Introduction
Gain-of-function mutations in the fibroblast growth factor receptor 3 gene (FGFR3) result in a group of skeletal dysplasias, such as prototypic achondroplasia (ACH: OMIM #100800) and lethal thanatophoric dysplasia (TD1: OMIM #187600). Hypochondroplasia (HCH: OMIM #146000) is the mildest of the FGFR3-associated skeletal dysplasias and is characterized by short stature with macrocephaly, brachydactyly, limited range of motion at the elbows, lumbar lordosis, and bowed legs. Radiological features of HCH are flared metaphyses, narrowed interpedicular distance, square ilia, and short femoral necks.
These clinical and radiological signs are generally less pronounced than those seen with ACH and may not be noticeable until early or middle childhood. Because GH replacement is effective in some HCH patients (1, 2), genetic analysis to assist early diagnosis and intervention may improve the prognosis of these patients. Here, we present such an example and report the identification of a novel mutation in FGFR3 in a familial case of HCH and the effectiveness of GH treatment in the elder sister.
Patient Report
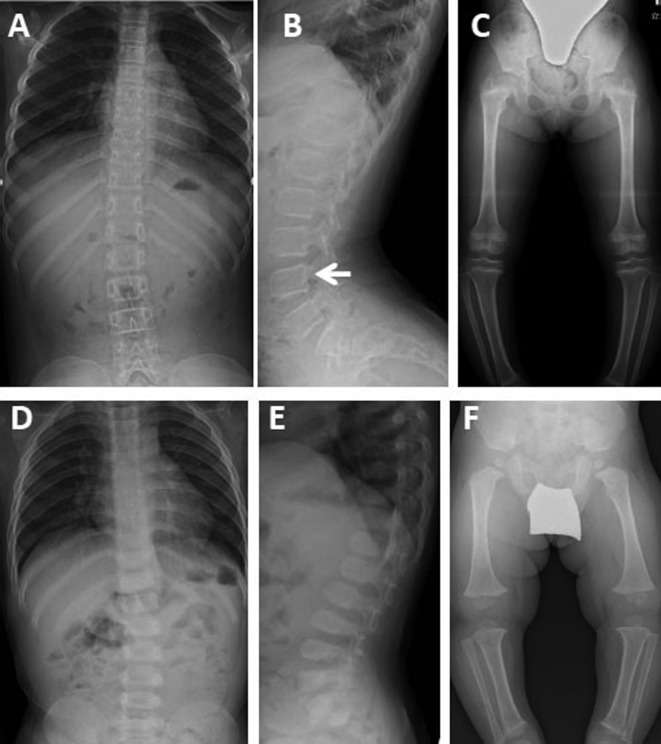
Patient 1: The patient was a 7-yr-old Japanese girl, who was born at full term after an uncomplicated pregnancy and delivery. At birth, her height and weight were 52.5 cm (+2.0 SD) and 2.9 kg (–0.1 SD), respectively. Her father was diagnosed with HCH based on clinical features and radiographs. She was referred to our hospital at 4 yr and 3 mo of age because of short stature. Her height was 86.0 cm (–3.9 SD) and her weight was 14.9 kg (–0.4 SD). The patient’s facial features were unremarkable, and her serum concentrations of insulin-like growth factor 1 (IGF-1) and thyroid hormone were normal. A radiological examination revealed appearances of the spine, pelvis, and legs that were consistent with HCH (Fig. 1A, B, C). She was diagnosed with HCH, and recombinant human GH therapy was started. Her growth responded well to GH treatment. During her last examination at 7 yr and 4 mo of age, her height was 106.5 cm (–2.8 SD).
Fig. 1.
Radiographs of the patients. Radiographs of Patient 1 at the age of 7 yr and 5 mo. Radiographs of the frontal and lateral spine (A, B): Mild thoracolumbar scoliosis is seen. The lumbar interpedicular distance is caudally decreased (L1/L4 0.9). The lumbar vertebral bodies are somewhat round, and lower lumbar vertebral bodies show mild posterior scalloping (arrow). Radiographs of the pelvis and legs (C): The iliac wings appear somewhat squared and the greater sciatic notches are somewhat short. The femoral necks are short. The long bones, particularly the tibia, are mildly broad with flared metaphyses. Genu varum is seen. Radiographs of Patient 2 at the age of 1 yr and 11 mo. Radiographs of the frontal and lateral spine, pelvis, and legs (D, E, F): The skeletal phenotype is similar to that of the patient’s elder sister (Patient 1) (L1/L4 1.1). However, the iliac hypoplasia, short greater sciatic notches, and metaphyseal flaring are more conspicuous.
Patient 2: The patient was a 23-mo-old Japanese boy who was the younger brother of Patient 1. He was born at full term after an uncomplicated pregnancy and delivery. At birth, his height and weight were 48.1 cm (–0.4 SD) and 3.29 kg (+0.7 SD), respectively. He was referred to our hospital at 1 yr and 11 mo of age because of short stature. His height was 73.7 cm (–3.6 SD) and his weight was 10.4 kg (–0.9 SD). He had frontal bossing, a mildly flattened nasal bridge, and short limbs. His serum concentrations of IGF-1 and thyroid hormone were normal. Radiographs of his spine, pelvis, and legs were consistent with HCH, and these findings were more conspicuous than those of his elder sister (Fig. 1D, E, F). HCH was diagnosed based on his short stature with relatively short upper arms and thighs, and the radiological findings.
Mutational Analysis
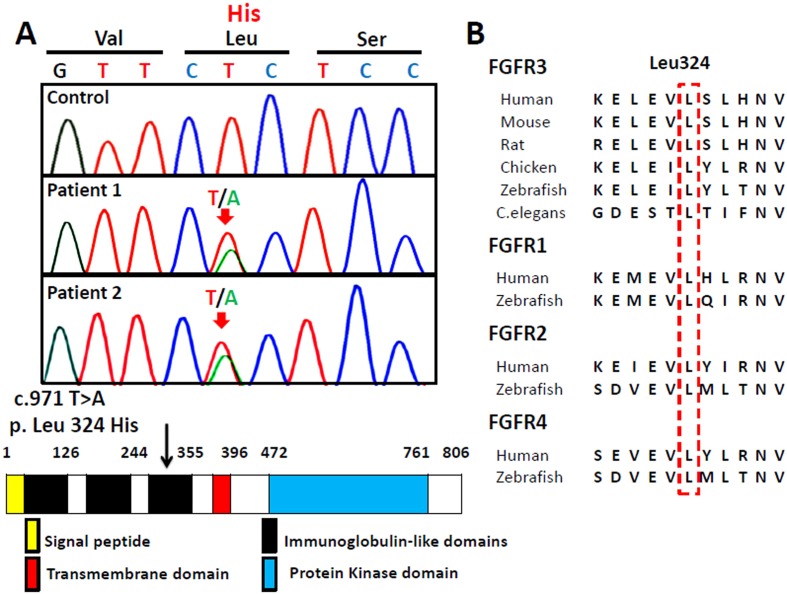
The study was approved by the Institutional Review Board of the Tokyo Metropolitan Children’s Medical Center (H24-94), and informed consent was obtained from the parents. Genomic DNA was extracted from the peripheral blood leukocytes of both patients. We used PCR-direct sequencing to examine all coding exons and flanking introns of FGFR3. A novel c.971T>A (p.Leu324His) missense mutation was identified in both patients (Fig. 2A). This mutation was not detected in any of the 150 healthy controls tested and was absent from various databases, including dbSNP, the 1,000 Genomes Project, Exome Variant Server, NHLBI Exome Sequencing Project, and Human Genetic Variation Database in Japanese. The mutation was located at the third immunoglobulin-like domain in the extracellular region of FGFR3. Leu324 was highly evolutionarily conserved, not only in all vertebrate FGFR3s but also in all the other FGFR families (Fig. 2B). Parental analysis was refused.
Fig. 2.
Identification of the sequence variation of FGFR3. A: Partial sequence of the PCR product, and schematic diagrams of the FGFR3 protein. The chromatogram represents a heterozygosity of histidine (CAC) in place of leucine (CTC) at codon 324, which is located at the third immunoglobulin-like domain in the extracellular region of FGFR3. The arrow indicates the mutated nucleotide. B: Leu324 is a highly evolutionarily conserved amino acid among several other species, not only in FGFR3 but also in the other three FGFRs.
Discussion
We describe a familial case of HCH with a missense mutation, p.Leu324His in FGFR3. The identified mutation is novel and has not been registered in the Human Genome Mutation Database (http://www.hgmd.cf.ac.uk/ac). Although the functional consequence of this mutation remains to be determined in vitro, we believe this mutation is pathological because (i) Leu324 is a highly conserved residue not only in all vertebrate FGFR3s but also in all other FGFR families and (ii) substituting Leu324 with Val has been reported by Saito et al. as being causative of the HCH phenotype (3).
Previous reports of patients with HCH have suggested an improvement in height velocity when treated with GH, particularly at the time of the pubertal growth spurt (1). Pinto et al. reported the natural history of growth in 40 untreated children with HCH and used this cohort as the control for a group of GH-treated HCH children (4). The 19 patients treated with GH in that study had an overall good response, with improvement in height and height velocities. Pinto et al. also concluded that GH treatment was well tolerated and effective in improving growth in HCH patients, particularly when started early. Therefore, genetic analysis can assist early diagnosis and intervention to improve the prognosis of patients with HCH.
Conflict of Interest
The authors have nothing to declare.
Acknowledgements
We thank the patients and their family for participation in this study. We also thank Kazue Kinoshita for technical assistance.
References
- 1.Appan S, Laurent S, Chapman M, Hindmarsh PC, Brook CG. Growth and growth hormone therapy in hypochondroplasia. Acta Paediatr Scand 1990;79: 796–803. doi: 10.1111/j.1651-2227.1990.tb11557.x [DOI] [PubMed] [Google Scholar]
- 2.Tanaka N, Katsumata N, Horikawa R, Tanaka T. The comparison of the effects of short-term growth hormone treatment in patients with achondroplasia and with hypochondroplasia. Endocr J 2003;50: 69–75. doi: 10.1507/endocrj.50.69 [DOI] [PubMed] [Google Scholar]
- 3.Saito T, Nagasaki K, Nishimura G, Takagi M, Hasegawa T, Uchiyama M. Radiological clues to the early diagnosis of hypochondroplasia in the neonatal period: report of two patients. Am J Med Genet A 2012;158A: 630–4. doi: 10.1002/ajmg.a.34424 [DOI] [PubMed] [Google Scholar]
- 4.Pinto G, Cormier-Daire V, Le Merrer M, Samara-Boustani D, Baujat G, Fresneau L, et al. Efficacy and safety of growth hormone treatment in children with hypochondroplasia: comparison with an historical cohort. Horm Res Paediatr 2014;82: 355–63. doi: 10.1159/000364807 [DOI] [PubMed] [Google Scholar]