Abstract
Transition metals such as iron, copper, zinc, or molybdenum are essential nutrients for plants. These elements are involved in almost every biological process, including photosynthesis, tolerance to biotic and abiotic stress, or symbiotic nitrogen fixation. However, plants often grow in soils with limiting metallic oligonutrient bioavailability. Consequently, to ensure the proper metal levels, plants have developed a complex metal uptake and distribution system, that not only involves the plant itself, but also its associated microorganisms. These microorganisms can simply increase metal solubility in soils and making them more accessible to the host plant, as well as induce the plant metal deficiency response, or directly deliver transition elements to cortical cells. Other, instead of providing metals, can act as metal sinks, such as endosymbiotic rhizobia in legume nodules that requires relatively large amounts to carry out nitrogen fixation. In this review, we propose to do an overview of metal transport mechanisms in the plant–microbe system, emphasizing the role of arbuscular mycorrhizal fungi and endosymbiotic rhizobia.
Keywords: metal homeostasis, plant–microbe interaction, arbuscular mycorrhiza, rhizobia, transition metals
Introduction
Iron, copper, zinc, and some other transition metals are essential nutrients for plants (Frausto da Silva and Williams, 2001; Clemens et al., 2002; Morrissey and Guerinot, 2009; Pilon, 2011; Olsen and Palmgren, 2014). It is estimated that a third of the proteins of a typical cell are metalloproteins (Dupont et al., 2006; Finkelstein, 2009), which participate in almost every biological process, from photosynthesis to seed production. However, plants often have to live in soils with low metal bioavailability (Alloway, 2008; White and Broadley, 2009). This prevalent metal deficiency limits plant growth and tolerance to stress, lowers yields, and reduces crop nutritional value. Consequently, human diet in many areas of the world does not include the minimum metal nutrient requirements, causing from minor immunological alterations to death (Grotz and Guerinot, 2006; Jamison et al., 2006; Alloway, 2008; White and Broadley, 2009; Akkermans et al., 2016). As a result, considerable effort has been made to increase plant metal uptake by using metal fertilizers (Larbi et al., 2010; Wei et al., 2012; López-Rayo et al., 2015), and developing new crop varieties with improved metal uptake capabilities (Hirschi, 2009; Masuda et al., 2012; Eggert and von Wiren, 2013).
However, when aiming to improve plant nutrition in a sustainable manner, the role of plant-associated microorganisms, the plant microbiome, should also be contemplated (East, 2013). Within a plant, different compartments can be identified, each with a different microbial community, such as the phyllosphere (leaves), the rhizosphere (the portion of soil directly affected by plant exudates), or the endosphere (the internal tissues of the plant). For almost a 100 years, since Hiltner’s pioneer work (Hiltner, 1904), the composition and role of the plant microbiome has been studied. These efforts have accelerated in recent years following the improvement of deep sequencing technologies and bioinformatics pipelines to process the vast amount of data obtained (Scholz et al., 2012). All these efforts have revealed that plants rely on their microbiome for a wide range of processes: from resisting pests to colonizing new environments (Haney and Ausubel, 2015; Santhanam et al., 2015; Vandenkoornhuyse et al., 2015). Arguably the most important role of the plant microbiome is to improve nutrient uptake (Li et al., 1991; Smith and Smith, 2011; Rana et al., 2012; Udvardi and Poole, 2013; Hart et al., 2014). Plant–microbe interactions enabled plant colonization of terrestrial environments by allowing the recovery of nutrients from soil (Field et al., 2015). Many of these symbiotic relationships, established in the early stages of plant evolution, have been maintained to date. As a result, we should study the plant-microbe holobiont (the host plant plus its associated microbiome) rather than the isolated plants, if we are to consider plant nutrient uptake in natural environments (Bordenstein and Theis, 2015; Vandenkoornhuyse et al., 2015).
This review intends to approach plant transition metal nutrition, contemplating how plants incorporate metals from soil either on their own or assisted by associated microorganisms (mainly arbuscular mycorrhiza), and how they are delivered to leaves, seeds, and, in the case of legumes, to endosymbiotic bacteria living within the root nodule cells. To do this, first we will describe some of the main protein families involved in metal transport and intracellular metal trafficking to have a conceptual frame to place the plethora of proteins involved in metal plant and plant-endoymbiont metal trafficking. For information on how plants protect themselves against toxic metal concentrations, please consider Clemens and Ma (2016), or Sharma et al. (2016) as two of the most recent reviews on the topic.
Proteins and Small Organic Molecules Involved in Transition Metal Transport
Cellular metal homeostasis requires a highly precise regulation to ensure that transition elements are kept at high enough levels to carry out their biological functions, but not so high that they can catalyze the production of free radicals, or displace less abundant elements in the core of metalloproteins. This balance is achieved by the coordinated action of ferroreductases that provide the metals in the correct oxidative state, together with the transporters that move them across membranes, the small soluble metal binding molecules and proteins that ferry metals among the different transporters and to apoproteins, and metal detoxifying molecules that buffer the cell against a sudden increase in transition metal levels (Figure 1). In this section, we will describe some of the key aspects of the different molecules involved in transition metal transport.
FIGURE 1.
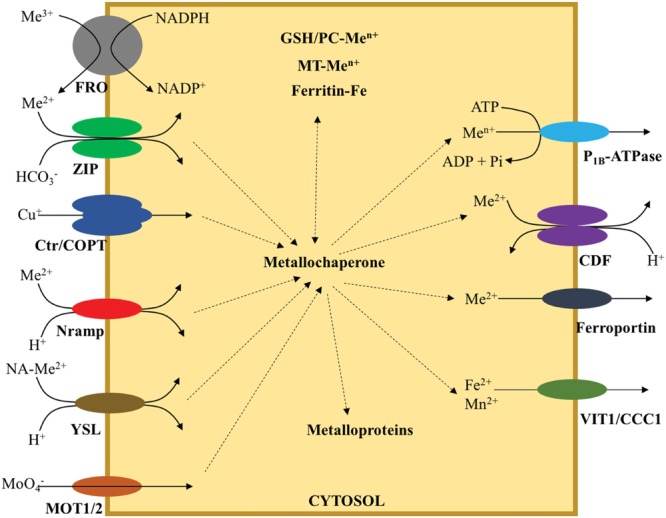
Main systems involved in metal transport and intracellular trafficking. Co-transported ion is indicated where known. Dotted arrows indicate exchange of metal ligands.
Ferroreductases
In the case of iron and copper are not translocated as a metal-chelator complex, the substrate is the reduced form (Fe2+/Cu+), rather than the more prevalent in aerobic environments oxidized state (Fe3+/Cu2+). Therefore, a mechanism must exist to reduce Fe3+/Cu2+. This process is carried out by members of the ferroreductase oxidase (FRO) family (Robinson et al., 1999; Connolly, 2014). FROs are membrane proteins with eight transmembrane domains and a large soluble domain with bound NADPH and FAD, in addition to an oxidoreductase motive (Connolly, 2014). Reducing electrons are provided by NADPH and directed toward the metals in a process that very likely requires two intramembrane heme groups.
Transition Metal Transporters
Once the metals are in the proper oxidative state or bound to the correct metallophore, they will be transported across the membrane by the different transition metal transporters. There are several families of them with distinct metal affinities and direction of transport. For the purposes of this review, we will classify them based on their direction of transport (into or out of the cytosol) since this will define their role in metal trafficking from soil to sink organs.
Transporters that Introduce Metals into the Cytosol
The best studied families are the ZIP, Ctr/COPT, Nramp, YSL, and MOT families. Their key features are:
-
•
ZIP transporters (Zinc resistance transporter, Iron-resistance transporter-like Proteins). These are a ubiquitous family of divalent metals transporters (mainly Fe2+, Zn2+, Ni2+, and Mn2+; Eide et al., 1996; Grotz et al., 1998; Guerinot, 2000; Mizuno et al., 2005). Although no structure is available of any ZIP transporter, computational modeling indicates that they act as homodimer, where each monomer has eight transmembrane domains (Antala et al., 2015). ZIP transporters contain a conserved cytosolic histidine-rich loop between transmembrane (TM) domains 3 and 4 in eukaryotes (Taylor and Nicholson, 2003), that seems to be responsible for metal specificity and transport rate (Antala et al., 2015). The energetics of transport has not been clearly defined yet, with authors proposing a HCO3- symport (Gaither and Eide, 2000; Liu et al., 2008), while others suggest that they work as channels (Lin et al., 2010). Members from this family include the transporters responsible for iron and zinc uptake from soil (Eide et al., 1996; Lin et al., 2009).
-
•
Ctr/COPT transporters (Copper transporter). They have only been found in eukaryotes, being known as COPT transporters in plants (Sancenón et al., 2003) and Ctr in animals and fungi (Kim et al., 2013). They work as homo- or heterotrimer of a three-transmembrane domain (Zhou and Thiele, 2001; Aller and Unger, 2006; Nose et al., 2006; De Feo et al., 2010), forming a channel responsible for specific Cu+ transport (Dancis et al., 1994; Eisses et al., 2005; Sinani et al., 2007). In plants, COPT proteins have been suggested to play a role in copper uptake from soil and delivery to pollen (Sancenón et al., 2004).
-
•
Nramp transporters (Natural Resistance-Associated Macro phage Protein). This family of transporters can be found in the three domains of life (Nevo and Nelson, 2006). They are a monomeric protein spanning 11 transmembrane domains (Ehrnstorfer et al., 2014). Transport is driven by a H+ symport (Gunshin et al., 1997). Nramp transporters have a wide range of metal substrates, typically Fe2+, Mn2+, Co2+, and Zn2+ (Nevo and Nelson, 2006). Metal binding sites are integrated by a carbonyl from a peptide bond in TM6, a Met in the same domain and two Asp from TM1, in a planar geometry (Ehrnstorfer et al., 2014). Some Nramp transporters have been proposed to be involved in iron and manganese uptake by the root epidermis (Cailliatte et al., 2010).
-
•
YSL transporters (Yellow Stripe-like proteins). Yellow stripe is a phenotype identified in maize where intervenal chlorotic (yellow) zones are observed (Beadle, 1929). This chlorosis is caused by deficient iron uptake, the result of the mutation of a root epidermal transporter (YS1; Beadle, 1929; Curie et al., 2001). Members of the YSL family can only be found in plants (Curie et al., 2008), although YSL belong to the larger OPT (oligopeptide transporter) family that is also present in fungi (Lubkowitz, 2011). YSLs transporters do not use free metals as substrate, but a complex of metals with nicotianamine (NA) or its derivatives (Curie et al., 2008). NA is a non-proteogenic amino acid that is synthesized from S-adenosyl-methionine by the enzyme NA synthase (NAS; Higuchi et al., 1999). Transport by YSL proteins is energized by a H+-symport (Schaaf et al., 2004). Additionally, at least some of the plant OPT transporters have also been associated by metal transport (Lubkowitz, 2011; Zhai et al., 2014; Bashir et al., 2015), although the identity of the metal complex transported still remains elusive. Not much is known about the structure of these proteins, with different models proposing a range of 11–16 transmembrane regions (Lubkowitz, 2011). In broad terms, YSL transporters are involved in metal uptake from soil in monocots and in long-distance metal distribution in both monocots and dicots (Conte and Walker, 2011).
-
•
MOT1 (Molybdate transporter type 1). In contrast to other transition metals, molybdenum is transported as the oxoanion molybdate. These transporters were first identified in parallel in Chlamydomonas reinhardtii and in A. thaliana and show high affinity for molybdate (Tejada-Jiménez et al., 2007; Tomatsu et al., 2007). They have been associated with Mo uptake in C. reinhardtii, while in A. thaliana its specific role still remains elusive since some authors suggest a role in molybdate uptake from soil, while other point to a role in mitochondrial molybdenum homeostasis (Tomatsu et al., 2007; Baxter et al., 2008). Other members of the family would be involved in molybdate storage. Aside from the MOT1 family, an additional family, MOT2, has been identified also involved in molybdate uptake in C. reinhardtii (Tejada-Jiménez et al., 2011). MOT2 family members role in higher plants has not been determined yet.
Transporters that Remove Metals Out of the Cytosol
The best studied families are the P1b-ATPases, CDF, ferroportins, and VIT/CCC1 families. Their key features are:
-
•
P1b-ATPases. They are a clade of the P-type superfamily of ATPases (which also includes the Na+/K+-ATPase, or the H+-ATPase; Axelsen and Palmgren, 1998). This clade is conserved in all three domains of life and is subdivided in subclades with different metal specificities (P1b-1 for Cu+, P1b-2 for Zn2+,…) (Argüello, 2003; Argüello et al., 2007). The transporter is a monomer with several transmembrane spanning domains (from 6 to 8). The last two cytosolic loops are enlarged and comprise the ATPase domain (closest to C-terminus) that drives transport, and the activator domain. Metal specificity of each subclade is determined by specific amino acids in the three transmembrane helices closest to C-terminus (Argüello, 2003; Argüello et al., 2007; González-Guerrero et al., 2008a; Raimunda et al., 2012). In addition, they frequently have cytosolic metal binding domains in N and/or C-termini with a regulatory function (Mandal and Argüello, 2003; Eren et al., 2007; González-Guerrero and Argüello, 2008). P1b-ATPases are involved in long-distance Cu+ and Zn2+ transport in plants, as well as metal transport into organnelle (Hussain et al., 2004; Andrés-Colás et al., 2006).
-
•
CDF transporters (Cation Diffusion Facilitator). Members of this family are present in all organisms (Kolaj-Robin et al., 2015). Their substrate are divalent metals such as Fe2+, Zn2+, or Mn2+, coupled to a H+ antiport (MacDiarmid et al., 2002; Rubio-Sanz et al., 2013; Gupta et al., 2014; Raimunda and Elso-Berberián, 2014). The functional transporter is a homodimer (Lu and Fu, 2007). The monomer has six transmembrane domains with a His-rich region in the cytosol between the fourth and the fifth transmembrane region, which is only present in eukaryotic CDF transporters (Kolaj-Robin et al., 2015). There are three metal binding domains in the protein: site I in the transmembrane region, site II in the membrane-cytosol interface and site III in the C- terminal domain, but only I and III seem to be directly involved in transport (Lu and Fu, 2007). Site I very likely defines the metal to be transported, which is coordinated by two Asp in TM2 and a His and Asp in TM5 in the Zn transporter YiiP. Site III facilitates dimerization, and would consequently, have a regulatory mechanism on metal transport. Most plant CDFs, known as MTPs, have been associated to metal detoxification, although others could play a role in long-distance metal transport (Ricachenevsky et al., 2013).
-
•
Ferroportins. They are only present in eukaryotes (Muckenthaler et al., 2008; Morrissey et al., 2009). The topology is predicted to comprise 11 transmembrane domains with a large extracytosolic loop between TM5 and 6 (Yeh et al., 2011). The metal substrates are Fe2+, Ni2+, and Co2+ (Schaaf et al., 2006; Muckenthaler et al., 2008; Morrissey et al., 2009), that are extruded out of the cytosol by a yet-to-be-determined mechanism. Ferroportins would be involved in iron/cobalt uploading of the xylem (Morrissey et al., 2009).
-
•
VIT1/CCC1. Fe2+ and Mn2+ has been identified as substrate (Li et al., 2001; Kim et al., 2006). Very little is known on their structure and transport mechanism, other than it is predicted to cross the membrane five times (Kim et al., 2006).
Soluble Metal Carriers
Unlike alkali or alkali-earth metals, transition elements are not kept “free,” hydrated, in the cytosol (O’Halloran and Culotta, 2000). This means that if they are not transported as metal-conjugates, they would have to bind a protein (metallochaperone) or to some small organic molecule as they are released by the metal transporter. It is estimated that at least a third of the metalloproteins of the cell obtain their metal cofactor from a metallochaperone (Foster et al., 2014). This has been clearly shown for copper, where several different proteins have been proposed to mediate metal delivery from the Ctr/COPT transporters to P-type ATPases or to apoproteins (O’Halloran and Culotta, 2000). Similar elements have been identified for Ni, clusters Fe-S, and molybdenum (Vergnes et al., 2006; Chan Chung and Zamble, 2011; Mapolelo et al., 2013). These observations indicate that at least in these cases, transition metal transport and delivery is mediated by specific protein–protein interactions, rather than by simple metal affinities and mass equilibria. However, for some other metals, specially for zinc, a labile metal pool has been proposed (Atkinson et al., 2010). Nevertheless, in these cases, transition metals are not free, hydrated, but bound to small organic molecules such as amino acids and organic acids (Sinclair and Krämer, 2012; Ma et al., 2014). In plants, some of these metal complexes are responsible for metal delivery by the vasculature and across simplastically disconnected tissues (Roschzttardtz et al., 2011; Álvarez-Fernández et al., 2014).
Metal Concentration Buffers/Metal Storage Proteins
When cytosolic metal concentrations rise above a certain level, metal binding capabilities of metallochaperones and organic molecules are overloaded. In that case, excessive metal levels have to be buffered by other means, since they could become toxic. Metallothioneins seem to be an universal solution to this problem (Blindauer and Leszczyszyn, 2010), since they are present in all three domains of life. These are cysteine-rich proteins that show high affinity for copper, zinc, or cadmium. Metallothioneins are kept in the cytosol. They have been predicted to have other functions based on the high number of cysteines, such as using internal disulphide bridges to control the oxidative state of the cell (Maret, 2011). Similarly participating in metal control in the cell is glutathione (GSH; Helbig et al., 2008), also based on the cysteine thiol in the tripeptide. To increase metal binding capabilities, plants and some fungi synthesize oligomers of GSH with the enzyme phytochelatin (PC) synthase (Ha et al., 1999). This enzyme uses the energy released by removing the peptide bond between Cys and Gly to condensate the γ-Glu-Cys dipeptide to the Glu of GSH. The resulting oligomer (typically of 2–10 units) is known as PC and can bind copper, cadmium, and zinc with high affinity in the cytosol (Cheng et al., 2005). Excess PC-metal complexes can be stored in vacuoles after being transported by ABC transporters (Ortiz et al., 1995; Song et al., 2010b).
The vacuole can also store other metals or other metal species. For instance, Zn-NA complexes are used to maintain cytosolic zinc homeostasis (Haydon et al., 2012). In the case of iron, the main reservoir are the vacuoles (Roschzttardtz et al., 2009; Lanquar et al., 2010), and the plastids (Terry and Abadía, 1986). In the later, iron is densely packed, in a quasi-crystalline structure by ferritins, oligomeric proteins that form a protein shell within which iron is stored (Briat et al., 2010).
Transition Metal Uptake From Soil
Transition metals are tightly bound to soil particles and have low solubility, specially in basic soils (Ruel and Bouis, 1998; Grotz and Guerinot, 2006). As a result, a fierce competition for these nutrients is established in the rhizosphere (Lugtenberg and Kamilova, 2009), where metal uptake efficiency is critical for proliferation. Plants have adapted to this environment using different and complementary strategies (Figure 2): (i) Using the metal solubilized by rhizospheric microorganisms, (ii) directly increasing metal solubility in their rhizosphere, and (iii) using mycorrhizal fungi to mine a wider soil area for metals.
FIGURE 2.
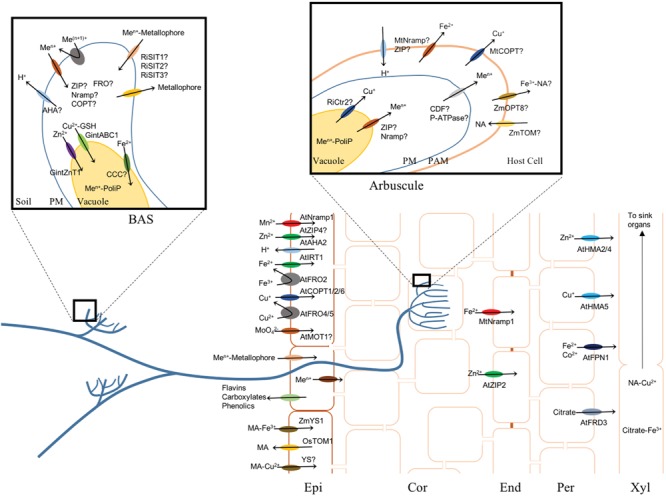
Diagram of the main transport processes in roots, using Strategy I or Strategy II or the mycorrhizal pathway (left inset for uptake by Branched Absorbing Structures, right inset for metal delivery by arbuscules). At is used as a prefix to indicate A. thaliana proteins, Os for Oryza sativa, Zm for Zea mays, Mt for M. truncatula, Gint for Glomus intraradices, Ri for Rhizophagus irregularis, and AMF for arbuscular mycorrhiza. Epi stands for Epidermis, Cor for Cortex, End for Endodermis, Per for Pericycle, Xyl for Xylem, PM for Plasma Membrane, PAM for Periarbuscular Membrane, and Men+ for a general metal.
Rhizospheric Microorganisms and Plant Metal Uptake
Bacteria can use both a reductive and a chelating strategy for metal uptake (Blindauer, 2008; Cornelis, 2010). In the chelating strategy, a wide array of metallophores such as enterobactin, pyoverdin, or anguibactin, are secreted and then, the metal-chelator complex is introduced though different metal transport complexes (Crosa, 1989; Bellenger et al., 2008; Kraepiel et al., 2009). The presence of these metal-chelator transporters does not necessarily indicate the ability of that particular bacteria of synthesizing the chelator. It is not uncommon for bacteria to be able to “steal” some of the metal complexes formed with chelators synthesized by other bacteria (Jurkevitch et al., 1992; Loper and Henkels, 1999; Cornelis and Matthijs, 2002).
Plants benefit from this scenario. As bacteria acidify the surrounding soil, some of the solubilized metal can be used by the plant (Zhang et al., 2009; Marschner et al., 2011). It can also use bacterial metallophores as sources of metals (Bar-Ness et al., 1992; Vansuyt et al., 2007). In other cases, root architecture is altered by rhizospheric bacteria increasing its exploratory capacity, and consequently, more access to nutrients is obtained (López-Bucio et al., 2007; Zamioudis et al., 2013). Rhizospheric bacteria can also directly affect plant metal uptake mechanism by means of volatile organic compounds (Zhang et al., 2009; Orozco-Mosqueda et al., 2013; Zamioudis et al., 2015). These molecules are able to induce the iron deficiency response in a FIT1-dependent manner (Zhang et al., 2009; Zamioudis et al., 2015), leading to upregulation of ferroreductase activity in A. thaliana roots as well as of iron mobilizing phenolics (Zamioudis et al., 2014, 2015). These volatiles are detected by the shoots, triggering the transmission of systemic iron deficiency signals (Zamioudis et al., 2015).
The relationship between plant and the microorganisms in its rhizosphere is dynamic. The plant microbiome can be altered to adapt to different developmental stages or to biotic and abiotic stresses (Carvalhais et al., 2015). This is achieved by changing the composition of root exudates (Chaparro et al., 2013; Liang et al., 2013). Similarly, metal deficiency also results in changes in the composition of root exudates (Kobayashi and Nishizawa, 2012; Sisó-Terraza et al., 2015). These changes could also be responsible for the variations observed in the plant microbiome of plants grown in metal deficient soils (Pii et al., 2016).
Transition Metal Uptake by the Root Epidermis
Plants have developed a number of mechanisms to obtain these nutrients directly from soil. This has been best studied in the case of iron uptake, where two approaches (Strategies I and II) are followed (Kobayashi and Nishizawa, 2012) (Figure 2). In Strategy I, plants lower the pH of the surrounding soil by extruding H+ by root epidermal H+-ATPases. Acidifying soils increases Fe3+ solubility and this is further increased by reducing it to Fe2+ with epidermal FRO proteins. Then, Fe2+ is transported into the cell by ZIP and Nramp transporters. In A. thaliana, the proteins carrying out these roles are mainly AHA2 (Santi and Schmidt, 2009), FRO2 (Robinson et al., 1999), and IRT1 (Eide et al., 1996). This process is very tightly controlled to prevent iron overload of the plant using at least three levels of regulation: transcriptional, posttranslational, and intracellular trafficking (Kobayashi and Nishizawa, 2012). At the transcriptional level, a number of bHLH factors control the expression of these genes (Colangelo and Guerinot, 2004; Sivitz et al., 2012). Ubiquitinization plays a role in controlling the protein levels of some of these transcription factors, as well as membrane recycling of IRT1 (Barberon et al., 2011). Furthermore, upon reduction of non-iron metal substrates of IRT1, this transporter polarly localizes to the soil-facing side of the epidermal plasma membrane (Barberon et al., 2014). Strategy I seems to be the most ancient one, since it is used by dicots and some monocots (rice; Ricachenevsky and Sperotto, 2014).
In Strategy II, carried out by monocots, iron is not transported as Fe2+, but as a complex with mugineic acids (MA; Kobayashi and Nishizawa, 2012), a NA-derivative with high affinity for Fe3+. Subsequently the complex is introduced into the plant by YSL transporters (Curie et al., 2001). For transport to occur, MAs have to be extruded to the rhizosphere mediated by TOM1-like transporters (Nozoye et al., 2011). This process is also under transcriptional control, being IDEF1, IDEF2, and IRO2 some of the key transcription factors involved (Ogo et al., 2006, 2008; Kobayashi et al., 2007). Strategy II is carried out by all monocots (Ricachenevsky and Sperotto, 2014).
However, the separation of the two strategies (reductive vs. chelating) is not as straightforward as originally thought. Many Strategy I plants release in their root exudates a number of molecules that can solubilize Fe3+ and form complexes that are subsequently introduced into the plant. These include phenolics, coumarins carboxylates, and flavins (Cesco et al., 2010; Fourcroy et al., 2013; Schmidt et al., 2014; Valentinuzzi et al., 2015). In addition, the flavins could also contribute to Fe3+ solubility by reducing it to Fe2+ (Sisó-Terraza et al., 2015).
Copper uptake from soil very likely follows similar strategies as for iron. In Arabidopsis, two FRO proteins, FRO4 and FRO5, are strong candidates for reducing Cu2+ to Cu+ (Bernal et al., 2012), which would be introduced into the plant via COPT transporters (candidates are COPT1, 2, or 6; Gayomba et al., 2013). Furthermore, Cu2+ can bind MA precursor NA and be transported by YSL proteins (DiDonato et al., 2004), supporting the existence of a Strategy II approach of copper uptake. Other elements that would not suffer oxidation changes under physiological conditions (Zn2+, Mn2+, …) would be transported by ZIP and Nramp transporters present in the plasma membrane of epidermal roots (Lin et al., 2009; Cailliatte et al., 2010).
Molybdenum is incorporated by plants as molybdate, instead of a cationic form. In green algae C. reinhardtii this is done by two molybdate transporters belonging to the MOT1 and the MOT2 families corresponding to a high affinity (7 nM) and low affinity (550 nM) system, respectively (Tejada-Jiménez et al., 2007, 2011). Higher plant genomes also encode for MOT1 and MOT2 orthologs. In A. thaliana AtMOT1 seems to be required for molybdate uptake from soil, either if this is done directly or indirectly still remains to be defined, since conflicting data has been reported of its membrane localization (Tomatsu et al., 2007; Baxter et al., 2008).
Transition Metal Uptake by Mycorrhizal Plants
Almost 90% of plants are able to establish an endosymbiotic relationship with fungi through their roots: the mycorrhiza (Smith and Read, 2008). Some of these fungi are basidiomycetes and ascomycetes that can establish a plethora of different types of mycorrhizal relationships: ectomycorrhiza, ectendomycorrhiza, arbutoid, orchioid, etc. (Peterson et al., 2004). However, the most common mycorrhiza (almost 85% of plants) is the arbuscular mycorrhiza established with fungi from the Glomeromycota phylum. Arbuscular mycorrhiza is formed through a complex and very regulated process (Gutjahr and Parniske, 2013). After spore germination, and detection of strigolactones, the germinating hyphae branches to maximize the chances of contacting the root epidermis. There, the fungus forms an appresorium that will allow penetration into the root cortex. Once in the cortex, the hyphae disperse though the intercellular spaces and at regular intervals, they penetrate into the cells, branching multiple times, and constituting an arbuscule. The arbuscules do not cross the plant cell plasma membrane, but establish a very close interface with a differentiated host plasma membrane cell, the periarbuscular membrane (Gutjahr and Parniske, 2013). Across these two membranes (arbuscular plasma membrane and periarbuscular membrane) nutrients are exchanged (Rausch et al., 2001; Javot et al., 2007). In addition, as the fungal intracellular mycelium and arbuscules develop, the fungal extracellular mycelium grows, producing branched absorbing structures (BAS) at regular intervals, which, when the conditions are right, they develop spores (Bago et al., 1998). In spite of sometimes reaching an area measured in square kilometers, the fungal colony is just one protoplasm, with millions of nuclei distributed at regular intervals in the coenocytic mycelium (Smith and Read, 2008). In natural environments plants greatly rely on arbuscular mycorrhizal fungi (AMF) to feed themselves (Smith and Read, 2008). It is estimated that they can transfer to their host over 90% of the phosphate, and over 50% of the fixed nitrogen in exchange of photosynthates (Smith and Smith, 2011). This nutrient exchange is critical for the symbiosis; otherwise the arbuscules are aborted (Javot et al., 2007; Helber et al., 2011; Breuillin-Sessoms et al., 2015).
The connection of AMF with plant transition metal nutrition has been known from very early on. Mosse (1957) showed that iron and copper content in apple seedlings increased upon mycorrhization. Further studies have shown a role of AMF in improving uptake of additional metals in several different plant species (Caris et al., 1998; Clark and Zeto, 2000; Cavagnaro, 2008). Experiments using radio-labeled metals and a mesh that created a fungal compartment, so that labeled metals could only be reached by fungal hyphae, proved that the host plant is able to recover metal from the soil through the mycorrhizal fungi (Caris et al., 1998). Further support for the existence of a mycorrhizal metal delivery pathway is that mycorrhizal plants diminish the expression levels of some root metal transporters compared to non-mycorrhizal roots. For instance, M. truncatula down-regulates a cortical ZIP protein upon mycorrhization (Burleigh et al., 2003). The specific contribution of the mycorrhizal pathway to plant metal nutrition has not been clearly determined, ranging from 20 to 50% (Ortas, 2012; Lehmann et al., 2014), although will greatly depend on soil type, fungal inoculum, and host. In tomato, for instance, zinc content of fruit was 50% higher in mycorrhizal plants (Cavagnaro et al., 2006). Metal transfer from AMF to their hosts is controlled to prevent metal overload of the host. For instance, when metal concentrations in soil are toxic, mycorrhizal plants accumulate less metals in their shoots than non-mycorrhizal ones (Diaz et al., 1996; Chen et al., 2003; Watts-Williams et al., 2013). Therefore, AMF act as metal buffers, increasing metal delivery to the plant under low metal levels, but decreasing metal uptake when toxic levels of metals are present.
Metal uptake by mycorrhizal fungi should be quite similar to free living fungi. Genomic evidence indicates that model AMF Rhizophagus irregularis genome encodes genes that are putatively involved in metallophore-metal uptake (RiSIT1, RiSIT2, and RiSIT3; Figure 2), as well as other that could be involved in metallophore syntheses (Tamayo et al., 2014). In addition, AMF genome encodes several different members of the Nramp, Ctr, and ZIP families of metal transporters (Tamayo et al., 2014), some of which could conceivably be involved in metal uptake from the soil, as it happens in other fungi (Dancis et al., 1994; Zhao and Eide, 1996; Cohen et al., 2000). Detailed expression and localization analyses of these genes is required to confirm this possibility.
The mechanism of nutrient delivery from AMF to the host is hypothesized to be mediated by vacuoles that act as carriers (Jin et al., 2005; Javot et al., 2006; González-Guerrero et al., 2008b), since a model based on nutrient diffusion would be too expensive given the energy required to establish gradients in the relative large distance from the extrarradical mycelium to the arbuscules. Vacuoles can be directed to different locations in an active way through the cytoskeleton (Allaway and Ashford, 2001). Phosphate that is incorporated by the BAS is delivered to the vacuoles where it is polymerized into polyphosphate fibers (Ezawa et al., 2004), that have a high density of negative charges. Other nutrients are loaded into the negatively charged vacuoles, helping to stabilize the charges (Jin et al., 2005; González-Guerrero et al., 2008b). This model for metal delivery to the host plant is supported in metal localization studies that show colocalization of phosphate and metals in vacuolar compartments (González-Guerrero et al., 2008b). Vacuoles would also play a role in metal detoxification. Vacuolar metal uploading has to be mediated by metal transporters. Two candidates are likely participating in this process: GintZnT1 and GintABC1. The former is a CDF family member that can transport Zn2+ out of the cytosol, that is able to reduce cytosolic zinc levels when expressed in yeast (González-Guerrero et al., 2005). It seems to be involved in early response to Zn2+ increase in the cytosol, as indicated by the upregulation of its expression in the early moments of exposure to moderate zinc concentrations. GintABC1 is an ABC transporter that is upregulated by increased copper, cadmium, and oxidative stress levels, being expressed at similar levels in the intraradical and extraradical mycelium (González-Guerrero et al., 2010). In addition, homologs of vacuolar iron transporter CCC1 and P-type ATPases have been identified in AMF genomes (Tamayo et al., 2014), being putative candidates for iron and copper vacuolar loading, respectively. All these genes could be responsible for metal loading of vacuoles. Under high metal concentrations, vacuoles would be diverted toward the spores (González-Guerrero et al., 2008b), explaining the protective effect that mycorrhiza has against toxic levels of metals in soils (Diaz et al., 1996; Ferrol et al., 2009).
Once the metal-loaded vacuole reaches the arbuscule, polyphosphate is hydrolized and then transferred to the host. As a result, the associated metals would be released into the cytosol by yet-to-be-determined metal transporters. Candidates for metal release are transporters of the Nramp, ZIP, and COPT families (Figure 2). Recently Tamayo et al. (2014) have proposed that RiCtr2, one of the three COPT transporters encoded in R. iregularis genome, could be moving Cu+ from the vacuole into the cytosol. This protein has the closest homology to a yeast vacuolar Ctr protein doing a similar function. Furthermore, it is expressed at the highest levels in the intraradical mycelium, where Cu+ would be released from the vacuole. However, further evidences (subcellular localization of the transporter, gene silencing by Host Induced Gene Silencing) are required to conclusively proof this role. The identity of the transporter(s) responsible for metal translocation across the arbuscule plasma membrane is also unknown, with candidates belonging to the metal efflux families indicated above.
Nutrient recovery from the periarbuscular space is mediated by transporters specific of infected cells for phosphate and ammonia (Javot et al., 2007; Guether et al., 2009). By analogy, it can be expected that metal transporters specific of the colonized cells are also mediating metal uptake. Transcriptomic analyses of these cells indicate that at least they specifically express a Ctr gene, and they up-regulate members of other metal uptake families (ZIP and Nramp; Gaude et al., 2011; Hogekamp and Küster, 2013). In maize, OPT8 could putatively be involved in iron recovery from the periarbuscular space, given that its expression is highly induced in mycorrhizal roots (Kobae et al., 2010). The role of this transporter is also supported by the co-upregulation of NAS genes (Kobae et al., 2010). Further detailed analyses of these proteins still remain to confirm this putative involvement, however, the already available data reflect an increased metal uptake at the cortical layers of mycorrhizal plants, consistent with a role of AMF in delivery metals to their host.
Although not as many as for arbuscular mycorrhiza, there are reports that indicate that ectomycorrhizal fungi are also able to buffer the plant from low or high metal levels (Bücking and Heyser, 1994; Langer et al., 2012). In these organisms, a vacuolar pathway is very likely used to deliver metals from soil to the host, as it is the case for K+ (Garcia et al., 2013). Once inside the root, within the hyphae of the Hartig net, metals and other nutrients will be delivered by a poorly characterized process.
Metal Delivery to Sink Organs
Once the metals are incorporated from soil they are transported to sink organs where the demand is higher. Arguably, photosynthesis is the plant physiological process with the highest transition metal requirements, at least during vegetative growth (Yruela, 2013). Consequently, an important part of the transition metals recovered from soil are directed to leaves. In legumes there are also additional metal sinks, the root nodules. In these organs, symbiotic nitrogen fixation is carried out, which also has relatively large transition metal requirements (Brear et al., 2013; González-Guerrero et al., 2014). Transition elements are also important for plant reproduction (Sancenón et al., 2004; Roschzttardtz et al., 2011; Mary et al., 2015), and as the plant flowers and produces seeds, metals are redirected to these new organs.
The Leaves
Once the metals are into the root cortex, either through the epidermis or through AMF, they symplastically and apoplastically reach the endodermis (Figure 2). Therefore, a number of metal transporters must exist to mediate metal release to the apoplast and subsequent apoplastic metal uptake and, at the pericycle, other metal transporters mediate their release into the xylem. However, there is not much information on transition metal transporters uploading or unloading metals to the apoplast. This is likely due to the lack of phenotype of mutants in genes that would play a role in endodermal transporters required for apoplast metal uptake. This seems to indicate that the symplastic metal translocation pathway is the most predominant or at least sufficient to satisfy most of the plant metal demands. In spite of this, localization studies of different metal transporters indicate that Zn2+ uptake from the root apoplast could be carried out by AtZIP2 in A. thaliana root endodermis (Milner et al., 2013). This is a Zn2+ uptake transporter that localizes mainly in the root stele. Nramp transporters could play a similar role. Recently, a M. truncatula iron transporter, MtNramp1, has been localized in the root endodermis (Tejada-Jiménez et al., 2015). When the encoding gene is knocked-out, neither growth alteration nor iron-deficiency phenotypes is observed, indicating that the apoplastic pathway for metal delivery to leaves is not essential. However, apolastic iron precipitates were observed in the root cortex, consistent with a role in apoplastic iron uptake.
More is known about how metals are released into the xylem. P-type ATPases play a role in metal extrusion into the xylem. In A. thaliana, HMA2 and HMA4 are the two Zn2+-ATPases that mediate long-distance Zn2+ transport (Eren and Argüello, 2004; Hussain et al., 2004), while HMA5 is likely involved in Cu+ translocation (Andrés-Colás et al., 2006). Iron and cobalt xylem loading is mediated by IRG/FPN transporters (Morrissey et al., 2009). However, at the pH present in the xylem, most metals tend to precipitate (Álvarez-Fernández et al., 2014). As a result, a number of metal binding molecules are present to allow for metal trafficking along the sap. In the case of iron, citrate seems to be the main responsible for maintaining metal solubility (Tiffin, 1970; López-Millán et al., 2000). This is evidenced by the detection of iron-citrate complexes in tomato xylem sap using high performance chromatographic methods (Rellán-Álvarez et al., 2010). Further support of the preeminent role of citrate as iron carrier in the xylem sap is the characterization of the FRD3 gene in Arabidopsis encoding a citrate efflux protein (Rogers and Guerinot, 2002; Durrett et al., 2007). FRD3 mutation results in iron deficiency in shoots and an accumulation of this metal in the roots, consistent with a major reduction of root to shoot transport. This transporter is not only involved in xylem citrate loading, but more generally in iron transport across symplastically disconnected tissues (Roschzttardtz et al., 2011). Copper seems to be primarily associated to deoxymugenic acid in monocots, or to its precursor NA in dicots (Álvarez-Fernández et al., 2014). The identity of zinc speciation in the xylem sap still remains elusive, being histidine, NA, citrate, or cysteine candidate molecules (Álvarez-Fernández et al., 2014).
Metal release from the xylem is not very well characterized. It has to go from the xylem into the mesophyll cells (Figure 3). Based on their expression pattern, ZIP transporters AtIRT3 and OsZIP4 might be involved in this process in the case of zinc (Olsen and Palmgren, 2014). TcZNT1 could be responsible also for zinc extrusion from the vasculature (Küpper and Kochian, 2010). In addition, YSL transporters would also be involved in metal unloading from the xylem (Conte and Walker, 2011), as indicated by the study of the tomato mutant chloronerva. This mutant shows intervenal chlorosis, the result of the mutation of gene encoding for a NA synthase gene (Ling et al., 1999). Due to this mutation, iron unloading from the xylem is impaired causing the chlorosis. Consequently, at this level iron speciation must change from iron-citrate to iron-NA, suggesting that YSL transporters would be mediating iron transfer to the xylem. This is supported by the expression pattern of AtYSL1, AtYSL2, and AtYSL3, with a maximum xylem-associated parenchyma cells, and the phenotype of the ysl1ysl3 mutant (DiDonato et al., 2004; Waters et al., 2006). Given the abundance of NA in these tissues (Stephan et al., 1990), and its affinity for multiple metals (Anderegg and Ripperger, 1989; von Wiren et al., 1999), it could be speculated that YSL transporters with different affinities for the different metal-NA complexes would be responsible for metal transfer from the xylem to the leaves.
FIGURE 3.
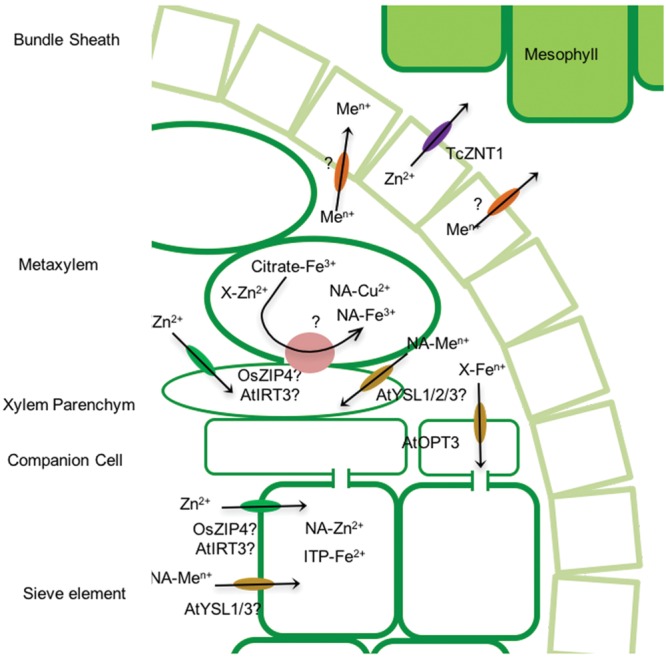
Metal delivery to the mesophyll cells. At is used as a prefix to indicate A. thaliana proteins, Os for Oryza sativa, and Tc for Thlaspi caerulescens. X is used to indicate an unknown metal chelator. Men+ stands for a general metal.
The Legume Nodules
Most legumes, in addition to the shoot, have other metal sinks: the root nodules. These are differentiated root organs that develop as the result of the interaction with specific soil bacteria species generally known as rhizobia (Rhizobium, Bradyrhizobium,…) (Elkan, 1981; Oldroyd, 2013; Downie, 2014). Rhizobia, upon detecting specific flavonoids released by the host plant, produce specific nod factors, that are detected by proteins of the LysM-receptor kinase family in the root epidermis (Oldroyd, 2013; Downie, 2014). This triggers a signaling cascade that results in curling of a root hair, invagination of the plasma membrane, that guides the rhizobia trapped by the root hair into the plant. As this is occurring, cells dedifferentiate in the root pericycle and develop a nodule primordia, in a process with many common aspects to lateral root development (Xiao et al., 2014). Rhizobia reach the nodule cortex, and are released into the host cell cytosol, in an endocytic-like process (Huisman et al., 2012). There, in the appropriate biochemical conditions, rhizobia differentiate into bacteroids (Kondorosi et al., 2013), surrounded by a plant-derived membrane, resulting in the symbiosomes (Clarke et al., 2014). Nodulation is a process that shares many common elements with mycorrhization (the chemical nature of nod and myc factors, or the common signaling pathway), as it very likely evolved from it (Parniske, 2008; Gutjahr and Parniske, 2013; Oldroyd, 2013).
In the symbiosomes N2 is converted into NH4+, a process catalyzed by the enzyme nitrogenase. This enzyme represents 10% of the total bacteroid proteins and has a unique iron-molybdenum cofactor (FeMoco) that with the assistance of two other Fe-S clusters is responsible for the reduction reaction (Miller et al., 1993). This process requires large amounts of energy and is very sensitive to O2, which poisons the enzyme. However, rhizobia are strict aerobes and cannot produce energy in anaerobic conditions. For this reason, the enzyme leghemoglobin (20% of the nodule protein) is critical for the reaction, since it is able to control O2 levels in the nodule (Appleby, 1984). It has a high affinity Fe-heme cofactor that keeps O2 concentrations below 1 μM (Soupène et al., 1995). This high affinity also requires the existence of high affinity cytochrome oxidases that can use O2 as electron acceptor when present at very low concentrations. This is cytochrome oxidase cbb3 that has a iron and copper cofactors (Preisig et al., 1996b). Other metalloenzymes are also critical for symbiotic nitrogen fixation, such as many of the detoxifiers of the free radicals that are produced in the nodule (Becana et al., 2010). The variety of metalloproteins and the high concentrations required of them, make the nodule one of the main metal sinks in legumes. In fact, metal bioavailability limits nodule appearance and development (Tang et al., 1991, 1992). To avoid this, nodulated plants typically induce their metal deficiency responses to ensure an adequate supply of metals to the nodule (Terry et al., 1991). This response also indicates that all these metals have to be provided by the host plant, rather than using any type of rhizobial metal reservoir.
Metal delivery to the nodule could, theoretically be through the epidermal layer (as in roots), delivered by the vasculature (as the shoot), or use pre-existing metal reserves. Studies of metal visualization using synchrotron based X-ray fluorescence in M. truncatula indeterminate type nodules indicate that iron, and likely the other elements, are delivered by the vasculature (Rodríguez-Haas et al., 2013). Indeterminate nodules are a type of nodule that has an apical meristem, and is, in theory, able to grow indefinitely. As it does, different developmental zones appear. Zone I is the meristematic region, Zone II is the zone where rhizobia reach the cells and differentiate into bacteroids, Zone III is the fixation zone, and Zone IV is the senescent one (Vasse et al., 1990). To this, some authors add the interzone, between Zones II and III, and Zone V, where the rhizobia act as saprobes (Timmers et al., 2000; Roux et al., 2014). By studying metal distribution along an indeterminate nodule, S-XRF analyses could study the different stages of nodule development. The results showed no metal storages either in the meristematic region or in the epidermis. In Zone II, iron accumulated in the apoplast, while in Zone III it was located in the infected cells, associated to symbiosomes. In Zone IV, the metal concentration in the infected cells diminished and they were relocalized to the vasculature. These observations were consistent with a model in which the metals were primarily delivered by the vasculature (Figure 4A) (Rodríguez-Haas et al., 2013). Ferroreductase activity in nodules is also increased (Slatni et al., 2011), what could indicate either that there is also some input from the nodule epidermis, or that metals have to be reduced within the nodule. The existence of a Fe2+ transporter in rhizobia infected nodule cells in zone II (Tejada-Jiménez et al., 2015), and the very likely use of citrate as an Fe3+ carrier to the nodule (Takanashi et al., 2013), would indicate that the induction of nodule ferroreductase activity observed is to facilitate metal upload by rhizobia-infected cells. No S-XRF analyses have been carried out in determinate-type nodules (as in soybean, without the meristematic region, and consequently, no clear zonation). However, the mutation of a nodule-specific citrate transporter (LjMATE1) results in iron accumulation in the vasculature and reduced nitrogen fixation rates (Takanashi et al., 2013), indicating that metal delivery in determinate type nodules would also be through the vessels.
FIGURE 4.
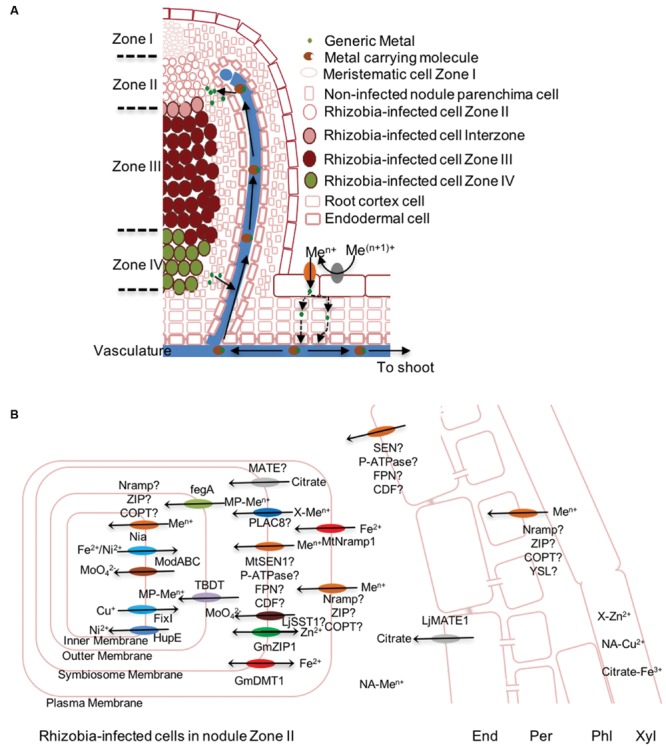
Metal transport in indeterminate nodules. (A) General overview of metal delivery and recovery to legume nodules. (B) Detail of transport process to deliver metals to symbiosomes in the nodule Zone II. Mt is used as a prefix to indicate M. truncatula proteins, Gm for G. max, and Lj for Lotus japonicus. X or MP are used to indicate an unkown metal chelator or a general metallophore, respectively. End stands for Endodermis, Per for Pericycle, Xyl for Xylem, Phl for Phloem, and Men+ for a general metal.
Once in the apoplast of Zone II, metals have to be incorporated by the cells (Figure 4B). It is to be expected that members of the ZIP, Nramp, YSL, and Ctr families are responsible for this. A M. truncatula Nramp transporter, MtNramp1, has been show to play a role in iron uptake by cells in Zone II (Tejada-Jiménez et al., 2015). This transporter is localized in the plasma membrane of these cells, and nramp1 mutant plants have reduced nitrogen fixation capabilities which are restored when the mutated gene is reintroduced or when the plants are watered with iron-fortified solutions. Zone II is the only region of the nodule where MtNramp1 is expressed, supporting the hypothesis that this area is where metals are released form the vessels. Nramp transporters can also transport other divalent metals, and consequently MtNramp1 could conceivably introduce other elements in the cells. However, no changes in the concentration of other elements where observed in either nodules or roots of nramp1 plants. Moreover, nitrogenase activity in these mutant lines was only reduced 60%, what indicates that iron must be uploaded through other systems, perhaps a yet-to-be-determined ZIP transporter.
In addition to citrate, NA should also be playing an important role in metal speciation in the nodule, since mutation in a NA synthase gene in M. truncatula results in a loss of nitrogenase activity in nodules (Avenhaus et al., 2016). This result indicates that during nodule maturation, metal speciation changes with an important effect on the functioning of the symbiosomes. It could be due to several different possibilities, such as NA being the intracellular metal carrier, NA being the metal donor for a specific intracellular transporter required for nitrogen fixation in the nodule, or NA mediating metal delivery to the nodule. More information on nodule metal speciation and in the specific localization of each metal species is required to draw further conclusions.
After being introduced into the rhizobia-infected cells, cytosolic metals have to be delivered to the same organelle as in a regular plant cell, and to symbiosomes. There is very little information on how this is done. It could merely rely in mass action effects and that metals are delivered to the organelle that has a bigger demand, and consequently a bigger “pull” on the metal reserves. Other possibility is a more directed way, either by establishing different pools accessible to only some acceptor, or by using different metal carrying proteins depending on its final destination. Identifying this mechanism is critical toward the current efforts to develop nitrogen fixing capabilities in non-legumes, since providing the metal cofactor in a timely manner is essential for nitrogenase assembly and function. These efforts will be greatly helped by the unequivocal identification of the proteins required for metal transport across the symbiosome. Seminal work by Moreau et al. (2001) and Kaiser et al. (2003) identified in Glycine max a ZIP and a Nramp transporter that were connected to metal transfer to the symbiosomes. However, the transporters belonging to these families that have been biochemically characterized transport metals toward the cytosol (Figure 1) (Nevo and Nelson, 2006; Lin et al., 2010). As a result, it could be speculated that GmZIP1 and GmDMT1 could more likely be involved in preventing metal overload of the symbiosomes by facilitating metal efflux from them. More recently, Hakoyama et al. (2012) have identified a sen1 mutant in G. max and in M. truncatula that is affected in symbiotic nitrogen fixation. SEN1 encodes a nodule-specific protein belonging to the VIT1/CCC1 family, which are located in organelle, transporting iron into their lumen. This would make SEN1 a good candidate to directly provide metal through the symbiosome membrane, although information of its subcellular localization has not been provided yet. It would also be expected a MATE transporter collaborating in iron transport across the symbiosome membrane. Guerinot et al. (1990) showed that B. japonicum prefers citrate as the chelator for iron uptake in free living conditions. If this is also valid for the nodule, we would expect a citrate-exporter, similar to A. thaliana FRD3, that would translocate citrate into the peribacteroid space. Zinc transport into the symbiosome could also potentially be carried out by proteins carrying he PLAC8 superfamily motif recently identified in the proteome of G. max nodules (Clarke et al., 2015), some of which have been suggested to play a role in zinc transport (Song et al., 2010a). In the case of copper, it would be expected that a P1b-ATPases would play this role. However, in the available transcriptomic data there is no ATPase upregulated in the nodule (Benedito et al., 2008; Roux et al., 2014). Similarly to copper, no molybdate transporter has been identified in the symbiosome membrane in spite of the importance of molybdenum in symbiotic nitrogen fixation. However, some sulfate transporters can also mediate molybdate transfer across membranes (Fitzpatrick et al., 2008). Consequently, symbiosome-specific sulfate transporter SST1 (Krussell et al., 2005) could also facilitate molybdenum delivery to bacteroids.
Once they cross the symbiosome membrane, metals are accumulated in the peribacteroideal space, as indicated by radiotracer studies of iron (LeVier et al., 1996). From there, metals have to cross the rhizobial outer membrane. Very little information is available on the identity of these metal transporters. Based on the study of metal uptake by free-living rhizobia or by pathogenic bacteria, it can be speculated that a metallophore-mediated system could be used (LeVier et al., 1996; Postle and Larsen, 2007; Aznar et al., 2014). Many of these complexes are substrate of the TonB-dependent transporters (TBDTs; Postle and Larsen, 2007), that can mediate the uptake of iron, zinc, cobalt, or nickel complexes (Chakraborty et al., 2007; Noinaj et al., 2010). Further support for metallophore role in metal transport across bacteroideal outer membrane is that R. leguminosarum vicibactin receptor is induced both in free-living bacteria grown under iron-limiting conditions, and in the infection zone of pea nodules (Yeoman et al., 2000), the area where metals are likely incorporated (Rodríguez-Haas et al., 2013). However, other systems are probably in place, since mutation of these transport systems does not show any major effect on symbiotic nitrogen fixation capabilities (Yeoman et al., 2000; Lynch et al., 2001).
From the bacteroideal periplasm, metals have to be transported into the cytosol. Molybdenum is introduced as a molybdate anion by the ModABC system (Delgado et al., 2006; Cheng et al., 2016). This transport complex is essential for nitrogenase maturation. Those rhizobia that also express hydrogenase require Ni2+ uptake, which is mediated by the transporter HupE (Brito et al., 2010). The identity of the transporters of other essential metals (iron, copper, zinc,…) has not been determined, what indicated that this might be a robust process where the mutation of single transport genes might not be enough to substantially reduce their transport. Metal efflux is also a very important process in bacteroids both to avoid metal toxicity and to metallate periplasmic proteins. In some cases, metal delivery by the plant systems could theoretically overload the bacterial metal homeostatic mechanisms, as seems to be indicated by the presence of a metal pool in the peribacteroideal space (LeVier et al., 1996). In this sense, bacteroids express P-type ATPases, CopA1 for Cu+, and Nia1 for Fe2+ and Ni2+ in the case of S. meliloti (Zielazinski et al., 2013; Patel et al., 2014). These systems are not critical for symbiotic nitrogen fixation, although, they are expressed in symbiotic conditions and they confer metal tolerance under free-living conditions. Another Cu+-ATPase, FixI is responsible for providing the Cu+ cofactor to high-affinity cytochrome oxidases (Preisig et al., 1996a). In some rhizobia, there has been a duplication of fixI, resulting in differential roles along the nodule (Patel et al., 2014). In addition, there is a third type of Cu+-ATPase, CopA3, which would be responsible for metallating some of the cuproenzymes involved in resisting the initial assault of the plant immune system in the early stages of nodulation (Patel et al., 2014).
Similar mechanisms of metal transport must be in place for other endosymbiotic interactions in which the microsymbiont is isolated from soil, as is the case of actinorhiza. In this sense, it has been shown that nodulated Alnus glutinosa plants allocate more molybdenum to roots (Bélanger et al., 2013; Pourhassan et al., 2015), consistent with the synthesis of FeMoCo for nitrogen fixation. In fact, control of nutrient delivery would be a possible mechanism of controlling the microbiont proliferation, since by limiting access to essential nutrients or encouraging it, growth rates can be modulated. However, very little is known on the specifics of modulation of metal transfer to other beneficial endosymbiotic bacteria.
The Seeds
Metal delivery to seeds and to younger leaves seem to be carried out through the phloem (Curie et al., 2008). At nodes in the stem and minor veins metal are very likely transferred from the xylem to the phloem (Andriunas et al., 2013). In the case of iron and A. thaliana this seems to be mediated by an OPT transporter, OPT3 (Figure 3) (Zhai et al., 2014). This transporter is localized in companion cells in minor veins and stem nodes, and its mutation results in iron accumulation in the vicinity of these veins, as well as in the xylem sap. As expected of a OPT transporter, it does not use iron as substrate but rather an iron-chelator complex to be determined. No information is available for how other transition elements are transferred from the xylem to the phloem.
In addition to transfer from the xylem, phloem also obtains metals from senescent organs. As the plant flowers, sink organs such as leaves and nodules (in the case of legumes) senesce and their nutrients are recycled. Alterations in NA levels results in reproductive abnormalities, indicating that this molecule participates in metal delivery to the flowers (Stephan et al., 1990; Takahashi et al., 2003). Consistent with this is the role of YSL transporters in metal seed loading. Expression of AtYSL1 and AtYSL3 in A. thaliana peaked during leaf senescence (Waters et al., 2006). Moreover, double mutant ysl1ysl3 plants had higher zinc and copper content in leaves, while reduced levels of iron, zinc, and copper where detected in their seeds. In rice, OsYSL2 and OsYSL16 would have a similar function (Ishimaru et al., 2010; Zheng et al., 2012). Legumes also recycle their metals from the nodules (Burton et al., 1998), very likely mediated by NA, as indicated by the identification of a NA synthase gene specific of senescent nodules that it is expressed in the vasculature (Hakoyama et al., 2009), and by the iron relocation observed in the senescent areas of nodules (Rodríguez-Haas et al., 2013). Overall, these and other data suggest that senescent organs are an important source of metals for flowering and embryo development (Hocking and Pate, 1977; Burton et al., 1998).
Although NA plays an important role in phloem loading, it does not seem to be the major iron chelating agent in it (Álvarez-Fernández et al., 2014). Iron is mostly associated to high molecular weight molecules, such as the iron transport protein ITP discovered in Ricinus communis (Krüger et al., 2002). In contrast zinc and copper seem to be mostly associated to NA (Álvarez-Fernández et al., 2014), although some complexes with higher molecular-weight molecules such as metallothioneins or metallochaperones can also be detected (Mira et al., 2001; Lattanzio et al., 2013).
Metals are also required for gametogenesis. Copper is needed for pollen tube development, as indicated by pollen abnormalities detected in copt1 A. thaliana plants (Sancenón et al., 2004). Iron, delivered as iron-citrate with the help of FRD3, is also essential for pollen development (Roschzttardtz et al., 2011). FRD3 could also play a role in embryogenesis, as citrate would solubilize the iron in the nutritive solution around the developing embryo (Roschzttardtz et al., 2011), although some other transporter ought to be involved since no developmental alteration was observed in frd3 embryos. Iron delivered to the embryos is directed to vascular tissues were it will be stored in vacuoles in endodermal cells (Roschzttardtz et al., 2009). In A. thaliana, AtVIT1 seems to be mediating the last step, introducing iron in these vacuoles (Kim et al., 2006). Upon germination, Nramp3 and Nramp4 could be responsible for remobilization of these iron reserves, as iron is stored in the vacuoles of endospermal cells to be later used as the seed germinates (Lanquar et al., 2005).
Conclusion
In the last three decades, we have gained a deep insight on what transporters are involved in root metal uptake and translocation to the shoot. We have also identified many of the metal-carrier molecules, as well as unveiled many of the complex regulatory pathways. More recently, as technology improved, the role of microbes in plant metal homeostasis is being better understood, as are the mechanisms mediating metal exchange with the endosymbionts. However, several other aspects have been insufficiently addressed. For instance, information on metal homeostasis in other, less studied, endosymbiosis is still lacking, very probably due to the difficulties of obtaining axenic cultures for some of them. More information is also required of the microbiome of plants growing under different levels of metal nutrition. In addition, although we know of multiple different metal transporters and some carrier proteins, their final destination, the identity of the metalloproteins that will use these metals, remains elusive. Consequently, we are missing a key element to better understand intracellular metal trafficking and use. At a systemic level we still need to determine which are the metal sensors, the signals that determine the plant metal nutritional levels, as well as to determine how the plant controls the shoot to root metal fluxes. This later aspect is especially important in legumes, since symbiotic nitrogen fixation is also an important metal sink and metal partitioning with leaves is critical to correctly balancing carbon and nitrogen fixation rates. Steps are being taken to tackle this question in the coming years. Improved methods for metalloproteomics are being developed, and elements involved in shoot-to-root metal transport in legumes are being unveiled to have a better understanding of metal partitioning in legumes, which together with improved metal imaging and metal speciation methods point toward obtaining a very clear picture on how plants use metals and the role that microorganisms have on plant metal homeostasis. This will allow us to select inoculants which will improve plant metal uptake, as well as cultivars with enhanced metal recovery capabilities from AMF or from senescent nodules, as well as increased delivery for symbiotic nitrogen fixation.
Author Contributions
VE wrote the section on plant metal uptake, ÁS the section on metal transport in the nodule, and MT-J the section on arbuscular mycorrhiza and the molybdenum transport sections. MG-G outlined the manuscript, wrote the remaining sections, and put together the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the members of the group “Plant Metal Homeostasis in Plant-Microbe Interactions” for their valuable input and criticism in preparing this review as well as Dr. Beatriz Jorrín for her help in preparing the figures. We would also like to apologize to those colleagues whose work, due to space limitations, we have not cited.
Footnotes
Funding. Funding was provided by ERC grant ERC-StG-2013-335284 and MINECO grant AGL-2012-32974 to MG-G.
References
- Akkermans M. D., van der Horst-Graat J. M., Eussen S. R. B. M., van Goudoever J. B., Brus F. (2016). Iron and vitamin D deficiency in healthy young children in Western-Europe despite current nutritional recommendations. J. Pediatr. Gastroenterol. Nutr. 62 635–642. 10.1097/MPG.0000000000001015 [DOI] [PubMed] [Google Scholar]
- Allaway W. G., Ashford A. E. (2001). Motile tubular vacuoles in extramatrical mycelium and sheath hyphae of ectomycorrhizal systems. Protoplasma 215 218–225. 10.1007/BF01280316 [DOI] [PubMed] [Google Scholar]
- Aller S. G., Unger V. M. (2006). Projection structure of the human copper transporter CTR1 at 6-A resolution reveals a compact trimer with a novel channel-like architecture. Proc. Natl. Acad. Sci. U.S.A. 103 3627–3632. 10.1073/pnas.0509929103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloway B. J. (2008). Zinc in Soils and Crop Nutrition 2nd Edn. Belgium: International Zinc Association and International Fertilizer Industry Association. [Google Scholar]
- Álvarez-Fernández A., Díaz-Benito P., Abadía A., López-Millán A. F., Abadía J. (2014). Metal species involved in long distance metal transport in plants. Front. Plant Sci. 5:105 10.3389/fpls.2014.00105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderegg G., Ripperger H. (1989). Correlation between metal complex formation and biological activity of nicotianamine analogues. J. Chem. Soc. Chem. Commun. 647–650. 10.1039/c39890000647 [DOI] [Google Scholar]
- Andrés-Colás N., Sancenón V., Rodríguez-Navarro S., Mayo S., Thiele D. J., Ecker J. R., et al. (2006). The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. Plant J. 45 225–236. 10.1111/j.1365-313X.2005.02601.x [DOI] [PubMed] [Google Scholar]
- Andriunas F. A., Zhang H. M., Xia X., Patrick J. W., Offler C. E. (2013). Intersection of transfer cells with phloem biology—broad evolutionary trends, function, and induction. Front. Plant Sci 4:221 10.3389/fpls.2013.00221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antala S., Ovchinnikov S., Kamisetty H., Baker D., Dempski R. E. (2015). Computation and functional studies provide a model for the structure of the zinc transporter hZIP4. J. Biol. Chem. 290 17796–17805. 10.1074/jbc.M114.617613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby C. A. (1984). Leghemoglobin and Rhizobium respiration. Annu. Rev. Plant Physiol. 35 443–478. 10.1146/annurev.pp.35.060184.002303 [DOI] [Google Scholar]
- Argüello J. M. (2003). Identification of ion-selectivity determinants in heavy-metal transport P-1B-type ATPases. J. Membr. Biol. 195 93–108. 10.1007/s00232-003-2048-2 [DOI] [PubMed] [Google Scholar]
- Argüello J. M., Eren E., González-Guerrero M. (2007). The structure and function of heavy metal transport P-1B-ATPases. Biometals 20 233–248. 10.1007/s10534-006-9055-6 [DOI] [PubMed] [Google Scholar]
- Atkinson A., Khalimonchuk O., Smith P., Sabic H., Eide D., Winge D. R. (2010). Mzm1 influences a labile pool of mitochondrial zinc important for respiratory function. J. Biol. Chem. 285 19450–19459. 10.1074/jbc.M110.109793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenhaus U., Cabeza R. A., Liese R., Lingner A., Dittert K., Salinas-Riester G., et al. (2016). Short-term molecular acclimation processes of legume nodules to increased external oxygen concentration. Front. Plant Sci. 6:1133 10.3389/fpls.2015.01133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsen K. B., Palmgren M. G. (1998). Evolution of substrate specificities in the P-type ATPase superfamily. J. Mol. Evol. 46 84–101. 10.1007/PL00006286 [DOI] [PubMed] [Google Scholar]
- Aznar A., Chen N. W. G., Rigault M., Riache N., Joseph D., Desmaële D., et al. (2014). Scavenging iron: a novel mechanism of plant immunity activation by microbial siderophores. Plant Physiol. 164 2167–2183. 10.1104/pp.113.233585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bago B., Azcón-Aguilar C., Goulet A., Piché Y. (1998). Branched absorbing structures (BAS): a feature of the extraradical mycelium of symbiotic arbuscular mycorrhizal fungi. New Phytol. 139 375–388. 10.1046/j.1469-8137.1998.00199.x [DOI] [Google Scholar]
- Barberon M., Dubeaux G., Kolb C., Isono E., Zelazny E., Vert G. (2014). Polarization of Iron-Regulated Transporter 1 (IRT1) to the plant-soil interface plays crucial role in metal homeostasis. Proc. Natl. Acad. Sci. U.S.A. 111 8293–8298. 10.1073/pnas.1402262111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberon M., Zelazny E., Robert S., Conéjéro G., Curie C., Friml J., et al. (2011). Monoubiquitin-dependent endocytosis of the Iron-Regulated Transporter 1 (IRT1) transporter controls iron uptake in plants. Proc. Natl. Acad. Sci. U.S.A. 108 E450–E458. 10.1073/pnas.1100659108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Ness E., Hadar Y., Chen Y., Shanzer A., Libman J. (1992). Iron uptake by plants from microbial siderophores: a study with 7-nitrobenz-2 oxa-1,3-diazole-desferrioxamine as fluorescent ferrioxamine B analog. Plant Physiol. 99 1329–1335. 10.1104/pp.99.4.1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir K., Ishimaru Y., Itai R. N., Senoura T., Takahashi M., An G., et al. (2015). Iron deficiency regulated OsOPT7 is essential for iron homeostasis in rice. Plant Mol. Biol. 88 1–12. 10.1007/s11103-015-0315-0 [DOI] [PubMed] [Google Scholar]
- Baxter I., Muthukumar B., Park H. C., Buchner P., Lahner B., Danku J., et al. (2008). Variation of molybdenum content across broadly distributed populations of Arabidopsis thaliana is controlled by a mitochondrial molybdenum transporter (MOT1). PLoS Genet. 4:e1000004 10.1371/journal.pgen.1000004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle G. W. (1929). Yellow stripe-A factor for chlorophyll deficiency in maize located in the Pr chromosome. Am. Nat. 63 189–192. 10.1086/280252 [DOI] [Google Scholar]
- Becana M., Matamoros M. A., Udvardi M. K., Dalton D. A. (2010). Recent insights into antioxidant defenses of legume root nodules. New Phytol. 188 960–976. 10.1111/j.1469-8137.2010.03512.x [DOI] [PubMed] [Google Scholar]
- Bélanger P. A., Bellenger J. P., Roy S. (2013). Strong modulation of nutrient distribution in Alnus glutinosa as a funcion of the actinorhizal symbiosis. Botany 91 218–224. 10.1139/cjb-2012-0184 [DOI] [Google Scholar]
- Bellenger J. P., Wichard T., Kustka A. B., Kraepiel A. M. L. (2008). Uptake of molybdenum and vanadium by a nitrogen-fixing soil bacterium using siderophores. Nat. Geosci. 1 243–246. 10.1007/s10534-009-9222-7 [DOI] [Google Scholar]
- Benedito V. A., Torres Jerez I., Murray J. D., Andriankaja A., Allen S., Kakar K., et al. (2008). A gene expression atlas of the model legume Medicago truncatula. Plant J. 55 504–513. 10.1111/j.1365-313X.2008.03519.x [DOI] [PubMed] [Google Scholar]
- Bernal M., Casero D., Singh V., Wilson G. T., Grande A., Yang H., et al. (2012). Transcriptome sequencing identifies SPL7-regulated copper acquisition genes FRO4/FRO5 and the copper dependence of iron homeostasis in Arabidopsis. Plant Cell 24 738–761. 10.1105/tpc.111.090431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blindauer C. A. (2008). Zinc-handling in cyanobacteria: an update. Chem. Biodivers. 5 1990–2013. 10.1002/cbdv.200890183 [DOI] [PubMed] [Google Scholar]
- Blindauer C. A., Leszczyszyn O. I. (2010). Metallothioneins: unparalleled diversity in structures and functions for metal ion homeostasis and more. Nat. Prod. Rep. 27 720–741. 10.1039/b906685n [DOI] [PubMed] [Google Scholar]
- Bordenstein S. R., Theis K. R. (2015). Host biology in light of the microbiome: ten principles of holobionts and hologenomes. PLoS Biol. 13:e1002226 10.1371/journal.pbio.1002226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brear E. M., Day D. A., Smith P. M. C. (2013). Iron: an essential micronutrient for the legume–rhizobium symbiosis. Front. Plant Sci. 4:359 10.3389/fpls.2013.00359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuillin-Sessoms F., Floss D. S., Gomez S. K., Pumplin N., Ding Y., Levesque-Tremblay V., et al. (2015). Suppression of the arbuscule degeneration in Medicago truncatula phosphate transporter4 mutant is dependent on the ammonium transporter 2 family protein AMT2;3. Plant Cell 27 1352–1366. 10.1105/tpc.114.131144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briat J. F., Duc C., Ravet K., Gaymard F. (2010). Ferritins and iron storage in plants. Biochim. Biophys. Acta 1800 806–814. 10.1016/j.bbagen.2009.12.003 [DOI] [PubMed] [Google Scholar]
- Brito B., Prieto R. I., Cabrera E., Mandrand-Berthelot M.-A., Imperial J., Ruiz-Argüeso T., et al. (2010). Rhizobium leguminosarum hupE encodes a nickel transporter required for hydrogenase activity. J. Bacteriol. 192 925–935. 10.1128/JB.01045-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bücking H., Heyser W. (1994). The effect of ectomycorrhizal fungi on Zn uptake and distribution in seedlings of Pinus sylvestris L. Plant Soil 167 203–212. 10.1007/BF00007946 [DOI] [Google Scholar]
- Burleigh S. H., Kristensen B. K., Benchmann I. E. (2003). A plasma membrane zinc transporter from Medicago truncatula is up-regulated in roots by Zn fertilization, yet down-regulated by arbuscular mycorrhizal colonization. Plant Mol. Biol. 52 1077–1088. 10.1023/A:1025479701246 [DOI] [PubMed] [Google Scholar]
- Burton J. W., Harlow C., Theil E. C. (1998). Evidence for reutilization of nodule iron in soybean seed development. J. Plant Nutr. 5 913–927. 10.1080/01904169809365453 [DOI] [Google Scholar]
- Cailliatte R., Schikora A., Briat J. F., Mari S., Curie C. (2010). High-affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. Plant Cell 22 904–917. 10.1105/tpc.109.073023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caris C., Hördt W., Hawkins H., Römheld V., George E. (1998). Studies of iron transport by arbuscular mycorrhizal hyphae from soil to peanut and sorghum plants. Mycorrhiza 8 35–39. 10.1007/s005720050208 [DOI] [Google Scholar]
- Carvalhais L. C., Dennis P. G., Badri D. V., Kidd B. N., Vivanco J. M., Schenk P. M. (2015). Linking jasmonic acid signaling, root exudates, and rhizosphere microbiomes. Mol. Plant Microbe Interact. 28 1049–1058. 10.1094/MPMI-01-15-0016-R [DOI] [PubMed] [Google Scholar]
- Cavagnaro T. R. (2008). The role of arbuscular mycorrhizas in improving plant zinc nutrition under low soil zinc concentrations: a review. Plant Soil 304 315–325. 10.1007/s11104-008-9559-7 [DOI] [Google Scholar]
- Cavagnaro T. R., Jackson L. E., Six J., Ferris H., Goyal S., Asami D., et al. (2006). Arbuscular mycorrhizas, microbial communities, nutrient availability, and soil aggregates in organic tomato production. Plant Soil 282 209–225. 10.1007/s11104-005-5847-7 [DOI] [Google Scholar]
- Cesco S., Neumann G., Tomasi N., Pinton R., Weisskopf L. (2010). Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 329 1–25. 10.1007/s11104-009-0266-9 [DOI] [Google Scholar]
- Chakraborty R., Storey E., van der Helm D. (2007). Molecular mechanism of ferricsiderophore passage through the outer membrane receptor proteins of Escherichia coli. Biometals 20 263–274. 10.1007/s10534-006-9060-9 [DOI] [PubMed] [Google Scholar]
- Chan Chung K. C., Zamble D. B. (2011). Protein interactions and localization of the Escherichia coli accessory protein HypA during nickel insertion to [NiFe] hydrogenase. J. Biol. Chem. 286 43081–43090. 10.1074/jbc.M111.290726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparro J. M., Badri D. V., Bakker M. G., Sugiyama A., Manter D. K., Vivanco J. M. (2013). Root exudation of phytochemicals in Arabidopsis follows specific Ppatterns that are developmentally programmed and correlate with soil microbial functions. PLoS ONE 8:e55731 10.1371/journal.pone.0055731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B. D., Li X. L., Tao H. Q., Christie P., Wong M. H. (2003). The role of arbuscular mycorrhiza in zinc uptake by red clover growing in a calcareous soil spiked with various quantities of zinc. Chemosphere 50 839–846. 10.1016/S0045-6535(02)00228-X [DOI] [PubMed] [Google Scholar]
- Cheng G., Karunakaran R., East A. K., Poole P. S. (2016). Multiplicity of sulfate and molybdate transporters and their role in nitrogen fixation in Rhizobium leguminosarum bv. viciae Rlv3841. Mol. Plant Microbe Interact. 29 143–152. 10.1094/MPMI-09-15-0215-R [DOI] [PubMed] [Google Scholar]
- Cheng Y., Yan Y. B., Liu J. (2005). Spectroscopic characterization of metal bound phytochelatin analogue (Glu–Cys)4–Gly. J. Inorg. Biochem. 99 1952–1962. 10.1016/j.jinorgbio.2005.06.016 [DOI] [PubMed] [Google Scholar]
- Clark R. B., Zeto S. K. (2000). Mineral acquisition by arbuscular mycorrhizal plants. J. Plant Nutr. 23 867–902. 10.1080/01904160009382068 [DOI] [Google Scholar]
- Clarke V. C., Loughin P. C., Day D. A., Smith P. M. (2014). Transport processes of the legume symbiosome membrane. Front Plant Sci 5:699 10.3389/fpls.2014.00699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke V. C., Loughlin P. C., Gavrin A., Chen C., Brear E. M., Day D. A., et al. (2015). Proteomic analysis of the soybean symbiosome identifies new symbiotic proteins. Mol. Cell Proteomics 14 1301–1322. 10.1074/mcp.M114.043166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S., Ma J. F. (2016). Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 67 489–512. 10.1146/annurev-arplant-043015-112301 [DOI] [PubMed] [Google Scholar]
- Clemens S., Palmgren M. G., Krämer U. (2002). A long way ahead: understanding and engineering plant metal accumulation. Trends Plant Sci. 7 309–315. 10.1016/S1360-1385(02)02295-1 [DOI] [PubMed] [Google Scholar]
- Cohen A., Nelson H., Nelson N. (2000). The family of SMF metal ion transporters in yeast cells. J. Biol. Chem. 275 33388–33394. 10.1074/jbc.M004611200 [DOI] [PubMed] [Google Scholar]
- Colangelo E. P., Guerinot M. L. (2004). The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. Plant Cell 16 3400–3412. 10.1105/tpc.104.024315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly E. L. (2014). The diverse roles of FRO family metalloreductases in iron and copper homeostasis. Front Plant Sci. 5:100 10.3389/fpls.2014.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte S. S., Walker E. L. (2011). Transporters contributing to iron trafficking in plants. Mol. Plant 4 464–476. 10.1093/mp/ssr015 [DOI] [PubMed] [Google Scholar]
- Cornelis P. (2010). Iron uptake and metabolism in pseudomonads. Appl. Microbiol. Biotechnol. 86 1637–1645. 10.1007/s00253-010-2550-2 [DOI] [PubMed] [Google Scholar]
- Cornelis P., Matthijs S. (2002). Diversity of siderophore-mediated iron uptake systems in fluorescent pseudomonads: not only pyoverdines. Environ. Microbiol. 4 787–798. 10.1046/j.1462-2920.2002.00369.x [DOI] [PubMed] [Google Scholar]
- Crosa J. H. (1989). Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol. Rev. 53 517–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curie C., Cassin G., Couch D., Divol F., Higuchi K., Le Jean M., et al. (2008). Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann. Bot. 103 1–11. 10.1093/aob/mcn207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curie C., Panaviene Z., Loulergue C., Dellaporta S. L., Briat J. F., Walker E. L. (2001). Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 409 346–349. 10.1038/35053080 [DOI] [PubMed] [Google Scholar]
- Dancis A., Yuan D. S., Haile D., Askwith C., Eide D., Moehle C., et al. (1994). Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell 76 393–402. 10.1016/0092-8674(94)90345-X [DOI] [PubMed] [Google Scholar]
- De Feo C., Mootien S., Unger V. (2010). Tryptophan scanning analysis of the membrane domain of CTR-copper transporters. J. Membr. Biol. 234 113–123. 10.1007/s00232-010-9239-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M. J., Tresierra-Ayala A., Talbi C., Bedmar E. J. (2006). Functional characterization of the Bradyrhizobium japonicum modA and modB genes involved in molybdenum transport. Microbiology 152 199–207. 10.1099/mic.0.28347-0 [DOI] [PubMed] [Google Scholar]
- Diaz G., Azcon-Aguilar C., Honrubia M. (1996). Influence of arbuscular mycorrhizae on heavy metals (Zn and Pb) uptake and growth of Lygeum spartum and Anthyllis cytisoides. Plant Soil 180 241–249. 10.1007/BF00015307 [DOI] [Google Scholar]
- DiDonato R. J., Roberts L. A., Sanderson T., Eisley R. B., Walker E. L. (2004). Arabidopsis yellow Stripe-Like2 (YSL2) a metal-regulated gene encoding a plasma membrane transporter of nicotianamine-metal complexes. Plant J. 39 403–414. 10.1111/j.1365-313X.2004.02128.x [DOI] [PubMed] [Google Scholar]
- Downie J. A. (2014). Legume nodulation. Curr. Biol. 24 R184–R190. 10.1016/j.cub.2014.01.028 [DOI] [PubMed] [Google Scholar]
- Dupont C. L., Yang S., Palenik B., Bourne P. E. (2006). Modern proteomes contain putative imprints of ancient shifts in trace metal geochemistry. Proc. Natl. Acad. Sci. U.S.A. 103 17822–17827. 10.1073/pnas.0605798103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrett T. P., Gassmann W., Rogers E. E. (2007). The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol. 144 197–205. 10.1104/pp.107.097162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- East R. (2013). Microbiome: soil science comes to life. Nature 501 S18–S19. 10.1038/501S18a [DOI] [PubMed] [Google Scholar]
- Eggert K., von Wiren N. (2013). Dynamics and partitioning of the ionome in seeds germinating seedlings of winter oilseed rape. Metallomics 5 1316–1325. 10.1039/c3mt00109a [DOI] [PubMed] [Google Scholar]
- Ehrnstorfer I. A., Geertsma E. R., Pardon E., Steyaert J., Dutzler R. (2014). Crystal structure of a SLC11 (NRAMP) transporter reveals the basis for transition-metal ion transport. Nat. Struct. Mol. Biol. 21 990–996. 10.1038/nsmb.2904 [DOI] [PubMed] [Google Scholar]
- Eide D., Broderius M., Fett J., Guerinot M. L. (1996). A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc. Natl. Acad. Sci. U.S.A. 93 5624–5628. 10.1073/pnas.93.11.5624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisses J. F., Chi Y., Kaplan J. H. (2005). Stable plasma membrane levels of hCTR1 mediate cellular copper uptake. J. Biol. Chem. 280 9635–9639. 10.1074/jbc.M500116200 [DOI] [PubMed] [Google Scholar]
- Elkan G. H. (1981). “The taxonomy of the Rhizobiaceae,” in Biology of the Rhizobiaceae Biology of the Rhizobiaceae ed. Atherly K. L. G. G. (Cambridge, MA: Academic Press; ) 1–14. [Google Scholar]
- Eren E., Argüello J. M. (2004). Arabidopsis HMA2, a divalent heavy metal-transporting PIB-type ATPase, is involved in cytoplasmic Zn2+ homeostasis. Plant Physiol. 136 3712–3723. 10.1104/pp.104.046292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren E., González-Guerrero M., Kaufman B. M., Argüello J. M. (2007). Novel Zn2+ coordination by the regulatory N-terminus metal binding domain of Arabidopsis thaliana Zn(2+)-ATPase HMA2. Biochemistry 46 7754–7764. 10.1021/bi7001345 [DOI] [PubMed] [Google Scholar]
- Ezawa T., Cavagnaro T. R., Smith S. E., Smith F. A., Ohtomo R. (2004). Rapid accumulation of polyphosphate in extraradical hyphae of an arbuscular mycorrhizal fungus as revealed by histochemistry and a polyphosphate kinase/luciferase system. New Phytol. 161 387–392. [DOI] [PubMed] [Google Scholar]
- Ferrol N., González-Guerrero M., Valderas A., Benabdellah K., Azcon-Aguilar C. (2009). Survival strategies of arbuscular mycorrhizal fungi in Cu-polluted environments. Phytochem. Rev. 8 551–559. 10.1007/s11101-009-9133-9 [DOI] [Google Scholar]
- Field K. J., Pressel S., Duckett J. G., Rimington W. R., Bidartondo M. I. (2015). Symbiotic options for the conquest of land. Trends Ecol. Evol. 30 477–486. 10.1016/j.tree.2015.05.007 [DOI] [PubMed] [Google Scholar]
- Finkelstein J. (2009). Metalloproteins. Nature 460 813–813. 10.1038/460813a [DOI] [PubMed] [Google Scholar]
- Fitzpatrick K. L., Tyerman S. D., Kaiser B. N. (2008). Molybdate transport through the plant sulfate transporter SHST1. FEBS Lett. 582 1508–1513. 10.1016/j.febslet.2008.03.045 [DOI] [PubMed] [Google Scholar]
- Foster A. W., Osman D., Robinson N. J. (2014). Metal preferences and metallation. J. Biol. Chem. 289 28095–28103. 10.1074/jbc.R114.588145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcroy P., Sisó-Terraza P., Sudre D., Savirón M., Reyt G., Gaymard F., et al. (2013). Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency. New Phytol. 201 155–167. 10.1111/nph.12471 [DOI] [PubMed] [Google Scholar]
- Frausto da Silva J. J. R., Williams R. J. P. (2001). The Biological Chemistry of the Elements. Oxford: Oxford University Press. [Google Scholar]
- Gaither L. A., Eide D. J. (2000). Functional expression of the human hZIP2 zinc transporter. J. Biol. Chem. 275 5560–5564. 10.1074/jbc.275.8.5560 [DOI] [PubMed] [Google Scholar]
- Garcia K., Delteil A., Conéjéro G., Becquer A., Plassard C., Sentenac H., et al. (2013). Potassium nutrition of ectomycorrhizal Pinus pinaster: overexpression of the Hebeloma cylindrosporum HcTrk1 transporter affects the translocation of both K+ and phosphorus in the host plant. New Phytol. 201 951–960. 10.1111/nph.12603 [DOI] [PubMed] [Google Scholar]
- Gaude N., Bortfeld S., Duensing N., Lohse M., Krajinski F. (2011). Arbuscule-containing and non-colonized cortical cells of mycorrhizal roots undergo extensive and specific reprogramming during arbuscular mycorrhizal development. Plant J. 69 510–528. 10.1111/j.1365-313X.2011.04810.x [DOI] [PubMed] [Google Scholar]
- Gayomba S. R., Jung H. I., Yan J., Danku J., Rutzke M. A., Bernal M., et al. (2013). The CTR/COPT-dependent copper uptake and SPL7-dependent copper deficiency responses are required for basal cadmium tolerance in A. thaliana. Metallomics 5 1262–1275. 10.1039/c3mt00111c [DOI] [PubMed] [Google Scholar]
- González-Guerrero M., Argüello J. M. (2008). Mechanism of Cu+ transporting ATPases: soluble Cu+-chaperones directly transfer Cu+to transmembrane transport sites. Proc. Natl. Acad. Sci. U.S.A. 105 5992–5997. 10.1073/pnas.0711446105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Guerrero M., Azcon-Aguilar C., Mooney M., Valderas A., MacDiarmid C. W., Eide D. J., et al. (2005). Characterization of a Glomus intraradices gene encoding a putative Zn transporter of the cation diffusion facilitator family. Fungal Genet. Biol. 42 130–140. 10.1016/j.fgb.2004.10.007 [DOI] [PubMed] [Google Scholar]
- González-Guerrero M., Benabdellah K., Valderas A., Azcon-Aguilar C., Ferrol N. (2010). GintABC1 encodes a putative ABC transporter of the MRP subfamily induced by Cu, Cd, and oxidative stress in Glomus intraradices. Mycorrhiza 20 137–146. 10.1007/s00572-009-0273-y [DOI] [PubMed] [Google Scholar]
- González-Guerrero M., Eren E., Rawat S., Stemmler T. L., Argüello J. M. (2008a). Structure of the two transmembrane Cu+ transport sites of the Cu+- ATPases. J. Biol. Chem. 283 29753–29759. 10.1074/jbc.M803248200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Guerrero M., Matthiadis A., Sáez Á., Long T. A. (2014). Fixating on metals: new insights into the role of metals in nodulation and symbiotic nitrogen fixation. Front. Plant Sci. 5:45 10.3389/fpls.2014.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Guerrero M., Melville L. H., Ferrol N., Lott J. N., Azcon-Aguilar C., Peterson R. L. (2008b). Ultrastructural localization of heavy metals in the extraradical mycelium and spores of the arbuscular mycorrhizal fungus Glomus intraradices. Can. J. Microbiol. 54 103–110. 10.1139/w07-119 [DOI] [PubMed] [Google Scholar]
- Grotz N., Fox T., Connolly E., Park W., Guerinot M. L., Eide D. (1998). Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc. Natl. Acad. Sci. U.S.A. 95 7220–7224. 10.1073/pnas.95.12.7220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotz N., Guerinot M. L. (2006). Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim. Biophys. Acta 1763 595–608. 10.1016/j.bbamcr.2006.05.014 [DOI] [PubMed] [Google Scholar]
- Guerinot M. L. (2000). The ZIP family of metal transporters. Biochim. Biophys. Acta 1465 190–198. 10.1016/S0005-2736(00)00138-3 [DOI] [PubMed] [Google Scholar]
- Guerinot M. L., Meidl E. J., Plessner O. (1990). Citrate as a siderophore in Bradyrhizobium japonicum. J. Bacteriol. 172 3298–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guether M., Neuhäuser B., Balestrini R., Dynowski M., Ludewig U., Bonfante P. (2009). A mycorrhizal-specific ammonium transporter from Lotus japonicus acquires nitrogen released by arbuscular mycorrhizal fungi. Plant Physiol. 150 73–83. 10.1104/pp.109.136390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunshin H., Mackenzie B., Berger U. V., Gunshin Y., Romero M. F., Boron W. F., et al. (1997). Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388 482–488. 10.1038/41343 [DOI] [PubMed] [Google Scholar]
- Gupta S., Chai J., Cheng J., D’Mello R., Chance M. R., Fu D. (2014). Visualizing the kinetic power stroke that drives proton-coupled Zn(II) transport. Nature 512 101–104. 10.1038/nature13382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutjahr C., Parniske M. (2013). Cell and developmental biology of arbuscular mycorrhiza symbiosis. Annu. Rev. Cell Dev. Biol. 29 593–617. 10.1146/annurev-cellbio-101512-122413 [DOI] [PubMed] [Google Scholar]
- Ha S. B., Smith A. P., Howden R., Dietrich W. M., Bugg S., O’Connell M. J., et al. (1999). Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell 11 1153–1163. 10.2307/3870806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakoyama T., Niimi K., Yamamoto T., Isobe S., Sato S., Nakamura Y., et al. (2012). The integral membrane protein SEN1 is required for symbiotic nitrogen fixation in Lotus japonicus nodules. Plant Cell Physiol. 53 225–236. 10.1093/pcp/pcr167 [DOI] [PubMed] [Google Scholar]
- Hakoyama T., Watanabe H., Tomita J., Yamamoto A., Sato S., Mori Y., et al. (2009). Nicotianamine synthase specifically expressed in root nodules of Lotus japonicus. Planta 230 309–317. 10.1007/s00425-009-0944-0 [DOI] [PubMed] [Google Scholar]
- Haney C. H., Ausubel F. M. (2015). Plant microbiome blueprints. Science 349 788–789. 10.1126/science.aad0092 [DOI] [PubMed] [Google Scholar]
- Hart M., Ehret D. L., Krumbein A., Leung C., Murch S., Turi C., et al. (2014). Inoculation with arbuscular mycorrhizal fungi improves the nutritional value of tomatoes. Mycorrhiza 25 359–376. 10.1007/s00572-014-0617-0 [DOI] [PubMed] [Google Scholar]
- Haydon M. J., Kawachi M., Wirtz M., Hillmer S., Hell R., Krämer U. (2012). Vacuolar nicotianamine has critical and distinct roles under iron deficiency and for zinc sequestration in Arabidopsis. Plant Cell 24 724–737. 10.1105/tpc.111.095042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helber N., Wippel K., Sauer N., Schaarschmidt S., Hause B., Requena N. (2011). A versatile monosaccharide transporter that operates in the arbuscular mycorrhiza fungus Glomus sp is crucial for the symbiotic relationship with plants. Plant Cell 23 3812–3823. 10.1105/tpc.111.089813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbig K., Bleuel C., Krauss G. J., Nies D. H. (2008). Glutathione and transition-metal homeostasis in Escherichia coli. J. Bacteriol. 190 5431–5438. 10.1128/JB.00271-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi K., Suzuki K., Nakanishi H., Yamaguchi H., Nishizawa N.-K., Mori S. (1999). Cloning of nicotianamine synthase genes, novel genes involved in the biosynthesis of phytosiderophores. Plant Physiol. 119 471–480. 10.1104/pp.119.2.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltner L. (1904). Über neuere erfahrungen und probleme auf dem gebiete der bodenbakteriologie unter bessonderer berücksichtigung der gründung und brache. Arb. Dtsch. Landwirtsch. Ges. Berl. 98 59–78. [Google Scholar]
- Hirschi K. D. (2009). Nutrient biofortification of food crops. Annu. Rev. Nutr. 29 401–421. 10.1146/annurev-nutr-080508-141143 [DOI] [PubMed] [Google Scholar]
- Hocking P. J., Pate J. S. (1977). Mobilization of minerals to developing seeds of legumes. Ann. Bot. 41 1259–1278. [Google Scholar]
- Hogekamp C., Küster H. (2013). A roadmap of cell-type specific gene expression during sequential stages of the arbuscular mycorrhiza symbiosis. BMC Genomics 14:306–318. 10.1186/1471-2164-14-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman R., Ovchinnikova E., Bisseling T., Limpens E. (2012). Endocytic Accommodation of Microbes in Plants, in Endocytosis in Plants. Berlin: Springer; 271–295. [Google Scholar]
- Hussain D., Haydon M. J., Wang Y., Wong E., Sherson S. M., Young J., et al. (2004). P-Type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell 16 1327–1339. 10.1105/tpc.020487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y., Masuda H., Bashir K., Inoue H., Tsukamoto T., Takahashi M., et al. (2010). Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J. 62 379–390. 10.1111/j.1365-313X.2010.04158.x [DOI] [PubMed] [Google Scholar]
- Jamison D. T., Breman J. G., Measham A. R., Alleyne G., Claeson M., Evans D. B., et al. (eds) (2006). Stunting, Wasting, and Micronutrient Deficiency Disorders. Washington, DC: World Bank Publications. [PubMed] [Google Scholar]
- Javot H., Penmetsa R. V., Terzaghi N., Cook D. R., Harrison M. J. (2007). A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. U.S.A. 104 1720–1725. 10.1073/pnas.0608136104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javot H., Pumplin N., Harrison M. J. (2006). Phosphate in the arbuscular mycorrhizal symbiosis: transport properties and regulatory roles. Plant Cell Environ. 30 310–322. 10.1111/j.1365-3040.2006.01617.x [DOI] [PubMed] [Google Scholar]
- Jin H., Pfeffer P. E., Douds D. D., Piotrowski E., Lammers P. J., Shachar-Hill Y. (2005). The uptake, metabolism, transport and transfer of nitrogen in an arbuscular mycorrhizal symbiosis. New Phytol. 168 687–696. 10.1111/j.1469-8137.2005.01536.x [DOI] [PubMed] [Google Scholar]
- Jurkevitch E., Hadar Y., Chen Y. (1992). Differential siderophore utilization and iron uptake by soil and rhizosphere bacteria. Appl. Environ. Microbiol. 8 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser B. N., Moreau S., Castelli J., Thomson R., Lambert A., Bogliolo S., et al. (2003). The soybean NRAMP homologue, GmDMT1, is a symbiotic divalent metal transporter capable of ferrous iron transport. Plant J. 35 295–304. 10.1046/j.1365-313X.2003.01802.x [DOI] [PubMed] [Google Scholar]
- Kim H., Wu X., Lee J. (2013). SLC31 (CTR) family of copper transporters in health and disease. Mol. Aspects Med. 34 561–570. 10.1016/j.mam.2012.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. A., Punshon T., Lanzirotti A., Li L., Alonso J. M., Ecker J. R., et al. (2006). Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 314 1295–1298. 10.1126/science.1132563 [DOI] [PubMed] [Google Scholar]
- Kobae Y., Tamura Y., Takai S., Banba M., Hata S. (2010). Localized expression of arbuscular mycorrhiza-inducible ammonium transporters in soybean. Plant Cell Physiol. 51 1411–1415. 10.1093/pcp/pcq099 [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Nishizawa N. K. (2012). Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 63 131–152. 10.1146/annurev-arplant-042811-105522 [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Ogo Y., Itai R. N., Nakanishi H., Takahashi M., Mori S., et al. (2007). The transcription factor IDEF1 regulates the response to and tolerance of iron deficiency in plants. Proc. Natl. Acad. Sci. U.S.A. 104 19150–19155. 10.1073/pnas.0707010104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaj-Robin O., Russell D., Hayes K. A., Pembroke J. T., Soulimane T. (2015). Cation diffusion facilitator family: structure and function. FEBS Lett. 589 1283–1295. 10.1016/j.febslet.2015.04.007 [DOI] [PubMed] [Google Scholar]
- Kondorosi E., Mergaert P., Kereszt A. (2013). A Paradigm for endosymbiotic life: cell differentiation of Rhizobium bacteria provoked by host plant factors. Annu. Rev. Microbiol. 67 611–628. 10.1146/annurev-micro-092412-155630 [DOI] [PubMed] [Google Scholar]
- Kraepiel A. M. L., Bellenger J. P., Wichard T., Morel F. M. M. (2009). Multiple roles of siderophores in free-living nitrogen-fixing bacteria. Biometals 22 573–581. 10.1007/s10534-009-9222-7 [DOI] [PubMed] [Google Scholar]
- Krüger C., Berkowitz O., Stephan U. W., Hell R. (2002). A metal-binding member of the late embryogenesis abundant protein family transports iron in the phloem of Ricinus communis L. J. Biol. Chem. 277 25062–25069. 10.1074/jbc.M201896200 [DOI] [PubMed] [Google Scholar]
- Krussell L., Krause K., Ott T., Desbrosses G., Krämer U., Sato S., et al. (2005). The sulfate transporter SST1 is crucial for symbiotic nitrogen fixation in Lotus japonicus root nodules. Plant Cell 17 1625–1636. 10.1105/tpc.104.030106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpper H., Kochian L. V. (2010). Transcriptional regulation of metal transport genes and mineral nutrition during acclimatization to cadmium and zinc in the Cd/Zn hyperaccumulator, Thlaspi caerulescens (Ganges population). New Phytol. 185 114–129. 10.1111/j.1469-8137.2009.03051.x [DOI] [PubMed] [Google Scholar]
- Langer I., Santner J., Krpata D., Fitz W. J., Wenzel W. W., Schweiger P. F. (2012). Ectomycorrhizal impact on Zn accumulation of Populus tremula L. grown in metalliferous soil with increasing levels of Zn concentration. Plant Soil 355 283–297. 10.1007/s11104-011-1098-y [DOI] [Google Scholar]
- Lanquar V., Lelièvre F., Bolte S., Hamès C., Alcon C., Neumann D., et al. (2005). Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 24 4041–4051. 10.1038/sj.emboj.7600864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanquar V., Ramos M. S., Lelievre F., Barbier-Brygoo H., Krieger-Liszkay A., Krämer U., et al. (2010). Export of vacuolar manganese by AtNRAMP3 and AtNRAMP4 is required for optimal photosynthesis and growth under manganese deficiency. Plant Physiol. 152 1986–1999. 10.1104/pp.109.150946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larbi A., Morales F., Abadía A., Abadía J. (2010). Changes in iron and organic acid concentrations in xylem sap and apoplastic fluid of iron-deficient Beta vulgaris plants in response to iron resupply. J. Plant Physiol. 167 255–260. 10.1016/j.jplph.2009.09.007 [DOI] [PubMed] [Google Scholar]
- Lattanzio G., Andaluz S., Matros A., Calvete J. J., Kehr J., Abadía A., et al. (2013). Protein profile of Lupinus texensis phloem sap exudates: searching for Fe- and Zn-containing proteins. Proteomics 13 2283–2296. 10.1002/pmic.201200515 [DOI] [PubMed] [Google Scholar]
- Lehmann A., Veresoglou S. D., Leifheit E. F., Rillig M. C. (2014). Arbuscular mycorrhizal influence on zinc nutrition in crop plants – A meta-analysis. Soil Biol. Biochem. 69 123–131. 10.1016/j.soilbio.2013.11.001 [DOI] [Google Scholar]
- LeVier K., Day D. A., Guerinot M. L. (1996). Iron uptake by symbisomes from soybean root nodules. Plant Physiol. 111 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Chen O. S., Ward D. M., Kaplan J. (2001). CCC1 is a transporter that mediates vacuolar iron storage in yeast. J. Biol. Chem. 276 29515–29519. 10.1074/jbc.M103944200 [DOI] [PubMed] [Google Scholar]
- Li X. L., Marschner H., George E. (1991). Acquisition of phosphorus and copper by VA-mycorrhizal hyphae and root-to-shoot transport in white clover. Plant Soil 136 49–57. 10.1007/BF02465219 [DOI] [Google Scholar]
- Liang C., Piñeros M. A., Tian J., Yao Z., Sun L., Liu J., et al. (2013). Low pH, aluminum, and phosphorus coordinately regulate malate exudation through Gmalmt1 to improve soybean adaptation to acid soils. Plant Physiol. 61 1347–1361. 10.1104/pp.112.208934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Chai J., Love J., Fu D. (2010). Selective electrodiffusion of zinc ions in a Zrt-. Irt-like Protein, ZIPB. J. Biol. Chem. 285 39013–39020. 10.1074/jbc.M110.180620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.-F., Liang H. M., Yang S.-Y., Boch A., Clemens S., Chen C. C., et al. (2009). Arabidopsis IRT3 is a zinc-regulated and plasma membrane localized zinc/iron transporter. New Phytol. 182 392–404. 10.1111/j.1469-8137.2009.02766.x [DOI] [PubMed] [Google Scholar]
- Ling H. Q., Koch G., Bäumlein H., Ganal M. W. (1999). Map-based cloning of chloronerva, a gene involved in iron uptake of higher plants encoding nicotianamine synthase. Proc. Natl. Acad. Sci. U.S.A. 96 7098–7103. 10.1073/pnas.96.12.7098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Li H., Soleimani M., Girijashanker K., Reed J. M., He L., et al. (2008). Cd2+ versus Zn2+ uptake by the ZIP8 -dependent symporter: kinetics, electrogenicity and trafficking. Biochem. Biophys. Res. Comm. 365 814–820. 10.1016/j.bbrc.2007.11.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loper J. E., Henkels M. D. (1999). Utilization of heterologous siderophores enhances levels of iron available to Pseudomonas putida in the rhizosphere. Appl. Environ. Microbiol. 65 5357–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J., Campos-Cuevas J. C., Hernández-Calderón E., Velásquez-Becerra C., Farías-Rodríguez R., Macías-Rodríguez L. I., et al. (2007). Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol. Plant Microbe Interact. 20 207–217. 10.1094/MPMI-20-2-0207 [DOI] [PubMed] [Google Scholar]
- López-Millán A. F., Morales F., Abadía A., Abadía J. (2000). Effects of iron deficiency on the composition of the leaf apoplastic fluid and xylem sap in sugar beet. Implications for iron and carbon transport. Plant Physiol. 124 873–884. 10.1104/pp.124.2.873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Rayo S., Nadal P., Lucena J. J. (2015). Reactivity and effectiveness of traditional and novel ligands for multi-micronutrient fertilization in a calcareous soil. Front. Plant Sci. 6:752 10.3389/fpls.2015.00752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Fu D. (2007). Structure of the zinc transporter YiiP. Science 317 1746–1748. 10.1126/science.1143748 [DOI] [PubMed] [Google Scholar]
- Lubkowitz M. (2011). The oligopeptide transporters: a small gene family with a diverse group of substrates and functions? Mol. Plant 4 407–415. 10.1093/mp/ssr004 [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Kamilova F. (2009). Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 63 541–556. 10.1146/annurev.micro.62.081307.162918 [DOI] [PubMed] [Google Scholar]
- Lynch D., O’Brien J., Welch T., Clarke P., ÓCuív P., Crosa J. H., et al. (2001). Genetic organization of the region encoding regulation, biosynthesis, and transport of rhizobactin 1021, a siderophore produced by Sinorhizobium meliloti. J. Bacteriol 183 2576–2585. 10.1128/JB.183.8.2576-2585.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., Chandrangsu P., Helmann T. C., Romsang A., Helmann J. D. (2014). Bacillithiol is a major buffer of the labile zinc pool in Bacillus subtilis. Mol. Microbiol. 94 756–770. 10.1111/mmi.12794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDiarmid C. W., Milanick M. A., Eide D. J. (2002). Biochemical properties of vacuolar zinc transport systems of Saccharomyces cerevisiae. J. Biol. Chem. 277 39187–39194. 10.1074/jbc.M205052200 [DOI] [PubMed] [Google Scholar]
- Mandal A. K., Argüello J. M. (2003). Functional roles of metal binding domains of the Archaeoglobus fulgidus Cu(+)-ATPase CopA. Biochemistry 42 11040–11047. 10.1021/bi034806y [DOI] [PubMed] [Google Scholar]
- Mapolelo D. T., Zhang B., Randeniya S., Albetel A. N., Li H., Couturier J., et al. (2013). Monothiol glutaredoxins and A-type proteins: partners in Fe–S cluster trafficking. Dalton Trans. 42 3107–3115. 10.1039/c2dt32263c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret W. (2011). Redox biochemistry of mammalian metallothioneins. J. Biol. Inorg. Chem. 16 1079–1086. 10.1007/s00775-011-0800-0 [DOI] [PubMed] [Google Scholar]
- Marschner P., Crowley D., Rengel Z. (2011). Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis - model and research methods. Soil Biol. Biochem. 43 883–894. 10.1016/j.soilbio.2011.01.005 [DOI] [Google Scholar]
- Mary V., Schnell Ramos M., Gillet C., Socha A. L., Giraudat J., Agorio A., et al. (2015). Bypassing iron storage in endodermal vacuoles rescues the iron mobilization defect in the natural resistance associated-macrophage protein 3 natural resistance associated-macrophage protein 4 double mutant. Plant Physiol. 169 748–759. 10.1104/pp.15.00380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Ishimaru Y., Aung M. S., Kobayashi T., Kakei Y., Takahashi M., et al. (2012). Iron biofortification in rice by the introduction of multiple genes involved in iron nutrition. Sci. Rep. 2:543 10.1038/srep00543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. W., Yu Z., Zarkadas C. G. (1993). The nitrogenase proteins of Rhizobium meliloti: purification and properties of the MoFe and Fe components. Biochim. Biophys. Acta 1163 31–41. 10.1016/0167-4838(93)90275-V [DOI] [PubMed] [Google Scholar]
- Milner M. J., Seamon J., Craft E., Kochian L. V. (2013). Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J. Exp. Bot. 64 369–381. 10.1093/jxb/ers315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira H., Martínez-García F., Peñarrubia L. (2001). Evidence for the plant-specific intercellular transport of the Arabidopsis copper chaperone CCH. Plant J. 25 521–528. 10.1046/j.1365-313x.2001.00985.x [DOI] [PubMed] [Google Scholar]
- Mizuno T., Usui K., Horie K., Nosaka S., Mizuno N., Obata H. (2005). Cloning of three ZIP/Nramp transporter genes from a Ni hyperaccumulator plant Thlaspi japonicum and their Ni2+-transport abilities. Plant Physiol. Biochem. 43 793–801. 10.1016/j.plaphy.2005.07.006 [DOI] [PubMed] [Google Scholar]
- Moreau S., Thomson R. M., Kaiser B. N., Trevaskis B., Guerinot M. L., Udvardi M. K., et al. (2001). GmZIP1 encodes a symbiosis-specific zinc transporter in soybean. J. Biol. Chem. 277 4738–4746. 10.1074/jbc.M106754200 [DOI] [PubMed] [Google Scholar]
- Morrissey J., Baxter I. R., Lee J., Li L., Lahner B., Grotz N., et al. (2009). The ferroportin metal efflux proteins function in iron and cobalt homeostasis in Arabidopsis. Plant Cell 21 3326–3338. 10.1105/tpc.109.069401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J., Guerinot M. L. (2009). Iron uptake and transport in plants: the good, the bad, and the ionome. Chem. Rev. 109 4553–4567. 10.1021/cr900112r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosse B. (1957). Growth and chemical composition of mycorrhizal and non-mycorrhizal apples. Nature 179 922–924. 10.1038/179922b013430740 [DOI] [Google Scholar]
- Muckenthaler M. U., Galy B., Hentze M. W. (2008). Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu. Rev. Nutr. 28 197–213. 10.1146/annurev.nutr.28.061807.155521 [DOI] [PubMed] [Google Scholar]
- Nevo Y., Nelson N. (2006). The NRAMP family of metal-ion transporters. Biochim. Biophys. Acta 1763 609–620. [DOI] [PubMed] [Google Scholar]
- Noinaj N., Guillier M., Barnard T. J., Buchanan S. K. (2010). TonB-dependent transporters: regulation, structure, and function. Annu. Rev. Microbiol. 64 43–60. 10.1146/annurev.micro.112408.134247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose Y., Rees E. M., Thiele D. J. (2006). Structure of the Ctr1 copper trans“PORE”ter reveals novel architecture. Trend Biochem. Sci. 31 604–607. 10.1016/j.tibs.2006.09.003 [DOI] [PubMed] [Google Scholar]
- Nozoye T., Nagasaka S., Kobayashi T., Takahashi M., Sato Y., Sato Y., et al. (2011). Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. J. Biol. Chem. 286 5446–5454. 10.1074/jbc.M110.180026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogo Y., Itai R. N., Nakanishi H., Inoue H., Kobayashi T., Suzuki M., et al. (2006). Isolation and characterization of IRO2, a novel iron-regulated bHLH transcription factor in graminaceous plants. J. Exp. Bot. 57 2867–2878. 10.1093/jxb/erl054 [DOI] [PubMed] [Google Scholar]
- Ogo Y., Kobayashi T., Nakanishi Itai R., Nakanishi H., Kakei Y., Takahashi M., et al. (2008). A NOVEL NAC TRANSCRIPTION FACTOR, IDEF2, that recognizes the Iron Deficiency-responsive Element 2 regulates the genes involved in iron homeostasis in plants. J. Biol. Chem. 283 13407–13417. 10.1074/jbc.M708732200 [DOI] [PubMed] [Google Scholar]
- O’Halloran T. V., Culotta V. C. (2000). Metallochaperones, an intracellular shuttle service for metal ions. J. Biol. Chem. 275 25057–25060. 10.1074/jbc.R000006200 [DOI] [PubMed] [Google Scholar]
- Oldroyd G. E. D. (2013). Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 11 252–263. 10.1038/nrmicro2990 [DOI] [PubMed] [Google Scholar]
- Olsen L. I., Palmgren M. G. (2014). Many rivers to cross: the journey of zinc from soil to seed. Front. Plant Sci. 5:30 10.3389/fpls.2014.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Mosqueda M. C., Macías-Rodríguez L. I., Santoyo G., Farías-Rodríguez R., Valencia-Cantero E. (2013). Medicago truncatula increases its iron-uptake mechanisms in response to volatile organic compounds produced by Sinorhizobium meliloti. Folia Microbiol. 58 579–585. 10.1007/s12223-013-0243-9 [DOI] [PubMed] [Google Scholar]
- Ortas I. (2012). Do maize and pepper plants depend on mycorrhizae in terms of phosphorus and zinc uptake? J. Plant Nutr. 35 1639–1656. 10.1080/01904167.2012.698346 [DOI] [Google Scholar]
- Ortiz D. F., Ruscitti T., McCue K. F., Ow D. W. (1995). Transport of metal-binding peptides by HMT1, a fission yeast ABC-type vacuolar membrane protein. J. Biol. Chem. 270 4721–4728. 10.1074/jbc.270.9.4721 [DOI] [PubMed] [Google Scholar]
- Parniske M. (2008). Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat. Rev. Microbiol. 6 763–775. 10.1038/nrmicro1987 [DOI] [PubMed] [Google Scholar]
- Patel S. J., Padilla-Benavides T., Collins J. M., Argüello J. M. (2014). Functional diversity of five homologous Cu+-ATPases present in Sinorhizobium meliloti. Microbiology 160 1237–1251. 10.1099/mic.0.079137-0 [DOI] [PubMed] [Google Scholar]
- Peterson R. L., Massicotte H. B., Melville L. H. (2004). Mycorrhizas: Anatomy and Cell Biology. Ottawa, ON: NRC Research Press. [Google Scholar]
- Pii Y., Borruso L., Brusetti L., Crecchio C., Cesco S., Mimmo T. (2016). The interaction between iron nutrition, plant species and soil type shapes the rhizosphere microbiome. Plant Physiol. Biochem. 99 39–48. 10.1016/j.plaphy.2015.12.002 [DOI] [PubMed] [Google Scholar]
- Pilon M. (2011). Moving copper in plants. New Phytol. 192 305–307. 10.1111/j.1469-8137.2011.03869.x [DOI] [PubMed] [Google Scholar]
- Postle K., Larsen R. A. (2007). TonB-dependent energy transduction between outer and cytoplasmic membranes. Biometals 20 453–465. 10.1007/s10534-006-9071-6 [DOI] [PubMed] [Google Scholar]
- Pourhassan N., Wichard T., Roy S., Bellenger J. P. (2015). Impact of elevated CO2 on metal homeostasis and the actinorhizal symbiosis in early successional alder shrubs. Environ. Exp. Bot. 109 168–176. 10.1016/j.envexpbot.2014.07.014 [DOI] [Google Scholar]
- Preisig O., Zufferey R., Hennecke H. (1996a). The Bradyrhizobium japonicum fixGHIS genes are required for the formation of the high-affinity cbb(3)-type cytochrome oxidase. Arch. Microbiol. 165 297–305. 10.1007/s002030050330 [DOI] [PubMed] [Google Scholar]
- Preisig O., Zufferey R., Thony-Meyer L., Appleby C. A., Hennecke H. (1996b). A high-affinity cbb3-type cytochrome oxidase terminates the symbiosis- specific respiratory chain of Bradyrhizobium japonicum. J. Bacteriol. 178 1532–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimunda D., Elso-Berberián G. (2014). Functional characterization of the CDF transporter SMc02724 (SmYiiP) in Sinorhizobium meliloti: roles in manganese homeostasis and nodulation. Biochim. Biophys. Acta 1838 1–33. 10.1016/j.bbamem.2014.09.005 [DOI] [PubMed] [Google Scholar]
- Raimunda D., Subramanian P., Stemmler T., Argüello J. M. (2012). A tetrahedral coordination of zinc during transmembrane transport by P-type Zn2+-ATPases. Biochim. Biophys. Acta 1818 1374–1377. 10.1016/j.bbamem.2012.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana A., Joshi M., Prasanna R., Shivay Y. S., Nain L. (2012). Biofortification of wheat through inoculation of plant growth promoting rhizobacteria and cyanobacteria. Eur. J. Soil Biol. 50 118–126. 10.1016/j.ejsobi.2012.01.005 [DOI] [Google Scholar]
- Rausch C., Daram P., Brunner S., Jansa J., Laloi M., Leggewie G., et al. (2001). A phosphate transporter expressed in arbuscule-containing cells in potato. Nature 414 462–470. 10.1038/35106601 [DOI] [PubMed] [Google Scholar]
- Rellán-Álvarez R., Giner-Martínez-Sierra J., Orduna J., Orera I., Rodríguez-Castrillón J. Á., García-Alonso J. I., et al. (2010). Identification of a tri-iron(iii), tri-citrate complex in the xylem sap of iron-deficient tomato resupplied with iron: new insights into plant iron long-distance transport. Plant Cell Physiol. 51 91–102. 10.1093/pcp/pcp170 [DOI] [PubMed] [Google Scholar]
- Ricachenevsky F. K., Menguer P. K., Sperotto R. A., Williams L. E., Fett J. P. (2013). Roles of plant metal tolerance proteins (MTP) in metal storage and potential use in biofortification strategies. Front. Plant Sci. 4:144 10.3389/fpls.2013.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricachenevsky F. K., Sperotto R. A. (2014). There and back again, or always there? The evolution of rice combined strategy for Fe uptake. Front. Plant Sci. 5:189 10.3389/fpls.2014.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N. J., Procter C. M., Connolly E. L., Guerinot M. L. (1999). A ferric-chelate reductase for iron uptake from soils. Nature 397 694–697. 10.1038/17800 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Haas B., Finney L., Vogt S., González-Melendi P., Imperial J., González-Guerrero M. (2013). Iron distribution through the developmental stages of Medicago truncatula nodules. Metallomics 5 1247–1253. 10.1039/c3mt00060e [DOI] [PubMed] [Google Scholar]
- Rogers E. E., Guerinot M. L. (2002). FRD3, a member of the multidrug and toxin efflux family, controls iron deficiency responses in Arabidopsis. Plant Cell 14 1787–1799. 10.1105/tpc.001495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschzttardtz H., Conéjéro G., Curie C., Mari S. (2009). Identification of the endodermal vacuole as the iron storage compartment in the Arabidopsis embryo. Plant Physiol. 151 1329–1338. 10.1104/pp.109.144444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschzttardtz H., Séguéla-Arnaud M., Briat J. F., Vert G., Curie C. (2011). The FRD3 citrate effluxer promotes iron nutrition between symplastically disconnected tissues throughout Arabidopsis development. Plant Cell 23 2725–2737. 10.1105/tpc.111.088088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux B., Rodde N., Jardinaud M. F., Timmers T., Sauviac L., Cottret L., et al. (2014). An integrated analysis of plant and bacterial gene expression in symbiotic root nodules using laser-capture microdissection coupled to RNA sequencing. Plant J. 77 817–837. 10.1111/tpj.12442 [DOI] [PubMed] [Google Scholar]
- Rubio-Sanz L., Prieto R. I., Imperial J., Palacios J. M., Brito B. (2013). Functional and expression analysis of the metal-inducible dmerf system from Rhizobium leguminosarum bv. viciae. Appl. Environ. Microbiol. 79 6414–6422. 10.1128/AEM.01954-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel M. T., Bouis H. E. (1998). Plant breeding: a long-term strategy for the control of zinc deficiency in vulnerable populations. Am. J. Clin. Nutr. 68 488S–494S. [DOI] [PubMed] [Google Scholar]
- Sancenón V., Puig S., Mateu-Andres I., Dorcey E., Thiele D. J., Peñarrubia L. (2004). The Arabidopsis copper transporter COPT1 functions in root elongation and pollen development. J. Biol. Chem. 279 15348–15355. 10.1074/jbc.M313321200 [DOI] [PubMed] [Google Scholar]
- Sancenón V., Puig S., Mira H., Thiele D., Peñarrubia L. (2003). Identification of a copper transporter family in Arabidopsis thaliana. Plant Mol. Biol. 51 577–587. 10.1023/A:1022345507112 [DOI] [PubMed] [Google Scholar]
- Santhanam R., Luu V. T., Weinhold A., Goldberg J., Oh Y., Baldwin I. T. (2015). Native root-associated bacteria rescue a plant from a sudden-wilt disease that emerged during continuous cropping. Proc. Natl. Acad. Sci. U.S.A. 112 E5013–E5020. 10.1073/pnas.1505765112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi S., Schmidt W. (2009). Dissecting iron deficiency-induced proton extrussion in Arabidopsis roots. New Phytol. 183 1072–1084. 10.1111/j.1469-8137.2009.02908.x [DOI] [PubMed] [Google Scholar]
- Schaaf G., Honsbein A., Meda A. R., Kirchner S., Wipf D., von Wiren N. (2006). AtIREG2 encodes a tonoplast transport protein involved in iron-dependent nickel detoxification in Arabidopsis thaliana roots. J. Biol. Chem. 281 25532–25540. 10.1074/jbc.M601062200 [DOI] [PubMed] [Google Scholar]
- Schaaf G., Ludewig U., Erenoglu B. E., Mori S., Kitahara T., Wiren, et al. (2004). ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. J. Biol. Chem. 279 9091–9096. 10.1074/jbc.M311799200 [DOI] [PubMed] [Google Scholar]
- Schmidt H., Günther C., Weber M., Spörlein C., Loscher S., Böttcher C., et al. (2014). Metabolome analysis of Arabidopsis thaliana roots identifies a key metabolic pathway for iron acquisition. PLoS ONE 9:e102444 10.1371/journal.pone.0102444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz M. B., Lo C.-C., Chain P. S. (2012). Next generation sequencing and bioinformatic bottlenecks: the current state of metagenomic data analysis. Curr. Opin. Biotechnol. 23 9–15. 10.1016/j.copbio.2011.11.013 [DOI] [PubMed] [Google Scholar]
- Sharma S. S., Dietz K. J., Mimura T. (2016). Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ. 39 1112–1126. 10.1111/pce.12706 [DOI] [PubMed] [Google Scholar]
- Sinani D., Adle D. J., Kim H., Lee J. (2007). Distinct mechanisms for Ctr1-mediated copper and cisplatin transport. J. Biol. Chem. 282 26775–26785. 10.1074/jbc.M703973200 [DOI] [PubMed] [Google Scholar]
- Sinclair S. A., Krämer U. (2012). The zinc homeostasis network of land plants. Biochim. Biophys. Acta 1823 1553–1567. 10.1016/j.bbamcr.2012.05.016 [DOI] [PubMed] [Google Scholar]
- Sisó-Terraza P., Rios J. J., Abadía J., Abadía A., Álvarez-Fernández A. (2015). Flavins secreted by roots of iron-deficient Beta vulgaris enable mining of ferric oxide via reductive mechanisms. New Phytol. 209 733–745. 10.1111/nph.13633 [DOI] [PubMed] [Google Scholar]
- Sivitz A. B., Hermand V., Curie C., Vert G. (2012). Arabidopsis bHLH100 and bHLH101 control iron homeostasis via a FIT-independent pathway. PLoS ONE 7:e44843 10.1371/journal.pone.0044843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatni T., Vigani G., Salah I. B., Kouas S., Dell’Orto M., Gouia H., et al. (2011). Metabolic changes of iron uptake in N2-fixing common bean nodules during iron deficiency. Plant Sci. 181 151–158. 10.1016/j.plantsci.2011.04.015 [DOI] [PubMed] [Google Scholar]
- Smith S. E., Read D. J. (2008). Mycorrhizal Symbiosis. Cambridge, MA: Academic Press. [Google Scholar]
- Smith S. E., Smith F. A. (2011). Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 62 227–250. 10.1146/annurev-arplant-042110-103846 [DOI] [PubMed] [Google Scholar]
- Song W. Y., Choi K. S., Kim D. Y., Geisler M., Park K., Vincenzetti V., et al. (2010a). Arabidopsis PCR2 is a zinc exporter involved in both zinc extrusion and long-distance zinc transport. Plant Cell 22 2237–2252. 10.1105/tpc.109.070185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W. Y., Park J., Mendoza-Cózatl D. G., Suter-Grotemeyer M., Shim D., Hörtensteiner S., et al. (2010b). Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc. Natl. Acad. Sci. U.S.A. 107 21187–21192. 10.1073/pnas.1013964107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soupène E., Foussard M., Boistard P., Truchet G., Batut J. (1995). Oxygen as a key developmental regulator of Rhizobium meliloti N2-fixation gene expression within the alfalfa root nodule. Proc. Natl. Acad. Sci. U.S.A. 92 3759–3763. 10.1073/pnas.92.9.3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan U. W., Scholz G., Rudolph A. (1990). Distribution of nicotianamine, a presumed symplast iron transporter, in different organs of sunflower and of a tomato wild type and its mutant chloronerva. Biochem. Physiol. Pflanz. 186 81–88. 10.1016/S0015-3796(11)80154-X [DOI] [Google Scholar]
- Takahashi M., Terada Y., Nakai I., Nakanishi H., Yoshimura E., Mori S., et al. (2003). Role of nicotianamine in the intracellular delivery of metals and plant reproductive development. Plant Cell 15 1263–1280. 10.1105/tpc.010256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanashi K., Yokosho K., Saeki K., Sugiyama A., Sato S., Tabata S., et al. (2013). LjMATE1: a citrate transporter responsible for iron supply to the nodule infection zone of Lotus japonicus. Plant Cell Physiol. 54 585–594. 10.1093/pcp/pct019 [DOI] [PubMed] [Google Scholar]
- Tamayo E., Gómez-Gallego T., Azcón-Aguilar C., Ferrol N. (2014). Genome-wide analysis of copper, iron and zinc transporters in the arbuscular mycorrhizal fungus Rhizophagus irregularis. Front. Plant Sci. 5:547 10.3389/fpls.2014.00547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C., Robson A. D., Dilworth M. J. (1991). Which stage of nodule initiation in Lupinus angustifolius L. is sensitive to iron deficiency? New Phytol. 117 243–250. 10.1111/j.1469-8137.1991.tb04905.x [DOI] [PubMed] [Google Scholar]
- Tang C., Robson A. D., Dilworth M. J., Kuo J. (1992). Microscopic evidence on how iron deficiency limits nodule initiation in Lupinus angustifolius L. New Phytol. 121 1–12. 10.1111/j.1469-8137.1992.tb02946.x [DOI] [PubMed] [Google Scholar]
- Taylor K. M., Nicholson R. I. (2003). The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim. Biophys. Acta 1611 16–30. 10.1016/S0005-2736(03)00048-8 [DOI] [PubMed] [Google Scholar]
- Tejada-Jiménez M., Castro-Rodríguez R., Kryvoruchko I., Lucas M. M., Udvardi M., Imperial J., et al. (2015). Medicago truncatula natural resistance-associated macrophage protein1 is required for iron uptake by rhizobia-infected nodule cells. Plant Physiol. 168 258–272. 10.1104/pp.114.254672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejada-Jiménez M., Galván A., Fernández E. (2011). Algae and humans share a molybdate transporter. Proc. Natl. Acad. Sci. U.S.A. 108 6420–6425. 10.1073/pnas.1100700108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejada-Jiménez M., Llamas A., Sanz-Luque E., Galván A., Fernández E. (2007). A high-affinity molybdate transporter in eukaryotes. Proc. Natl. Acad. Sci. U.S.A. 104 20126–20130. 10.1073/pnas.0704646104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N., Abadía J. (1986). Function of iron in chloroplasts. J. Plant Nutr. 9 609–646. 10.1080/01904168609363470 [DOI] [Google Scholar]
- Terry R. E., Soerensen K. U., Jolley V. D., Brown J. C. (1991). The role of active Bradyrhizobium japonicum in iron stress response of soy-beans. Plant Soil 130 225–230. 10.1007/BF00011877 [DOI] [Google Scholar]
- Tiffin L. O. (1970). Translocation of iron citrate and phosphorus in xylem exudate of soybean. Plant Physiol. 45 280–283. 10.1104/pp.45.3.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers A. C. J., Soupène E., Auriac M. C., de Billy F., Vasse J., Boistard P., et al. (2000). Saprophytic intracellular rhizobia in alfalfa nodules. Mol. Plant Microbe Interact. 13 1204–1213. 10.1094/MPMI.2000.13.11.1204 [DOI] [PubMed] [Google Scholar]
- Tomatsu H., Takano J., Takahashi H., Watanabe-Takahashi A., Shibagaki N., Fujiwara T. (2007). An Arabidopsis thaliana high-affinity molybdate transporter required for efficient uptake of molybdate from soil. Proc. Natl. Acad. Sci. U.S.A. 104 18807–18812. 10.1073/pnas.0706373104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvardi M., Poole P. S. (2013). Transport and metabolism in legume-rhizobia symbioses. Annu. Rev. Plant Biol. 64 781–805. 10.1146/annurev-arplant-050312-120235 [DOI] [PubMed] [Google Scholar]
- Valentinuzzi F., Pii Y., Vigani G., Lehmann M., Cesco S., Mimmo T. (2015). Phosphorus and iron deficiencies induce a metabolic reprogramming and affect the exudation traits of the woody plant Fragaria × ananassa. J. Exp. Bot. 66 6483–6495. 10.1093/jxb/erv364 [DOI] [PubMed] [Google Scholar]
- Vandenkoornhuyse P., Quaiser A., Duhamel M., Le Van A., Dufresne A. (2015). The importance of the microbiome of the plant holobiont. New Phytol. 206 1196–1206. 10.1111/nph.13312 [DOI] [PubMed] [Google Scholar]
- Vansuyt G., Robin A., Briat J.-F., Curie C., Lemanceau P. (2007). Iron acquisition from fe-pyoverdine by Arabidopsis thaliana. Mol. Plant Microbe Iteract. 20 441–447. 10.1094/MPMI-20-4-0441 [DOI] [PubMed] [Google Scholar]
- Vasse J., de Billy F., Camut S., Truchet G. (1990). Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J. Bacteriol. 172 4295–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnes A., Pommier J., Toci R., Blasco F., Giordano G., Magalon A. (2006). NarJ chaperone binds on two distinct sites of the aponitrate reductase of Escherichia coli to coordinate molybdenum cofactor insertion and assembly. J. Biol. Chem. 281 2170–2176. 10.1074/jbc.M505902200 [DOI] [PubMed] [Google Scholar]
- von Wiren N., Klair S., Bansal S., Briat J. F., Khodr H., Shioiri T., et al. (1999). Nicotianamine chelates both Fe(III) and Fe(II), implications for metal transport in plants. Plant Physiol. 119 1107–1114. 10.1104/pp.119.3.1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters B. M., Chu H.-H., DiDonato R. J., Roberts L. A., Eisley R. B., Lahner B., et al. (2006). Mutations in Arabidopsis Yellow Stripe-Like1 and Yellow Stripe-Like3 reveal their roles in metal ion homeostasis and loading of metal ions in seeds. Plant Physiol. 141 1446–1458. 10.1104/pp.106.082586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts-Williams S. J., Patti A. F., Cavagnaro T. R. (2013). Arbuscular mycorrhizas are beneficial under both deficient and toxic soil zinc conditions. Plant Soil 371 299–312. 10.1007/s11104-013-1670-8 [DOI] [Google Scholar]
- Wei Y., Shohag M. J. I., Yang X., Yibin Z. (2012). Effects of foliar iron application on iron concentration in polished rice grain and its bioavailability. J. Agric. Food Chem. 60 11433–11439. 10.1021/jf3036462 [DOI] [PubMed] [Google Scholar]
- White P. J., Broadley M. R. (2009). Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 182 49–84. 10.1111/j.1469-8137.2008.02738.x [DOI] [PubMed] [Google Scholar]
- Xiao T. T., Schilderink S., Moling S., Deinum E. E., Kondorosi E., Franssen H., et al. (2014). Fate map of Medicago truncatula root nodules. Development 141 3517–3528. 10.1242/dev.110775 [DOI] [PubMed] [Google Scholar]
- Yeh K. Y., Yeh M., Glass J. (2011). Interactions between ferroportin and hephaestin in rat enterocytes are reduced after iron ingestion. Gastroenterology 141 292–299 299.e1 10.1053/j.gastro.2011.03.059 [DOI] [PubMed] [Google Scholar]
- Yeoman K. H., Wisniewski-Dye F., Timony C., Stevens J. B., deLuca N. G., Downie J. A., et al. (2000). Analysis of the Rhizobium leguminosarum siderophore-uptake gene fhuA: differential expression in free-living bacteria and nitrogen-fixing bacteroids and distribution of an fhuA pseudogene in different strains. Microbiology 146 829–837. 10.1099/00221287-146-4-829 [DOI] [PubMed] [Google Scholar]
- Yruela I. (2013). Transition metals in plant photosynthesis. Metallomics 5 1090–1109. 10.1039/c3mt00086a [DOI] [PubMed] [Google Scholar]
- Zamioudis C., Hanson J., Pieterse C. M. J. (2014). β-Glucosidase BGLU42 is a MYB72-dependent key regulator of rhizobacteria-induced systemic resistance and modulates iron deficiency responses in Arabidopsis roots. New Phytol. 204 368–379. 10.1111/nph.12980 [DOI] [PubMed] [Google Scholar]
- Zamioudis C., Korteland J., Van Pelt J. A., van Hamersveld M., Dombrowski N., Bai Y., et al. (2015). Rhizobacterial volatiles and photosynthesis-related signals coordinate MYB72 expression in Arabidopsis roots during onset of induced systemic resistance and iron-deficiency responses. Plant J. 84 309–322. 10.1111/tpj.12995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamioudis C., Mastranesti P., Dhonukshe P., Blilou I., Pieterse C. M. J. (2013). Unraveling root developmental programs initiated by beneficial Pseudomonas spp. bacteria. Plant Physiol. 162 304–318. 10.1104/pp.112.212597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z., Gayomba S. R., Jung H.-I., Vimalakumari N. K., Piñeros M., Craft E., et al. (2014). OPT3 Is a phloem-specific iron transporter that is essential for systemic iron signaling and redistribution of iron and cadmium in Arabidopsis. Plant Cell 26 2249–2264. 10.1105/tpc.114.123737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Sun Y., Xie X., Kim M.-S., Dowd S. E., Paré P. W. (2009). A soil bacterium regulates plant acquisition of iron via deficiency-inducible mechanisms. Plant J. 58 568–577. 10.1111/j.1365-313X.2009.03803.x [DOI] [PubMed] [Google Scholar]
- Zhao H., Eide D. (1996). The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proc. Natl. Acad. Sci. U.S.A. 93 2454–2458. 10.1073/pnas.93.6.2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Yamaji N., Yokosho K., Ma J. F. (2012). YSL16 Is a phloem-localized transporter of the copper-nicotianamine complex that is responsible for copper distribution in rice. Plant Cell 24 3767–3782. 10.1105/tpc.112.103820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Thiele D. J. (2001). Identification of a novel high affinity copper transport complex in the fission yeast Schizosaccharomyces pombe. J. Biol. Chem. 276 20529–20535. 10.1074/jbc.M102004200 [DOI] [PubMed] [Google Scholar]
- Zielazinski E. L., González-Guerrero M., Subramanian P., Stemmler T. L., Argüello J. M., Rosenzweig A. C. (2013). Sinorhizobium meliloti Nia is a P1B-5-ATPase expressed in the nodule during plant symbiosis and is involved in Ni and Fe transport. Metallomics 5 1614–1623. 10.1039/c3mt00195d [DOI] [PMC free article] [PubMed] [Google Scholar]


