Abstract
Blood pressure (BP) exhibits different variabilities and surges with different time phases, from the shortest beat-by-beat to longest yearly changes. We hypothesized that the synergistic resonance of these BP variabilites generates an extraordinarily large dynamic surge in BP and triggers cardiovascular events (the resonance hypothesis). The power of pulses is transmitted to the peripheral sites without attenuation by the large arteries, in individuals with stiffened arteries. Thus, the effect of a BP surge on cardiovascular risk would be especially exaggerated in high-risk patients with vascular disease. Based on this concept, our group recently proposed a new theory of systemic hemodynamic atherothromboltic syndrome (SHATS), a vicious cycle of hemodynamic stress and vascular disease that advances organ damage and triggers cardiovascular disease. Clinical phenotypes of SHATS are large-artery atherothombotic diseases such as stroke, coronary artery disease, and aortic and pheripheral artery disease; small-artery diseases, and microcirculation-related disease such as vascular cognitive dysfunction, heart failure, and chronic kidney disease. The careful consideration of BP variability and vascular diseases such as SHATS, and the early detection and management of SHATS, will achieve more effective individualized cardiovascular protection. In the near future, information and communication technology-based 'anticipation medicine' predicted by the changes of individual BP values could be a promising approach to achieving zero cardiovascular events.
Keywords: Hypertension, Masked hypertension, Blood pressure, Remote sensing technology
Introduction
Hypertension is a major risk factor for cardiovascular disease, as are metabolic, thrombotic, and inflammatory risk factors. As the association between blood pressure (BP) and cardiovascular events is strong and stroke is more common than myocardial infarction in eastern Asian countries than Western countries,1),2) strict BP control is important in Asian countries.3),4) The diagnosis and management of hypertension is based on the average of BP values measured several times, and the current guidelines for the management of hypertension stresses the importance of out-of-office BP, and the BP control status as assessed by the average of clinic, home, or 24-hr BP values.5) Clinical evidence clearly demonstrated that systolic BP, especially the 24-hr average of systolic BP values measured by ambulatory BP monitoring (ABPM), is the most powerful predictor of cardiovascular events.6),7),8),9),10),11),12),13),14)
As BP per se is a cyclic physical stress on the cardiovascular system, the association between BP and cardiovascular disease cannot be completely explained theoretically, by the increase in the average of BP values. Even when the average value of one of these BP measures is well below the recommended threshold of the target BP level, there is still a blind spot in the disease management; namely, exaggerated BP variability still poses a risk of organ damage and cardiovascular events.15),16) In particular, the impact of BP variability will be increasingly important in the area of wearable continuous BP monitoring.
Indeed, the probability of cardiovascular events increases, along with increase in BP; however, we are not able to predict when and where the cardiovascular events will occur. In this review, we would like to stress the importance of BP variability based on the 'synergistic resonance hypothesis' of BP variability17) and the new disease concept of the vicious cycle of hemodynamic BP stress and vascular disease, i.e., 'SHATS' as a trigger of cardiovascular events.6),16),18),19),20),21),22)
The synergistic resonance hypothesis of BP variability
BP exerts a continuous vertical physical force on cardiac and arterial walls, generated by cyclic cardiac contractions. The different shapes of the continuous wave form of one cycle may produce different stresses on the cardiovascular system, even when systolic and diastolic BP show the same values. The shape of the time-to-time pulse wave form changes due to cardiac function and vascular tonus, differ in the central cardiovascular system and at each peripheral site where atherosclerotic plaques exist.
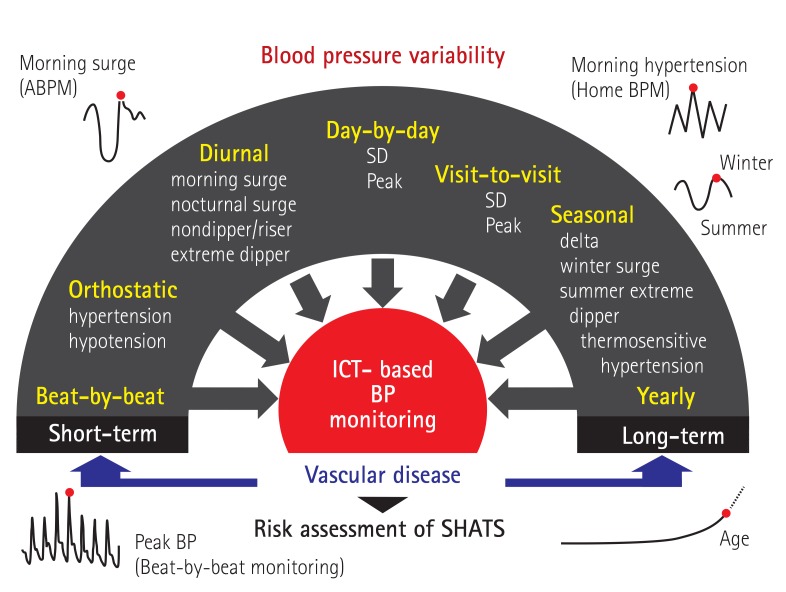
BP exhibits various degrees of variability with different time phases, from the shortest beat-by-beat variability, diurnal variability changes modified by position, diet, alcohol, psychological stress, and physical activity; day-by-day variability, visit-to-visit variability, seasonal variability (in which BP is higher winter and lower in summer), and the longest yearly variability of BP (Fig. 1).16) There is growing evidence that variability of each of these types of BP is associated with organ damage and cardiovascular disease.15),16),23),24),25),26),27),28),29),30),31),32),33),34),35),36),37),38),39),40),41),42),43),44),45) Each type of BP variability increases with aging due to baroreflex dysfunction and small artery remodeling. Although most of these phenotypes of BP variability are partly correlated with each other,18),46),47),48) the degree of augmentation of each type of BP variability differs from person to person.
Fig. 1. Information and communication technology (ICT)-based assessment of different types of BP variability and vascular damage in SHATS. ABPM: ambulatory blood pressure monitoring, BPM: blood pressure monitoring, SD: standard deviation, BP: blood pressure, SHATS: systemic hemodynamic atherothrombotic syndrome. Kario K., Hypertension 2015; 65:1163-9.
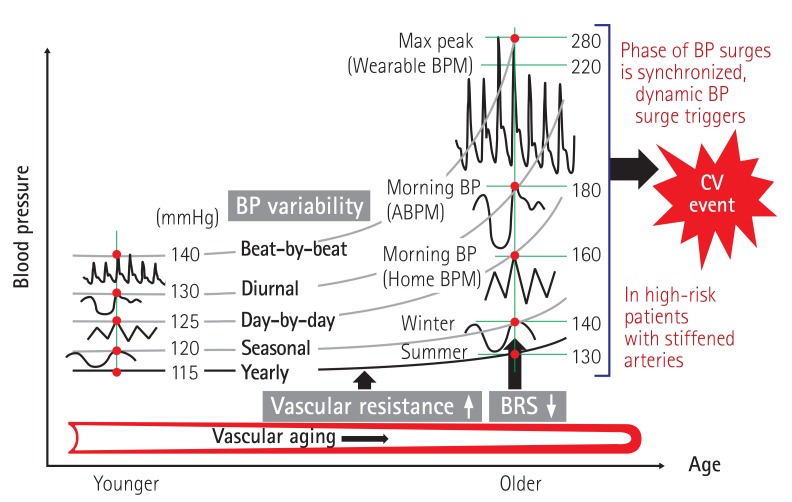
We hypothesized that the resonance of each type of BP variability with different time phases synergistically generates an extraordinarily large dynamic surge which would trigger a cardiovascular event (i.e., the 'resonance hypothesis of BP variability') (Fig. 2).17)
Fig. 2. The synergistic resonance hypothesis of BP variability. ABPM: ambulatory blood pressure monitoring, BPM: blood pressure monitoring, CV: cardiovascular, BRS: baroreceptor sensitivity. Kario K, Am J Hypertens 2016; 29:14-6.
Systemic hemodynamic atherothrombotic syndrome
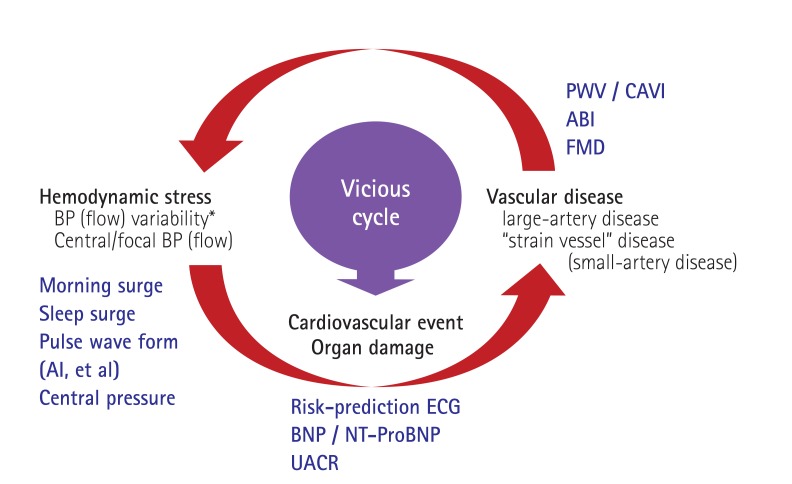
We recently proposed the concept of a vicious cycle between hemodynamic stress (increased variability of BP and blood flow) and vascular disease that damages organs and contributes to cardiovascular events: SHATS (Fig. 3).6),16),18),19),20),21),22) In SHATS, the pulse was directly transmitted from the central artery to peripheral artery without diminishment, in individuals with stiffened arteries; this is because stiffened large arteries cannot absorb the power.
Fig. 3. Concept of systemic hemodynamic atherothrombotic syndrome (SHATS). The SHATS is a disease condition that accelerates the risk of organ damage and cardiovascular events via a vicious cycle of hemodynamic stress and vascular disease. AI: augmentation index., ECG: electrocardiography, BNP: B-type natriuretic peptide, NT-ProBNP: N-Terminal-ProBNP, UACR: urinary albumin/creatinine ratio, PWV: pulse wave velocity, CAVI: cardio ankle vascular index, ABI: ankle-brachial index, FMD: flow-mediated dilatation. Kario K., Nature Review Nephrol 2013;9:726-38.
The novel contribution of SHATS is its synergistic consideration of the different BP variabilities and hemodynamic stress in relation to vascular disease. That is, SHATS is defined by both vascular (one or more clinical/subclinical vascular diseases) and BP components (one or more phenotypes of BP variability), but the precise definition and criteria of SHATS are not yet clearly established. Nonetheless, the concept emphasizes that clinicians should recognize the synergistic risk posed by exaggerated BP variability and vascular disease in clinical practice. Different aspects of the phenotypes of BP variability in SHATS can be detected by home BP monitoring (HBPM), ambulatory blood pressure monitoring (ABPM), an active standing test, etc.
Vascular aging leads to increased vascular resistance and a reduction of baroreceptor sensitivity through both small and large artery remodeling. Baroreceptor sensitivity, which is largely determined by vascular stiffness and sympathetic tonus, can be expected to be the key mechanisms of exaggerated BP variability in SHATS.
The clinical relevance of SHATS differs between younger and older subjects. SHATS is clinically important for predicting future sustained hypertension in younger subjects. The early detection of SHATS may raise the alert for the prevention of organ damage in the early stages. In older adults, SHATS is important as a direct risk for triggering cardiovascular events. The suppression of SHATS leads directly to a reduction in the risk of cardiovascular events.
Targets of systemic hemodynamic atherothrombotic syndrome
Arteries with different sizes and microcirculation organ interaction are the target of SHATS. Large-artery disease will augment the impact of exaggerated BP variability on atherosclerotic and small artery-related cardiovascular events. An increase in large artery stiffness decreases the attenuation of BP pulses transmitted to the peripheral arteries.
The first target of SHATS is advanced vulnerable plaques in high-risk patients. Their exaggerated BP variability — especially BP surges — triggers plaque rupture due to the mechanical vertical stress from BP and the shear stress from the exaggerated variability of the blood flow, resulting in the onset of cardiovascular events.
The second target of SHATS is the small arteries that branch out in a perpendicular manner from the large arteries. These so-called "strain vessels" are exposed to high pressure, and their strong vascular tone must be preserved so that large pressure gradients can be provided to the capillaries by the parent vessels.49) For example, the nearer that the large artery (e.g., the arcuate arteries) is, the greater the pressure overload will be in the glomeruli's afferent arterioles.49) The primary source of microalbuminuria is the glomeruli in the cortex close to the outer medulla's arcuate arteries. The strain vessels are located in retinal and coronary arteries, renal juxtamedullary afferent arterioles, and the cerebral perforating arteries.49)
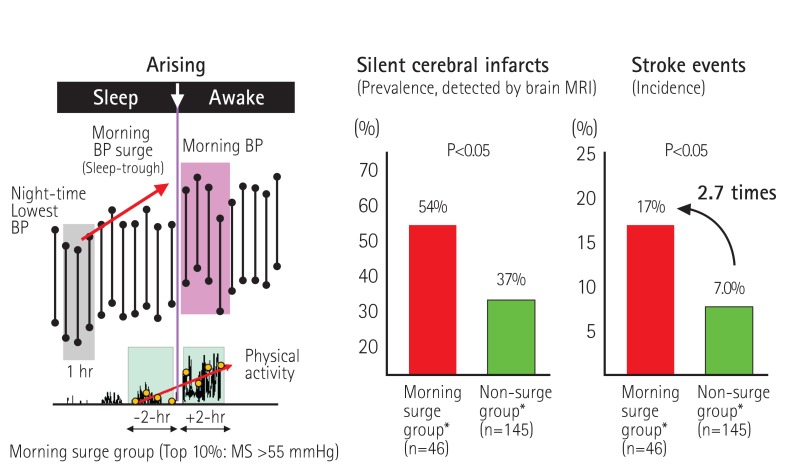
The majority of cerebral hemorrhages and lacunar infarctions take place in small perforating arteries. In one of the studies conducted by our group, the brain MRI studies of elderly individuals with hypertension, silent cerebral infarcts (SCIs), particularly multiple SCIs, were more frequently detected in the individuals who showed exaggerated morning BP surges, as compared to a normal surge group (Fig. 4).11) A sub-analysis of the Ohasama Study also showed that cerebral hemorrhages occurred more frequently in the exaggerated morning surge patients than in the normal surge group.50) The analysis revealed that exaggerated BP variability presents a risk of branch atheromatous diseases (which is frequently observed in proximal lesions of perforating cerebral arteries such as the paramedian pontine arteries and lenticulostriate arteries). An investigation demonstrated that branch atheromatous diseases are associated with metabolic risk factors for advanced atherosclerotic large-artery disease.51) In patients with increased stiffness of the large arteries, the focal pressure on the strain vessels' proximal portion is higher
Fig. 4. Prevalence of MRI-detected silent cerebral infarcts and incidence of stroke events during a 42-month period, in elderly hypertensive patients with or without morning surge in blood pressure — the Jichi Medical University ABPM Study, Wave 1. *Matching for age and 24-hr systolic BP. Kario K. et al., Circulation 2003;107:1401-6.
The final target of SHATS is microcirculation-related organ damage, such as vascular dementia, heart failure, chronic kidney disease, and sarcopenia, all age-related diseases. White-matter disease and SCI are the predisposing conditions of clinical stroke, cognitive dysfunction, and vascular depression; they are present in the elderly,52) and are found to be advanced in patients with increased pulse wave velocity.
Evidence suggests the clinical involvement of SHATS in organ damage, based on the finding of a synergistic association between BP variability and vascular disease. In our group's 2014 investigation of a visit-to-visit BP variability in the elderly, we observed that the delta systolic BP (the peak systolic BP minus the lowest systolic BP over a 12-month period), a measure of visit-to-visit variability of office BP and vascular disease (intima-media thickness and beta-stiffness of carotid artery evaluated by carotid echography), had a synergistic impact on the cognitive impairment evaluated by the reduction of Mini-Mental State Examination (MMSE) scores in the very elderly patients with the mean age >80 years, and one or more cardiovascular risk factors.39)
In addition, a synergistic protection of the end organs was found between BP and vascular disease. In our titration of doxazosin to target home BP <135/85 mmHg by home BP monitoring, not only the reduction of home BP, but also the reduction of pulse wave velocity (PWV) was important for reducing microalbuminuria.53) The reduction of urinary albumin excretion was not observed in the subjects without a reduction in PWV, even when their home BP levels were significantly decreased.
Mechanisms underlying the vicious cycle of systemic hemodynamic atherothrombotic syndrome
The three BP measures of SHATS, all closely related, are an increase in BP variability, an increase in central pressure, and a decrease in baroreceptor sensitivity. The causative mechanisms that underlie SHATS may be impaired vascular and neural components of baroreflex due to decreased carotid dispensability, and increased central sympathetic activity. Large-artery disease and small-artery remodeling may also contribute to BP variability increases.
Two important components of pulsatile hemodynamics are arterial stiffness and pressure wave reflections. Ambulatory BP measurements, including those of morning blood pressure surges, can be used to determine pulsatile hemodynamics based on the effects of arterial stiffness and wave reflections. The different phenotypes of BP variability may be identified by the degree of activation of the central and peripheral neurohumoral status and the related cardiovascular reactivity of the phenotypes in each specific condition.
A suppression of the vicious cycle of SHATS may be achieved by using an invasive approach towards the arteries, reducing the exaggerated BP variability and reflection pulse waves. Stenting the carotid artery significantly reduced the day-by-day variability of home BP values in patients with carotid artery disease.52) After percutaneous transluminal angioplasty, the reduction of left ventricular hypertrophy (LVH) and microalbuminuria were correlated with the augmentation index of the carotid artery.53) The neuromodulation by renal denervation and baroreceptor sensitization may therefore be an effective treatment for the BP variability observed in individuals with SHATS.
Morning BP surge and evidence
An exaggerated morning BP surge, specifically potentiated by neurohumoral activation in the morning, is the BP variability phenotype of SHATS. It is well known that cardiovascular events occur more frequently in the morning, and that BP levels increase upon waking in the morning (the morning surge). At the Jichi Medical University ABPM (JMU-ABPM) study of elderly hypertensive patients, we were the first to demonstrate that both ABPM-defined measures of morning surge in systolic BP (i.e., the sleep-trough and pre-awakening surge) were independently associated with future clinical stroke events, independent of age or average 24-hr BP level (Fig. 4).11) Numerous studies have described the strong predictive ability of morning BP surge as a risk for cardiovascular disease (stroke, coronary artery disease, cardiovascular disease, total mortality) being independent of the 24-hr BP level in both hypertensive outpatients and community-dwelling subjects,1),11),50),54),55),56) although some studies did not find that this predictive ability was present independently of the 24-hr BP level, and a few studies have struck a discordant note.57),58)
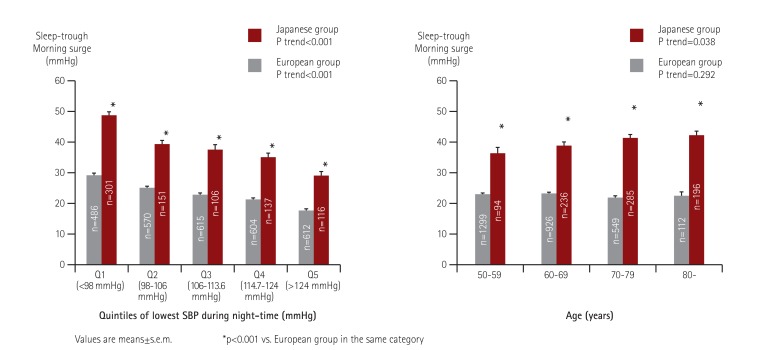
The morning BP surge is positively correlated with inflammatory biomarkers and other types of BP variability such as orthostatic hypertension and increased daytime ambulatory BP variability. The morning BP surge is associated with target organ damage such as left ventricular hypertrophy and albuminuria, and large- and small-artery diseases such as carotid atherosclerosis, arterial stiffness, albuminuria, and silent cerebrovascular disease, all being independent of the 24-hr BP level. In our international collaboration ABPM study, the Asian subjects had more extended morning BP surges compared to the Western subjects (Fig. 5).59) The steeper association between office BP and cardiovascular disease (particularly stroke) in Asian populations may be partly explained by their exaggerated morning BP surge.
Fig. 5. Ethnic differences in the degree of morning BP surge: the ARTEMIS study (811 Japanese and 2,887 Caucasians). Hoshide S. et al., Hypertension 2015;66:750-6.
The morning BP surge would be potentiated by a synergistic resonance of various components of BP variability, resulting in the morning-onset cardiovascular events. The previous studies did not strictly refer to the onset time of cardiovascular events. In our 2003 study of morning hypertension, the stroke events were demonstrated to be significantly more likely to occur in the morning.11) A stroke may occur in the morning even among high-risk hypertensives whose home and ambulatory BP levels are well-controlled.
In SHATS, synergistic resonance between the morning BP surge and different types of BP variability may occur and could trigger a cardiovascular event. In previous ABPM studies, the morning BP surge was exaggerated in the winter, especially in elderly patients (winter morning surge in BP)60) and on Mondays (Monday morning surge in BP).61) These changes in morning BP surge may contribute to the increase in cardiovascular events in the winter in the elderly, and on Mondays in working adults. Maximum systolic BP, one of the measures of day-by-day home BP monitoring most frequently observed in the morning, has been significantly associated with measures of cardiovascular remodeling (left ventricular mass index, carotid intima-media thickness) even in hypertensives with a well-controlled average of home BP <135/85 mmHg.25) In addition, the increased SD of morning BP is a significant independent predictor of cardiovascular death.23)
Thus, organ damage and the triggering of a cardiovascular event may be more likely when an unstable morning BP surge is synergistically augmented by the resonance of other phenotypes of BP variability compared to stable and reproducible morning BP surges. Other BP phenotypes are more closely associated with the morning BP surge. These include: (1) increased SD of daytime ambulatory BP values, (2) circadian BP variation, particularly that with extreme dipping of the nocturnal BP values (i.e., a nocturnal systolic BP drop >20%), and (3) orthostatic hypertension (i.e., a 15-20 mmHg increase in systolic BP due to standing up).18),46,47,48)
Morning hypertension
Morning BP per se is a stronger risk factor for organ damage and cardiovascular events than the BP values of other periods. Our group first defined morning hypertension as having the ABPM-measured morning BP values (the 2-hr average of BP values just after arising) >135 mmHg for systolic BP, or >85 mmHg for diastolic BP, regardless of office BP values, and we stressed the importance of controlling morning hypertension in clinical practice.62),63)
The BP-lowering effect of a once-daily morning use of an antihypertensive drug is minimal the next morning just before any other medication is taken. Thus, the prevalence of 'masked morning hypertension,' defined as normotension in office BP and hypertension in morning BP, increases after a conventional office BP-guided treatment of hypertension. During the past decade, we have persistently recommended home morning BP-guided antihypertensive treatment for hypertensive patients. We compared the prevalence of uncontrolled masked hypertension in two different studies performed at a 10-year interval. The first of the two studies, the Jichi Morning Hypertension Research (J-MORE) study performed 10 years ago, demonstrated that the prevalence of uncontrolled masked morning hypertension was 52% in the subjects with well-controlled clinic BP (<140 mmHg).64) However, in the recent J-HOP study,65) this prevalence had fallen to approx. 38%. Although the prevalence of masked uncontrolled morning hypertension could not be simply compared between the 2 studies, this trend suggests that it is feasible to achieve an effective control rate of the morning BP by targeting morning BP.
The HONEST Study: Latest home BP monitoring evidence
We recently published the main results of the Home Blood Pressure Measurement with Olmesartan-Naïve Patients to Establish Standard Target Blood Pressure (HONEST) study, the largest-scale prospective, real-world observational study of 21,591 outpatients with essential hypertension in Japan.66) The results of the study demonstrated that on-treatment morning home BP is much more important than office BP during antihypertensive treatment. In the HONEST study, the patients received olmesartan-based treatment throughout the treatment period. Both office and home BP values were comparably reduced by olmesartan, in both the patients treated with monotherapy and in those treated with combination therapy with other classes of antihypertensive drugs. The primary endpoint was major cardiovascular events (stroke, myocardial infraction, and angina pectoris with coronary intervention) and sudden death.
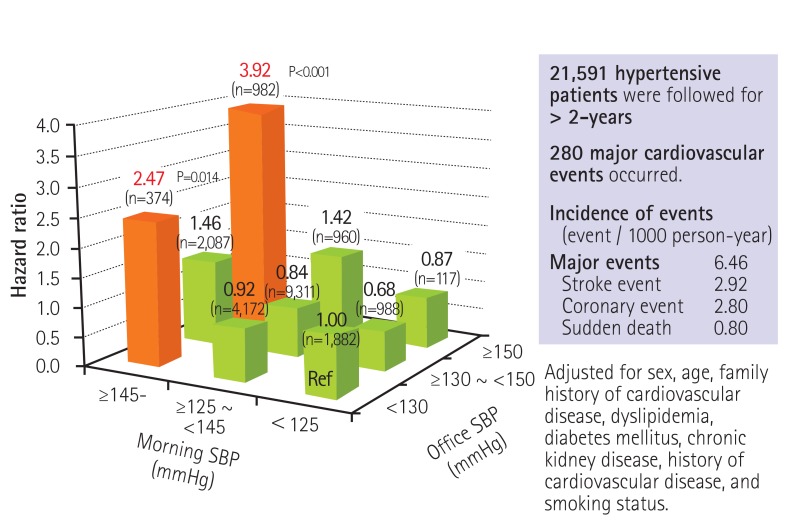
During the mean follow-up period of 2.02 years, cardiovascular events occurred in 280 patients (incidence, 6.46/1,000 patient years). The risk for the primary endpoint was significantly higher in the patients with on-treatment morning home systolic BP values >145 to 155 mmHg (hazard ratio [HR] 1.83) and >155 mmHg (HR 5.03) compared to the patients with corresponding values <125 mmHg, and compared to those with on-treatment office BP >150 to 160 mmHg (HR 1.69) and >160 mmHg (HR 4.38) than those with <130 mmHg. The morning home BP value associated with the minimum risk was 124 mmHg by a spline regression analysis, and the office systolic BP associated with the minimum risk was 131 mmHg. There is no J-curve phenomenon in home BP until 100 mmHg, whereas a slight increase in the cardiovascular risk around 100 mmHg was observed for office BP. When morning BP was controlled at <125 mmHg, there was no increase in cardiovascular risk even when the office systolic BP was increased to >150 mmHg. Instead, the cardiovascular risk was increased in patients with morning home BP>145 mmHg and office BP<130 mmHg (HR 2.47), as compared to those with morning home BP<125 mmHg and office BP&130 mmHg (Fig. 6). Thus, the risk of cardiovascular events was high in the patients with masked uncontrolled morning hypertension, although their office BP was not increased.
Fig. 6. On-treatment morning home and office BP values and cardiovascular events in medicated hypertensive patients (the HONEST Study). Kario K. et al., Hypertension 2014; 64: 989-96.
These real-world findings emphasize the importance of HBPM in clinical practice. Based on this evidence, it is essential to control morning home systolic BP to <145 mmHg as a first step, even in patients with controlled office BP. Then, achieving morning home systolic BP<135 mmHg, i.e., the target home BP level of the JSH2014 and other international guidelines, is the second step; the ultimate goal is to reach around 125 mmHg, for the home BP-guided management of hypertension.6),67)
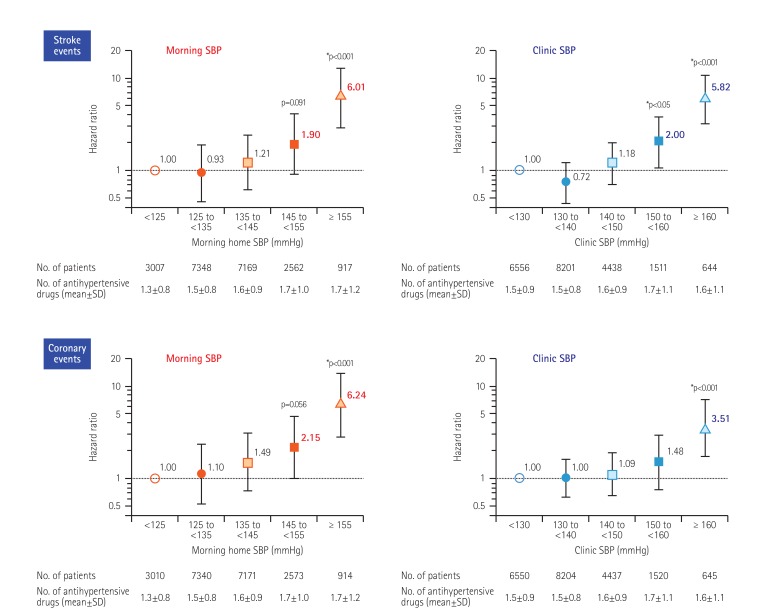
In addition, as few studies have evaluated out-of-office BP measurements as predictors of coronary artery disease (CAD) events, we recently investigated whether morning home BP could be a predictor of coronary artery disease, and we compared this ability with the ability to predict a stroke.68) Morning home BP was a strong predictor of future CAD events as well as stroke events, and it may be superior to office BP. In that study population, there was an occurrence of 127 stroke events (2.92/1000 patient-years) and 121 CAD events (2.78/1000 patient-years). The HR of stroke was 6.01 between patients with morning home systolic BP≥155 mmHg and those with morning home systolic BP<125 mmHg, and 5.82 between patients with office systolic BP≥160 mmHg and those with office systolic BP<130 mmHg; morning home systolic BP predicted stroke events similarly to office systolic BP (Fig. 7). The HR of CAD events for morning home systolic BP≥155 mmHg was 6.24, and the HR for office systolic BP≥160 mmHg was 3.51; therefore, compared with morning home systolic BP, office systolic BP may underestimate the CAD risk. In addition, there does not appear to be a J-curve in the relationship between morning HBP and stroke or CAD events. Thus, a determination of morning home BP levels could be used to detect the risks of both stroke and CAD, whereas office BP underestimates the risk of CAD, indicating that a home morning BP-guided antihypertensive strategy is more effective in clinical practice.
Fig. 7. On-treatment BP and coronary artery disease (CAD) vs. stroke risk: Hazard ratio* HONEST Study (21,591 hypertensives patients were followed for >2 yr). *Adjusted for sex, age, family history of cardiovascular disease (CVD), dyslipidemia, diabetes mellitus, chronic kidney disease, history of CVD, and smoking status. Kario K. et al., J Am Coll Cardiol 2016;67:1519-27.
Renal denervation
Sympathetic nervous activity exhibits marked diurnal variation with a morning peaks, and morning BP is closely associated with alpha-adrenergic hyperactivity.69)
Bedtime dosing of an alpha-blocker, doxazosin, significantly reduced morning BP to a greater degree than the BP values during other time-periods.70),71) In two studies of patients with uncontrolled hypertension, it was demonstrated that percutaneous catheter-based renal denervation (RDN) by radiofrequency ablation of the renal artery nerves, reduced both BP and central sympathetic activity.72),73),74),75) We recently published the results of the SYMPLICITY HTN-Japan trial, which is the first prospective, randomized controlled trial comparing RDN with standard pharmacotherapy for the treatment of resistant hypertension (systolic BP ≥160 mmHg on ≥3 antihypertensive drugs, including a diuretic for ≥6 weeks) in Asia.76) Unfortunately, the results could not demonstrate statistically significant effectiveness. It may partly be small number of the study patients since the trial was terminated before completion.
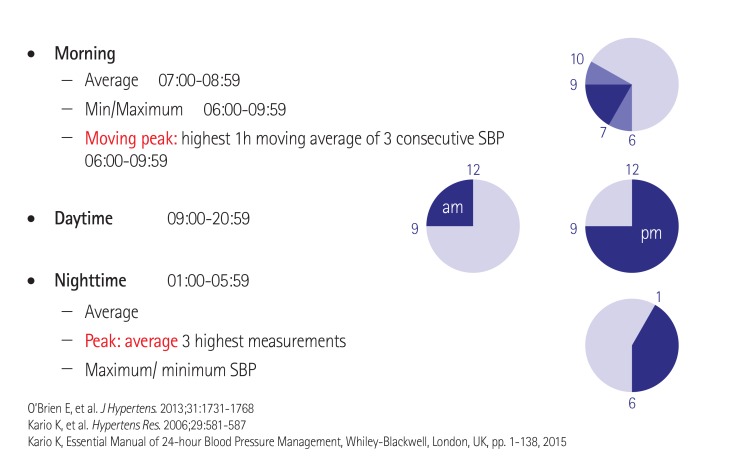
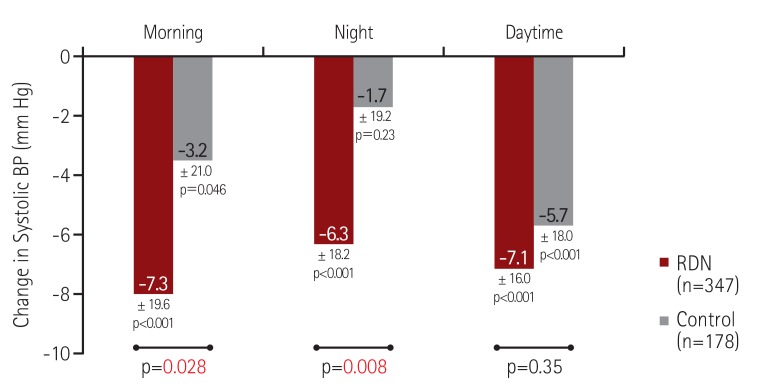
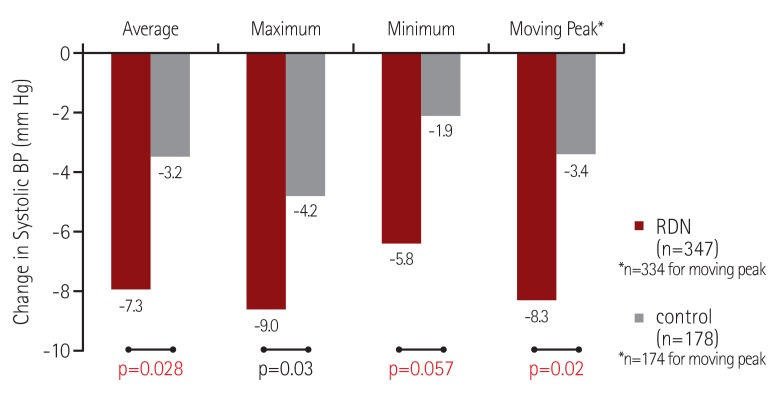
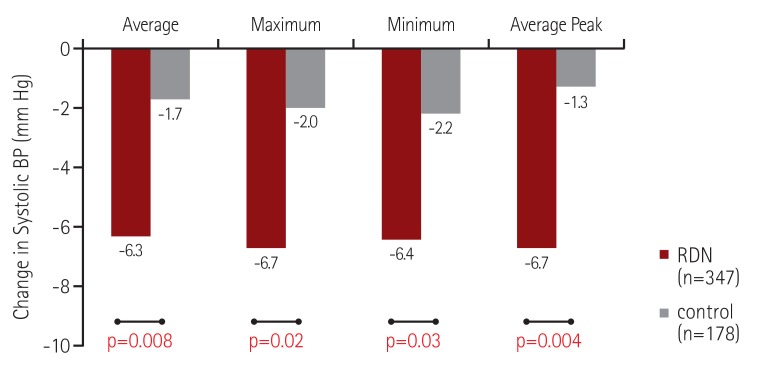
In our combined analysis of the SYMPLICITY HTN-377) and SYMPLICITY HTN-Japan77) studies, we evaluated the effect of RDN on ambulatory BP during discrete time periods, particularly early morning and nocturnal phases. We used more specific windows to define morning and nighttime BP levels that closely match the morning rising hours and the nighttime sleeping hours (Fig. 8). Compared with the control group at 6 months post-randomization, RDN was associated with a significantly greater reduction in both morning and nocturnal systolic BP levels but not daytime systolic BP in treatment-resistant hypertensive patients, in both the original HTN-3 cohort and in a pooled cohort of HTN-3 and HTN-Japan patients (Fig. 9).78) Additionally, the patients who underwent RDN had significantly lower maximum and moving peak morning systolic BP values compared to the control patients (Fig. 10A), as well as maximum, minimum, and mean peak nighttime systolic BP (Fig. 10B).
Fig. 8. Ambulatory BP definitions by 24-hr clock. Kario K. et al., Hypertension 2015; 66:1130-7.
Fig. 9. Differential time-dependent ambulatory systolic BP reduction 6-month after RDN (pooled data of SYMPLICITY HTN-J+3). RDN: renal denervation. Kario K. et al., Hypertension 2015; 66:1130-7.
Fig. 10A. Morning systolic BP reduction 6 months after RDN (pooled data of SYMPLICITY HTN-J+3). BP: blood pressure, RDN: renal denervation. Kario K. et al., Hypertension 2015;66:1130-7.
Fig. 10B. Nighttime systolic BP reduction 6 months after RDN (pooled data of SYMPLICITY HTN-J+3). BP: blood pressure, RDN: renal denervation. Kario K. et al., Hypertension 2015;66:1130-7.
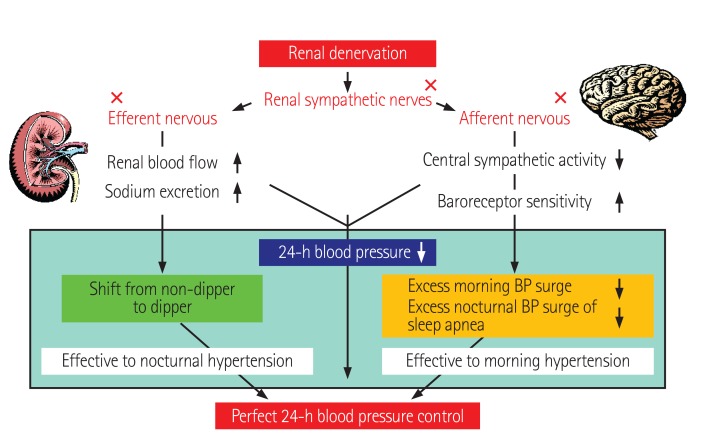
Thus, the more sensitive analysis of the response to RDN restricted to select diurnal periods when sympathetic tone was high indicated a significant impact of RDN on ambulatory BP. Renal denervation would be effective against nocturnal hypertension via increased sodium excretion (efferent nervous ablation) and against morning hypertension and nocturnal peaks via an increase in baroreceptor sensitivity due to central sympatholytic activity (afferent nervous ablation), resulting in perfect 24-hr BP control which might reduce the cardiovascular risk in hypertensive patients (Fig. 11).16),19)
Fig. 11. Hypothesis of 'perfect' 24-h BP control by renal denervation in resistant hypertension. BP: blood pressure. Kario K., Essential Manual of 24-hour Blood Pressure Management from Morning to Nocturnal Hypertension. London, UK; Wiley-Blackwell; 2015. p. 1-138.
Regarding the reduction of all of the measures of the nocturnal BP profile, including the average peak and maximum nighttime BP values, we expect an effect of concomitant obstructive sleep apnea syndrome (OSAS), which increases the sympathetic nervous activity, to result in non-dippers with diminished nocturnal BP falls during sleep or risers with higher nocturnal BP than daytime BP. 79) In addition, we recently developed information technology-based trigger nocturnal BP monitoring (ITNP) which can selectively measure hypoxia-induced BP peaks,6),22),79),80),81),82),83) and we started the nationwide SPREAD (Sleep Pressure and Disordered Breathing in Resistant Hypertension and Cardiovascular Disease) study of high-risk patients with a history of cardiovascular events or OSAS and resistant hypertension. We frequently observed an exaggerated nocturnal BP surge triggered by periodic hypoxia-induced sympathetic overdrive in OSAS patients, and this exaggerated surge may partly explain an increase in the risk of sleep-onset cardiovascular events.
In the HTN-J study, we used ITNP to investigate the effect of RDN on the nocturnal BP profile in OSAS patients who were already enrolled in the SPREAD study.84) The results of the HTN-J study demonstrated that catheter-based RDN significantly reduced hypoxia-induced nocturnal systolic BP peaks by 10 mmHg. Thus, RDN might be an effective approach to reduce sleep-onset cardiovascular events in high-risk patients with OSAS regardless of continuous positive airway pressure (CPAP) treatment, by suppressing the hypoxia-induced nocturnal BP peaks. This HTN-J data also indicate that hypertension with OSAS might be a promising candidate for a high-risk target of RDN. In our post-hoc analysis of HTN-3, renal denervation significantly reduced peak nocturnal BPs.85)
The Global SYMPLICITY Registry in South Korea (GSR Korea) demonstrated that the effectiveness of renal denervation may be greater in Asians than in Caucasians.86) In Japan, the prevalence of resistant hypertensive patients among all of the hypertensive patients is 4.1% from our Japan Ambulatory Blood Pressure (JAMP) study.14) Based on this prevalence, the number of resistant hypertensive individuals in Japan would be 1870000 patients. We are soon starting the clinical trial of renal denervation using the new device.87)
Perspectives
BP variability is the master biomarker of human health care, since it is not only a modifiable risk factor of organ damage and cardiovascular disease but also a sensor of cardiovascular dysregulation that is affected by individualized characteristics and stressors of daily behavioral factors and environmental conditions. The synergistic resonance of BP surges with different time phases generates a large dynamic BP surge. In SHATS, the power of the exaggerated pulse transmits peripheral vulnerable plaques and/or microcirculation without attenuation, triggering the onset of a cardiovascular event and enhancing age-related disease. Practically, strict morning BP control guided by home BP monitoring is recommended, especially in individuals with vascular disease. In the future, innovations in the design of wearable continuous surge BP monitoring devices and the simultaneous ICT-based assessment of the resonance of all of the BPV phenotypes will change the management of hypertension, resulting in the ultimate personalized prevention of cardiovascular events.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Lawes CM, Bennett DA, Parag V, et al. Asia Pacific Cohort Studies Collaboration. Blood pressure indices and cardiovascular disease in the Asia Pacific region: a pooled analysis. Hypertension. 2003;42:69–75. doi: 10.1161/01.HYP.0000075083.04415.4B. [DOI] [PubMed] [Google Scholar]
- 2.Ueshima H, Sekikawa A, Miura K, et al. Cardiovascular disease and risk factors in Asia: a selected review. Circulation. 2008;118:2702–2709. doi: 10.1161/CIRCULATIONAHA.108.790048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoshide S, Wang J, Park SH, et al. Treatment considerations of clinical physician on hypertension management in Asia. Curr Hypertens Rev. 2016;12:164–168. doi: 10.2174/1573402111666150812143155. [DOI] [PubMed] [Google Scholar]
- 4.Park JB, Kario K, Wang JG. Systolic hypertension: an increasing clinical challenge in Asia. Hypertens Res. 2015;38:227–236. doi: 10.1038/hr.2014.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimamoto K, Ando K, Fujita T, et al. Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014) Hypertens Res. 2014;37:253–390. doi: 10.1038/hr.2014.20. [DOI] [PubMed] [Google Scholar]
- 6.Kario K. Essential manual of 24-hour blood pressure management from morning to nocturnal hypertension. London, UK: Wiley-Blackwell; 2015. pp. 1–138. [Google Scholar]
- 7.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354:2368–2374. doi: 10.1056/NEJMra060433. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien E, Parati G, Stergiou G, et al. European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–1768. doi: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- 9.Imai Y, Ohkubo T, Sakuma M, et al. Predictive power of screening blood pressure, ambulatory blood pressure and blood pressure measured at home for overall and cardiovascular mortality: a prospective observation in a cohort from Ohasama, northern Japan. Blood Press Monit. 1996;1:251–254. [PubMed] [Google Scholar]
- 10.Kario K, Shimada K, Schwartz JE, Matsuo T, Hoshide S, Pickering TG. Silent and clinically overt stroke in older Japanese subjects with white-coat and sustained hypertension. J Am Coll Cardiol. 2001;38:238–245. doi: 10.1016/s0735-1097(01)01325-0. [DOI] [PubMed] [Google Scholar]
- 11.Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. doi: 10.1161/01.cir.0000056521.67546.aa. [DOI] [PubMed] [Google Scholar]
- 12.Clement DL, De Buyzere ML, De Bacquer DA, et al. Office versus Ambulatory Pressure Study Investigators. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–2415. doi: 10.1056/NEJMoa022273. [DOI] [PubMed] [Google Scholar]
- 13.Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of "masked" hypertension and "white-coat" hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46:508–515. doi: 10.1016/j.jacc.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 14.Kario K, Okura A, Okawara Y, Tomitani N, Ikemoto T, Hoshide S. Impact of introducing catheter-based renal denervation into Japan for hypertension management: Estimation of number of target patients and clinical relevance of ambulatory blood pressure reduction. Curr Hypertens Rev. 2016;12:156–163. doi: 10.2174/1573402112666160302102841. [DOI] [PubMed] [Google Scholar]
- 15.Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol. 2013;10:143–155. doi: 10.1038/nrcardio.2013.1. [DOI] [PubMed] [Google Scholar]
- 16.Kario K. Prognosis in relation to blood pressure variability: Pro side of the argument. Hypertension. 2015;65:1163–1169. doi: 10.1161/HYPERTENSIONAHA.115.04800. [DOI] [PubMed] [Google Scholar]
- 17.Kario K. New insight of morning blood pressure surge into the triggers of cardiovascular disease-synergistic resonance of blood pressure variability. Am J Hypertens. 2016;29:14–16. doi: 10.1093/ajh/hpv114. [DOI] [PubMed] [Google Scholar]
- 18.Kario K. Orthostatic hypertension-a new haemodynamic cardiovascular risk factor. Nat Rev Nephrol. 2013;9:726–738. doi: 10.1038/nrneph.2013.224. [DOI] [PubMed] [Google Scholar]
- 19.Kario K. Proposal of a new strategy for ambulatory blood pressure profile-based management of resistant hypertension in the era of renal denervation. Hypertens Res. 2013;36:478–484. doi: 10.1038/hr.2013.19. [DOI] [PubMed] [Google Scholar]
- 20.Kario K. Systemic hemodynamic atherothrombotic syndrome: a blind spot in the current management of hypertension. J Clin Hypertens (Greenwich) 2015;17:328–331. doi: 10.1111/jch.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kario K. Morning surge in blood pressure: a phenotype of systemic hemodynamic atherothrombotic syndrome. Am J Hypertens. 2015;28:7–9. doi: 10.1093/ajh/hpu179. [DOI] [PubMed] [Google Scholar]
- 22.Kario K, Hamasaki H. Nocturnal Blood Pressure Surge Behind Morning Surge in Obstructive Sleep apnea syndrome: another phenotype of systemic hemodynamic atherothrombotic syndrome. J Clin Hypertens (Greenwich) 2015;17:682–685. doi: 10.1111/jch.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kikuya M, Ohkubo T, Metoki H, et al. Day by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis: the Ohasama study. Hypertension. 2008;52:1045–1050. doi: 10.1161/HYPERTENSIONAHA.107.104620. [DOI] [PubMed] [Google Scholar]
- 24.Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 25.Matsui Y, Ishikawa J, Eguchi K, Shibasaki S, Shimada K, Kario K. Maximum value of home blood pressure: a novel indicator of target organ damage in hypertension. Hypertension. 2011;57:1087–1093. doi: 10.1161/HYPERTENSIONAHA.111.171645. [DOI] [PubMed] [Google Scholar]
- 26.Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension. 2011;57:160–166. doi: 10.1161/HYPERTENSIONAHA.110.162255. [DOI] [PubMed] [Google Scholar]
- 27.Matsui Y, O'Rourke MF, Hoshide S, Ishikawa J, Shimada K, Kario K. Combined effect of angiotensin II receptor blocker and either a calcium channel blocker or diuretic on day-by-day variability of home blood pressure: the Japan Combined Treatment With Olmesartan and a Calcium-Channel Blocker Versus Olmesartan and Diuretics Randomized Efficacy Study. Hypertension. 2012;59:1132–1138. doi: 10.1161/HYPERTENSIONAHA.111.189217. [DOI] [PubMed] [Google Scholar]
- 28.Eguchi K, Hoshide S, Schwartz JE, Shimada K, Kario K. Visit-to-visit and ambulatory blood pressure variability as predictors of incident cardiovascular events in patients with hypertension. Am J Hypertens. 2012;25:962–968. doi: 10.1038/ajh.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Visit-to-visit blood pressure variations: new independent determinants for cognitive function in the elderly at high risk of cardiovascular disease. J Hypertens. 2012;30:1556–1563. doi: 10.1097/HJH.0b013e3283552735. [DOI] [PubMed] [Google Scholar]
- 30.Nagai M, Hoshide S, Nishikawa M, Shimada K, Kario K. Sleep duration and insomnia in the elderly: associations with blood pressure variability and carotid artery remodeling. Am J Hypertens. 2013;26:981–989. doi: 10.1093/ajh/hpt070. [DOI] [PubMed] [Google Scholar]
- 31.Nagai M, Kario K. Visit-to-visit blood pressure variability, silent cerebral injury, and risk of stroke. Am J Hypertens. 2013;26:1369–1376. doi: 10.1093/ajh/hpt167. [DOI] [PubMed] [Google Scholar]
- 32.Yokota K, Fukuda M, Matsui Y, Hoshide S, Shimada K, Kario K. Impact of visit-to-visit variability of blood pressure on deterioration of renal function in patients with non-diabetic chronic kidney disease. Hypertens Res. 2013;36:151–157. doi: 10.1038/hr.2012.145. [DOI] [PubMed] [Google Scholar]
- 33.Kawai T, Ohishi M, Ito N, et al. Alteration of vascular function is an important factor in the correlation between visit-to-visit blood pressure variability and cardiovascular disease. J Hypertens. 2013;31:1387–1395. doi: 10.1097/HJH.0b013e328360f796. [DOI] [PubMed] [Google Scholar]
- 34.Kawai T, Ohishi M, Kamide K, et al. Differences between daytime and nighttime blood pressure variability regarding systemic atherosclerotic change and renal function. Hypertens Res. 2013;36:232–239. doi: 10.1038/hr.2012.162. [DOI] [PubMed] [Google Scholar]
- 35.Fukui M, Ushigome E, Tanaka M, et al. Home blood pressure variability on one occasion is a novel factor associated with arterial stiffness in patients with type 2 diabetes. Hypertens Res. 2013;36:219–225. doi: 10.1038/hr.2012.177. [DOI] [PubMed] [Google Scholar]
- 36.Endo K, Kario K, Koga M, et al. Impact of early blood pressure variability on stroke outcomes after thrombolysis: the SAMURAI rt-PA Registry. Stroke. 2013;44:816–818. doi: 10.1161/STROKEAHA.112.681007. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka E, Koga M, Kobayashi J, et al. Blood pressure variability on antihypertensive therapy in acute intracerebral hemorrhage: the Stroke Acute Management with Urgent Risk-factor Assessment and Improvement-intracerebral hemorrhage study. Stroke. 2014;45:2275–2279. doi: 10.1161/STROKEAHA.114.005420. [DOI] [PubMed] [Google Scholar]
- 38.Wei FF, Li Y, Zhang L, et al. Beat-to-beat, reading-to-reading, and day-to-day blood pressure variability in relation to organ damage in untreated Chinese. Hypertension. 2014;63:790–796. doi: 10.1161/HYPERTENSIONAHA.113.02681. [DOI] [PubMed] [Google Scholar]
- 39.Nagai M, Hoshide S, Nishikawa M, Masahisa S, Kario K. Visit-to-visit blood pressure variability in the elderly: associations with cognitive impairment and carotid artery remodeling. Atherosclerosis. 2014;233:19–26. doi: 10.1016/j.atherosclerosis.2013.11.071. [DOI] [PubMed] [Google Scholar]
- 40.Palatini P, Reboldi G, Beilin LJ, et al. Added predictive value of nighttime blood pressure variability for cardiovascular events and mortality: the Ambulatory Blood Pressure-International Study. Hypertension. 2014;64:487–493. doi: 10.1161/HYPERTENSIONAHA.114.03694. [DOI] [PubMed] [Google Scholar]
- 41.Kagitani H, Hoshide S, Kario K. Optimal indicators of home BP variability in perimenopausal women and associations with albuminuria and reproducibility: The J-HOT home BP study. Am J Hypertens. 2015;28:586–594. doi: 10.1093/ajh/hpu210. [DOI] [PubMed] [Google Scholar]
- 42.Rakugi H, Ogihara T, Saruta T, et al. Preferable effects of olmesartan/calcium channel blocker to olmesartan/diuretic on blood pressure variability in very elderly hypertension: COLM study subanalysis. J Hypertens. 2015;33:2165–2172. doi: 10.1097/HJH.0000000000000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shibasaki S, Hoshide S, Eguchi K, Ishikawa J, Kario K Japan Morning Surge-Home Blood Pressure (J-HOP) Study Group. Increase trend in home blood pressure on a single occasion is associated With B-type natriuretic peptide and the estimated glomerular filtration rate. Am J Hypertens. 2015;28:1098–1105. doi: 10.1093/ajh/hpu295. [DOI] [PubMed] [Google Scholar]
- 44.Liu Z, Zhao Y, Zhang H, et al. Excessive variability in systolic blood pressure that is self-measured at home exacerbates the progression of brain white matter lesions and cognitive impairment in the oldest old. Hypertens Res. 2016;39:245–253. doi: 10.1038/hr.2015.135. [DOI] [PubMed] [Google Scholar]
- 45.Kario K, Tomitani N, Matsumoto Y, et al. Research and development of information and communication technology-based home blood pressure monitoring from morning to nocturnal hypertension. Ann Glob Health. 2016;82:254–273. doi: 10.1016/j.aogh.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Kario K, Eguchi K, Nakagawa Y, Motai K, Shimada K. Relationship between extreme dippers and orthostatic hypertension in elderly hypertensive patients. Hypertension. 1998;31:77–82. doi: 10.1161/01.hyp.31.1.77. [DOI] [PubMed] [Google Scholar]
- 47.Kario K, Eguchi K, Hoshide S, et al. U-curve relationship between orthostatic blood pressure change and silent cerebrovascular disease in elderly hypertensives: orthostatic hypertension as a new cardiovascular risk factor. J Am Coll Cardiol. 2002;40:133–141. doi: 10.1016/s0735-1097(02)01923-x. [DOI] [PubMed] [Google Scholar]
- 48.Kario K. Orthostatic hypertension: a measure of blood pressure variation for predicting cardiovascular risk. Circ J. 2009;73:1002–1007. doi: 10.1253/circj.cj-09-0286. [DOI] [PubMed] [Google Scholar]
- 49.Ito S, Nagasawa T, Abe M, Mori T. Strain vessel hypothesis: a viewpoint for linkage of albuminuria and cerebro-cardiovascular risk. Hypertens Res. 2009;32:115–121. doi: 10.1038/hr.2008.27. [DOI] [PubMed] [Google Scholar]
- 50.Metoki H, Ohkubo T, Kikuya M, Asayama K, Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. Prognostic significance for stroke of a morning pressor surge and a nocturnal blood pressure decline: the Ohasama study. Hypertension. 2006;47:149–154. doi: 10.1161/01.HYP.0000198541.12640.0f. [DOI] [PubMed] [Google Scholar]
- 51.Nakase T, Yoshioka S, Sasaki M, Suzuki A. Clinical evaluation of lacunar infarction and branch atheromatous disease. J Stroke Cerebrovasc Dis. 2013;22:406–412. doi: 10.1016/j.jstrokecerebrovasdis.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Kario K, Pickering TG. Blood pressure variability in elderly patients. Lancet. 2000;355:1645–1646. doi: 10.1016/S0140-6736(05)72549-6. [DOI] [PubMed] [Google Scholar]
- 53.Matsui Y, Eguchi K, Shibasaki S, et al. Japan morning Surge-1 Study Group. Impact of arterial stiffness reduction on urinary albumin excretion during antihypertensive treatment: the Japan morning Surge-1 study. J Hypertens. 2010;28:1752–1760. doi: 10.1097/HJH.0b013e32833a3981. [DOI] [PubMed] [Google Scholar]
- 54.Kario K. Morning surge in blood pressure and cardiovascular risk – evidence and perspectives. Hypertension. 2010;56:765–773. doi: 10.1161/HYPERTENSIONAHA.110.157149. [DOI] [PubMed] [Google Scholar]
- 55.Li Y, Thijs L, Hansen TW, et al. International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes Investigators. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension. 2010;55:1040–1048. doi: 10.1161/HYPERTENSIONAHA.109.137273. [DOI] [PubMed] [Google Scholar]
- 56.Luo Y, Wang YL, Wu YB, et al. Association between the rate of the morning surge in blood pressure and cardiovascular events and stroke. Chin Med J (Engl) 2013;126:510–514. [PubMed] [Google Scholar]
- 57.Verdecchia P, Angeli F, Mazzotta G, et al. Day-night dip and early-morning surge in blood pressure in hypertension: prognostic implications. Hypertension. 2012;60:34–42. doi: 10.1161/HYPERTENSIONAHA.112.191858. [DOI] [PubMed] [Google Scholar]
- 58.Bombelli M, Fodri D, Toso E, et al. Relationship among morning blood pressure surge, 24-hour blood pressure variability, and cardiovascular outcomes in a white population. Hypertension. 2014;64:943–950. doi: 10.1161/HYPERTENSIONAHA.114.03675. [DOI] [PubMed] [Google Scholar]
- 59.Hoshide S, Kario K, de la Sierra A, et al. Ethnic differences in the degree of morning blood pressure surge and in its determinants between Japanese and European hypertensive subjects: data from the ARTEMIS study. Hypertension. 2015;66:750–756. doi: 10.1161/HYPERTENSIONAHA.115.05958. [DOI] [PubMed] [Google Scholar]
- 60.Kario K. Caution for winter morning surge in blood pressure: a possible link with cardiovascular risk in the elderly. Hypertension. 2006;47:139–140. doi: 10.1161/01.HYP.0000199162.89857.7a. [DOI] [PubMed] [Google Scholar]
- 61.Murakami S, Otsuka K, Kubo Y, et al. Repeated ambulatory monitoring reveals a Monday morning surge in blood pressure in a community-dwelling population. Am J Hypertens. 2004;17:1179–1183. doi: 10.1016/j.amjhyper.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 62.Kario K. Clinician's manual on early morning risk management in hypertension. London, UK: Science Press; 2004. pp. 1–68. [Google Scholar]
- 63.Kario K, Ishikawa J, Pickering TG, et al. Morning hypertension: the strongest independent risk factor for stroke in elderly hypertensive patients. Hypertens Res. 2006;29:581–587. doi: 10.1291/hypres.29.581. [DOI] [PubMed] [Google Scholar]
- 64.Kario K, Eguchi K, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: response. Circulation. 2003;108:e72–e73. doi: 10.1161/01.CIR.0000089096.13946.CB. [DOI] [PubMed] [Google Scholar]
- 65.Hoshide S, Kario K, Yano Y, et al. J-HOP study group. Association of morning and evening blood pressure at home with asymptomatic organ damage in the J-HOP Study. Am J Hypertens. 2014;27:939–947. doi: 10.1093/ajh/hpt290. [DOI] [PubMed] [Google Scholar]
- 66.Kario K, Saito I, Kushiro T, et al. Home blood pressure and cardiovascular outcomes in patients during antihypertensive therapy: primary results of HONEST, a large-scale prospective, real-world observational study. Hypertension. 2014;64:989–996. doi: 10.1161/HYPERTENSIONAHA.114.04262. [DOI] [PubMed] [Google Scholar]
- 67.Kario K. Benefits of strict blood-pressure lowering in hypertension. Nat Rev Cardiol. 2016;13:125–126. doi: 10.1038/nrcardio.2016.13. [DOI] [PubMed] [Google Scholar]
- 68.Kario K, Saito I, Kushiro T, et al. Morning home blood pressure is a strong predictor of coronary artery disease. J Am Coll Cardiol. 2016;67:1519–1527. doi: 10.1016/j.jacc.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 69.Panza JA, Epstein SE, Quyyumi AA. Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N Engl J Med. 1991;325:986–990. doi: 10.1056/NEJM199110033251402. [DOI] [PubMed] [Google Scholar]
- 70.Kario K, Pickering TG, Hoshide S, et al. Morning blood pressure surge and hypertensive cerebrovascular disease: role of the alpha adrenergic sympathetic nervous system. Am J Hypertens. 2004;17:668–675. doi: 10.1016/j.amjhyper.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 71.Kario K, Matsui Y, Shibasaki S, et al. Japan Morning Surge-1 (JMS-1) Study Group. Japan Morning Surge-1 (JMS-1) Study Group. An alpha-adrenergic blocker titrated by self-measured blood pressure recordings lowered blood pressure and microalbuminuria in patients with morning hypertension: the Japan Morning Surge-1 Study. J Hypertens. 2008;26:1257–1265. doi: 10.1097/HJH.0b013e3282fd173c. [DOI] [PubMed] [Google Scholar]
- 72.Krum H, Schlaich MP, Sobotka PA, et al. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet. 2014;383:622–629. doi: 10.1016/S0140-6736(13)62192-3. [DOI] [PubMed] [Google Scholar]
- 73.Esler MD, Krum H, Schlaich M, et al. Symplicity HTN-2 Investigators. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126:2976–2982. doi: 10.1161/CIRCULATIONAHA.112.130880. [DOI] [PubMed] [Google Scholar]
- 74.Hering D, Lambert EA, Marusic P, et al. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61:457–464. doi: 10.1161/HYPERTENSIONAHA.111.00194. [DOI] [PubMed] [Google Scholar]
- 75.Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361:932–934. doi: 10.1056/NEJMc0904179. [DOI] [PubMed] [Google Scholar]
- 76.Kario K, Ogawa H, Okumura K, et al. SYMPLICITY HTN-Japan Investigators. SYMPLICITY HTN-Japan—first randomized controlled trial of catheterbased renal denervation in Asian patients. Circ J. 2015;79:1222–1229. doi: 10.1253/circj.CJ-15-0150. [DOI] [PubMed] [Google Scholar]
- 77.Bhatt DL, Kandzari DE, O'Neill WW, et al. SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 78.Kario K, Bhatt DL, Brar S, Cohen SA, Fahy M, Bakris GL. Effect of Catheter-Based Renal Denervation on Morning and Nocturnal Blood Pressure: Insights From SYMPLICITY HTN-3 and SYMPLICITY HTN-Japan. Hypertension. 2015;66:1130–1137. doi: 10.1161/HYPERTENSIONAHA.115.06260. [DOI] [PubMed] [Google Scholar]
- 79.Kario K. Obstructive sleep apnea syndrome and hypertension: ambulatory blood pressure. Hypertens Res. 2009;32:428–432. doi: 10.1038/hr.2009.56. [DOI] [PubMed] [Google Scholar]
- 80.Shirasaki O, Kuwabara M, Saito M, Tagami K, Washiya S, Kario K. Development and clinical application of a new technique for detecting 'sleep blood pressure surges' in sleep apnea patients based on a variable desaturation threshold. Hypertens Res. 2011;34:922–928. doi: 10.1038/hr.2011.52. [DOI] [PubMed] [Google Scholar]
- 81.Kario K, Kuwabara M, Hoshide S, Nagai M, Shimpo M. Effects of nighttime single-dose administration of vasodilating vs. sympatholytic antihypertensive agents on sleep blood pressure in hypertensive patients with sleep apnea syndrome. J Clin Hypertens (Greenwich) 2014;16:459–466. doi: 10.1111/jch.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuwabara M, Kario K. Development of a triggered nocturnal blood pressure monitoring which detects nighttime blood pressure surges in sleep apnea syndrome. Curr Hypertens Rev. 2016;12:27–31. doi: 10.2174/1573402112666160114093500. [DOI] [PubMed] [Google Scholar]
- 83.Yoshida T, Kuwabara M, Hoshide S, Kario K. Recurrence of stroke caused by nocturnal hypoxia-induced blood pressure surge in a young adult male with severe obstructive sleep apnea syndrome. J Am Soc Hypertens. 2016;10:201–204. doi: 10.1016/j.jash.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 84.Kario K, Ikemoto T, Kuwabara M, Ishiyama H, Saito K, Hoshide S. Catheter-based renal denervation reduces hypoxia-triggered nocturnal blood pressure peak in obstructive sleep apnea syndrome. J Clin Hypertens (Greenwich) 2015 doi: 10.1111/jch.12759. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kario K, Bhatt DL, Kandzari DE, et al. Impact of renal denervation on patients with obstructive sleep apnea and resistant hypertension: insights from the SYMPLICITY HTN-3 trial. Circ J. 2016;80:1404–1412. doi: 10.1253/circj.CJ-16-0035. [DOI] [PubMed] [Google Scholar]
- 86.Kim BK, Böhm M, Mahfoud F, et al. Renal denervation for treatment of uncontrolled hypertension in an Asian population: results from the Global SYMPLICITY Registry in South Korea (GSR Korea) J Hum Hypertens. 2016;30:315–321. doi: 10.1038/jhh.2015.77. [DOI] [PubMed] [Google Scholar]
- 87.Kandzari D, Kario K, Mahfoud F, et al. The SPYRAL HTN global clinical trial program: rationale and design for studies of renal denervation in the absence (SPYRAL HTN OFF-MED) and presence (SPYRAL HTN ON-MED) of antihypertensive medications. Am Heart J. 2016;171:82–91. doi: 10.1016/j.ahj.2015.08.021. [DOI] [PubMed] [Google Scholar]