Abstract
An open-label study with one treatment arm was conducted to investigate changes in health-related biomarkers (blood pressure and liver enzyme activity) and the safety of 4 weeks of consuming a purple-fleshed sweet potato beverage in Caucasian subjects. Twenty healthy adults, 18–70 years of age, with a body mass index >25 kg/m2, elevated blood pressure and elevated levels of liver function biomarkers consumed two cartons of purple-fleshed sweet potato beverage (125 ml, including 117 mg anthocyanin per carton) daily for 4 weeks. Hematology, serum clinical profile, dipstick urinalysis and blood pressure were determined before consumption, at 2 and 4 weeks of consumption and after a 2-week washout period. A trend was found toward lowering systolic blood pressure during the treatment period (p=0.0590). No significant changes were found in diastolic blood pressure throughout the study period. Systolic blood pressure was significantly lower after 4 weeks of consumption compared with before consumption (p=0.0125) and was significantly higher after the 2-week washout period compared with after consumption (p=0.0496). The serum alanine aminotransferase level significantly increased over time, but aspartate aminotransferase and γ-glutamyltransferase levels stayed within the normal range of reference values. Safety parameters of the blood and urine showed no clinically relevant changes. The consumption of a purple-fleshed sweet potato beverage for 4 weeks resulted in no clinically relevant changes in safety parameters of the blood and urine and showed a trend toward lowering systolic blood pressure.
Keywords: anthocyanin, blood pressure, Caucasians, liver function biomarker, safety parameter, sweet potato
INTRODUCTION
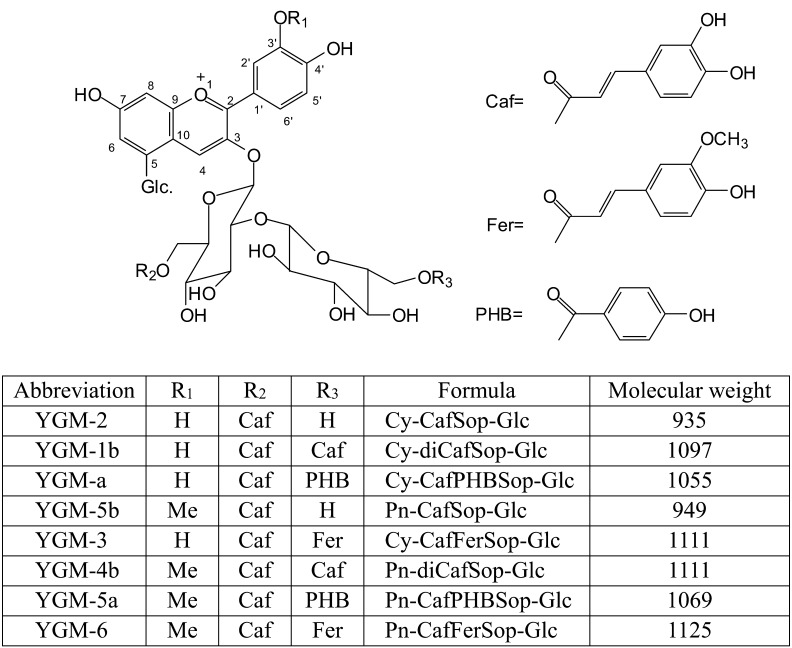
Anthocyanins, naturally occurring pigments in vegetables, fruits and flowers, are promising functional food materials owing to their beneficial effects on human health [1,2,3]. Ayamurasaki is a cultivar of sweet potato (Ipomoea batatas (L.) Lam) that has purple flesh, a high anthocyanin content and high productivity and was developed just over a decade ago in Japan by cross-breeding a line containing anthocyanins with a cultivar lacking β-amylase activity [4]. The chemical structures of the major anthocyanins in the purple-fleshed sweet potato are monoacylated and diacylated forms of cyanidin and peonidin, as shown in Fig. 1 [5, 6]. Anthocyanin pigments in purple-fleshed sweet potatoes have high stability against heat and ultraviolet irradiation owing to their acylated forms, which is an advantage when they are used in food additives as natural colorants [7]. In addition to their use as natural colorants, purple-fleshed sweet potatoes are processed to produce concentrated juice, paste and flour. Many foods containing processed sweet potatoes, such as noodles, bread, jams, chips, confections, beverages and alcoholic beverages, are manufactured and commercially available in Japan [8].
Fig. 1.
Chemical structure of anthocyanins from purple-fleshed sweet potato (Ipomoea batatas cultivar Ayamurasaki).
Me: methyl; Cy: cyanidin; Pn: peonidin; Caf: (E)-cafferic acid; Sop: sophoroside; PHB: p-hydroxybenzoic acid; Fer: (E)-ferulic acid; Glc: glucopyranoside.
Despite the complex chemical structure of anthocyanins, studies have confirmed that when an anthocyanin-rich extract is administered to rats [9,10,11] or a beverage prepared from purple-fleshed sweet potato is consumed by humans [10,11,12], two components of anthocyanins, YGM-2 and YGM-5b, are rapidly absorbed into the body (detectable in the blood) and rapidly excreted together with non-acylated anthocyanins in the urine. Previous studies have shown that the purple-fleshed sweet potato or beverages derived from it containing anthocyanins show biological activity, such as antimutagenicity in vitro [13] and antihyperglycemic effects through α-glucosidase inhibition [14] and antiatherosclerotic effects [15] in animal models. Moreover, beneficial effects on hypertension and hepatitis have been observed in both animal models [16, 17] and clinical trials [18, 19]. However, most previous studies on the effects of purple-fleshed sweet potato consumption were conducted with Japanese individuals, and their results may not be generally applicable to other populations because of ethnic differences in the development of chronic disease, body composition, and anthropometry as well as in food consumption patterns. Therefore, we conducted an investigation of the effects of a purple-fleshed sweet potato beverage in Caucasian subjects with elevated blood pressure and elevated levels of liver function biomarkers, namely γ-glutamyltransferase (GGT), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activity. The effects of 4 weeks of purple-fleshed sweet potato beverage consumption on blood pressure and liver function were investigated, and safety was evaluated by determining the occurrence of adverse events and the hematological and biochemical parameters in blood and urine samples.
MATERIALS AND METHODS
Ethical considerations
The study was conducted according to the current version of the World Medical Association Declaration of Helsinki (revised in Edinburgh, October 2000), the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Guidelines for Good Clinical Practice, the requirements as described in the European Union (EU) Clinical Trials Directive 2001/20/EC transposed in the revised version of the Dutch Medical Research Involving Human Subjects Act and current national regulations. The protocol was submitted to an independent ethics committee, namely the Medical Ethics Committee METC Brabant in Tilburg, The Netherlands, and approval was received on October 15, 2008. An amendment of the protocol was approved on October 31, 2008.
All subjects provided written informed consent to participate in the study.
Subjects
Subjects were recruited from the Utrecht-Zeist area of the Netherlands, and the study was conducted at the Netherlands Organization for Applied Scientific Research (TNO), located in Zeist, The Netherlands. Eligible individuals were healthy and aged 18–70 years on day 1 of the study. Among the inclusion criteria were body mass index (BMI) >25 kg/m2; systolic blood pressure 130–160 mm Hg or diastolic blood pressure 85–95 mm Hg; and a GGT level >55 IU/l for men and >38 IU/l for women, an AST level >38 IU/l for men and >32 IU/l for women or an ALT level >30 IU/l for men and >28 IU/l for women. Among the exclusion criteria were any concomitant medication (e.g., angiotensin I converting enzyme inhibitory drugs or other antihypertensive medication) with the exception of occasional use of a paracetamol tablet, food allergy or food intolerance and alcohol consumption >28 units/week for men and >21 units/week for women. Seventeen men and three women participated in the study, and all but one subject, who did not appear for blood pressure measurement and blood and urine sampling on day 15 because of illness unrelated to the study, completed the entire intervention and washout periods.
Test beverage
A purple-fleshed sweet potato beverage (trade name, Ayamurasaki), which is commercially available in Japan, was obtained from Yakult Honsha Co., Ltd. (Tokyo, Japan). The purple-fleshed sweet potato beverage was a mixture of concentrated purple-fleshed sweet potato juice with enzymatically degraded starch and fiber [9] and taste-modifying materials (lemon juice and flavorings). One carton of beverage (125 mL) contained 0.4 g protein, >0.1 g fat, 15.9 g nonfibrous carbohydrates, 0.4 g fiber, 3.2 g sodium and 117 mg anthocyanin in the form of delphinidin, as determined by the colorimetric method. Beverages were stored at ambient temperature (15–25°C). Using high-performance liquid chromatography for anthocyanin analysis in the purple-fleshed sweet potato [20], we confirmed that the total content of eight major anthocyanins, as shown in Fig. 1, decreased by only 6% during the intervention period.
Study design
The study was designed to be open-label and non-comparative. All subjects received the same single treatment. The pre-study screening was performed at least 2 weeks before starting the treatment. The study period comprised 6 weeks: 4 weeks during which the purple-fleshed sweet potato beverage was consumed daily and a subsequent 2-week washout period. Subjects visited the study site (TNO) in a fasted state four times during the study period, on days 1, 15, 29 and 43, for blood pressure measurement and blood and urine collection. At each visit, they completed a questionnaire on well-being. They were advised to maintain their habitual food intake and lifestyle throughout the study period but were restricted from taking part in sports or heavy physical activity and from consuming alcohol the day before visiting the study site. The purple-fleshed sweet potato beverage was provided in ready-to-drink paper cartons, each containing 125 ml of the purple-fleshed sweet potato beverage; one carton was consumed in the morning or after visiting the study site, and the second was consumed in the evening. Subjects were asked to consume a total of 56 beverages each during the study period. In total, 1120 cartons were used in this study, with only six cartons (0.5%) not consumed; therefore, treatment compliance was considered very good.
Blood pressure measurement
Blood pressure was measured using an automated digital sphygmomanometer (HEM-1025, Omron Healthcare Co., Ltd., Kyoto, Japan) at the study site. In brief, the blood pressure cuff was placed around the upper left arm while subjects sat in a chair at a table with the feet positioned flat on the floor and with the back straight and resting against the chair. The left arm was supported at the level of the heart on the table. The first measurement was taken after approximately 3 min of rest in this position. Subjects were instructed not to move or speak during the measurement. Measurements were taken in duplicate. The value of the last measurement was used for analysis.
Blood collection, preparation and storage
For hematology, blood was collected in tubes (2 ml) containing tripotassium EDTA as an anticoagulant, stored at room temperature and analyzed on the day of collection. For serum analysis, blood was collected in tubes containing a clot activator with gel to separate the serum and packed cells after centrifugation. After the samples were centrifuged (3,000 × g for 20 min at 4°C), serum was collected and used for serum chemistry, and an aliquot was used for measurement of insulin by an immunoenzymatic assay. Serum samples were stored at or below –20°C.
Hematological and serobiochemical parameters
Red blood cell count, platelets, reticulocytes, white blood cell (WBC) count, differential WBC count, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), MCH concentration (MCHC), hematocrit and hemoglobin were measured using an ADVIA 120 hematology analyzer (Bayer Diagnostics, Pittsburgh, PA, USA). The conventional serum biochemical markers ALT, AST, GGT, glucose, urea, albumin, creatinine, total bilirubin, total cholesterol, high-density lipoprotein cholesterol and triacylglycerides were measured using an automatic biochemical analyzer (AU400, Olympus, Tokyo, Japan). The low-density lipoprotein cholesterol level was calculated by Friedewald’s equation.
Urine parameters
Spot urine samples were stored at room temperature and analyzed by dipstick on the day of collection. The urinalysis included the following tests: protein, glucose, leukocytes, erythrocytes, nitrite, pH, ketones, bilirubin and urobilinogen. A microscopic inspection of urine sediment was done if the dipstick test indicated values above the normal range for leukocytes, blood or protein.
Statistical analysis
All data are expressed as means ± standard deviation for each experimental period. Changes over time in blood pressure, hematological and serobiochemical parameters were analyzed by analysis of variance (ANOVA). If the ANOVA indicated a significant time effect, comparisons between means of the parameters at each time point were performed using a two-sided, paired Student t-test. The SAS statistical software (version 8.2, SAS Institute Inc., Cary, NC, USA) was used for statistical analysis. In all statistical tests performed, the null hypothesis (no time effect) was rejected at the 0.05 level of probability (α = 5%). In this study, outliers were defined as subjects having an absolute residual three times higher than the root mean square error of the measurements and were removed before statistical analysis.
RESULTS
Baseline characteristics of the subjects
The baseline characteristics of the subjects were as follows: age, 52 ± 13 years; body weight, 97.1 ± 15.5 kg; height, 1.78 ± 0.07 m; BMI, 30.6 ± 4.1 kg/m2; systolic blood pressure, 141 ± 16 mmHg; diastolic blood pressure, 90 ± 6 mmHg; ALT, 39 ± 16 IU/l; AST, 32 ± 12 IU/l; and GGT, 53 ± 29 IU/l.
Blood pressure
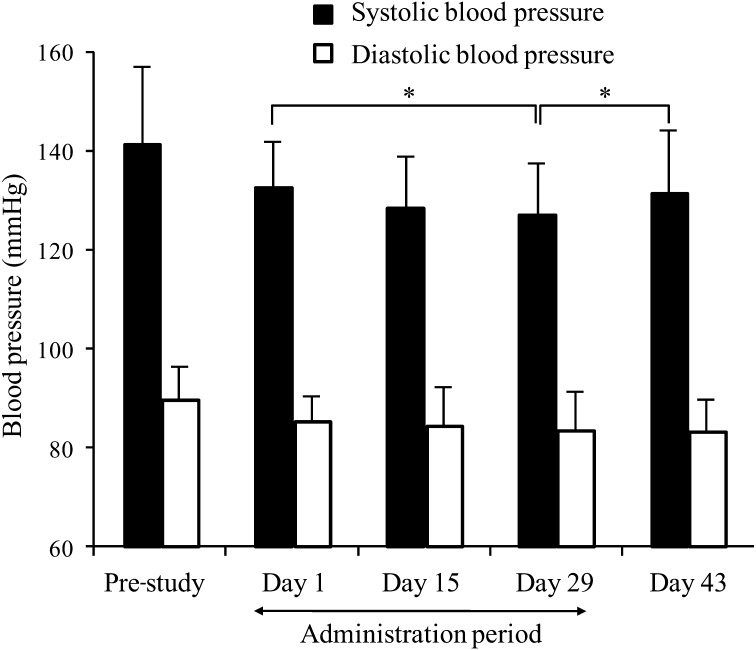
A trend was found toward lowering systolic blood pressure during the treatment period (p=0.0590). No significant changes were found in diastolic blood pressure over time. Systolic blood pressure was significantly lower on day 29 compared with day 1 (p=0.0125) and significantly higher on day 43 compared with day 29 (p=0.0496) (Fig. 2).
Fig. 2.
Changes in blood pressure among participants consuming the purple-fleshed sweet potato beverage.
Data are expressed as means ± standard deviation (n=20). *p<0.05 (paired t-test).
Liver function biomarkers
A significant increase in ALT level over time was found (p<0.0001), as shown in Table 1. All time points differed significantly (day 1 versus day 29, p<0.0001; day 1 versus day 43, p<0.0001; day 15 versus day 29, p=0.0012; day 15 versus day 43, p<0.0001; and day 29 versus day 43, p=0.0001), except for day 1 versus day 15 (p=0.2297). A trend toward significance was found in AST level during the treatment period (p=0.0732). The AST level was significantly lower on day 15 compared with day 29 (p=0.0319) and day 43 (p=0.0209). For the GGT level, no significant changes were found during the study period after removing one outlier with a value of 94.5 IU/l on day 43.
Table 1. Changes in hematological parameters in subjects consuming the purple-fleshed sweet potato beverage over a 4-week study period with a 2-week washout period.
| Pre-studya | Day 1 | Day 15 | Day 29 | Day 43 | p-valuec | |
|---|---|---|---|---|---|---|
| ALT (IU/l) | 39 ± 16 | 22 ± 10 | 24 ± 10 | 29 ± 14 | 34 ± 14 | <0.0001 |
| AST (IU/l) | 32 ± 12 | 28 ± 5 | 27 ± 6 | 30 ± 8 | 30 ± 7 | 0.0732 |
| GGT (IU/l) | 53 ± 29 | 51 ± 26 | 49 ± 24 | 52 ± 26 | 55 ± 31b | 0.5672 |
a Pre-study data are shown for information purposes but were not included in the statistical analysis.
b One outlier with a value of 94.5 IU/l on day 43 was removed.
c A p-value of less than 0.05 was considered to be statistically significant, and a p-value between 0.05 and 0.1 was considered a trend toward significance.
Blood parameters
Significant overall changes with time were found for reticulocytes, the WBC to monocyte ratio, MCV, MCHC, glucose and albumin, as shown in Table 2. As for the results of partial tests between days, a significant increase in reticulocytes was observed on day 43 compared with day 1 (p=0.0002), day 15 (p<0.0001) and day 29 (p<0.0001). The WBC to monocyte ratio was significantly lower on day 15 compared with the three other measurement days (day 1, p=0.0013; day 29, p=0.0272; and day 43, p=0.0370). After day 1, an increase in MCV was observed; the value on day 1 was significantly lower compared with those on day 15 (p<0.0001) and day 29 (p<0.0001), and the value on day 43 was significantly higher than that on day 1 (p<0.0001). An opposite effect was found for MCHC. After day 1, a decrease in MCHC was observed; the value on day 1 was significantly higher compared with those on day 15 (p<0.0001) and day 29 (p<0.0001), and the value on day 43 was significantly lower than that on day 1 (p<0.0001). For albumin, a decrease over time was observed during the 4-week consumption period; the difference between day 1 and day 15 was not significant (p=0.0533), but the value on day 29 was significantly lower compared with those on day 1 (p<0.0001), day 15 (p=0.0049) and day 43 (p=0.0319). There was also a significant difference between the values on day 1 and day 43 (p=0.0072).
Table 2. Changes in serobiochemical parameters in subjects consuming the purple-fleshed sweet potato beverage over a 4-week study period with a 2-week washout period.
| Day 1 | Day 15 | Day 29 | Day 43 | p-valuec | |
|---|---|---|---|---|---|
| RBC (tera/l) | 4.96 ± 0.29 | 4.93 ± 0.24 | 4.93 ± 0.28 | 4.88 ± 0.28 | 0.1567 |
| Platelets (giga/l) | 257 ± 56 | 260 ± 64 | 265 ± 56 | 266 ± 60 | 0.3403 |
| Reticulocytesa (%) | 1.01 ± 0.22 | 1.02 ± 0.15 | 1.00 ± 0.22 | 1.16 ± 0.21 | <0.0001 |
| Leucocytesa (giga/l) | 6.6 ± 1.4 | 7.0 ± 1.8 | 7.0 ± 1.5 | 6.6 ± 1.2 | 0.1465 |
| WBC: lymphocytesa (%) | 34.7 ± 6.1 | 32.3 ± 8.4 | 34.1 ± 7.9 | 35.5 ± 6.8 | 0.1489 |
| WBC: neutrophilsa (%) | 55.1 ± 7.3 | 58.2 ± 9.1 | 55.7 ± 9.4 | 54.3 ± 7.5 | 0.2037 |
| WBC: monocytes (%) | 5.8 ± 1.1 | 5.2 ± 0.8 | 5.6 ± 1.3 | 5.6 ± 1.3 | 0.0121 |
| WBC: eosinophilsa (%) | 3.4 ± 1.9 | 3.5 ± 1.6 | 3.6 ± 2.1 | 3.7 ± 2.1 | 0.7138 |
| WBC: basophils (%) | 0.9 ± 0.2 | 0.9 ± 0.3 | 1 ± 0.4 | 0.9 ± 0.2 | 0.4189 |
| MCV (fl) | 83.5 ± 2.9 | 84.7 ± 3.3 | 85.0 ± 3.3 | 84.9 ± 3.4 | <0.0001 |
| MCH (fmol) | 1.84 ± 0.08 | 1.83 ± 0.09 | 1.83 ± 0.09 | 1.83 ± 0.08 | 0.5769 |
| MCHC (mmol/l) | 22.02 ± 0.63 | 21.61 ± 0.66 | 21.57 ± 0.56 | 21.61 ± 0.63 | <0.0001 |
| Hematocrit (l/l) | 0.414 ± 0.028 | 0.417 ± 0.023 | 0.419 ± 0.027 | 0.414 ± 0.027 | 0.2885 |
| Hemoglobin (mmol/l) | 9.1 ± 0.7 | 9.0 ± 0.6 | 9.0 ± 0.7 | 9.0 ± 0.6 | 0.1253 |
| Glucose (mmol/l) | 6.1 ± 0.8 | 6.1 ± 0.9 | 6.1 ± 0.9 | 6.3 ± 0.9 | 0.0407 |
| Urea (mmol/l) | 5.9 ± 1.3 | 6.0 ± 1.2 | 5.7 ± 1.5 | 6.0 ± 0.9 | 0.629 |
| Albumin (g/l) | 46 ± 3 | 45 ± 3 | 44 ± 2 | 45 ± 2 | <0.0001 |
| Creatinine (μmol/l) | 89 ± 12 | 90 ± 13 | 90 ± 11 | 89 ± 12 | 0.9757 |
| Total bilirubina (μmol/l) | 11 ± 5 | 10 ± 3 | 10 ± 4 | 10 ± 4 | 0.3441 |
| Cholesterol (mmol/l) | 5.9 ± 0.7 | 5.8 ± 1.2 | 5.8 ± 1.0 | 5.9 ± 1.0 | 0.2233 |
| HDL-cholesterola (mmol/l) | 1.21 ± 0.26 | 1.19 ± 0.31 | 1.2 ± 0.28 | 1.23 ± 0.28 | 0.1739 |
| LDL-cholesterola (mmol/l) | 3.9 ± 0.7 | 3.9 ± 1.0 | 3.8 ± 1.0 | 3.9 ± 0.9 | 0.2342 |
| Triacylglyceridesb (mmol/l) | 1.7 ± 0.7 | 1.6 ± 0.6 | 1.6 ± 0.6 | 1.7 ± 0.7 | 0.902 |
| Insulina,b (mU/l) | 12.6 ± 8.5 | 13.8 ± 11.1 | 13.2 ± 9.1 | 13.0 ± 8.5 | 0.315 |
a Parameters for which outliers were found and removed.
b Statistical analysis based on log-transformed data.
c A p-value of less than 0.05 was considered to be statistically significant.
HDL: high-density lipoprotein; LDL: low-density lipoprotein; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; MCV: mean corpuscular volume; RBC: red blood cell; WBC: white blood cell.
Urine parameters
For eight urine parameters (protein, glucose, nitrite, ketones, bilirubin, urobilirubin, sediment crystals and sediment casts), the ANOVA test was not performed because there was no variation in the data. Mainly zero values were observed, except for ketones, for which only one non-zero value was found (data not shown).
For the other nine parameters (leukocytes, erythrocytes, pH, sediment erythrocytes, sediment leukocytes, sediment epithelium, sediment-amorphous material, sediment bacteria and sediment mucus), a nonparametric Kruskal-Wallis analysis was performed because there were very few variations in the data; that is, mainly zero values and limited non-zero values were observed. The statistical analyses revealed no significant differences over time for the nine urine parameters.
Safety
No serious adverse events were reported during the study. Nine adverse events were reported by eight participants who completed the study. Of these adverse events, the intensities of seven of the events were mild, the intensity of one was moderate, and the intensity of one was severe. The severe adverse event was the common cold or flu. After the study, none of the adverse events were present. Of the nine adverse events, one event was not related to the treatment, six events were unlikely related to the treatment, and two events (nausea and change in bowel habits) were considered possibly related to the treatment.
DISCUSSION
Sweet potato is generally available in Europe and has a history of safe use for human consumption. The main difference between the normal sweet potato and the purple-fleshed sweet potato is the presence of anthocyanins. Anthocyanins are widely distributed in fruits, beans, cereals, vegetables and red wine, and humans ingest a considerable amount of anthocyanins daily in plant-based diets [21,22,23]. Besides their natural presence in foods, anthocyanins are also listed as a colorant by EU legislation (product E163). Nevertheless, Europeans have little or no experience in the consumption of the acylated anthocyanins in purple-fleshed sweet potatoes, the chemical structures of which are markedly different from the anthocyanins consumed already in the European region.
In this study, we therefore evaluated the effects of daily consumption of 250 ml of a purple-fleshed sweet potato beverage for 4 weeks in 20 Caucasian subjects from Europe. No serious adverse events were reported, and only two of nine such events, relating to nausea and change in bowel habits, were possibly related to the treatment. These adverse events could be attributed to soluble fiber enzymatically degraded in the purple-fleshed sweet potato beverage because sweet potatoes are rich in soluble fiber and are well known as foodstuffs that promote bowel movements. In a previous study, Japanese subjects also reported this change in bowel habits as an adverse event, although this adverse event was considered an advantage for subjects with bowel difficulties [18]. This study also demonstrated a significant change over time in several blood and urine parameters, although no notable overall changes were observed in hematology, clinical chemistry or urine parameters. Furthermore, no clinically relevant changes in individual hematology, clinical chemistry or urine parameters in Caucasian subjects were considered to be related to purple-fleshed sweet potato beverage consumption, as has also been found previously in Japanese subjects. These findings suggest that daily intake of the purple-fleshed sweet potato beverage is safe for Caucasian populations in Europe.
Previous studies demonstrated that the serum levels of liver function biomarkers in healthy Japanese volunteers decreased after consuming a purple-fleshed sweet potato beverage for 8 weeks. After 4 weeks, GGT and AST levels significantly decreased by 18% (–19.0 IU/l) and 15% (–5.4 IU/l), respectively, whereas no change from baseline to 10 weeks was observed for ALT levels [19]. Suda and coworkers included subjects with borderline levels of one or more liver function biomarkers (GGT, >80 IU/l; AST, 42–99 IU/l; ALT, 42–99 IU/l) [19]. When we applied these reference values determined for the Japanese population to the Caucasian population in the present study, only eight of 119 volunteers who provided informed consent had elevated or borderline liver function biomarker levels, whereas 60 of the 119 volunteers met the blood pressure criteria. Every county and region applies its own reference values for clinical laboratory tests because the values differ for ethnic population groups; therefore, in the present study, the following reference values were applied to our Caucasian subjects: GGT >55 IU/l, AST >38 IU/l and ALT >30 IU/l for men and GGT >38 IU/l, AST >32 IU/l and ALT >28 IU/l for women. These reference values were determined at TNO using the local blood bank, yielding 17 subjects that met the criteria. In Caucasian subjects consuming the purple-fleshed sweet potato beverage dairy for 4 weeks, no significant changes over time were found for GGT; the mean level of GGT fluctuated only slightly by 5–11%, and the mean levels during the study were below the reference values. The AST level on day 15 was 10% lower than those on days 29 and 43, but the fluctuation seen during the study was still within the normal reference range. On the other hand, the ALT level increased significantly from day 15 to day 43. In the statistical analyses, the values at pre-study screening were not included, although a 43% decrease from baseline to day 1 (38.8 to 22.0 IU/l) was observed. Furthermore, the ALT level at the end of the study was 12% lower compared with that before the study. Taking into consideration these observations, our study could not provide a clear explanation for the heightening effect on the ALT level due to consuming of the purple-fleshed sweet potato beverage. Our results indicate that the purple-fleshed sweet potato beverage did not show clinically relevant effects on normal liver function among our Caucasian subjects. The difference between these findings and those reported for Japanese subjects could be attributed to the differences between ethnic groups and subject characteristics. There is a clear difference between the liver function reference values used in the Netherlands and Japan and the population with borderline or higher levels of liver function was small among our Caucasian subjects, illustrating different liver enzyme activities due to ethnicity and/or genetics [24].
We observed a trend toward lowering systolic blood pressure among the Caucasian participants of this study. This is consistent with a lowering effect on systolic blood pressure shown previously in mildly hypertensive Japanese volunteers who consumed a purple-fleshed sweet potato beverage (with a different anthocyanin content from that used in this study) every day for 44 days in an open-label, non-comparative study [18]. It should, however, be realized that blood pressure may decrease even in the absence of any treatment, such as in a blinded comparative study [25].
In a spontaneously hypertensive rat (SHR) model, anthocyanin-rich extracts from Ipomoea batatas cultivar Ayamurasaki produced a significant decrease in systolic blood pressure (p<0.05) at 2 hours after administration, and the decrease was prolonged over 8 hours [17]. An antihypertensive effect was also observed during long-term ingestion (0.1% and 0.2% diet for 8 weeks) of an anthocyanin-rich extract in the SHR model, whereas after stopping feeding of the anthocyanin-rich extract, the systolic blood pressure was at almost the same level as that of an SHR group fed a control diet [17]. In the present human intervention study, an increase in systolic blood pressure was also observed in the washout period from day 29 to day 43 (p=0.0496). These findings might suggest that the acylated anthocyanins in purple-fleshed sweet potato contributed to the reduction in blood pressure by vasodilation and that this is an acute effect (present only during consumption).
In conclusion, ingestion of the purple-fleshed sweet potato beverage for 4 weeks in Caucasian individuals produced no clinically relevant changes in safety parameters in blood and urine and showed a trend toward lowering systolic blood pressure. In this study, we could not examine gender differences in the effect of consuming the purple-fleshed sweet potato beverage because there were more male subjects than female subjects, with the ratio being 17 to 3. Further studies are needed in order to discuss the gender differences.
Acknowledgments
This study was supported by a Grant-in-Aid for Research and Development Projects for Application in Promoting New Policy of Agriculture, Forestry and Fisheries (Grant Number: 1922) from the Ministry of Agriculture, Forestry and Fisheries (MAFF) of Japan.
REFERENCES
- 1.Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. 2007. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res 51: 675–683. [DOI] [PubMed] [Google Scholar]
- 2.Seeram NP. 2008. Berry fruits: compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. J Agric Food Chem 56: 627–629. [DOI] [PubMed] [Google Scholar]
- 3.Cassidy A, Mukamal KJ, Liu L, Franz M, Eliassen AH, Rimm EB. 2013. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 127: 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshinaga M. 1995. New cultivar “Ayamurasaki” for colorant production. Sweet Potato Res Front 2: 1. [Google Scholar]
- 5.Goda Y, Shimizu T, Kato Y, Nakamura M, Maitani T, Yamada T, Terahara N, Yamaguchi M. 1997. Two acylated anthocyanins from purple sweet potato. Phytochemistry 44: 183–186. [DOI] [PubMed] [Google Scholar]
- 6.Terahara N, Shimizu T, Kato Y, Nakamura M, Maitani T, Yamaguchi M, Goda Y. 1999. Six diacylated anthocyanins from the storage roots of purple sweet potato, Ipomoea batatas. Biosci Biotechnol Biochem 63: 1420–1424. [DOI] [PubMed] [Google Scholar]
- 7.Odake K, Hatanaka A, Kajiwara T, Muroi T, Nishiyama K, Yamakawa O, Terahara N, Yamaguchi M. 1994. Evaluation methods and breeding of purple sweet potato “Yamakawamurasaki” (Ipomoea batatas POIR.) for raw material of food colorants. J Jpn Soc Food Sci Technol 41: 287–293(in Japanese). [Google Scholar]
- 8.Suda I, Oki T, Masuda M, Kobayashi M, Nishiba Y, Furuta S. 2003. Physiological functionality of purple-fleshed sweet potatoes containing anthocyanins and their utilization in foods. Jpn Agric Res Q 37: 167–173. [Google Scholar]
- 9.Suda I, Oki T, Masuda M, Nishiba Y, Furuta S, Matsugano K, Sugita K, Terahara N. 2002. Direct absorption of acylated anthocyanin in purple-fleshed sweet potato into rats. J Agric Food Chem 50: 1672–1676. [DOI] [PubMed] [Google Scholar]
- 10.Harada K, Kano M, Takayanagi T, Yamakawa O, Ishikawa F. 2004. Absorption of acylated anthocyanins in rats and humans after ingesting an extract of Ipomoea batatas purple sweet potato tuber. Biosci Biotechnol Biochem 68: 1500–1507. [DOI] [PubMed] [Google Scholar]
- 11.Kano M, Takayanagi T, Harada K, Makino K, Ishikawa F. 2005. Antioxidative activity of anthocyanins from purple sweet potato, Ipomoea batatas cultivar Ayamurasaki. Biosci Biotechnol Biochem 69: 979–988. [DOI] [PubMed] [Google Scholar]
- 12.Oki T, Suda I, Terahara N, Sato M, Hatakeyama M. 2006. Determination of acylated anthocyanin in human urine after ingesting a purple-fleshed sweet potato beverage with various contents of anthocyanin by LC-ESI-MS/MS. Biosci Biotechnol Biochem 70: 2540–2543. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimoto M, Okuno S, Yoshinaga M, Yamakawa O, Yamaguchi M, Yamada J. 1999. Antimutagenicity of sweetpotato (Ipomoea batatas) roots. Biosci Biotechnol Biochem 63: 537–541. [DOI] [PubMed] [Google Scholar]
- 14.Matsui T, Ebuchi S, Kobayashi M, Fukui K, Sugita K, Terahara N, Matsumoto K. 2002. Anti-hyperglycemic effect of diacylated anthocyanin derived from Ipomoea batatas cultivar Ayamurasaki can be achieved through the α-glucosidase inhibitory action. J Agric Food Chem 50: 7244–7248. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki K, Makino K, Iwadate E, Deguchi Y, Ishikawa F. 2008. Anthocyanins from purple sweet potato Ipomoea batatas cultivar Ayamurasaki suppress the development of atherosclerotic lesions and both enhancements of oxidative stress and soluble vascular cell adhesion molecule-1 in apolipoprotein E-deficient mice. J Agric Food Chem 56: 11485–11492. [DOI] [PubMed] [Google Scholar]
- 16.Suda I, Furuta S, Nishiba Y, Yamakawa O, Matsugano K, Sugita K. 1997. Reduction of liver injury induced by carbon tetrachloride in rats administered purple-colored sweet potato juice. J Jpn Soc Food Sci Technol 44: 315–318(in Japanese). [Google Scholar]
- 17.Kobayashi M, Oki T, Masuda M, Nagai S, Fukui K, Matsugano K, Suda I. 2005. Hypotensive effect of anthocyanin-rich extract from purple-fleshed sweet potato cultivar “Ayamurasaki” in spontaneously hypertensive rats. J Jpn Soc Food Sci Technol 52: 41–44(in Japanese). [Google Scholar]
- 18.Suda I, Yamakawa O, Matsugano K, Sugita K, Takeguma Y, Irisa K, Tokumaru F. 1998. Change of serum γ-GPT, GOT and GPT levels in hepatic function-weakling subjects by ingestion of high anthocyanin sweet potato juice. J Jpn Soc Food Sci Technol 45: 611–617(in Japanese). [Google Scholar]
- 19.Suda I, Ishikawa F, Hatakeyama M, Miyawaki M, Kudo T, Hirano K, Ito A, Yamakawa O, Horiuchi S. 2008. Intake of purple sweet potato beverage affects on serum hepatic biomarker levels of healthy adult men with borderline hepatitis. Eur J Clin Nutr 62: 60–67. [DOI] [PubMed] [Google Scholar]
- 20.Terahara N, Oki T, Matsui T, Fukui K, Sugita K, Matsumoto K, Suda I. 2007. Simultaneous determination of major anthocyanins in purple sweet potato. J Jpn Soc Food Sci Technol 54: 33–38(in Japanese). [Google Scholar]
- 21.Kong JM, Chia LS, Goh NK, Chia TF, Brouillard R. 2003. Analysis and biological activities of anthocyanins. Phytochemistry 64: 923–933. [DOI] [PubMed] [Google Scholar]
- 22.Williams CA, Grayer RJ. 2004. Anthocyanins and other flavonoids. Nat Prod Rep 21: 539–573. [DOI] [PubMed] [Google Scholar]
- 23.He J, Giusti MM. 2010. Anthocyanins: natural colorants with health-promoting properties. Annu Rev Food Sci Technol 1: 163–187. [DOI] [PubMed] [Google Scholar]
- 24.Yamaori S, Yamazaki H, Iwano S, Kiyotani K, Matsumura K, Saito T, Parkinson A, Nakagawa K, Kamataki T. 2005. Ethnic differences between Japanese and Caucasians in the expression levels of mRNAs for CYP3A4, CYP3A5 and CYP3A7: lack of co-regulation of the expression of CYP3A in Japanese livers. Xenobiotica 35: 69–83. [DOI] [PubMed] [Google Scholar]
- 25.Seppo L, Jauhiainen T, Poussa T, Korpela R. 2003. A fermented milk high in bioactive peptides has a blood pressure-lowering effect in hypertensive subjects. Am J Clin Nutr 77: 326–330. [DOI] [PubMed] [Google Scholar]