Abstract
Schizophrenia is a chronic psychiatric illness. Disruption of the dopaminergic system has been suggested to be the pathogenic cause of this disease. The effect of Bifidobacterium longum BB536 (BB536) on schizophrenic behavior was investigated in an animal model. Daily administration of BB536 (109 CFU/mouse, p.o. for 2 weeks) was found to reduce rearing behavior augmented by the dopamine receptor agonist apomorphine and to decrease the resting level of plasma corticosterone and the ratio of kynurenine to tryptophan. These results suggest the potential of BB536 for supplemental treatment of the symptoms of schizophrenia.
Keywords: Bifidobacterium longum BB536, schizophrenia, rearing behavior, apomorphine, corticosterone, tryptophan, kynurenine
Increasing evidence suggests that the microbiota-gut-brain axis plays a key role in the regulation of stress and psychiatric disorders [1]. Schizophrenia is a chronic, severe and disabling psychiatric illness that affects approximately 1% of the population worldwide and is characterized by positive, negative and cognitive symptoms [2]. Although the pathogenic mechanism of schizophrenia remains unclear, one mechanism that has been suggested is dopaminergic system disruption. A therapy for schizophrenia is the administration of neuroleptics, which selectively block D2 dopamine receptors [3]. Immune abnormalities may also play a pathological role in some forms of schizophrenia [4]. In paranoid schizophrenics, but not catatonic schizophrenics, white blood cell activity is suppressed compared with that found in healthy volunteers [5]. Mittleman et al. found a relative skewing of cerebrospinal fluid profiles toward type 2 cytokines and found that humoral immunity is involved in childhood-onset schizophrenia [6]. Stress and mild inflammation are also presumed relevant in schizophrenia [7, 8]. Probiotics have been investigated as adjunctive treatments for patients with schizophrenia; however, the results have been inconsistent [9, 10]. The purpose of this study was to examine the effect of Bifidobacterium longum BB536 (BB536), a probiotic strain originally isolated from the human intestine, on schizophrenia. BB536 has been shown to have efficacy in relieving Japanese cedar pollinosis, probably through modulation of a T-helper type 2-skewed immune response [11]. This study focused on the rearing behavior of mice treated with apomorphine (Apo), a dopamine receptor agonist. This animal model has previously been used to assess the effects of neuroleptics used as therapeutic drugs for schizophrenia [12, 13]. Corticosterone (Cor) is a stress-related hormone, and the ratio of kynurenine (Kyn) to tryptophan (Trp) is associated with inflammation [14, 15]. Biochemical analysis of these parameters was also performed to investigate the possible mechanism of action of BB536.
Four-week-old male ddY mice were purchased from Japan SLC (Shizuoka, Japan). The mice were housed individually for 2 weeks while they received the probiotic (109 CFU/mouse, p.o.) or vehicle control once daily. To control for the repeated handling necessary for oral administration, another group of mice did not receive the probiotic or vehicle. All mice had free access to food and water, except during the behavioral tests. The animal experiments were approved by the Institutional Animal Care and Use Committee of Morinaga Milk Industry Co., Ltd., and were performed in accordance with the Guide for the Care and Use of Laboratory Animals of Morinaga Milk Industry Co., Ltd. Commercially available lyophilized live bifidobacteria powder of BB536 (Morinaga Milk Industry, Tokyo, Japan) and Apo hydrochloride (Sigma-Aldrich Chemical, St. Louis, MO, USA) were used in these experiments. The probiotic BB536 and Apo were suspended and dissolved in saline (Otsuka Pharmaceutical, Tokyo, Japan) immediately before use, respectively. BB536 was administered orally at a volume of 0.2 ml/mouse, whereas Apo was injected subcutaneously at a volume of 10 ml/kg. For behavioral assessment, each mouse was placed in a cylindrical observation cage that was unfamiliar to them immediately after subcutaneous injection of 0.5 mg/kg Apo. The number of rearing behaviors that the mouse exhibited was measured over 1 min at 10, 20, 30, 40, 50 and 60 min after injection. For biochemical analysis, trunk blood was collected from the mice either immediately after removal from the home cage (no acute stress condition) or after being placed in the unfamiliar observation cage without Apo injection for 15 min (acute stress condition). For measurements of plasma corticosterone, a blood sample was collected after rapid decapitation. Due to revision of the American Veterinary Medical Association Guidelines for the Euthanasia of Animals in 2013, blood was collected by cardiac punctuation under isoflurane inhalation anesthesia for plasma tryptophan and kynurenine measurements. The blood samples were transferred to heparinized tubes (Terumo Medical, Somerset, NJ, USA). The tubes were kept in an ice bath until centrifugation at 4,000 rpm for 5 min at 4°C. Plasma was collected and stored immediately in a freezer, with storage continuing until the time of assay. Cor (Enzo Life Sciences International, Plymouth Meeting, PA, USA), Trp (Immundiagnostik, Bensheim, Germany) and L-Kyn (Immundiagnostik, Bensheim, Germany) plasma levels were measured using ELISA kits according to the manufacturer’s instructions. A group of 8 mice was used for the experiment, and the data are expressed as the means ± SEM. The statistical significance of differences between the two groups was evaluated by the Mann-Whitney U test, with a p value < 5% being considered to indicate a significant difference.
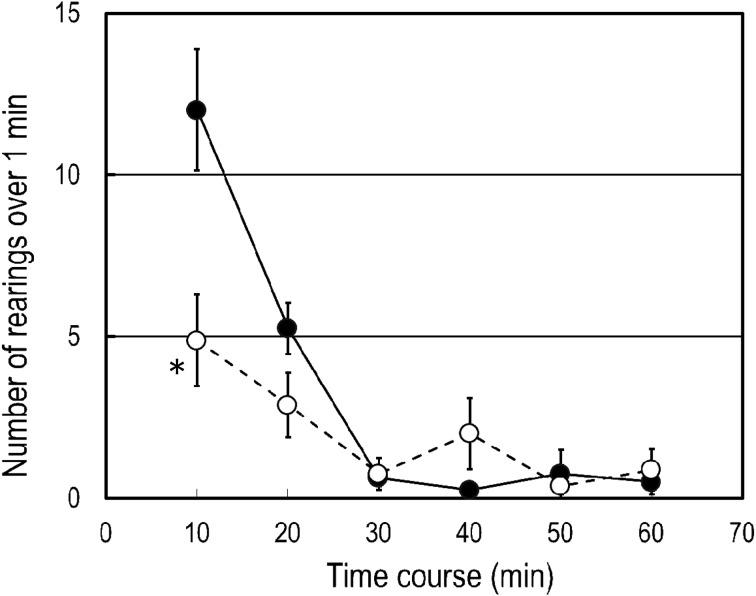
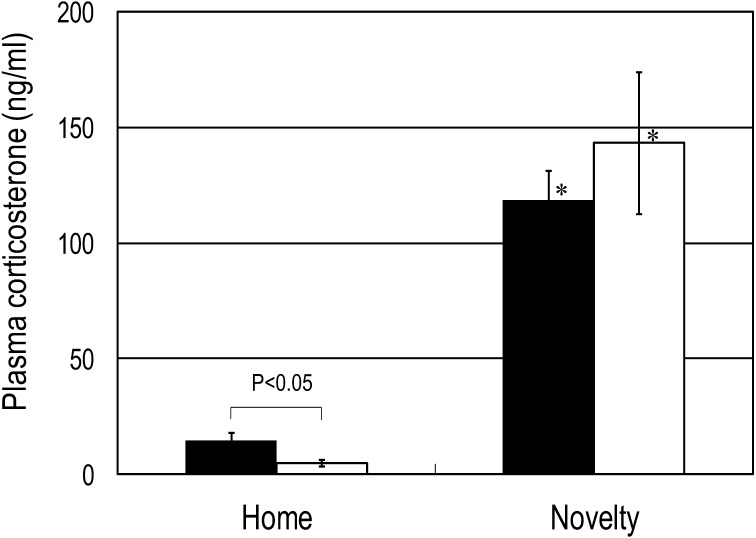
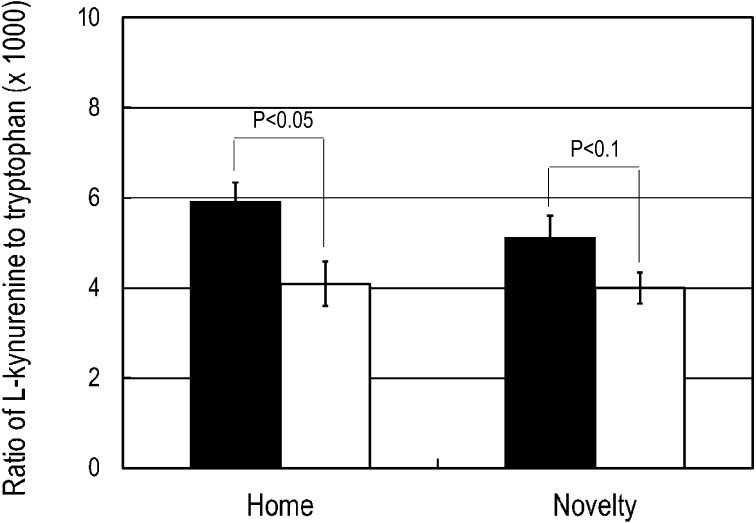
We observed characteristic rearing behavior induced with subcutaneous injection of 0.5 mg/kg Apo in mice in an unfamiliar environment. In mice that were not repeatedly handled for oral administration of BB536 or the vehicle, the number of rearing behaviors exhibited over 1 min at 10 min after Apo administration was 5.8 ± 0.9. Repeated handling for oral administration of the vehicle increased the number of rearing behaviors to 12.0 ± 1.9. However, mice that received repeated oral administration of the probiotic BB536 exhibited 4.9 ± 1.4 rearing behaviors over 1 min, which was significantly lower (p<0.05) than that observed in the mice that received the vehicle (Fig. 1). For the mice resting in the home cage, the plasma Cor level was significantly lower (p<0.05) among those treated with BB536 than among those treated with the vehicle. The Cor level rose significantly (p<0.001) in response to the novel environment stress in both the vehicle and BB536 groups. However, after exposure to acute stress, the Cor level was not significantly different between the BB536 and vehicle groups (Fig. 2). A significant decrease (p<0.05) and a marginal (p<0.1) decrease in the ratio of Kyn to Trp were observed in the resting mice and the mice exposed to the novel acute stress, respectively (Fig. 3).
Fig. 1.
Effect of repeated oral administration of BB536 on apomorphine-induced rearing behavior in mice.
Mice received the probiotic (open circles) or vehicle control (closed circles) for 2 weeks and were subcutaneously injected with apomorphine. The number of rearing behaviors that the mouse exhibited after injection was measured. *p<0.05 when compared with the vehicle-treated group.
Fig. 2.
Effect of repeated oral administration of BB536 on plasma corticosterone in mice.
Mice received the probiotic (open columns) or vehicle control (closed columns) for 2 weeks. Blood samples were collected from the mice either immediately after removal from the home cage (home) or 15 min after being placed in the unfamiliar observation cage (novelty), and the plasma levels of corticosterone were determined. *p<0.001 when compared with the home group.
Fig. 3.
Effect of repeated oral administration of BB536 on the ratio of kynurenine to tryptophan in mice.
Mice received the probiotic (open columns) or vehicle control (closed columns) for 2 weeks. Blood samples were collected from the mice either immediately after removal from the home cage (home) or 15 min after being placed in the unfamiliar observation cage (novelty), and the plasma levels of kynurenine and tryptophan were determined.
Both genetic and environmental factors have been shown to impact vulnerability to schizophrenia [16]. In children, daily stressors and negative life events increase the risk of developing the disease [17]. In animals, postweaning social isolation induces behavioral abnormalities, and this is considered to be a model of psychiatric disorders, including schizophrenia [17]. However, Niwa et al. reported that mild stress induced by isolation for three weeks affected animal behaviors and biochemical markers, but only among animals with a relevant genetic risk factor for neuropsychiatric disorders [18]. The observation of a delayed increase in the level of plasma Cor, a stress-related hormone in animals, via repeated handling for 6 days indicates the possibility of gradual sensitization of the stress axis in animals [19]. In the present study, for the mice that were not repeatedly handled, a significant difference in rearing behavior was not observed between the isolated mice and the group-housed mice (data not shown). However, repeated handling for oral administration of the vehicle increased Apo-induced rearing behavior in mice housed in isolation for two weeks (12.0 ± 1.9 vs. 5.8 ± 0.9). Therefore, the experimental conditions used in this study generated chronic stress that promoted schizophrenia-like behavior and enabled assessment of the effects of BB536 on schizophrenia-like behavior.
Desbonnet et al. found that administration of the probiotic B. infantis in drinking water improved depression-like behavior in the rat maternal separation model [20] and found no such effect in rats not separated [21]. Bercik et al. observed normalization of anxiety-like behavior with administration of B. longum NCC3001 (ATCC BAA-999, a synonym of BB536) in Trichuris muris-infected mice [22]. In the present experiment, the number of rearing behaviors induced by Apo was significantly suppressed by repeated oral administration of BB536. These results indicate that BB536 may effectively decrease schizophrenia-related behaviors. A case report by Nagamine et al. showed that an probiotic preparation composed of a mixture of Streptococcus faecalis, Clostridium butyricum and Bacillus mesentericus improved the negative symptoms of schizophrenia [9]. However, a randomized placebo-controlled trial conducted by Dickerson et al. showed that the adjunctive treatment of a probiotic combination of Lactobacillus rhamnosus and B. animalis did not have a significant effect on the positive and negative syndrome scale total score of schizophrenia, although patients in the probiotic group were less likely to develop severe bowel difficulty [10]. The discrepancies in the efficacies of the probiotics used in these studies may be due to the differences in the bacterial species used.
Increased serum cortisol level has been reported in patients with schizophrenia [23]. Desbonnet et al. found that administration of the probiotic B. infantis in drinking water tended to decrease the Cor level in rats, although this decrease was not statistically significant [21]. In the present study, the resting plasma Cor level was significantly lowered by repeated oral administration of BB536. Therefore, the effects of this probiotic may be at least partially due to stress relief. In rats, orally administered L. farciminis has been reported to suppress the increase in Cor level induced by 2 h of restraint [24]. Sudo et al. found that plasma Cor elevation in response to restraint stress is substantially higher in germfree mice than in specific pathogen-free mice and that the acute response in germfree mice is attenuated by reconstitution with B. infantis [25]. Exposure to a novel environment has been shown to markedly increase the Cor level in animals [14]. The Cor plasma level also rose in mice exposed to a novel environment in the present study. However, the Cor level after exposure to a novel environment was not different between the mice treated with BB536 and those treated with the vehicle, even though the resting Cor level was different between the two groups. The discrepancies between our acute stress test results and those of other researchers may be due to differences in the stresses applied. Our data also indicate that the probiotic BB536 may be more effective at alleviating a chronic stressor or trait (perpetual) condition than an acute stressor or state (transitory) condition. Tryptophan is not only a precursor of serotonin but is also degraded by an enzymatic cascade known as the Kyn pathway. This cascade is associated with psychiatric disorders, including schizophrenia. Decreased Trp has been reported in the serum of patients with schizophrenia [26]. Recently, Fukushima et al. reported an increase in serum Kyn in patients with schizophrenia [27]. The probiotic used in the present study significantly reduced the ratio of Kyn to Trp at baseline and marginally reduced it after exposure to the novel environment. These results also possibly suggest that the Kyn pathway is relevant in the effects of the probiotic. Skewed immune balance and mild inflammation are also presumed relevant in schizophrenia [8]. BB536 has been shown to have efficacy in modulation of a T-helper type 2-skewed immune response [11]. In addition, B. longum BB536 has also been demonstrated to possess anti-inflammatory effects [28]. We postulate that the immune modulating and anti-inflammatory effects are relevant to the effects of B. longum BB536. However, further study is needed to elucidate the details of these mechanisms.
Available treatments for schizophrenia are largely palliative and empirical and produce significant short- and long-term side effects. Therefore, investigation of alternative treatments with a rational basis for use in schizophrenia is warranted. Our results suggest the potential of the probiotic BB536 for supplementary treatment of some forms of the disease.
REFERENCES
- 1.Dinan TG, Cryan JF. 2012. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology 37: 1369–1378. [DOI] [PubMed] [Google Scholar]
- 2.Bitanihirwe BKY, Woo TUW. 2011. Oxidative stress in schizophrenia: an integrated approach. Neurosci Biobehav Rev 35: 878–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seeman P. 1987. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse 1: 133–152. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz MJ, Müller N, Riedel M, Ackenheil M. 2001. The Th2-hypothesis of schizophrenia: a strategy to identify a subgroup of schizophrenia caused by immune mechanisms. Med Hypotheses 56: 483–486. [DOI] [PubMed] [Google Scholar]
- 5.Alevizos BH, Stefanis CN. 1980. Enzyme activity of G-6-PD, γ-GT and lysozyme in white cells of schizophrenics. Neuropsychobiology 6: 333–340. [DOI] [PubMed] [Google Scholar]
- 6.Mittleman BB, Castellanos FX, Jacobsen LK, Rapoport JL, Swedo SE, Shearer GM. 1997. Cerebrospinal fluid cytokines in pediatric neuropsychiatric disease. J Immunol 159: 2994–2999. [PubMed] [Google Scholar]
- 7.Cullen AE, Fisher HL, Roberts RE, Pariante CM, Laurens KR. 2014. Daily stressors and negative life events in children at elevated risk of developing schizophrenia. Br J Psychiatry 204: 354–360. [DOI] [PubMed] [Google Scholar]
- 8.Debnath M, Berk M. 2014. Th17 pathway-mediated immunopathogenesis of schizophrenia: mechanisms and implications. Schizophr Bull 40: 1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagamine T, Sato N, Seo G. 2012. Probiotics reduce negative symptoms of schizophrenia: a cace report. Int Med J 19: 67–68. [Google Scholar]
- 10.Dickerson FB, Stallings C, Origoni A, Katsafanas E, Savage CLG, Schweinfurth LAB, Goga J, Khushalani S, Yolken RH. 2014. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao JZ, Kondo S, Yanagisawa N, Takahashi N, Odamaki T, Iwabuchi N, Miyaji K, Iwatsuki K, Togashi H, Enomoto K, Enomoto T. 2006. Probiotics in the treatment of Japanese cedar pollinosis: a double-blind placebo-controlled trial. Clin Exp Allergy 36: 1425–1435. [DOI] [PubMed] [Google Scholar]
- 12.Protais P, Costentin J, Schwartz JC. 1976. Climbing behavior induced by apomorphine in mice: a simple test for the study of dopamine receptors in striatum. Psychopharmacology (Berl) 50: 1–6. [DOI] [PubMed] [Google Scholar]
- 13.Orikasa S, Iwatsuki K. 2012. Haloperidol-like effects of α-lactalbumin, lactoferrin and lysozyme in laboratory animals. Milk Sci 61: 1–9. [Google Scholar]
- 14.Seggie JA, Brown GM. 1975. Stress response patterns of plasma corticosterone, prolactin, and growth hormone in the rat, following handling or exposure to novel environment. Can J Physiol Pharmacol 53: 629–637. [DOI] [PubMed] [Google Scholar]
- 15.Miura H, Ozaki N, Sawada M, Isobe K, Ohta T, Nagatsu T. 2008. A link between stress and depression: shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress 11: 198–209. [DOI] [PubMed] [Google Scholar]
- 16.Lewis DA, Lieberman JA. 2000. Catching up on schizophrenia: natural history and neurobiology. Neuron 28: 325–334. [DOI] [PubMed] [Google Scholar]
- 17.Geyer MA, Ellenbroek B. 2003. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry 27: 1071–1079. [DOI] [PubMed] [Google Scholar]
- 18.Niwa M, Jaaro-Peled H, Tankou S, Seshadri S, Hikida T, Matsumoto Y, Cascella NG, Kano S, Ozaki N, Nabeshima T, Sawa A. 2013. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science 339: 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longordo F, Fan J, Steimer T, Kopp C, Lüthi A. 2011. Do mice habituate to “gentle handling?” A comparison of resting behavior, corticosterone levels and synaptic function in handled and undisturbed C57BL/6J mice. Sleep 34: 679–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. 2010. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 170: 1179–1188. [DOI] [PubMed] [Google Scholar]
- 21.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. 2008. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res 43: 164–174. [DOI] [PubMed] [Google Scholar]
- 22.Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA, Lu J, Khan WI, Corthesy-Theulaz I, Cherbut C, Bergonzelli GE, Collins SM. 2010. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 139: 2102–2112.e1. [DOI] [PubMed] [Google Scholar]
- 23.Guest PC, Schwarz E, Krishnamurthy D, Harris LW, Leweke FM, Rothermundt M, van Beveren NJM, Spain M, Barnes A, Steiner J, Rahmoune H, Bahn S. 2011. Altered levels of circulating insulin and other neuroendocrine hormones associated with the onset of schizophrenia. Psychoneuroendocrinology 36: 1092–1096. [DOI] [PubMed] [Google Scholar]
- 24.Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V. 2012. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 37: 1885–1895. [DOI] [PubMed] [Google Scholar]
- 25.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. 2004. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 558: 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaryura-Tobias JA, Chang A, Neziroglu F. 1978. A study of relationships of serum glucose, insulin, free fatty acids, and free and total tryptophan to mental illness. Biol Psychiatry 13: 243–254. [PubMed] [Google Scholar]
- 27.Fukushima T, Iizuka H, Yokota A, Suzuki T, Ohno C, Kono Y, Nishikiori M, Seki A, Ichiba H, Watanabe Y, Hongo S, Utsunomiya M, Nakatani M, Sadamoto K, Yoshio T. 2014. Quantitative analyses of schizophrenia-associated metabolites in serum: serum D-lactate levels are negatively correlated with gamma-glutamylcysteine in medicated schizophrenia patients. PLoS ONE 9: e101652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomosada Y, Villena J, Murata K, Chiba E, Shimazu T, Aso H, Iwabuchi N, Xiao JZ, Saito T, Kitazawa H. 2013. Immunoregulatory effect of bifidobacteria strains in porcine intestinal epithelial cells through modulation of ubiquitin-editing enzyme A20 expression. PLoS ONE 8: e59259. [DOI] [PMC free article] [PubMed] [Google Scholar]