1988 Mar 15;263(8):3811–3817. [PubMed] [Google Scholar]
- Levay G., Pongracz K., Bodell W. J. Detection of DNA adducts in HL-60 cells treated with hydroquinone and p-benzoquinone by 32P-postlabeling. Carcinogenesis. 1991 Jul;12(7):1181–1186. doi: 10.1093/carcin/12.7.1181. [DOI] [PubMed] [Google Scholar]
- Lewis J. G., Stewart W., Adams D. O. Role of oxygen radicals in induction of DNA damage by metabolites of benzene. Cancer Res. 1988 Sep 1;48(17):4762–4765. [PubMed] [Google Scholar]
- Lutz W. K., Schlatter C. Mechanism of the carcinogenic action of benzene: irreversible binding to rat liver DNA. Chem Biol Interact. 1977 Aug;18(2):241–245. doi: 10.1016/0009-2797(77)90010-2. [DOI] [PubMed] [Google Scholar]
- Maltoni C., Ciliberti A., Cotti G., Conti B., Belpoggi F. Benzene, an experimental multipotential carcinogen: results of the long-term bioassays performed at the Bologna Institute of Oncology. Environ Health Perspect. 1989 Jul;82:109–124. doi: 10.1289/ehp.8982109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzullo M., Bartoli S., Bonora B., Colacci A., Grilli S., Lattanzi G., Niero A., Turina M. P., Parodi S. Benzene adducts with rat nucleic acids and proteins: dose-response relationship after treatment in vivo. Environ Health Perspect. 1989 Jul;82:259–266. doi: 10.1289/ehp.8982259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellack-Walker P., Blumer J. L. DNA damage in L5178YS cells following exposure to benzene metabolites. Mol Pharmacol. 1986 Jul;30(1):42–47. [PubMed] [Google Scholar]
- Pongracz K., Bodell W. J. Detection of 3'-hydroxy-1,N6-benzetheno-2'-deoxyadenosine 3'-phosphate by 32P postlabeling of DNA reacted with p-benzoquinone. Chem Res Toxicol. 1991 Mar-Apr;4(2):199–202. doi: 10.1021/tx00020a012. [DOI] [PubMed] [Google Scholar]
- Pongracz K., Kaur S., Burlingame A. L., Bodell W. J. Detection of (3'-hydroxy)-3,N4-benzetheno-2'-deoxycytidine-3'-phosphate by 32P-postlabeling of DNA reacted with p-benzoquinone. Carcinogenesis. 1990 Sep;11(9):1469–1472. doi: 10.1093/carcin/11.9.1469. [DOI] [PubMed] [Google Scholar]
- Reddy M. V., Blackburn G. R., Irwin S. E., Kommineni C., Mackerer C. R., Mehlman M. A. A method for in vitro culture of rat Zymbal gland: use in mechanistic studies of benzene carcinogenesis in combination with 32P-postlabeling. Environ Health Perspect. 1989 Jul;82:239–247. doi: 10.1289/ehp.8982239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. V., Randerath K. Nuclease P1-mediated enhancement of sensitivity of 32P-postlabeling test for structurally diverse DNA adducts. Carcinogenesis. 1986 Sep;7(9):1543–1551. doi: 10.1093/carcin/7.9.1543. [DOI] [PubMed] [Google Scholar]
- Rickert D. E., Baker T. S., Bus J. S., Barrow C. S., Irons R. D. Benzene disposition in the rat after exposure by inhalation. Toxicol Appl Pharmacol. 1979 Jul;49(3):417–423. doi: 10.1016/0041-008x(79)90441-1. [DOI] [PubMed] [Google Scholar]
- Rinsky R. A., Smith A. B., Hornung R., Filloon T. G., Young R. J., Okun A. H., Landrigan P. J. Benzene and leukemia. An epidemiologic risk assessment. N Engl J Med. 1987 Apr 23;316(17):1044–1050. doi: 10.1056/NEJM198704233161702. [DOI] [PubMed] [Google Scholar]
- Robertson M. L., Eastmond D. A., Smith M. T. Two benzene metabolites, catechol and hydroquinone, produce a synergistic induction of micronuclei and toxicity in cultured human lymphocytes. Mutat Res. 1991 Jul;249(1):201–209. doi: 10.1016/0027-5107(91)90147-g. [DOI] [PubMed] [Google Scholar]
- Sadler A., Subrahmanyam V. V., Ross D. Oxidation of catechol by horseradish peroxidase and human leukocyte peroxidase: reactions of o-benzoquinone and o-benzosemiquinone. Toxicol Appl Pharmacol. 1988 Mar 30;93(1):62–71. doi: 10.1016/0041-008x(88)90025-7. [DOI] [PubMed] [Google Scholar]
- Sammett D., Lee E. W., Kocsis J. J., Snyder R. Partial hepatectomy reduces both metabolism and toxicity of benzene. J Toxicol Environ Health. 1979 Sep;5(5):785–792. doi: 10.1080/15287397909529789. [DOI] [PubMed] [Google Scholar]
- Schlosser M. J., Kalf G. F. Metabolic activation of hydroquinone by macrophage peroxidase. Chem Biol Interact. 1989;72(1-2):191–207. doi: 10.1016/0009-2797(89)90027-6. [DOI] [PubMed] [Google Scholar]
- Smith M. T., Yager J. W., Steinmetz K. L., Eastmond D. A. Peroxidase-dependent metabolism of benzene's phenolic metabolites and its potential role in benzene toxicity and carcinogenicity. Environ Health Perspect. 1989 Jul;82:23–29. doi: 10.1289/ehp.898223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder R., Jowa L., Witz G., Kalf G., Rushmore T. Formation of reactive metabolites from benzene. Arch Toxicol. 1987;60(1-3):61–64. doi: 10.1007/BF00296948. [DOI] [PubMed] [Google Scholar]
- Subrahmanyam V. V., Kolachana P., Smith M. T. Metabolism of hydroquinone by human myeloperoxidase: mechanisms of stimulation by other phenolic compounds. Arch Biochem Biophys. 1991 Apr;286(1):76–84. doi: 10.1016/0003-9861(91)90010-g. [DOI] [PubMed] [Google Scholar]
- Thomas D. J., Sadler A., Subrahmanyam V. V., Siegel D., Reasor M. J., Wierda D., Ross D. Bone marrow stromal cell bioactivation and detoxification of the benzene metabolite hydroquinone: comparison of macrophages and fibroblastoid cells. Mol Pharmacol. 1990 Feb;37(2):255–262. [PubMed] [Google Scholar]
- Thompson D. C., Trush M. A. Enhancement of the peroxidase-mediated oxidation of butylated hydroxytoluene to a quinone methide by phenolic and amine compounds. Chem Biol Interact. 1989;72(1-2):157–173. doi: 10.1016/0009-2797(89)90025-2. [DOI] [PubMed] [Google Scholar]
- Tunek A., Högstedt B., Olofsson T. Mechanism of benzene toxicity. Effects of benzene and benzene metabolites on bone marrow cellularity, number of granulopoietic stem cells and frequency of micronuclei in mice. Chem Biol Interact. 1982 Mar 15;39(2):129–138. doi: 10.1016/0009-2797(82)90116-8. [DOI] [PubMed] [Google Scholar]