Abstract
Background and Objectives
In Kawasaki disease (KD), high dose intravenous immunoglobulin (IVIG) significantly lowers the coronary complications. However, some patients either do not respond to initial therapy or develop coronary complications. We aimed to identify the predictive factors for unresponsiveness to initial IVIG therapy and coronary artery dilatation (CAD; defined by Z-score≥2.5) in the acute phase and convalescent phase.
Subjects and Methods
A retrospective review was conducted of 703 patients with KD, admitted to Gachon University Gil Medical Center between January 2005 and June 2013. The patients were divided into two groups—IVIG responders vs. non-responders—based on the IVIG treatments, and presence of fever after treatment. Further, these groups were divided into two subgroups based on their CAD.
Results
Among the 703 patients with KD, the rate of non-responders to initial IVIG was 16.8%. Serum total bilirubin, platelet count, and neutrophil proportion were independent predictive parameters of unresponsiveness (p<0.05). CAD was found in 234 patients (33.3%) in the acute phase, and in 32 patients (4.6%) in the convalescent phase. Male gender, fever duration, serum C-reactive protein, and white blood cell count were related to CAD (p<0.05). CAD was detected more frequently in non-responders than in the responders (47.5% vs. 31.5%, p=0.001). Kobayashi, Egami, and Sano scoring systems applied to our study population reflected low sensitivities (28.0-33.9%).
Conclusion
Several independent parameters were related to unresponsiveness to the initial IVIG or CAD. These parameters might be helpful in establishing more focused and careful monitoring of high-risk KD patients in Korea.
Keywords: Kawasaki disease, Intravenous immunoglobulins, Coronary arteries, Children
Introduction
In developed countries, Kawasaki disease (KD), an acute, systemic vasculitis first reported in 1967, is a leading cause of acquired heart disease in children.1) In most patients, the standard treatment of intravenous immunoglobulin (IVIG) and aspirin diminishes the inflammation, enough to prevent the development of coronary artery complications.2) However, about 10-20% of KD patients have persistent or recrudescent fever, despite the initial IVIG therapy.2),3) These patients are at increased risk of developing coronary artery complications.4) Therefore, many studies have been conducted with the focus on early identification of patients who do not respond to IVIG. The Kobayashi,5) Egami,6) and Sano7) scoring systems have identified factors such as patient age, fever duration, proportion of neutrophils, platelet count, and the serum levels of sodium, C-reactive protein (CRP), total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT).5),6),7) Nevertheless, there is no current consensus regarding the factors for predicting patients not responsive to IVIG. Thus, the aim of this study was to identify the predictive factors for nonresponse to IVIG and coronary artery dilatation (CAD), by means of a large-scale, single-center study in Korea.
Subjects and Methods
Subjects
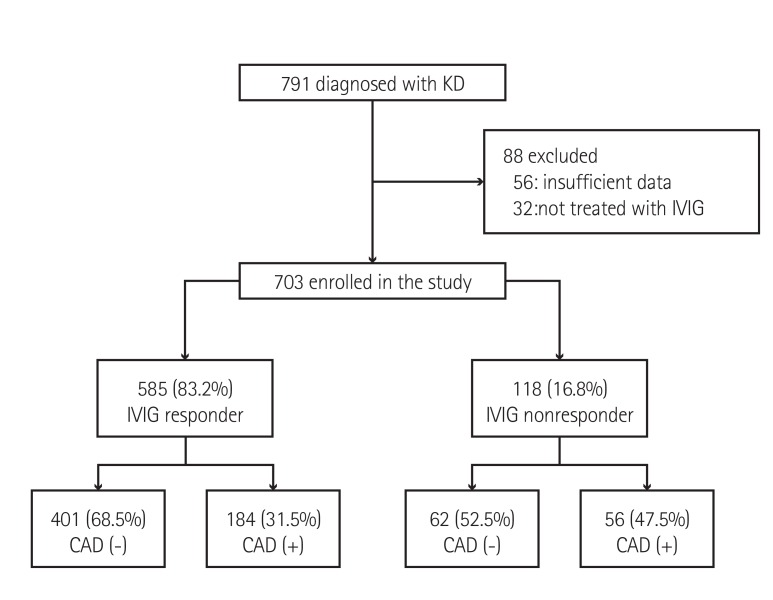
The subjects of this study were patients diagnosed with KD and treated at the Department of Pediatrics, Gachon University Gil Medical Center, Incheon, Korea, from January 2005 to June 2013. During the study period, 791 patients were diagnosed with KD, 88 of who were excluded due to the following reasons: the data were insufficient in 56 patients, and 32 patients had no fever, and therefore, had not received IVIG treatment (Fig. 1). The remaining 703 patients were analyzed in this study. The enrolled patients had both typical and atypical KD. We reviewed the medical records of all the enrolled patients retrospectively, including clinical characteristics, laboratory findings at the time of admission, response to treatment, and echocardiographic findings. The laboratory data were converted to categorical variables based on references from the previous scoring systems (Kobayashi,5) Egami,6) and Sano7)). The patients were classified into two groups, based to their response to IVIG treatment: responders and non-responders. The responder group included patients who received a single dose of IVIG (2 g/kg) treatment and had no reappearance of fever 48 hours after IVIG, while the non-responder group included patients who received more than one dose of IVIG due to persistent or recrudescent fever. The patients were divided further, according to the presence of CAD, in order to identify the potential predictive factors of CAD.
Fig. 1. Flowchart of enrollment and classification of the subjects. KD: Kawasaki disease, IVIG: intravenous immunoglobulin, CAD: coronary artery dilatation.
Ethics statement
This study was approved by the Institutional Review Board (IRB) of Gachon University Gil Medical Center (IRB No. GBIRB2015-175).
Echocardiography
Two-dimensional echocardiography was performed at the time of diagnosis (defined as acute phase) and repeated approximately 6-8 weeks after diagnosis (defined as convalescent phase). Internal diameters of the right coronary artery (RCA), left main coronary artery (LMCA), left anterior descending artery (LAD), and left circumflex artery (LCX) were measured and converted to Z-scores based on the patients' body weight, using the Montreal calculator.8) At the time of data collection, we re-measured the internal diameters of the coronary arteries of all the patients and obtained the mean values of the previously measured and re-measured values to increase the reliability of CAD. CAD was defined as Z-score>2.5 in the RCA, LMCA, LAD, and/or LCX, and it was classified into small (2.5≤Z<5), large (5≤Z<10), or giant (Z≥10) dilatation.9)
Statistical analysis
All data were analyzed with the Statistical Package for the Social Sciences for Windows software, version 12.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as either mean±standard deviation, or the number (percentage). The difference between the two groups were analyzed using an unpaired t-test or a Chi-square test. Multivariate logistic regression analysis was used to determine the independent predictive parameters of nonresponse to IVIG and CAD. These results were expressed as an adjusted odds ratio with a 95% confidence interval (CI). A 95% CI that did not include 1.0 was interpreted as statistically significant. Values were considered significantly different at p<0.05.
Results
The ages of the 703 patients at diagnosis ranged from 2 months to 9 years (mean age, 31.9 months), and the male to female ratio was 1.3:1.0. All the enrolled patients were treated initially with 2 g/kg IVIG, and medium-dose aspirin (50 mg/kg/day) was administered for one week, after which the dose was reduced to 5 mg/kg/day. The initial IVIG was infused within the mean 5.8 days of illness, where illness day 1 was defined as the first day of fever.
Prediction of intravenous immunoglobulin non-responders
Among the 703 patients, 118 (16.8%) did not respond to the initial IVIG. All of the IVIG non-responders were administered additional IVIG doses (113 patients with one additional dose and 5 patients with two additional doses); in addition, 47 patients (39.8%) were given steroids and 2 patients (1.7%) were given oral methotrexate.
Comparison of the clinical characteristics and laboratory findings are shown in Table 1. Total duration of fever and duration of fever after IVIG treatment were longer in the non-responder group (p<0.001 and p<0.001). There were no differences in gender, age, or duration of fever before IVIG treatment between the IVIG responders and non-responders. Serum levels of total bilirubin, AST, and ALT, and proportion of neutrophils were significantly higher (p<0.05), and serum level of sodium was significantly lower, in the non-responder group (p<0.001). There were no significant differences in serum levels of albumin and CRP, or the platelet count between the two groups. When the variables were categorized, serum sodium, total bilirubin, AST, ALT, albumin, platelet count, and proportion of neutrophils were significantly different between the groups (p<0.05), but no significant differences in CRP or white blood cell (WBC) count were found. The most comparable cutoff value between the two groups for each variable is presented in Table 1.
Table 1. Clinical characteristics and laboratory findings of IVIG responders and non-responders.
| Responders (n=585) | Non-responders (n=118) | p* | |
|---|---|---|---|
| Clinical characteristics | |||
| Male sex (%) | 339 (57.9) | 62 (52.5) | 0.946 |
| Age (month) | 31.62±22.71 | 33.31±21.97 | 0.457 |
| Fever duration before IVIG (day) | 5.87±1.87 | 5.65±2.16 | 0.265 |
| Fever duration after IVIG (day) | 0.52±0.89 | 3.23±2.28 | <0.001 |
| Total fever duration (day) | 6.38±1.96 | 8.86±3.06 | <0.001 |
| Laboratory findings (continuous variables) | |||
| Sodium (mmol/L) | 137.18±2.62 | 136.16±2.55 | <0.001 |
| Total bilirubin (mg/dL) | 0.56±0.63 | 1.02±1.03 | <0.001 |
| AST (IU/L) | 70.36±129.25 | 118.89±214.53 | 0.019 |
| ALT (IU/L) | 73.03±124.87 | 125.51±175.94 | 0.002 |
| Albumin (g/dL) | 4.13±1.22 | 3.91±0.48 | 0.056 |
| CRP (mg/dL) | 6.47±6.05 | 7.45±5.93 | 0.106 |
| Hct (%) | 33.03±2.73 | 32.79±3.26 | 0.402 |
| WBC (×103/µL) | 13.19±5.24 | 13.75±6.34 | 0.370 |
| Platelet (×103/µL) | 351.32±120.23 | 333.45±160.52 | 0.253 |
| Neutrophil (%) | 56.78±18.47 | 64.23±17.83 | <0.001 |
| Laboratory findings (categorical variables) | |||
| Sodium<134 (mmol/L) | 46 (7.9) | 16 (13.6) | 0.047 |
| Total bilirubin ≥0.9 (mg/dL) | 65 (11.1) | 40 (33.9) | <0.001 |
| AST≥80 (IU/L) | 94 (16.1) | 44 (37.3) | <0.001 |
| ALT≥45 (IU/L) | 191 (32.6) | 62 (52.5) | <0.001 |
| Albumin<3.5 (g/dL) | 38 (6.5) | 21 (17.8) | <0.001 |
| CRP≥8 (mg/dL) | 175 (29.9) | 46 (39.0) | 0.053 |
| Hct<31 (%) | 120 (20.5) | 31 (26.3) | 0.165 |
| WBC>12 (×103/µL) | 323 (55.2) | 65 (55.1) | 0.980 |
| Platelet<300 (×103/µL) | 203 (34.7) | 54 (45.8) | 0.023 |
| Neutrophil≥80 (%) | 49 (8.4) | 25 (21.2) | <0.001 |
Data are presented as either mean±standard deviation or percentage. *Analyzed by an unpaired t-test or a Chi-square test. IVIG: intravenous immunoglobulin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, CRP: C-reactive protein, Hct: hematocrit, WBC: white blood cell
Multivariate logistic regression analysis was conducted using the significantly different variables mentioned above. Total bilirubin≥0.9 mg/dL (odds ratio [OR]=2.443; 95% CI: 1.364-4.374; p=0.003), platelet count<300×103/µL (OR=1.532; 95% CI: 1.007–2.332; p=0.046), and proportion of neutrophils≥80% (OR=1.812; 95% CI: 1.008-3.257; p=0.047) were significantly different between the two groups (Table 2).
Table 2. Multivariate logistic regression analysis for predictors of IVIG non-responders.
| OR (95% CI) | p | |
|---|---|---|
| Sodium<134 (mmol/L) | 0.907 (0.454–1.812) | 0.782 |
| Total bilirubin≥0.9 (mg/dL) | 2.443 (1.364–4.374) | 0.003 |
| AST≥80 (IU/L) | 1.704 (0.901–3.225) | 0.101 |
| ALT≥45 (IU/L) | 1.011 (0.559–1.829) | 0.970 |
| Albumin<3.5 (g/dL) | 1.676 (0.857–3.276) | 0.131 |
| Platelet<300 (×103/µL) | 1.532 (1.007–2.332) | 0.046 |
| Neutrophil≥80 (%) | 1.812 (1.008–3.257) | 0.047 |
IVIG: intravenous immunoglobulin, OR: odds ratio, CI: confidence interval, AST: aspartate aminotransferase, ALT: alanine aminotransferase
When the Kobayashi,5) Egami,6) and Sano7) scoring systems were applied to the patients in this study, the mean scores were significantly higher in the IVIG non-responder group (Table 3). The sensitivity of each scoring system was low (31.4%, 33.9%, and 28.0%, respectively) and the specificity was high (87.0%, 87.0%, and 91.6%, respectively).
Table 3. Comparison of the risk scores by Kobayashi,5) Egami,6) and Sano7) scoring systems.
| Responders (n=585) | Non-responders (n=118) | p* | |
|---|---|---|---|
| Kobayashi score5) | 1.73±1.61 | 2.60±2.03 | <0.001 |
| Low risk (0-3) | 509 (87.0)† | 81 (68.6) | |
| High risk (4-6) | 76 (13.0) | 37 (31.4)‡ | |
| Egami score6) | 1.22±1.09 | 1.84±1.38 | <0.001 |
| Low risk (0-2) | 509 (87.0)† | 78 (66.1) | |
| High risk (3-5) | 76 (13.0) | 40 (33.9)‡ | |
| Sano score7) | 0.52±0.70 | 0.91±0.92 | <0.001 |
| Low risk (0-1) | 536 (91.6)† | 85 (72.0) | |
| High risk (2-3) | 49 (8.4) | 33 (28.0)‡ |
Data are presented as either mean±standard deviation or n (%). *Analyzed by an unpaired t-test. †Specificity and ‡sensitivity of each scoring system. The parameters of Kobayashi score5) are as follows: 1) sodium ≤133 mmol/L, 2 points, 2) days of illness at initial treatment≤4 days, 2 points, 3) AST≥100 IU/L, 2 points, 4) % of neutrophil≥80, 2 points, 5) CRP≥10 mg/dL, 1 point, 6) age≤12 months, 1 point, and 7) platelet count≤300×103/mm3, 1 point. The parameters of Egami score6) are as follows: 1) ALT≥80 IU/L, 2 points, 2) illness days≤4 days, 1 point, 3) CRP≥8 mg/dL, 1 point, 4) age≤6 months, 1 point, and 5) platelet count≤300×103/mm3, 1 point. The Sano scoring system7) is as follows: 1) AST≥200 IU/L, 1 point, 2) CRP≥7 mg/dL, 1 point, and 3) total bilirubin≥0.9 mg/dL, 1 point. AST: aspartate aminotransferase, ALT: alanine aminotransferase, CRP: C-reactive protein
Prediction of coronary artery dilatation
CAD was found in 234 patients (33.3%) in the acute phase and in 32 patients (4.6%) in the convalescent phase. Initial echocardiographs were performed at the mean 6.3 days of illness. The more problematic arteries were the LMCA and RCA: LMCA and RCA dilatations were identified in 75.8% and 31.0%, respectively, of the 240 patients. The patients were further divided into two groups, based on the presence of CAD. When the clinical characteristics were compared, the CAD group had a significantly higher proportion of males, lower mean age, and longer total fever duration, than the group of patients without CAD (p<0.05) (Table 4). Significant differences in serum levels of sodium, total bilirubin, and CRP, WBC count, platelet count, and proportion of neutrophils were found between the two groups (p<0.05). When compared as categorical variables, only serum CRP level and WBC count were significantly higher in the patients with CAD (p<0.05).
Table 4. Clinical characteristics and laboratory findings of CAD (-) and CAD (+) groups.
| CAD (-) group (n=463) | CAD (+) group (n=240) | p* | |
|---|---|---|---|
| Clinical characteristics | |||
| Male sex (%) | 245 (52.9) | 156 (65.0) | 0.002 |
| Age (month) | 33.61±23.65 | 28.61±20.02 | 0.003 |
| Fever duration before IVIG (day) | 5.84±1.87 | 5.83±2.01 | 0.943 |
| Fever duration after IVIG (day) | 0.81±1.18 | 1.28±2.16 | 0.002 |
| Total fever duration (day) | 6.65±2.15 | 7.10±2.73 | 0.027 |
| Laboratory findings (continuous variables) | |||
| Sodium (mmol/L) | 137.23±2.65 | 136.58±2.57 | 0.002 |
| Total bilirubin (mg/dL) | 0.59±0.63 | 0.73±0.89 | 0.031 |
| AST (IU/L) | 83.10±167.30 | 69.64±100.32 | 0.184 |
| ALT (IU/L) | 79.02±142.33 | 87.34±123.21 | 0.443 |
| Albumin (g/dL) | 4.07±0.39 | 4.14±1.85 | 0.434 |
| CRP (mg/dL) | 5.85±5.18 | 8.14±7.19 | <0.001 |
| Hct (%) | 33.13±2.72 | 32.70±3.01 | 0.053 |
| WBC (×103/µL) | 12.65±5.13 | 14.51±5.81 | <0.001 |
| Platelet (×103/µL) | 338.93±122.89 | 366.45±135.54 | 0.007 |
| Neutrophil (%) | 56.82±19.05 | 60.36±17.39 | 0.014 |
| Laboratory findings (categorical variables) | |||
| Sodium<135 (mmol/L) | 66 (14.3) | 47 (19.6) | 0.068 |
| Total bilirubin ≥0.9 (mg/dL) | 61 (13.2) | 44 (18.3) | 0.069 |
| AST≥100 (IU/L) | 73 (15.8) | 40 (16.7) | 0.758 |
| ALT≥45 (IU/L) | 156 (33.7) | 97 (40.4) | 0.078 |
| Albumin <3.5 (g/dL) | 34 (7.3) | 25 (10.4) | 0.163 |
| CRP≥7 (mg/dL) | 147 (31.7) | 111 (46.3) | <0.001 |
| Hct<31 (%) | 91 (19.7) | 60 (25.0) | 0.102 |
| WBC>12 (×103/µL) | 231 (49.9) | 157 (65.4) | <0.001 |
| Platelet<300 (×103/µL) | 181 (39.1) | 76 (31.7) | 0.053 |
| Neutrophil≥80 (%) | 45 (9.7) | 29 (12.1) | 0.333 |
Data are presented as either mean±standard deviation or n (%). *Analyzed by an unpaired t-test or a Chi-square test. CAD: coronary artery dilatation, IVIG: intravenous immunoglobulin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, CRP: C-reactive protein, Hct: hematocrit, WBC: white blood cell
Multivariate logistic regression analysis showed significant differences in the male gender (OR=1.685; 95% CI: 1.212-2.342; p=0.002), fever duration of 8 or more days (OR=1.521; 95% CI: 1.076-2.151; p=0.018), CRP≥7 mg/dL (OR=1.668; 95% CI: 1.197-2.325; p=0.003), and WBC count>12×103/µL (OR=1.740; 95% CI: 1.247-2.429; p=0.001) between the two groups (Table 5).
Table 5. Multivariate logistic regression analysis for predictors of coronary artery dilatation.
| OR (95% CI) | p | |
|---|---|---|
| Male sex | 1.685 (1.212-2.342) | 0.002 |
| Total fever duration≥8 (day) | 1.521 (1.076-2.151) | 0.018 |
| CRP≥7 (mg/dL) | 1.668 (1.197-2.325) | 0.003 |
| WBC>12 (×103/µL) | 1.740 (1.247-2.429) | 0.001 |
OR: odds ratio, CI: confidence interval, CRP: C-reactive protein, WBC: white blood cell
Relationship between intravenous immunoglobulin non-responders and coronary artery dilatation
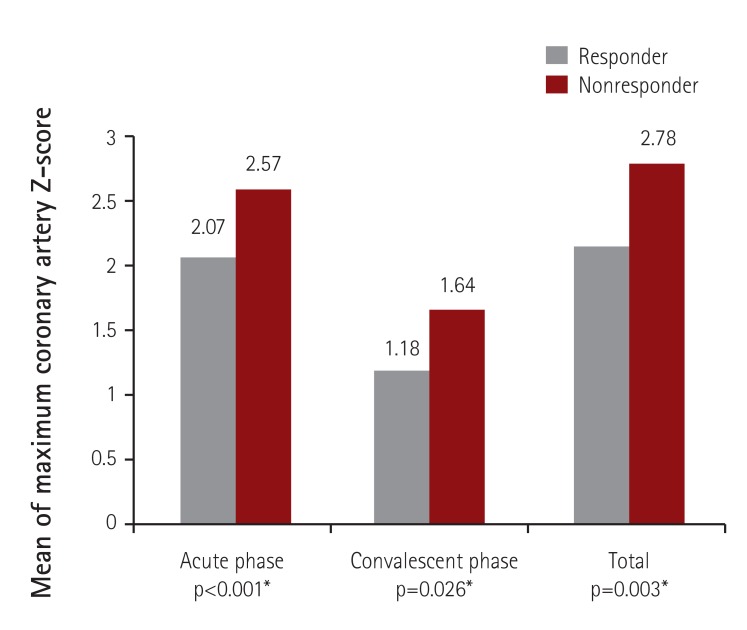
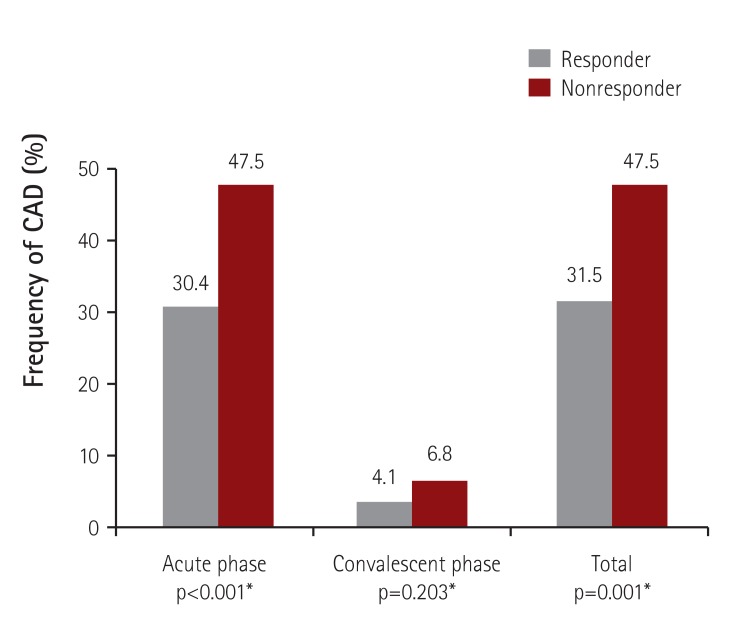
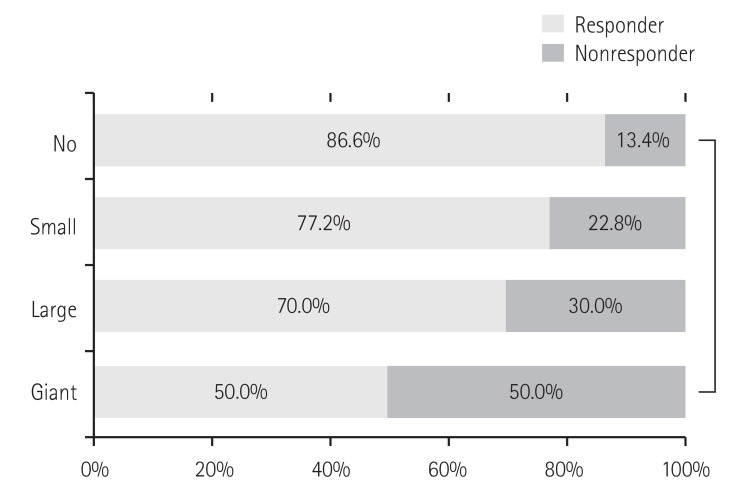
The mean values of maximum coronary artery Z-scores were significantly higher in the non-responders during both the acute and convalescent periods (Fig. 2). CAD was found in 47.5% (56/118) of the IVIG non-responders, which was more frequent than in the IVIG responders (31.5%; 184/585) (Fig. 3). Regarding CAD severity based on maximum Z-score value (small 2.5≤Z<5, large 5≤Z<10, and giant Z≥10), the rate of IVIG nonresponse was higher in patients with giant CAD compared to patients with small or large CAD: the rates of IVIG nonresponse in patients with small, large, and giant CAD were 22.8%, 30.0%, and 50.0%, respectively (p<0.001) (Fig. 4). Among the 10 patients with large CAD, 7 belonged to the responder group, and 9 had CAD in the acute phase. There were 2 patients with giant CAD in this study. One of those patients was a non-responder who received two additional IVIG treatments, and the other patient was a responder treated with IVIG on the fourth day of fever onset, who had an RCA Z-score of 10.8 in the initial echocardiography checked the following day.
Fig. 2. Mean values of maximum coronary artery Z-scores in IVIG responders and nonresponders. *Analyzed by an unpaired t-test. IVIG: intravenous immunoglobulin.
Fig. 3. Frequency of CAD (Z-score≥2.5) in IVIG responders and nonresponders. *Analyzed by a Chi-square test. CAD: coronary artery dilatation, IVIG: intravenous immunoglobulin.
Fig. 4. Severity of CAD in IVIG responders and nonresponders. *Analyzed by a Chi-square test with linear by linear association. No dilatation (Z<2.5) in 463 (65.9%), small dilatation (2.5≤Z<5) in 228 (32.4%), large dilatation (5≤Z<10) in 10 (1.4%), and giant dilatation (Z≥10) in 2 (0.3%). CAD: coronary artery dilatation, IVIG: intravenous immunoglobulin.
Discussion
According to a recent nationwide survey in Korea in 2011, the incidence of KD has continued to increase, to 134.4 per 100000 children<5 years of age.10) The survey also reported that 11.6% of patients did not respond to initial IVIG treatment and that CAD was noted in 16.4% of patients, based on the Japanese Ministry of Health and Welfare criteria.
The effectiveness of IVIG in the treatment of KD has previously been proven. However, despite the timely introduction of IVIG, 10-20% of patients do not respond to the treatment. These refractory patients are at an increased risk of developing coronary artery complications. Therefore, the screening of high-risk patients and aggressive management are important in the prevention of coronary artery complications.
From the results of our study, we identified a few independent predictive parameters for unresponsiveness to initial IVIG treatment in KD: serum total bilirubin, platelet count, and proportion of neutrophils. Most of the risk factors are related to severe inflammatory reactions that are not suppressed by initial IVIG treatment. In IVIG non-responder patients, fever represents ongoing inflammatory reactions in vessels and may reflect defects in the inhibition of immunologic stimuli.11)
In the USA, Tremoulet et al.12) mentioned a high percentage of band-form neutrophils, long duration of illness, high gamma-glutamyl transferase levels, and low age-adjusted hemoglobin as risk factors. Ashouri et al.13) reported a high band count percentage, low albumin, and the presence of coronary artery lesions as risk predictors. Fu et al.14) identified risk factors such as total bilirubin, albumin, CRP, neutrophil, and fever duration in Chinese patients. In Korea, Cha et al.15) identified elevated bilirubin level, elevated AST level, high percentage of polymorphonuclear cells, and low platelet count as predictors of IVIG nonresponse. Do et al.16) reported that low albumin, low sodium, and a high proportion of neutrophils were significantly related to IVIG resistance. Park et al.17) reported that ALT and total bilirubin were independent predictors of IVIG resistance. These study results show that so far, there is no consensus regarding the risk factors of IVIG nonresponse. Many confounding factors may influence the statistical analyses, such as patient age distribution, number of patients, duration of fever, ethnicity, and degree of inflammation. In this respect, the current study displays strength by including a large population of KD patients.
In Japan, Kobayashi et al.5) Egami et al.6) and Sano et al.7) proposed scoring systems for predicting IVIG non-responders using various parameters. However, Sleeper et al.18) concluded that these Japanese scoring systems were not suitable for the North American population. When these systems were applied to the current study, the specificities were high (87.0-91.6%), but the sensitivities were low (28.0-33.9%). In a previous study, Choi et al.19) applied the Kobayashi scoring system to Korean patients, and obtained a specificity of 80.0% and a sensitivity of 35.4%. These results are thought to be due to genetic differences in immune mechanisms between the Japanese and Korean populations. Accordingly, Choi et al.19) tried to develop a new scoring system, including male gender, cervical lymphadenopathy, changes in the extremities, platelet counts, total bilirubin, alkaline phosphatase, lactate dehydrogenase, and CRP, to validate the effectiveness of the system.
Significant coronary artery abnormalities develop in about 20% of untreated KD patients, and treatment with IVIG in the acute phase of the disease reduces this risk to 5-10%.20) CAD occurs much more frequently in IVIG non-responders than in responders.6),21) In the current study, CAD developed in 47.5% of the IVIG non-responders. This proportion is somewhat similar to the proportions found in previous studies: 44.4% and 48.6%.15),22) Previous studies identified fever duration, age, gender, erythrocyte sedimentation rate, CRP, interleukin-6, WBC count, platelet count, hemoglobin, and albumin as the predictive factors of CAD.23),24),25),26) In the current study, male gender, long fever duration, and high CRP level and WBC count were identified as risk factors for CAD.
Most of the CAD cases in this study were transient ectasia rather than persistent CAD (86.7% vs. 13.3%). Unexpectedly, 33.3% of the patients developed CAD in their acute phases. Whether this transient ectasia can be included in CAD is a controversial topic. It was found in this study that the risk factors of CAD, including transient ectasia in the acute phase, emphasized the importance of earlier use of echocardiography in the initial workup, and the association between coronary artery abnormalities in the acute phase and long-term complications. In addition, 70% of the large CAD patients and one giant CAD patient (50%) were in the responder group. Given these results, we emphasize the importance of early echocardiography examinations and subsequent follow ups. KD-associated coronary artery abnormalities, such as ectasia, aneurysms, stenosis, and calcification in early childhood, can develop into long-term complications in young adulthood, including myocardial infarction, arrhythmia, and even sudden death.27) In some cases, the consequences of coronary vasculitis and myocarditis due to KD may not even manifest in childhood. For example, a recent case report showed that 2 patients developed acute myocardial infarction in their 30s, even though during childhood, their coronary arteries were apparently normal more than one year after acute KD.28)
Interestingly, there were no overlapping predictive parameters between IVIG non-responders and CAD in this study, although there was a significant correlation between the two factors. The objective of the efforts to identify the predictive parameters is to detect high-risk patients earlier and treat them more aggressively, in order to reduce coronary artery complications. For these patients, subsequent IVIG or immunomodulatory agents, such as corticosteroid, infliximab, and cyclophosphamide, can be considered. According to the studies of Ogata et al.29) and Chen et al.,30) a combination of corticosteroid with IVIG as an initial treatment strategy could reduce the risk of coronary artery abnormalities.
This study has several limitations. First, this was a retrospective, single-center study, so we could not obtain enough data on the clinical characteristics and laboratory findings. Therefore, we could not present the proportion of atypical KD in the enrolled patients or the results of echocardiography in the sub-acute phases. Second, many patients were excluded because of the lack of adequate data or loss to follow up, thus reducing statistical power. Third, we could not exclude patients with the co-morbidity of other febrile illnesses.
In conclusion, several independent parameters were related to non-responders to initial IVIG treatment and CAD, which might be helpful in establishing more focused monitoring and careful follow up of KD patients. Moreover, advanced and modified initial therapy can be considered for these high-risk KD patients. The results of our large-scale study may be beneficial in selecting patients who are at risk of IVIG unresponsiveness or coronary artery complications.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967;16:178–222. [PubMed] [Google Scholar]
- 2.Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease US/Canadian Kawasaki Syndrome study group. Pediatr Infect Dis J. 1998;17:1144–1148. doi: 10.1097/00006454-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Wallace CA, French JW, Kahn SJ, Sherry DD. Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics. 2000;105:E78. doi: 10.1542/peds.105.6.e78. [DOI] [PubMed] [Google Scholar]
- 4.Koren G, Lavi S, Rose V, Rowe R. Kawasaki disease: review of risk factors for coronary aneurysms. J Pediatr. 1986;108:388–392. doi: 10.1016/s0022-3476(86)80878-2. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi T, Inoue Y, Takeuchi K, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–2612. doi: 10.1161/CIRCULATIONAHA.105.592865. [DOI] [PubMed] [Google Scholar]
- 6.Egami K, Muta H, Ishii M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. 2006;149:237–240. doi: 10.1016/j.jpeds.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 7.Sano T, Kurotobi S, Matsuzaki K, et al. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr. 2007;166:131–137. doi: 10.1007/s00431-006-0223-z. [DOI] [PubMed] [Google Scholar]
- 8.Dallaire F, Dahdah N. New equations and a critical appraisal of coronary artery Z scores in healthy children. J Am Soc Echocardiogr. 2011;24:60–74. doi: 10.1016/j.echo.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Manlhiot C, Millar K, Golding F, McCrindle BW. Improved classification of coronary artery abnormalities based only on coronary artery z-scores after Kawasaki disease. Pediatr Cardiol. 2010;31:242–249. doi: 10.1007/s00246-009-9599-7. [DOI] [PubMed] [Google Scholar]
- 10.Kim GB, Han JW, Park YW, et al. Epidemiologic features of Kawasaki disease in South Korea: data from nationwide survey, 2009-2011. Pediatr Infect Dis J. 2014;33:24–27. doi: 10.1097/INF.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 11.Matsubara T, Ichiyama T, Furukawa S. Immunological profile of peripheral blood lymphocytes and monocytes/macrophages in Kawasaki disease. Clin Exp Immunol. 2005;141:381–387. doi: 10.1111/j.1365-2249.2005.02821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tremoulet AH, Best BM, Song S, et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. 2008;153:117–121. doi: 10.1016/j.jpeds.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashouri N, Takahashi M, Dorey F, Mason W. Risk factors for nonresponse to therapy in Kawasaki disease. J Pediatr. 2008;153:365–368. doi: 10.1016/j.jpeds.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Fu PP, Du ZD, Pan YS. Novel predictors of intravenous immunoglobulin resistance in Chinese children with Kawasaki disease. Pediatr Infect Dis J. 2013;32:e319–e323. doi: 10.1097/INF.0b013e31828e887f. [DOI] [PubMed] [Google Scholar]
- 15.Cha S, Yoon M, Ahn Y, Han M, Yoon KL. Risk factors for failure of initial intravenous immunoglobulin treatment in Kawasaki disease. J Korean Med Sci. 2008;23:718–722. doi: 10.3346/jkms.2008.23.4.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Do YS, Kim KW, Chun JK, Cha BH, Namgoong MK, Lee HY. Predicting factors for refractory Kawasaki disease. Korean Circ J. 2010;40:239–242. doi: 10.4070/kcj.2010.40.5.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park HM, Lee DW, Hyun MC, Lee SB. Predictors of nonresponse to intravenous immunoglobulin therapy in Kawasaki disease. Korean J Pediatr. 2013;56:75–79. doi: 10.3345/kjp.2013.56.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sleeper LA, Minich LL, McCrindle BM, et al. Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J Pediatr. 2011;269:831–835.e3. doi: 10.1016/j.jpeds.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi MH, Park CS, Kim DS, Kim KH. Prediction of intravenous immunoglobulin nonresponse Kawasaki disease in Korea. Korean J Pediatr Infect Dis. 2014;21:29–36. [Google Scholar]
- 20.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–1733. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 21.Cho KH, Kang SJ. Clinically useful predictors of resistance to intravenous immunoglobulin and prognosis of coronary artery lesions in patients with incomplete Kawasaki disease. Korean Circ J. 2014;44:328–335. doi: 10.4070/kcj.2014.44.5.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashino K, Ishii M, Iemura M, Akagi T, Kato H. Re-treatment for immune globulin-resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy. Pediatr Int. 2001;43:211–217. doi: 10.1046/j.1442-200x.2001.01373.x. [DOI] [PubMed] [Google Scholar]
- 23.Beiser AS, Takahashi M, Baker AL, Sundel RP, Newburger JW. A predictive instrument for coronary artery aneurysms in Kawasaki disease. US Multicenter Kawasaki Disease Study Group. Am J Cardiol. 1998;81:1116–1120. doi: 10.1016/s0002-9149(98)00116-7. [DOI] [PubMed] [Google Scholar]
- 24.Honkanen VE, McCrindle BW, Laxer RM, Feldman BM, Schneider R, Silverman ED. Clinical relevance of the risk factors for coronary artery inflammation in Kawasaki disease. Pediatr Cardiol. 2003;24:122–126. doi: 10.1007/s00246-002-0063-1. [DOI] [PubMed] [Google Scholar]
- 25.Gersony WM. Predicting coronary aneurysms in Kawasaki disease. Am J Cardiol. 1998;81:1162–1164. doi: 10.1016/s0002-9149(98)00115-5. [DOI] [PubMed] [Google Scholar]
- 26.Park MJ, Jeon IS, Tchah H, Cho KH, Jung MJ, Choi DY. Predictive indicators of coronary artery complications in Kawasaki disease. Korean J Pediatr. 2009;52:1161–1166. [Google Scholar]
- 27.Singh S, Aulakh R, Kawasaki T. Kawasaki disease and the emerging coronary artery disease epidemic in India: is there a correlation? Indian J Pediatr. 2014;81:328–332. doi: 10.1007/s12098-013-1229-y. [DOI] [PubMed] [Google Scholar]
- 28.Kawai H, Takakuwa Y, Naruse H, et al. Two cases with past Kawasaki disease developing acute myocardial infarction in their thirties, despite being regarded as at low risk for coronary events. Heart Vessels. 2015;30:549–553. doi: 10.1007/s00380-014-0541-4. [DOI] [PubMed] [Google Scholar]
- 29.Ogata S, Ogihara Y, Honda T, Kon S, Akiyama K, Ishii M. Corticosteroid pulse combination therapy for refractory Kawasaki disease: a randomized trial. Pediatrics. 2012;129:e17–e23. doi: 10.1542/peds.2011-0148. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Dong Y, Yin Y, Krucoff MW. Intravenous immunoglobulin plus corticosteroid to prevent coronary artery abnormalities in Kawasaki disease: a meta-analysis. Heart. 2013;99:76–82. doi: 10.1136/heartjnl-2012-302126. [DOI] [PubMed] [Google Scholar]