Abstract
The regulatory dynamics of mitochondria comprises well orchestrated distribution and mitochondrial turnover to maintain the mitochondrial circuitry and homeostasis inside the cells. Several pieces of evidence suggested impaired mitochondrial dynamics and its association with the pathogenesis of neurodegenerative disorders. We found that chronic exposure of synthetic xenoestrogen bisphenol A (BPA), a component of consumer plastic products, impaired autophagy-mediated mitochondrial turnover, leading to increased oxidative stress, mitochondrial fragmentation, and apoptosis in hippocampal neural stem cells (NSCs). It also inhibited hippocampal derived NSC proliferation and differentiation, as evident by the decreased number of BrdU- and β-III tubulin-positive cells. All these effects were reversed by the inhibition of oxidative stress using N-acetyl cysteine. BPA up-regulated the levels of Drp-1 (dynamin-related protein 1) and enhanced its mitochondrial translocation, with no effect on Fis-1, Mfn-1, Mfn-2, and Opa-1 in vitro and in the hippocampus. Moreover, transmission electron microscopy studies suggested increased mitochondrial fission and accumulation of fragmented mitochondria and decreased elongated mitochondria in the hippocampus of the rat brain. Impaired mitochondrial dynamics by BPA resulted in increased reactive oxygen species and malondialdehyde levels, disruption of mitochondrial membrane potential, and ATP decline. Pharmacological (Mdivi-1) and genetic (Drp-1siRNA) inhibition of Drp-1 reversed BPA-induced mitochondrial dysfunctions, fragmentation, and apoptosis. Interestingly, BPA-mediated inhibitory effects on NSC proliferation and neuronal differentiations were also mitigated by Drp-1 inhibition. On the other hand, Drp-1 inhibition blocked BPA-mediated Drp-1 translocation, leading to decreased apoptosis of NSC. Overall, our studies implicate Drp-1 as a potential therapeutic target against BPA-mediated impaired mitochondrial dynamics and neurodegeneration in the hippocampus.
Keywords: hippocampus, mitochondria, mitophagy, neural stem cell (NSC), neurodegeneration, neurogenesis, neurotoxin, xenobiotic, Mitochondrial dynamics, neurotoxicity
Introduction
Bisphenol A (BPA),3 a synthetic xenoestrogen and neurotoxicant, is one of the most widely used chemical components among the human population worldwide. The extensive usage of BPA in the manufacturing of plastic packaging materials and its release inside the food because of the alteration in the physiological conditions and consequential health hazards are some issues that need to be addressed. The major route of BPA exposure in humans is through the ingestion of BPA contaminated food, beverages, and water. Our recent study suggests that the adverse consequences of BPA vary with the duration of its exposure, such as activation of autophagic flux takes place after the transient exposure of BPA in the hippocampus region of the rat brain to ameliorate its neurotoxicity (1). Contrarily, prolonged and chronic exposure of BPA impairs autophagy as well as neurogenesis in the hippocampus, leading to neurodegeneration and cognitive impairments (1–3). Several studies have reported the association between toxic effects of BPA and pathogenesis of neurodegenerative disorders and other diseases (4–7). BPA-induced neurotoxicity corroborates with mitochondrial dysfunctions and apoptosis (7). Exposure to BPA in adult rats reduces sperm quality with disruption of the hypothalamic-pituitary-testicular axis (8). BPA exposure causes abnormalities of liver function and hepatic damage associated with mitochondrial apoptosis (9). Further, low doses of BPA and exposure during maternal stage induced lipid accumulation and production of reactive oxygen species in the hepatic and testis mitochondria, respectively (10, 11). Prenatal BPA exposure also impairs the mitochondria in the heart of neonatal rats (12). BPA diglycidyl ether induced Bax/Bid-dependent apoptosis and mitochondrial release of apoptosis-inducing factor cytochrome c and Smac/DIABLO (13). Most of these studies highlight the effects of BPA-induced apoptosis, reactive oxygen species (ROS) generation, antioxidant depletion, and mitochondrial dysfunction. However, to date, no study has reported elucidating the cellular and molecular mechanism of effects of BPA on mitochondrial dynamics and its association with autophagy in the brain.
Mitochondrial dynamics involves the fusion and fission of mitochondria, which helps to maintain mitochondrial circuitry and homeostasis (14–18). Several genes/proteins are responsible for maintaining the dynamic equilibrium of mitochondrial pool inside the brain (19–21). Dynamin-related proteins, such as Dnm-1, comprise the family of various proteins like Drp-1 and Fis-1, which are responsible for mitochondrial fission, and Opa-1, Mfn-1, and Mfn-2 for mitochondrial fusion, thereby maintaining the overall mitochondrial circuitry (14–18, 22). The processes of fusion and fission are finely regulated inside the eukaryotic cells to maintain the mitochondrial morphology (14, 16–18, 23). Dysregulation in the process of fission may enhance mitochondrial fragmentation, whereas impaired fusion may lead to uneven mitochondrial elongation (17, 22). Balanced fission and fusion are not only responsible for providing proper shape to the mitochondria but also maintain the mitochondrial functions (18). Intriguingly, when mitochondrial dynamics is impaired, it leads to accumulation of mitochondrial population having autonomous organelles with impaired function, because of the deficiency of interaction with each other (20). Hence, fusion is attributed as a more crucial process in the maintenance of mitochondrial functions (24). Several lines of evidence suggested that mitochondrial fission plays a crucial role in apoptosis (25, 26). The activity of Drp-1 and Fis-1 regulates mitochondrial fragmentation, and inhibition of these molecules also decreases apoptosis (25–27). Any interference or disruption in mitochondrial dynamics including both the fission and fusion processes may lead to pathogenesis of neurodegenerative disorders such as Parkinson disease, Alzheimer disease, Huntington disease, Charcot-Marie-Tooth disease, etc. (17, 21, 28–33). In addition, during apoptosis mitochondria exhibit major structural alterations including mitochondrial fragmentation and cristae remodeling (34), which eventually leads to cytosolic translocation of cytochrome c and up-regulation of apoptosis (34).
In our previous study, we reported that long term exposure of BPA in hippocampal neuronal cells impairs autophagy by decreasing the levels of Lamp-2 and up-regulating p62 and cleaved caspase-3 (1). Herein, we delineate that chronic BPA exposure not only impairs autophagy but also causes Drp-1-dependent impaired mitochondrial dynamics and apoptosis in the rat brain hippocampus. We found that BPA exposure increased the levels of Drp-1 and was associated with impaired autophagy and enhanced apoptosis. In addition, genetic and pharmacological inhibition of Drp-1 mitigated BPA-induced neurotoxicity. Interestingly, BPA-mediated inhibitory effects on neural stem cell (NSC) proliferation and neuronal differentiations were also mitigated by Drp-1 inhibition. Therefore, targeting Drp-1 to maintain mitochondrial dynamics may create a new avenue for therapeutic approach against environmental xenoestrogen-induced neurodegeneration.
Results
Chronic Exposure of BPA Reduced Autophagy and Enhanced Oxidative Stress and Apoptosis in the Hippocampus Region of the Rat Brain
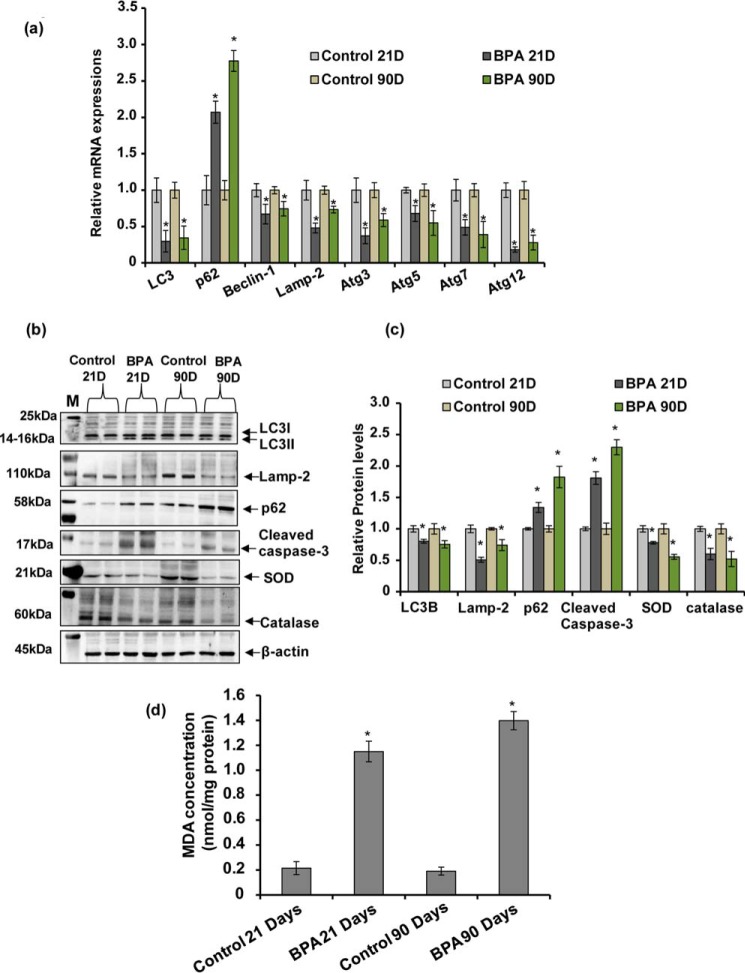
Our previous study suggested that short term BPA exposure during PND 14–21 up-regulated the expression and level of autophagy genes and proteins in the rat brain (1). However, in the human population long term chronic exposure of BPA takes place. Therefore, in the present study we studied the effects of chronic BPA exposure from gestational day (GD) 6 to PND 21 and from PNDs 21 to 90 on the expression and levels of autophagy genes and proteins, respectively. We observed that chronic BPA exposure during both the time points significantly decreased the expression of LC3, Beclin-1, Lamp-2, Atg3, Atg5, Atg7, and Atg12 and up-regulated the expression of autophagic cargo receptor p62 in the hippocampus region of the rat brain (Fig. 1a). BPA exposure also decreased the protein levels of LC3B, Lamp-2, superoxide dismutase, and catalase and up-regulated the levels of cleaved caspase-3 and SQSTM1/p62 proteins at PNDs 21 and 90 (Fig. 1, b and c). Next, we found that MDA levels were significantly enhanced in the hippocampus after BPA exposure in comparison with control rats (Fig. 1d). These results suggest that chronic exposure of BPA during gestational, lactational, and adulthood periods reduces autophagy, resulting in increased oxidative stress and apoptosis in the hippocampus.
FIGURE 1.
Chronic exposure of BPA reduced autophagy and enhanced oxidative stress and apoptosis in the hippocampus region of the rat brain. a, effects of BPA treatment (40 μg/kg body weight, oral) at PNDs 21 and 90 on the expression of autophagy genes in the hippocampus region of the brain was studied by quantitative RT-PCR. β-Actin served as a housekeeping gene for normalization. The data are expressed as means ± S.E. (n = 6 rats/group). *, p < 0.05 versus control. b and c, Western blotting analysis of the levels of LC3B, Lamp-2, p62, cleaved caspase-3, superoxide dismutase (SOD), and catalase proteins in the hippocampus. M, marker. Quantification of relative protein density after normalization with β-actin. d, lipid peroxidation (MDA levels) in the hippocampus region of the rat brain. The values are means ± S.E. (n = 6 rats/group). *, p < 0.05 versus control.
BPA Increased Mitochondrial Fragmentation in the Hippocampus Region of the Rat Brain
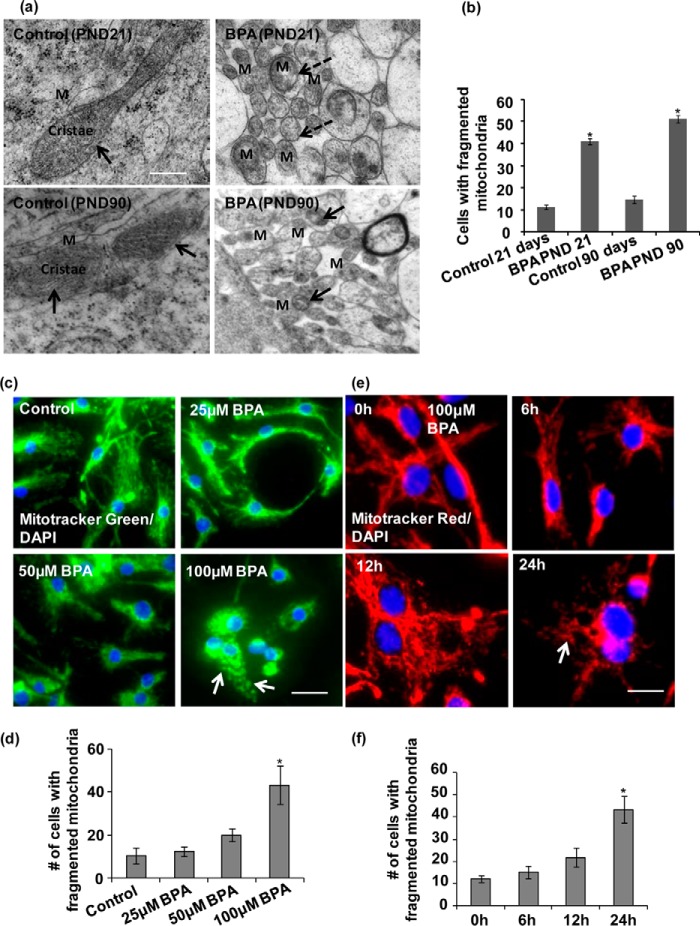
In our previous study, we found that acute exposure of BPA enhanced mitochondrial dysfunction and mitophagy (1). In the present study, we observed that chronic exposure of BPA enhanced apoptotic cell death through inhibition of autophagy. As autophagy and mitochondrial dynamics are interlinked processes (35, 36), we studied the effects of chronic BPA exposure on mitochondrial fragmentation in the hippocampus. Through transmission electron microscopy (TEM), we observed marked fragmentation of mitochondria, with abnormal cristae depletion, after BPA exposure at both time points: PNDs 21 and 90 (Fig. 2, a and b). The elongated mitochondria were significantly decreased, and the number of fragmented and round mitochondria was enhanced in the brain hippocampus after BPA exposure. However, the accumulation of fragmented mitochondria was remarkably observed after BPA exposure, suggesting the alteration in the mitochondrial morphology (Fig. 2, a and b).
FIGURE 2.
BPA increased mitochondrial fragmentation in the hippocampus region of the rat brain and hippocampal NSC-derived neuron cultures. a, to study the effects of BPA on mitochondrial dynamics including mitochondrial morphology and fragmentation, ultrastructural analysis of mitochondria was carried out by TEM in the neurons of the hippocampus region (including dentate gyrus and CA regions) of the rat brain. Several fragmented and spherical mitochondria with depleted cristae were observed in BPA-treated rats in comparison with control. The elongated mitochondria were significantly decreased, and the number of fragmented mitochondria was enhanced in the brain hippocampus after BPA exposure. M, mitochondria. Arrows show healthy elongated mitochondria. The dotted arrow showing round, small, and fragmented mitochondria. Scale bar, 500 nm. b, quantification of TEM images. The cells with fragmented mitochondria were quantified. The values are means ± S.E. (n = 3 rats/group). *, p < 0.05 versus control. c–f, hippocampal NSC-derived neuronal cultures were treated with various concentrations of BPA (25, 50, and 100 μm) for different time periods. Staining of mitochondria with MitoTracker Red or Green suggest dose-dependent and time-dependent increases in mitochondrial fragmentation in BPA-treated hippocampal NSC-derived neuron cultures. The mitochondrial morphology was found to be altered to punctuated structures after 100 μm BPA exposure. DAPI was used for counterstaining of the nucleus. Arrows show fragmented mitochondria. Scale bar, 20 μm. Means ± S.E. (n = 3 independent experiments). *, p < 0.05 versus control.
Further, we examined the mitochondrial morphology after BPA exposure in hippocampal NSC-derived neurons in vitro (Fig. 2, c–f). The dose- and time-dependent analysis of BPA exposure on mitochondrial structure was carried out. Through MitoTracker fluorescence localization, we observed that BPA dose-dependently increased mitochondrial fragmentation, with significant mitochondrial morphology alterations only at 100 μm (Fig. 2, c and d). Similarly, BPA treatment caused significant mitochondrial fragmentation at 100 μm concentration after 12 h of exposure, as evident from the presence of increased number of fragmented mitochondria (Fig. 2, e and f). Hence, these results suggest that BPA-induced neurotoxicity is corroborated with the mitochondrial fragmentation and further results in mitochondrial dysfunction in the hippocampus.
BPA-induced Alterations in the Expression and the Levels of Mitochondrial Fusion-Fission Proteins in Hippocampal NSC-derived Neuron Cultures and the Hippocampus
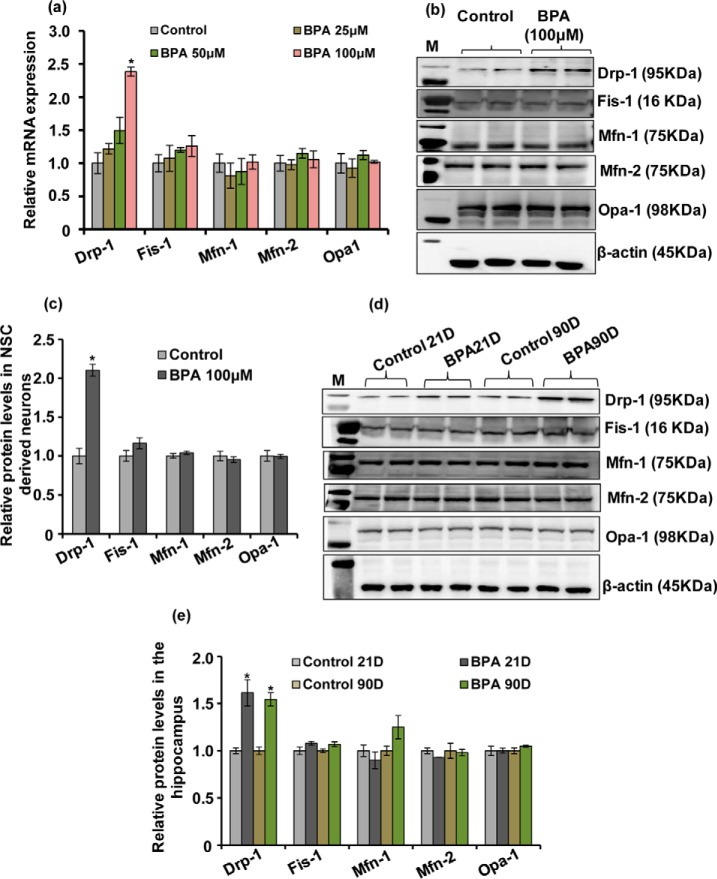
Because BPA increased mitochondrial fragmentation, we next studied the mechanistic insights underlying the alteration of mitochondrial dynamics in response to long term BPA exposure in the hippocampus. Several genes are responsible for maintaining the mitochondrial pool inside the brain by fusion and fission processes (19–21). To examine whether BPA alters the expression of mitochondrial dynamic genes and protein levels, hippocampal NSC-derived neurons were treated with various non-cytotoxic concentrations of BPA (i.e. 25, 50, and 100 μm). We investigated the effects of BPA on the gene expression and levels of mitochondrial fusion proteins (i.e.Mfn-1, Mfn-2, and Opa-1) and fission proteins (i.e. Drp-1 and Fis-1) (Fig. 3, a–e). The expression of Drp-1 was significantly increased at 100 μm concentration of BPA, whereas the expression of Mfn-1, Mfn-2, and Opa-1 was not significantly altered (Fig. 3a). Further, BPA (100 μm) also up-regulated the levels of Drp-1 but did not alter the levels of other mitochondrial dynamin proteins such as Mfn-1, Mfn-2, and Opa-1 (Fig. 3, b and c).
FIGURE 3.
BPA induced alterations in the expression and the levels of mitochondrial fusion-fission proteins in hippocampal NSC-derived neuron cultures and in the hippocampus. a, hippocampal NSC-derived neuronal cultures were treated with various concentrations of BPA (25, 50, and 100 μm), and the expression of mitochondrial dynamin family genes was studied by quantitative RT-PCR. b and c, Western blotting analysis of mitochondrial dynamin proteins levels in BPA-treated (100 μm) NSC-derived neuronal cultures. M, marker. Relative protein levels were quantified after normalization with β-actin. The values are means ± S.E. (n = 3 independent experiments). *, p < 0.05 versus control. d and e, Western blotting analysis of levels of mitochondrial dynamin-related proteins in the entire hippocampus after BPA treatment (40 μg/kg body weight, oral) at PNDs 21 and 90. M, marker. Quantification of relative protein density after normalization with β-actin. The values are means ± S.E. (n = 6 rats/group). *, p < 0.05 versus control.
Next, we also found that BPA (40 μg/kg body weight) exposure during both PNDs 21 and 90 caused significant increase in the levels of Drp-1 in the hippocampal tissue (Fig. 3, d and e). Interestingly, the levels of Mfn-1, Mfn-2, and Opa-1 were not significantly altered even after BPA exposure (Fig. 3, d and e). Hence, these results suggest the involvement of Drp-1 in BPA-induced increase in mitochondrial fragmentation in the brain.
BPA-mediated Increased Apoptosis and Inhibition of NSC Proliferation and Differentiation Were Mitigated by NAC
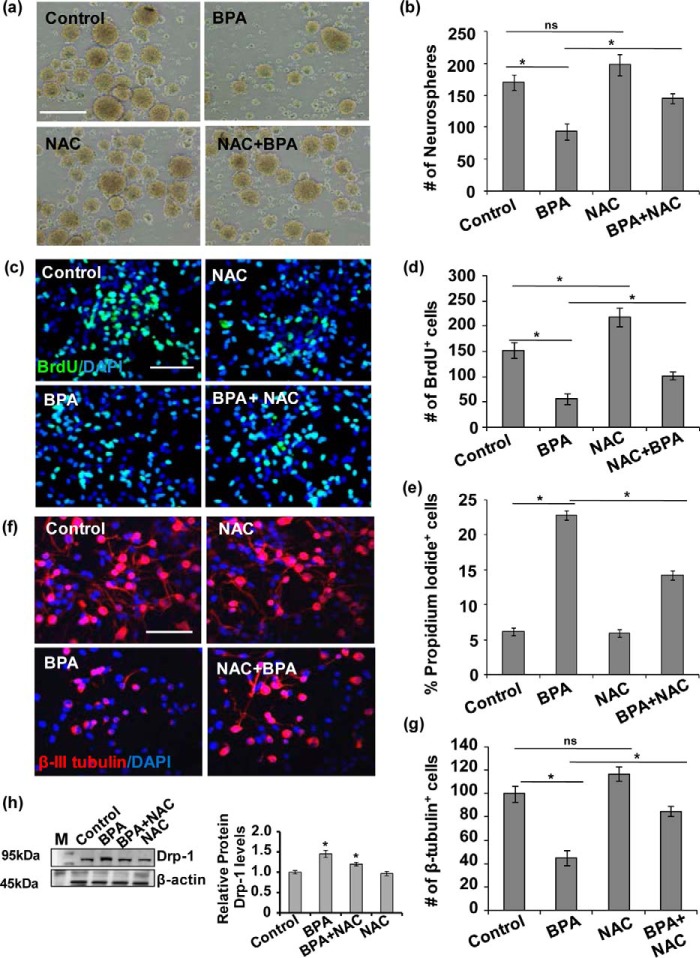
Our previous studies demonstrated that BPA exposure inhibits NSC proliferation and differentiation and also causes neurodegeneration in the hippocampus (1–3). In the present study, we found increased mitochondrial fission, increased oxidative stress, and apoptosis. Therefore, next we studied the effects of oxidative stress on BPA-mediated inhibition of NSC proliferation and differentiation and apoptosis in vitro. Further, to study the effects of BPA on NSC in perception with the increased apoptosis, accompanied by impaired mitochondrial dynamics, we treated NSC cultures with BPA for 24 h in the presence and absence of NAC. BPA significantly reduced the number of neurospheres (93 ± 13, 54%) as compared with control cultures (170 ± 12.1, 100%, p < 0.05), suggesting potent inhibitory effects of BPA on multipotent NSC (Fig. 4, a and b). Similarly, BPA also significantly reduced the number of BrdU-positive cells (56 ± 10.6, 37%) in hippocampal NSC cultures as compared with control (152 ± 15.6, 100%) (Fig. 4, c and d). Interestingly NAC alone treatment significantly increased the number of BrdU-positive cells (218 ± 18.7, 143%), with no significant effect on neurospheres number (198 ± 17, 116%) as compared with control. The treatment of NAC significantly increased the number of neurospheres (145 ± 8, 85%) and BrdU-positive cells (102 ± 7.8, 68%) in BPA + NAC-treated group as compared with the group treated with BPA alone (93 ± 13 and 56 ± 10.64 respectively) (Fig. 4, a and d).
FIGURE 4.
BPA-mediated increased oxidative stress resulted in inhibition of NSC proliferation and differentiation in vitro. a and b, effects of BPA on proliferation of NSC in neurospheres. Neurosphere growth kinetics was done by counting the number of neurospheres. The number and size of NSC-derived neurospheres were decreased by BPA as compared with control. The values are expressed as means ± S.E. (n = 3 cultures). *, p < 0.05. ns, not significant. Scale bar, 100 μm. c and d, primary hippocampal NSC-derived neuronal cultures were treated with BPA (100 μm) in the presence/absence of antioxidant NAC (10 mm), and NSC proliferation was studied by BrdU immunocytochemistry. BPA treatment alters proliferation of the hippocampus NSC in the presence of NAC. Representative immunofluorescent images and quantitative analysis suggested that BPA significantly decreased BrdU+ cells (proliferation marker), but NAC ameliorated the inhibitory action of BPA on proliferation. DAPI was used for counterstaining of the nucleus. The values are means ± S.E. (n = 3 independent experiments). *, p < 0.05. e, the number of apoptotic cells expressed as percentages PI-positive cells as compared with control as studied flow cytometry. The values are expressed as means ± S.E. (n = 3 independent experiments). *, p < 0.05. f and g, BPA treatment also alters neuronal differentiation potential of the hippocampus NSC. Representative immunofluorescent images and quantitative analysis suggested that BPA significantly decreased β-tubulin-III+ neuronal cells (neuronal differentiation marker), but NAC ameliorated the inhibitory action of BPA on differentiation of hippocampus NSC. The values are means ± S.E. (n = 3 independent experiments). *, p < 0.05. ns, not significant. Scale bar, 100 μm. h, Drp-1 protein levels in the presence of NAC in BPA-treated hippocampal NSC-derived neurons culture. The values are means ± S.E. (n = 3 independent experiments). M, marker. *, p < 0.05 versus control.
BPA significantly increased the number of PI-positive cells, which was blocked through inhibition of oxidative stress by NAC in NSC-derived neurons (Fig. 4e). In addition, we found that BPA significantly reduced the number of NSC-derived β-III tubulin+ neurons (45 ± 6.6) as compared with control (100 ± 6.7) (Fig. 4, f and g). Interestingly, NAC alone treatment showed no significant effect on the number of β-III tubulin+ neurons (117 ± 6) as compared with control. On the other hand, pretreatment of NAC diminished BPA cytotoxicity and enhanced the number of β-III tubulin+ neurons (85 ± 4.5) as compared with BPA-treated group. Thus, NAC exposure alleviates BPA-mediated decrease in proliferation and also increases the number of β-III tubulin+ cells. These results suggest that BPA treatment inhibits NSCs proliferation and differentiation and induces apoptosis because of increased oxidative stress, which can be mitigated by NAC.
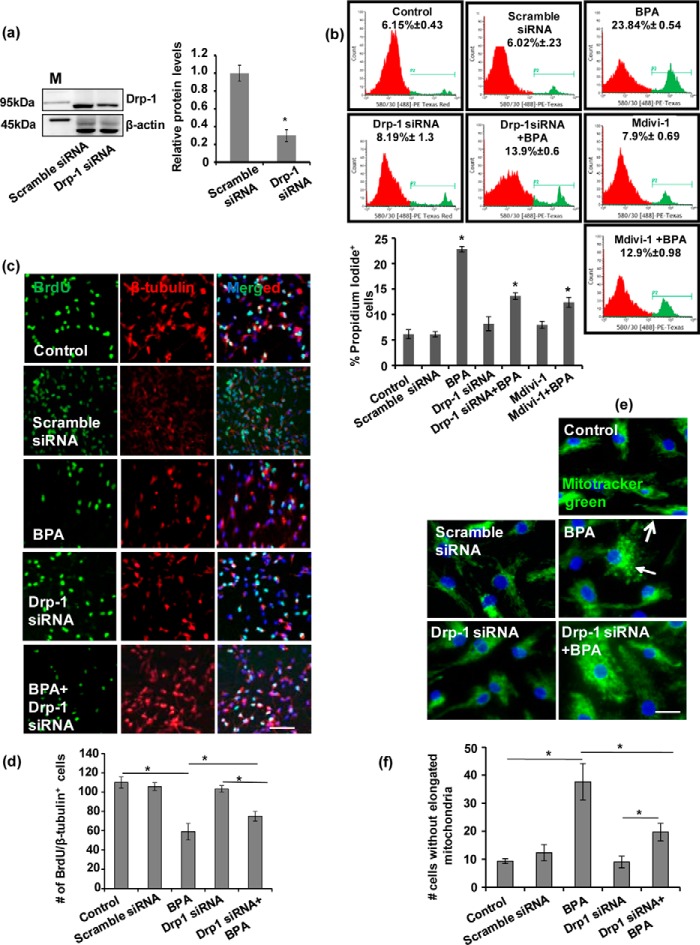
Drp-1 Is Involved in the Regulation of BPA-induced Mitochondrial Fragmentation, Apoptosis, and Decrease in Hippocampal NSC Neuronal Differentiation
The mitochondrial dysfunction impairs mitochondrial dynamics, thereby disrupting the fusion-fission equilibrium inside the cells (14, 15, 23). In mammals, both the Drp-1 and Fis-1 are chiefly involved in the process of mitochondrial fission (17, 18, 37). Accumulated evidence suggested that Drp-1 is crucially involved in inducing apoptosis-mediated cell death (25, 38–41). Additionally, mitochondrial translocation of Drp-1 from the cytosol leads to the mitochondrial fission and apoptosis (25, 41–43). Intriguingly, silencing the expression of Drp-1 results with the inhibition of mitochondrial fission and also delayed cellular apoptosis (42). Because ROS generation causes mitochondrial dysfunction, we studied whether NAC actually blocks Drp1 to explore the role of Drp-1 in BPA-mediated generation of ROS. We found inhibition of ROS generation by NAC significantly reduced Drp-1 levels in BPA + NAC-treated NSC-derived neuron cultures as compared with BPA (Fig. 4h).
Further, we examined the role of Drp-1 inhibition on NSC viability, apoptosis, and neuronal differentiation potential in hippocampal NSC culture (Fig. 5, a–d). Drp-1 knockdown by Drp-1 siRNA significantly reduced Drp-1 protein levels as compared with scramble siRNA (Fig. 5a). We studied the effects of BPA exposure on number of PI-positive neuronal cells in the presence and absence of Drp-1 siRNA and Drp-1 pharmacological inhibitor, Mdivi-1 (Fig. 5b). Scramble siRNA, Drp-1 siRNA, and Mdivi-1 did not show any significant effect on the number of PI-positive cells in NSC cultures. BPA significantly increased the apoptosis, as evident from increased number of PI-positive cells. BPA-mediated induction of apoptosis significantly reduced by Drp-1 siRNA and Mdivi-1 in NSC culture, where a reduced number of PI-positive cells was observed as compared with the group treated with BPA alone. These results suggest that BPA-mediated apoptosis was significantly blocked by the inhibition of Drp-1 (Fig. 5b).
FIGURE 5.
Drp-1 is involved in the regulation of BPA-induced mitochondrial fragmentation, apoptosis, and decrease in neuronal differentiation potential of hippocampal NSC. a, Drp-1 siRNA significantly decreased the Drp-1 protein levels in hippocampal NSC cultures. M, marker. b, hippocampal NSC cultures were preincubated with Mdivi-1 (pharmacological inhibitor of Drp-1) and transfected with Drp-1 siRNA followed by treatment with BPA (100 μm) for 24 h. PI staining was done through flow cytometry to study the number of PI-positive cells (apoptotic cells). BPA-mediated induction of apoptosis significantly reduced by Drp-1 siRNA and Mdivi-1. The values are means ± S.E. (n = 3 independent experiments). *, p < 0.05 versus control. c and d, effects of BPA on NSC neuronal differentiation after Drp-1 inhibition. Hippocampal NSC were co-labeled with BrdU (proliferation marker) and β-III tubulin (neuronal marker). Quantification of number of BrdU/β-III tubulin co-labeled cells suggests that Drp-1 inhibition significantly increased neuronal differentiation in BPA-treated NSC cultures. DAPI was used for counterstaining of the nucleus. Arrows show BrdU/β-III tubulin co-labeled cells. The values are means ± S.E. (n = 3 independent experiments). *, p < 0.05 versus control. Scale bar, 100 μm. e and f, effects of BPA on mitochondrial morphology were observed using staining of mitochondria with MitoTracker Green in hippocampal NSC-derived neuron cultures. BPA-induced fragmented mitochondria were alleviated significantly after Drp-1 knockdown. Arrows show fragmented mitochondria. The values are means ± S.E. (n = 3 independent experiments). *, p < 0.05 versus control. Scale bar, 20 μm.
Similarly, the role of Drp-1 in BPA-mediated inhibition of NSC proliferation and differentiation was studied by immunofluorescence analysis of proliferating cells and neuronal differentiation in hippocampal NSC cultures (Fig. 5, c and d). The co-localization of BrdU/β-III tubulin+ neurons was studied both in the presence and absence of Drp-1siRNA followed by BPA exposure (Fig. 5, c and d). BPA significantly inhibited the number of BrdU/β-III tubulin co-immunolabeled cells, suggesting decreased neuronal differentiation in hippocampal NSC cultures (Fig. 5, c and d). Drp-1 knockdown showed no significant effect on neuronal differentiation. Similarly, scramble siRNA did not affect neuronal differentiation (data not shown). Interestingly, a reduced number of BrdU/β-III tubulin+ cells was significantly increased in Drp-1 siRNA + BPA group, suggesting reversal of BPA-mediated inhibition of neuronal differentiation by Drp-1 siRNA. These results implicate the role of Drp-1 in the regulation of neuronal differentiation. Further, these findings also suggest that inhibitory effects of BPA on NSC differentiation can be mitigated by Drp-1 knockdown.
In addition, we studied the role of Drp-1 in BPA-mediated impairment in mitochondrial dynamics including mitochondrial morphology and mitochondrial fragmentation in hippocampal NSC-derived neurons. Drp-1 and scramble siRNA showed no significant effect on mitochondrial fragmentation (Fig. 5, e and f). BPA significantly induced mitochondrial fragmentation, as evident from the presence of reduced number of cells with elongated mitochondria. We found that BPA-induced fragmented mitochondria were alleviated significantly after Drp-1 knockdown. (Fig. 5, e and f). Taken together, these results suggest that the BPA-induced rise in the levels of Drp-1 corroborates with enhanced apoptosis, mitochondrial fission, and inhibition of NSC neuronal differentiation in hippocampal NSC. Further, inhibition of Drp-1 reversed all these BPA-mediated inhibitory effects in NSC culture.
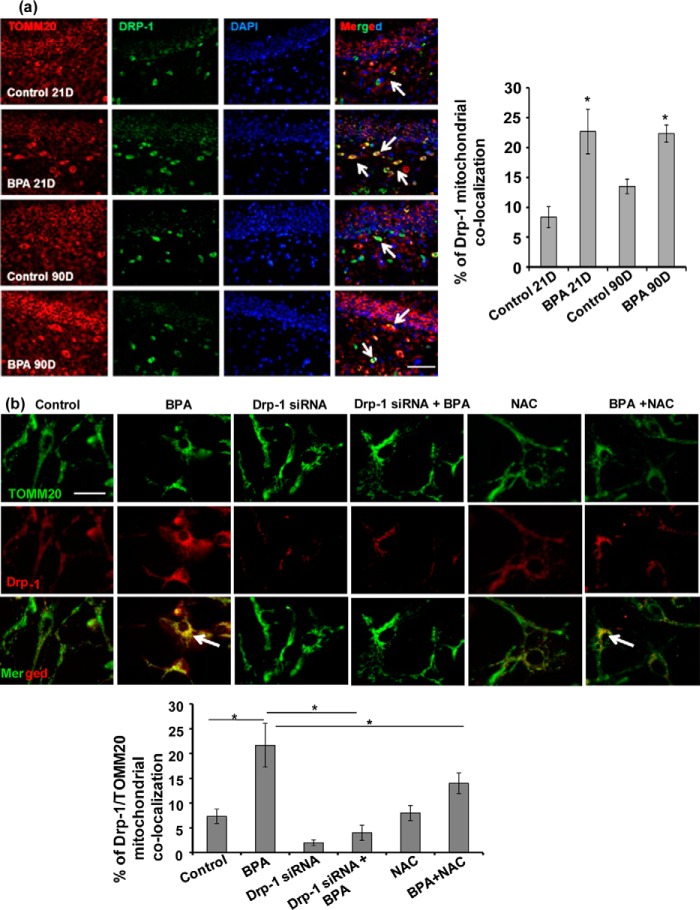
BPA Neurotoxicity Mediated via Enhanced Drp-1 Mitochondrial Translocation in the Hippocampus
Our previous studies depicted that BPA induces apoptosis and also impairs autophagy/mitophagy in the hippocampus and NSC-derived neurons in culture (1–3). However, in the present study we observed that BPA-induced cytotoxicity results from alteration in the mitochondrial dynamics involving enhanced mitochondrial fission and increased levels of mitochondrial fission protein Drp-1. Several previous studies suggested that translocation of Drp-1 in the mitochondria enhances fission and apoptosis (25, 41–44). Similarly, enhanced Drp-1 mitochondrial translocation is also associated with enhanced neurodegeneration and pathogenesis of neurodegenerative disorders (25, 45, 46). We therefore hypothesized whether increased apoptosis and enhanced mitochondrial fragmentation by BPA is associated with mitochondrial translocation of Drp-1. We studied co-localization of Drp-1 and TOMM20 (mitochondrial protein), in terms of the percentage of Drp-1 mitochondrial translocation in the hippocampus region of the brain (Fig. 6a) and hippocampus NSC-derived neuron cultures (Fig. 6b). We observed that BPA treatment increased Drp-1 and TOMM20 co-localization in the hippocampal NSC-derived neurons in vitro and also in the rat brain hippocampus, suggesting increased Drp-1 mitochondrial translocation (Fig. 6, a and b). BPA exposure both during PNDs 21 and 90 enhanced the co-localization of Drp-1 and TOMM20 (mitochondrial protein) in the hippocampus region (Fig. 6a), suggesting increased Drp-1 mitochondrial translocation. Further, in hippocampal NSC-derived neuron cultures, BPA treatment (100 μm) increased Drp-1 mitochondrial translocation by enhancing Drp-1 and TOMM20 co-localization (Manders co-localization coefficient; M = 0.21) as compared with control (M = 0.073) (Fig. 6b). Conversely, Drp-1 silencing inhibits BPA-induced Drp-1 mitochondrial translocation in the hippocampal NSC-derived neurons (M = 0.043). In addition role of NAC on Drp-1 co-localization was also studied in the presence and absence of BPA. NAC along with BPA decreases Drp-1 co-localization with mitochondrial protein TOMM20 (M = 0.15). On the other hand treatment of NAC alone causes no significant changes in Drp-1 mitochondrial co-localization as compared with control (M = 0.048). Thus, suggesting the significant role of NAC in mitigating BPA-mediated Drp-1 mitochondrial translocation. These results suggest that increased translocation of Drp-1 in the mitochondria is involved in BPA-mediated neurotoxicity.
FIGURE 6.
BPA neurotoxicity mediated via enhanced Drp-1 mitochondrial translocation in the hippocampus and hippocampal NSC-derived neurons. a, BPA treatment increased Drp-1 mitochondrial translocation in hippocampal (dentate gyrus) neurons of the rat brain as evident from increased co-localization of Drp-1 with mitochondrial protein TOMM20. BPA exposure both during PNDs 21 and 90 enhanced the co-localization of Drp-1 and TOMM20 (mitochondrial protein) in the hippocampus region. Arrows show Drp-1/TOMM20 co-labeled cells. The values are means ± S.E. (n = 6 rats/group). *, p < 0.05 versus control. Scale bar, 100 μm. b, Drp-1 silencing and NAC inhibited BPA-induced Drp-1 mitochondrial translocation as evident from reduced co-localization of Drp-1 with mitochondrial protein TOMM20 in the hippocampal NSC-derived neurons in culture. The values are means ± S.E. (n = 3 independent experiments). *, p < 0.05 versus control. Scale bar, 20 μm.
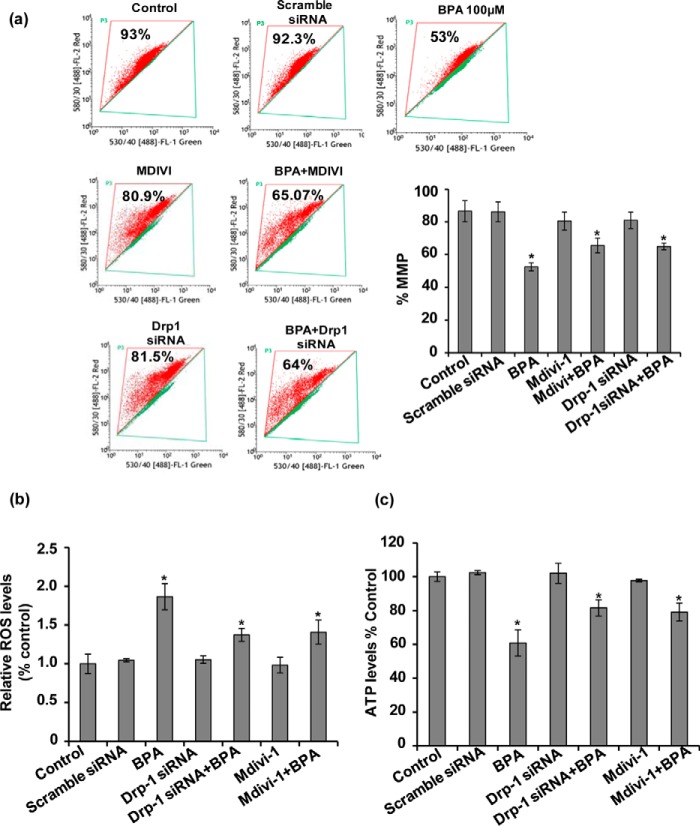
BPA-induced Mitochondrial Fragmentation Resulted in Mitochondrial Dysfunction and Bioenergetic Deficits in the Hippocampal Neurons
Impaired mitochondrial fragmentation and mitochondrial cristae depletion causes accumulation of defective mitochondria unable to produce energy, which ultimately leads to neurodegeneration (1, 25, 47). We hypothesized whether increased mitochondrial fragmentation and cristae depletion by BPA leads to accumulation of defective mitochondria and mitochondrial dysfunction. We assessed the effects of BPA on the alteration of mitochondrial functions in the hippocampal NSC-derived neurons. We found that BPA exposure for 24 h significantly enhanced ROS generation, reduced mitochondrial membrane potential and ATP levels (Fig. 7, a–c). Contrarily, pharmacological and genetic inhibition of Drp-1 by Mdivi-1 and Drp-1siRNA alleviated BPA-induced neurotoxicity. Drp-1 inhibition significantly blocked BPA-induced ROS generation, loss in mitochondrial membrane potential, and decline in ATP levels (Fig. 7, a–c), suggesting that Drp-1 knockdown ameliorates BPA-induced mitochondrial dysfunctions in the hippocampal neurons (Fig. 7, a–c).
FIGURE 7.
BPA-induced mitochondrial fragmentation resulted in mitochondrial dysfunction and bioenergetic deficits in the hippocampal NSC cultures. a–c, the effects of BPA on the alteration of mitochondrial functions in hippocampal NSC cultures were studied. BPA exposure for 24 h resulted in significantly enhanced ROS generation, loss in membrane potential, and decline in ATP levels. Pharmacological and genetic inhibition of Drp-1 using Mdivi-1 and Drp-1 siRNA alleviated BPA-induced neurotoxicity. The values are expressed as means ± S.E. (n = 3 cultures). **, p < 0.05 versus control.
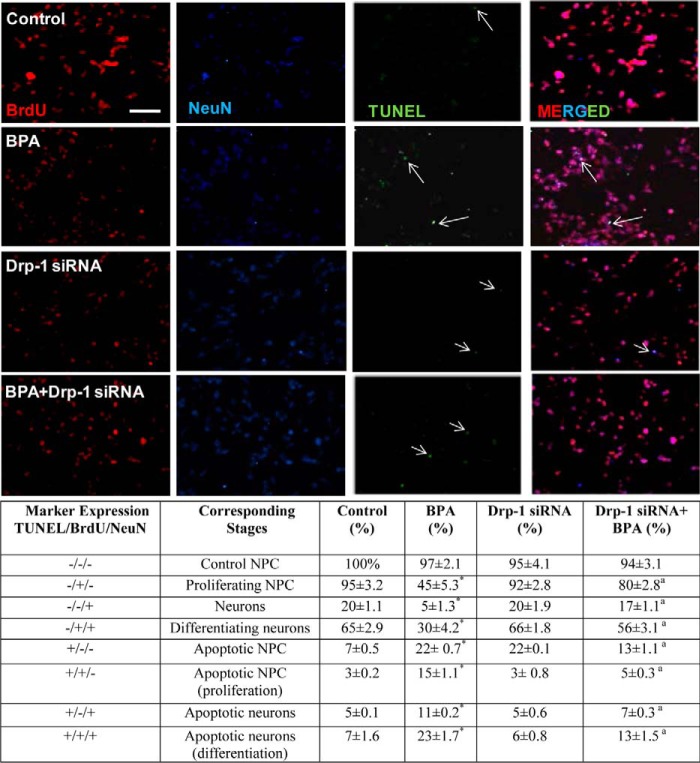
Effect of BPA on Number of Proliferating and Differentiating Cells Undergoing Apoptosis
We have validated the effect of BPA on NSC proliferation and neuronal differentiation. However, the decrease in the NSC proliferation and differentiation in response to BPA exposure could be due to up-regulation of apoptosis after BPA exposure. Thus, to verify this, we performed TUNEL assay and through immunocytochemistry studied co-localization of TUNEL-positive cells with BrdU and NeuN (Fig. 8). We observed that at 100 μm BPA treatment, only 22% cells were TUNEL-positive, which were also co-localized with BrdU and NeuN. Interestingly, in the presence of Drp-1 siRNA this co-localization between TUNEL-BrdU-NeuN positive cells was decreased to 13%. On the other hand, we found that at 100 μm BPA exposure 50% cells exhibits decrease in neuronal differentiation as is evident from significantly reduced number of BrdU/NeuN co-labeled cells. In the presence of Drp-1 siRNA, this decrease in co-localization was further mitigated. These results suggest that BPA-mediated inhibition of NSC proliferation and neuronal differentiation is independent of increased apoptosis by BPA.
FIGURE 8.
Effects of BPA on proliferating and differentiating cells undergoing apoptosis in hippocampal NSC cultures. To study the effects of BPA on proliferating and differentiating cells undergoing apoptosis in hippocampal NSC cultures, TUNEL assay was carried out. BPA exposure for 24 h resulted in significantly increased number of TUNEL-positive cells co-localized with BrdU and NeuN. Genetic inhibition of Drp-1 using Drp-1 siRNA alleviated BPA-induced increased co-localization of TUNEL-positive cell with neuronal and proliferation marker. However, when only BrdU and NeuN co-localized cells were counted after BPA exposure, it was found that BPA treatment further decreased the number of BrdU and NeuN co-localized cell, and the effect was alleviated in the presence of Drp-1 siRNA. The values are expressed as means ± S.E. (n = 3 cultures). *, p < 0.05 versus control; a, versus BPA. Scale bar, 20 μm.
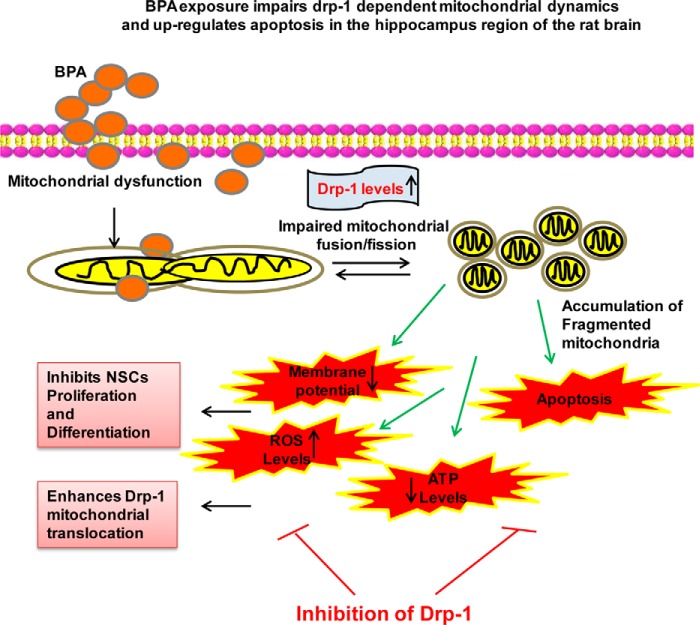
On the basis of our experimental studies, we proposed a schematic model elucidating the plausible mechanism(s) of BPA-mediated Drp-1-dependent impaired mitochondrial dynamics and resulting mitochondrial dysfunctions (Fig. 9). BPA exposure enhanced oxidative stress and up-regulated the expression and levels of Drp-1 and its mitochondrial translocation. Increased levels of Drp-1 resulted in enhanced mitochondrial fragmentation and apoptosis, thereby lowering the energy content, i.e. ATP depletion. Increased mitochondrial fission and impaired function resulted in inhibition of NSC proliferation and neuronal differentiation. Further, pharmacological and genetic inhibition of Drp-1 mitigated BPA-mediated ROS generation, mitochondrial fragmentation, mitochondrial dysfunctions, ATP depletion, and apoptosis. Drp-1 knockdown also reversed BPA-mediated inhibition of NSC and differentiation.
FIGURE 9.
Schematic representation of effects of BPA on mitochondrial dynamics. BPA exposure impairs Drp-1-dependent mitochondrial dynamics and up-regulates apoptosis in the hippocampus region of the rat brain. Drp-1 inhibition leads to reduced NSC proliferation and differentiation.
Discussion
Our recent studies suggested that BPA exposure inhibits hippocampal neurogenesis and myelination potential, leading to enhanced neurodegeneration and impaired learning and memory abilities (1–3, 48). Further, we also reported that BPA impairs autophagy/mitophagy and enhances apoptosis in the hippocampus of the rat brain (1). Consequences of impaired autophagy may be associated with the enhanced apoptotic cell death and neurodegeneration by the elevation in the levels of ROS and decline in the antioxidant levels (1). However, the mechanism of reduced NSC proliferation and neuronal differentiation and increased neurodegeneration by BPA remained obscured. Therefore, intriguingly, our present study provided evidence that BPA inhibited NSC proliferation and differentiation and induced neurodegeneration by disrupting the mitochondrial dynamics in the rat brain hippocampus. The impaired mitochondrial dynamics was associated with the increase in the levels of Drp-1 with insignificant changes in other mitochondrial dynamin-related proteins. The mitochondria are most dynamic organelles frequently involved in the process of fusion and fission forming an interconnecting tubular network and also individual entity (49). However, during apoptosis, mitochondria undergo fragmentation, which results in enhanced accumulation of smaller mitochondria (49). We have observed that Drp-1 significantly involved in regulating the mitochondrial shape, their fragmentation, and also functions. We found that Drp-1 contributes in the regulation of BPA-mediated mitochondrial fragmentation and enhanced fission. Additionally, Drp-1 knockdown mitigated BPA-induced mitochondrial fragmentation, apoptosis, and mitochondrial dysfunctions. Drp-1 knockdown also reversed inhibitory effects of BPA on NSC proliferation and neuronal differentiation. Our findings suggested that targeting Drp-1 may help in elucidating the potential mechanism of BPA-induced neurodegeneration.
Autophagy involves the degradation and recycling of proteins, initiating with sequestration of cytoplasmic components into double-membrane vesicles followed by autophagosome-lysosome fusion (50). Accumulated evidence suggested an interlink between autophagy and mitochondrial proteins turnover (35, 51). Herein, we observed that prolonged BPA exposure impairs autophagy and enhances oxidative stress and apoptosis in the hippocampus region of the rat brain. Chronic exposure of BPA down-regulated autophagy proteins including LC3B, Lamp-2, and antioxidant enzymes such as catalase and superoxide dismutase, whereas it up-regulated the levels of cleaved caspase-3 and p62 (1). The p62 is an autophagic substrate and cytosolic protein involved both in the formation and degradation of the accumulated redundant proteins (52). However, p62 can selectively sequester the aggregated proteins and help in their degradation (53, 54). Further, an increase in the levels of p62 infers disrupted autophagic flux, because p62 acts as an autophagic substrate. We observed decreased levels of proteins involved in autophagy and oxidative stress regulation and increased levels of lipid peroxidation and p62 protein. These results substantiated by a study, where p62 levels were increased in association with increased oxidative stress and apoptosis (1, 54). Taken together, these results suggest that BPA neurotoxicity is associated with impaired autophagy and antioxidant levels and increased apoptosis in response to BPA exposure.
In our earlier studies we observed that BPA inhibits NSC proliferation and neuronal differentiation and induced neurodegeneration through increased oxidative stress (1–3, 48). Because NSC proliferation and differentiation largely depend on energy production from the healthy mitochondria, we studied the effects of oxidative stress and mitochondrial dysfunction on BPA-mediated inhibition of NSC proliferation and differentiation and apoptosis in vitro. BPA significantly increased apoptosis in hippocampal NSC, which was blocked by inhibition of oxidative stress using an antioxidant NAC. Similarly, inhibition of oxidative stress mitigated BPA-mediated decrease in proliferation and the number of β-III tubulin+ neurons.
Because mitochondrial dynamics and autophagy/mitophagy are interlinked processes, we next studied the effects of BPA on mitochondrial dynamics including fusion and fission. Mitochondrial dynamics is a complex process, which maintains mitochondrial homeostasis through dynamic regulation of mitochondrial fusion and fission (14–18). Mitochondrial fusion and fission are regulated by family of dynamin-related proteins (Dnm-1), including fission proteins Drp-1 and Fis-1, and fusion proteins Opa-1, Mfn-1, and Mfn-2 (14–19, 21, 22). Dysregulation in mitochondrial fission leads to excessive mitochondrial fragmentation, whereas impaired fusion causes uneven mitochondrial elongation (20, 22). Therefore, an optimum balance between fission and fusion is critical not only to maintain normal shape of mitochondria but also to maintain mitochondrial functions (18). Any defect in this balance causes accumulation of defective mitochondria, unable to produce energy, leading to neurodegeneration as observed in neurodegenerative disorders including Parkinson disease, Huntington disease, and Alzheimer disease (17, 21, 28–33).
We observed that BPA exposure led to marked mitochondrial fragmentation in the hippocampal NSC-derived neurons and in the hippocampus region of the rat brain. Using TEM, it was observed that BPA exposure increased the number of fragmented mitochondria with cristae depletion and decreased elongated mitochondria. BPA significantly altered mitochondrial morphology from elongated and tubular to small and rounded structures. Thus, increases in mitochondrial fragmentation and altered mitochondrial dynamics are associated with BPA-induced neurotoxicity. Several lines of evidence suggested that mitochondrial fission plays a crucial role in apoptosis (25, 26).Therefore, increased apoptosis in our study caused by BPA could be attributed to enhanced accumulation of defective mitochondria in the cell caused by increased mitochondrial fission. Interestingly, BPA significantly increased the levels of only Drp-1, with no effects on Fis-1, Mfn-1, Mfn-2, and Opa-1 in vitro and in the hippocampal tissue, suggesting involvement of Drp-1 in BPA-induced increase in mitochondrial fragmentation in the brain. Therefore, BPA-mediated Drp-1 up-regulation caused pronounced mitochondrial fragmentation and their accumulation with altered mitochondrial morphology.
Next, we studied the specific role of Drp-1 in BPA-induced mitochondrial fragmentation, enhanced mitochondrial fission, and apoptosis. The activity of Drp-1 regulates mitochondrial fragmentation, and inhibition of it decreases mitochondrial fission and apoptosis (25–27, 42). Drp-1 is crucially involved in the induction of mitochondrial fission, apoptotic cell death, and neurodegeneration (38–41, 46) through its translocation from the cytosol to mitochondria (34, 41, 42). Studies suggested that autophagy is critically involved in the control of Drp-1 levels (35). Moreover, treatment with the autophagy inhibitors in the HEK-293T cells up-regulated the Drp-1 levels, and Atg7 knockdown also increased levels of Drp-1 (35). BPA significantly enhanced Drp-1 mitochondrial translocation in the hippocampal NSC-derived neurons in vitro and also in the hippocampus. We found enhanced co-localization of Drp-1 and TOMM20 (mitochondrial protein) in the hippocampus region after BPA exposure both during PNDs 21 and 90, suggesting increased Drp-1 mitochondrial translocation, which was blocked by Drp-1 siRNA. We found that BPA-mediated apoptosis was significantly blocked by the inhibition of Drp-1 through Drp-1 siRNA and Mdivi-1. Similarly, inhibitory effects of BPA on neuronal differentiation were mitigated by Drp-1 knockdown. Interestingly, BPA-induced fragmented mitochondria were alleviated significantly after Drp-1 knockdown in hippocampal NSC cultures. Further, BPA significantly enhanced ROS generation and reduced mitochondrial membrane potential and ATP levels, and these negative effects were blocked by Drp-1 inhibition, suggesting that Drp-1 knockdown ameliorates BPA-induced mitochondrial dysfunctions in the hippocampal neurons. These results imply that BPA-mediated enhanced levels of Drp-1 corroborate with increased apoptosis, mitochondrial fission, and inhibition of neuronal differentiation in hippocampal NSC. Our findings are substantiated by a study, where Drp-1 inhibition reduces aberrant mitochondrial fission and neurotoxicity in neuronal culture (55). Similarly, ethanol, a well established neurotoxicant also induced mitochondrial fission through enhanced Drp-1 mitochondrial translocation (56). In addition, manganese disrupted the mitochondrial network toward to an exacerbated fragmentation by enhancing the levels of Drp-1 (41, 57). Inhibition of excessive mitochondrial fission reduces aberrant autophagy and prevents neurodegeneration (58). The observed alterations in mitochondrial dynamics by BPA in the present study could also be explained on the basis of alterations in the Wnt/β-catenin pathway (59, 60). In our earlier studies we found that BPA exposure down-regulated the expression and levels of Wnt proteins (2). We found that BPA exposure decreases neurogenesis, thereby decreasing NSC proliferation and neuronal differentiation by impairing the Wnt/β-catenin pathway (2). The Wnt pathway plays an essential role during the development, and Wnt components participate as key players in the development of the central nervous system (61). Previous evidence suggests that Wnt proteins act as a potent modulator of mitochondrial dynamics and initiate the process of mitochondrial fission and fusion in the hippocampal neurons of rodents (59, 60). Therefore, increased mitochondrial fission by BPA observed in the present study could also be due to impaired expression and levels of Wnt proteins. Taken together, results of the present study suggest that Drp-1 is critically involved in BPA-induced impaired autophagy turnover, oxidative stress, mitochondrial fragmentation, inhibition of NSC proliferation, and differentiation and apoptosis in the hippocampus.
In conclusion, in the line of the above discussions, we conclude that BPA-induced neurotoxicity is corroborated with impaired autophagy and mitochondrial dynamics. BPA-mediated impaired mitochondrial dynamics is associated with inhibition of NSC proliferation and differentiation. Interestingly, BPA-mediated induction of mitochondrial fragmentation and impaired mitochondrial dynamics is Drp-1-dependent. Inhibition of Drp-1 reversed the deleterious effects of BPA on mitochondrial dynamics and neuronal differentiation. Thus, Drp-1 is a possible target for mitigation of BPA-mediated neurotoxicity and neurodegeneration. However, the effects of BPA on impaired mitochondrial dynamics need to be explored in further depth in context with the overall mitochondrial biogenesis and mitochondrial protein import in the brain.
Experimental Procedures
Materials and Methods
BPA (4,4′-(propane-2,2-diyl) diphenol), EGF, FGF2, BDNF, mitochondrial fission inhibitor, Mdivi-1, 2′,7′-dichlorofluorescin diacetate, and mitochondrial membrane potential kit (JC-1) from Sigma-Aldrich. Primary antibodies such as anti-mouse Drp-1 (ab56788, lot no. GR130216–1), anti-rabbit Opa-1 (ab42364, lot no. GR78795–6), anti-mouse Mfn-1 (ab57602, lot no. GR169819–3), and anti-mouse Mfn-2 (ab56889, lot no. 955856) were obtained from Abcam. Anti-rabbit neuronal nuclei (NeuN) (Merck Millipore, ABN78, lot no.2040920), mouse anti-BrdU (Santa Cruz Biotechnology, sc-32323, lot no. H2813), anti-rabbit Fis-1 (Santa Cruz Biotechnology, sc-98900, lot no. J1512), and anti-mouse anti-β-actin (Sigma-A5441, lot no. 123M4887V). Secondary antibodies such as Alexa Flour 594 goat anti-rabbit IgG, Alexa Flour 488 anti-mouse IgG, anti-rabbit IgG peroxidase antibody, anti-mouse IgG peroxidase antibody, MitoTracker, neurobasal medium, B-27, and N-2 supplement were obtained from Gibco. siRNA was from Dharmacon, and Cell Titer-Glo Luminescent cell viability assay kit for ATP measurement was obtained from Promega.
Animals and BPA Treatment
Wistar rats were obtained from an animal breeding colony of the Council of Scientific and Industrial Research-Indian Institute of Toxicology Research. The rats were kept in a 12-h light/dark cycle with ad libitum water and pellet diet. The experimental animals were handled and experiments with BPA were performed according to the guidelines of the Council of Scientific and Industrial Research-Indian Institute of Toxicology Research ethical committee for animal experiments (ethical approval number IITR/IAEC/28/13). The animals were divided into the following groups: (i) the vehicle control group, which received daily single oral gavage of vehicle (corn oil), once daily from gestational day 6 to PND 21; (ii) the BPA (40 μg) PND 21 group, which received daily single oral gavage of BPA in corn oil (40 μg/kg body weight) daily from gestational day 6 to PND 21 and adult rats from PNDs 21 to PND; (iii) the vehicle control group, which received daily single oral gavage of vehicle (corn oil), once daily from PNDs 21 to 90; and (iv) the BPA (40 μg) PND 90 group, which received daily single oral gavage of BPA in corn oil (40 μg/kg body weight) daily from PNDs 21 to 90.
The dose of BPA was selected on the basis of our earlier studies, in which BPA caused neurochemical alterations in the brain that were associated with impaired neurobehavioral functions in the rats (1–3). We treated pregnant rats from gestational day 6 to PND 21 (during gestational and lactational period; total 37 days), ensuring BPA exposure to the fetus and offspring, because BPA crosses the placental barrier and also passes through breast milk. In another group, rats were treated with BPA during postnatal period from PNDs 21 to 90 (70 days total). After respective treatments, offspring (PND 21) and adult rats (PND 90) from both the control and BPA-treated groups were sacrificed for neurochemical and histochemical analysis.
Hippocampal NSC Culture
Hippocampus-derived NSC culture was carried out following our previous studies (2, 3, 62). Briefly, pregnant Wistar rats were deeply anesthetized with a mixture of ketamine and xylazine (3:1 ratio) and dissected cautiously to remove the embryos at embryonic day 12. The hippocampal region was further microdissected from these embryos. Hippocampal tissues were collected in sterile Hanks' balanced salt solution and passed through syringes thrice. These tiny tissue pieces were then transferred to tubes containing 0.05% trypsin/EDTA at 37 °C for 30 min followed by addition of 0.5 mg/ml soybean trypsin inhibitor and then mild trituration and centrifugation. The cells were resuspended in proliferation medium containing neurobasal medium, 2 mm l-glutamine, 1% antibiotic antimycotic, 2% B-27, 1% N-2 supplement, and 20 ng/ml each of EGF and FGF2. Cell-containing flasks were placed in CO2 incubator at 37 °C, where neurosphere formation started after 4–5 days of culture.
BPA Treatment and Neurosphere Growth Kinetics Assay
To study the effects of BPA on multipotent NSC proliferation and neurosphere formation, the neurosphere growth kinetics assay was performed as described in our previous studies (2, 62). Briefly, hippocampal single-cell suspension was plated in a 12-well plate at a density of 5 × 104 cells/well in neurobasal medium containing B-27, N-2 supplement, basic FGF, and EGF. After 5 days, neurosphere cultures containing multipotent NSC were treated with non-cytotoxic concentration of BPA (100 μm) dissolved in DMSO for 24 h in the presence of basic FGF and EGF. The non-cytotoxic concentration of BPA was determined by trypan blue and PI uptake analysis. Next, the effects of antioxidant NAC (10 mm) on the number of neurospheres in BPA-treated NSC cultures were studied. The number of neurospheres was analyzed in all the groups using a Nikon Eclipse Ti-S inverted fluorescent microscope attached with Nikon CCD camera and NIS Elements BR imaging software (Nikon, Japan).
BrdU Incorporation Assay for NSC Proliferation Analysis
To see the effects of BPA on NSC proliferation, neurospheres were triturated to make single cell suspension of NSCs, because neurospheres are clusters of thousands of multipotent NSC. In brief, 1 × 105 cells were plated in chamber slides containing proliferation medium and treated with BPA for 24 h followed by NAC (10 mm) for 30 min. After respective treatments, cells were treated with BrdU (25 μm) for 4 h followed by fixation in 4% paraformaldehyde. The cells were treated with 1 n HCl for 10 min at 37 °C, followed by neutralization with borate buffer for 5 min at room temperature. Nonspecific binding sites were blocked using blocking buffer (0.1% Tween 20 and 3% BSA in PBS) followed by overnight incubation of cells in primary anti-BrdU antibody (1:250). After three washings, the cells were incubated in Alexa 488-conjugated secondary antibody for 2 h at room temperature and counterstained with nuclear stain DAPI. Immunofluorescence BrdU-positive cells were observed in inverted fluorescent microscope. The unbiased quantification of BrdU-positive cells was carried out in 10 microscopic fields using Nikon Eclipse Ti-S inverted fluorescent microscope attached with Nikon CCD camera and NIS Elements BR imaging software (Nikon, Japan).
Neural Stem Cells Derived Neuron Culture/Neuronal Differentiation
Hippocampal NSC cultures were differentiated into neurons following slightly modified protocol (63, 64). Hippocampal NSC were plated in poly-l-lysine-coated 6-well plates and chamber slides in neuronal differentiation medium containing 1% N-2, 2% B-27, 20 ng/ml FGF2, and 100 ng/ml BDNF. Further, the cells were grown with these factors for 14 days by the continuous replenishment of media at every second day. After 14 days the cells were processed for immunocytochemical localization of neuronal marker β-III tubulin. We found ∼90% of cell population was β-III tubulin-positive neurons after differentiation. Neuronal cells grown in flasks were treated with non-cytotoxic concentration of BPA (100 μm) for 24 h. To study the levels of mitochondrial dynamin-related proteins, neuronal cells were grown in the presence of BPA (100 μm) for 24 h. Further, in other groups the neuronal cells were treated/transfected in the presence/absence of pharmacological inhibitor of Drp-1 i.e. Mdivi-1 (25 μm), and Drp-1 siRNA (25 nm), respectively. After respective treatments, the cells were analyzed for mitochondrial dynamics (mitochondrial shape, morphology, and protein levels) by immunofluorescence studies, flow cytometry, and Western blotting.
Flow Cytometry for Cell Viability
The viability of hippocampal NSC-derived neurons was studied by flow cytometry using PI staining as described earlier (1, 65, 66). After respective treatment with Mdivi-1 and transfection with Drp-1 siRNA in the presence and absence of BPA, the cells were pelleted. After resuspension in PBS, the cells were incubated with 500 μl of PI (1 μg/μl) for 30 min on ice in the dark. In each experiment, minimum 10,000 cells were counted for PI-positive cells using BD FACScanto II flow cytometer attached with BD FACS Diva software (Becton-Dickinson).The results are expressed as percentages of control.
Mitochondrial Membrane Potential Assessment
The mitochondrial membrane potential of neuronal cells after various treatments was assessed using JC-1 kit following the manufacturer's instructions.
siRNA Transfection in Hippocampal NSC
Rat fetal hippocampal NSCs were transiently transfected with Drp-1 siRNA (25 nm) and scramble siRNA using a Neon Transfection Device as per the manufacturer's instructions. Briefly, 2.5 × 105 cells were plated in a 6-well plate. The cells diluted in 500 μl of neuronal medium were transfected with 25 nm of siRNA into a sterile 1.5-ml microcentrifuge tube, followed by gentle mixing. For transfection, we used a pulse voltage of 1500 V, a pulse width of 10 ms, and three pulses. Lastly, to determine the transfection efficiency, we performed immunoblotting and identified knockdown levels using densitometry analysis 24 h post-transfection. We found ∼70% knockdown of Drp-1 protein levels. Further, immunocytochemical visualization of Drp-1 in the cells was done in a Nikon Eclipse Ti-S inverted fluorescent microscope attached with Nikon Digital Sight Ds-Ri1 CCD camera with NIS Elements BR imaging software.
Oxidative Stress and MDA Levels
ROS and lipid peroxidation in neuronal cells were assessed using 2′,7′-dichlorofluorescin diacetate and MDA kit, respectively. Briefly, 10,000 neuronal cells/well in 96-well plates were treated with 100 μm BPA for 12 h and incubated with dichloro-dihydro-fluorescein diacetate (DCFH-DA) (10 μm) for 30 min at 37 °C. Fluorescence intensity was studied at excitation/emission wavelengths of 485/530 nm, respectively, using a spectrofluorometer. Further, lipid peroxidation was assessed in terms of MDA levels using lipid peroxidation assay kit as per the manufacturer's instructions.
Gene Expression Analysis by Quantitative RT-PCR
To study the expression of genes involved in autophagy and mitochondrial dynamics, quantitative RT-PCR analysis was carried out following our earlier published studies (1, 67). The relative expression was calculated using the ΔΔCt method.
Immunoblot Protein Level Analysis
Hippocampal tissue/cells were lysed with cell lytic MT mammalian tissue lysis/extraction reagent. The membranes were blocked with western blocker solution (Sigma-Aldrich). The blots were incubated overnight with primary antibodies LC3B (1:1000), Lamp-2 (1:1000), p62 (1:1000), cleaved caspase-3 (1:1000), catalase (1:500), superoxide dismutase (1:500), Drp-1 (1:1000), Fis-1 (1:500), Mfn-1 (1:1000), Mfn-2 (1:10,000), Opa-1 (1:1000), and β-actin (1:10,000). Densitometry analysis of the protein bands was carried out using Scion Image for Windows (National Institutes of Health).
Immunofluorescence Study
After respective treatments, rats were deeply anesthetized with ketamine and xylazine mixture and perfused with normal saline followed by 4% paraformaldehyde. The brains were removed and postfixed in 4% paraformaldehyde for 24 h and then transferred to gradient of sucrose (10, 20, and 30% in PBS). 30-μm thin serial coronal sections covering the entire hippocampus were cut using a freezing microtome (Slee Mainz Co.). Sections were kept for 2 h in blocking buffer containing 3% normal goat serum (NGS), 0.5% BSA, and 0.1% Triton X-100. Sections were then incubated for 24 h with primary antibodies Drp-1 (1:500) and TOMM20 (1:500) at 4 °C followed by incubation in Alexa Fluor 594- or 488-linked secondary antibodies (1:200) for 2 h at room temperature. The slides were analyzed under a Nikon Eclipse Ti-S inverted fluorescent microscope.
The quantification of co-localized cells in the hippocampus region was carried out following our earlier studies (1–3, 62, 68). In brief, unbiased stereological methods were applied, where a person blind to the experimental groups carried out cell quantification on coded slides. Labeled cells were counted in every sixth section in one in six series, with a total of six sections per rat analyzed. The region of interest (hippocampus) was identified at 10×, whereas the cells were quantified at 600×. The data were expressed as the percentage of Drp-1 and TOMM20 co-localization.
Immunocytochemistry
To evaluate the effects of BPA on NSC proliferation and differentiation, immunocytochemistry was performed. In brief, NSCs were plated in chamber slides, and siRNA-mediated Drp-1 knockdown was done followed by BPA treatment. The cells were then fixed with 4% paraformaldehyde for 30 min, washed, and incubated in blocking buffer (2% BSA and 0.1% Tween 20) for 1 h at room temperature. Further, the cells were incubated overnight at 4 °C with primary antibodies rabbit β-tubulin III (1:250), mouse anti-BrdU (1:200), mouse anti-TOMM20 (1:400), and rabbit anti Drp-1 (1:250). The cells were then incubated with Alexa Fluor 594- or 488-conjugated secondary antibodies. The cells were mounted in DAPI-containing antifade mounting medium. Fluorescent images were acquired using an inverted fluorescent microscope. The quantification of proliferating and differentiating cells was carried out following our previously published studies (2, 3, 62, 68). Co-localization of Drp-1 and TOMM20, in terms of % Drp-1 mitochondrial translocation was quantified using ImageJ (National Institutes of Health) employing the JACoP plugin (1, 69). Mander's co-localization coefficient (M) showing the percentage of Drp-1 and TOMM20 co-localization (M = 1 regarded as perfect correlation) was obtained from the data from at least three independent experiments.
TUNEL Assay
The in vitro detection of apoptosis in hippocampal NSC cultures was carried out with the cell death detection fluorescein kit (Roche Applied Science) as per the manufacturer's protocol and visualized under a fluorescence microscope. Briefly, the hippocampal NSC were plated in chamber slide and treated with Drp-1 siRNA followed by BPA treatment at 100 μm concentration for 24 h and kept at 37 °C inside CO2 incubator. The TUNEL/BrdU/NeuN-positive cells were counted in 10 randomly selected microscopic fields.
Mitochondrial Morphology
The effects of different concentrations of BPA (25, 50, and 100 μm) at different time intervals (0, 6, 12, and 24 h) on mitochondrial morphology were studied using fluorescence microscopy based localization of MitoTracker Green and Red dyes. The quantification of elongated and round shaped mitochondria was done using National Institutes of Health ImageJ software as described earlier (70).
ATP Measurement Assay
ATP levels were assessed using an ATP determination kit (Promega) following the manufacturer's instructions. In brief, 1 × 106 neuronal cells were plated, reaction buffer containing the substrate and luciferin was added and mixed, and luminescence was measured by microplate reader. The ATP levels in the samples were calculated using an ATP standard curve, and the results are expressed as ATP levels percentage of control.
TEM Analysis
To study the effects of BPA on mitochondrial dynamics including mitochondrial fusion, fission, and fragmentation ultrastructural analysis of mitochondria was carried out by TEM in the neurons of the hippocampus region (including dentate gyrus and CA regions) of the rat brain following method as described earlier (62). Mitochondrial fragmentation in the hippocampus was quantified, where minimum of 50 cells from 6 animals were evaluated.
Statistical Analysis
We carried out statistical analysis using GraphPad InStat statistical analysis software (San Diego, CA). The mean significant difference (p < 0.05) among various experimental groups was calculated using one-way analysis of variance and Tukey-Kramer post hoc test.
Author Contributions
S. A. and R. K. C. conceived and coordinated the study, performed experiments, analyzed data, and wrote the paper. S. A., S. K. T., B. S., and A. Y. designed, performed, and analyzed the experiments shown in Figs. 1–9. S. A. and L. K. S. C. performed experiments and analyzed the data shown in Fig. 2. S. A., R. S. R., and P. K. performed experiments and analyzed data shown in Figs. 5 and 7. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgment
We are thankful to the Director of the Council of Scientific and Industrial Research-Indian Institute of Toxicology Research for constant support during this study.
This work was supported by the Council of Scientific and Industrial Research-Network Grant InDepth BSC0111, a Lady Tata Memorial Trust Young Scientist Grant (to R. K. C.), and Senior Research Fellowships from the Council of Scientific and Industrial Research, New Delhi (to S. A. and B. S.). The authors declare that they have no conflicts of interest with the contents of this article.
- BPA
- bisphenol A
- NSC
- neural stem cell
- TEM
- transmission electron microscopy
- NAC
- N-acetyl cysteine
- PI
- propidium iodide
- PND
- postnatal day
- MDA
- malondialdehyde.
References
- 1. Agarwal S., Tiwari S. K., Seth B., Yadav A., Singh A., Mudawal A., Chauhan L. K., Gupta S. K., Choubey V., Tripathi A., Kumar A., Ray R. S., Shukla S., Parmar D., and Chaturvedi R. K. (2015) Activation of autophagic flux against xenoestrogen bisphenol-A-induced hippocampal neurodegeneration via AMP kinase (AMPK)/mammalian target of rapamycin (mTOR) pathways. J. Biol. Chem. 290, 21163–21184 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2. Tiwari S. K., Agarwal S., Seth B., Yadav A., Ray R. S., Mishra V. N., and Chaturvedi R. K. (2015) Inhibitory effects of bisphenol-A on neural stem cells proliferation and differentiation in the rat brain are dependent on Wnt/β-catenin pathway. Mol. Neurobiol. 52, 1735–1757 [DOI] [PubMed] [Google Scholar]
- 3. Tiwari S. K., Agarwal S., Tripathi A., and Chaturvedi R. K. (2016) Bisphenol-A mediated inhibition of hippocampal neurogenesis attenuated by curcumin via canonical Wnt pathway. Mol. Neurobiol. 53, 3010–3029 [DOI] [PubMed] [Google Scholar]
- 4. Masuo Y., and Ishido M. (2011) Neurotoxicity of endocrine disruptors: possible involvement in brain development and neurodegeneration. J. Toxicol. Environ. Health B Crit. Rev. 14, 346–369 [DOI] [PubMed] [Google Scholar]
- 5. Huang B., Jiang C., Luo J., Cui Y., Qin L., and Liu J. (2014) Maternal exposure to bisphenol A may increase the risks of Parkinson's disease through down-regulation of fetal IGF-1 expression. Med. Hypotheses 82, 245–249 [DOI] [PubMed] [Google Scholar]
- 6. Egnaczyk G. F., Greis K. D., Stimson E. R., and Maggio J. E. (2001) Photoaffinity cross-linking of Alzheimer's disease amyloid fibrils reveals interstrand contact regions between assembled β-amyloid peptide subunits. Biochemistry 40, 11706–11714 [DOI] [PubMed] [Google Scholar]
- 7. Ooe H., Taira T., Iguchi-Ariga S. M., and Ariga H. (2005) Induction of reactive oxygen species by bisphenol A and abrogation of bisphenol A-induced cell injury by DJ-1. Toxicol. Sci. 88, 114–126 [DOI] [PubMed] [Google Scholar]
- 8. Wisniewski P., Romano R. M., Kizys M. M., Oliveira K. C., Kasamatsu T., Giannocco G., Chiamolera M. I., Dias-da-Silva M. R., and Romano M. A. (2015) Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic-pituitary-testicular axis. Toxicology 329, 1–9 [DOI] [PubMed] [Google Scholar]
- 9. Xia W., Jiang Y., Li Y., Wan Y., Liu J., Ma Y., Mao Z., Chang H., Li G., Xu B., Chen X., and Xu S. (2014) Early-life exposure to bisphenol a induces liver injury in rats involvement of mitochondria-mediated apoptosis. PLoS One 9, e90443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huc L., Lemarié A., Guéraud F., and Héliès-Toussaint C. (2012) Low concentrations of bisphenol A induce lipid accumulation mediated by the production of reactive oxygen species in the mitochondria of HepG2 cells. Toxicol. in Vitro 26, 709–717 [DOI] [PubMed] [Google Scholar]
- 11. Xie M., and Li F. (2014) [Effects of bisphenol A exposure during lactation on testicular mitochondria in male mouse offspring]. Wei Sheng Yan Jiu 43, 962–966 [PubMed] [Google Scholar]
- 12. Jiang Y., Liu J., Li Y., Chang H., Li G., Xu B., Chen X., Li W., Xia W., and Xu S. (2014) Prenatal exposure to bisphenol A at the reference dose impairs mitochondria in the heart of neonatal rats. J. Appl. Toxicol. 34, 1012–1022 [DOI] [PubMed] [Google Scholar]
- 13. Fehlberg S., Trautwein S., Göke A., and Göke R. (2002) Bisphenol A diglycidyl ether induces apoptosis in tumour cells independently of peroxisome proliferator-activated receptor-gamma, in caspase-dependent and -independent manners. Biochem. J. 362, 573–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen H., and Chan D. C. (2009) Mitochondrial dynamics–fusion, fission, movement, and mitophagy–in neurodegenerative diseases. Hum. Mol. Genet. 18, R169–R176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan D. C. (2007) Mitochondrial dynamics in disease. New Engl. J. Med. 356, 1707–1709 [DOI] [PubMed] [Google Scholar]
- 16. Chen H., and Chan D. C. (2006) Critical dependence of neurons on mitochondrial dynamics. Curr. Opin. Cell Biol. 18, 453–459 [DOI] [PubMed] [Google Scholar]
- 17. Chan D. C. (2006) Mitochondrial fusion and fission in mammals. Annu. Rev. Cell Dev. Biol. 22, 79–99 [DOI] [PubMed] [Google Scholar]
- 18. Chen H., and Chan D. C. (2005) Emerging functions of mammalian mitochondrial fusion and fission. Hum. Mol. Genet. 14, R283–R289 [DOI] [PubMed] [Google Scholar]
- 19. Chen H., and Chan D. C. (2004) Mitochondrial dynamics in mammals. Curr. Top. Dev. Biol. 59, 119–144 [DOI] [PubMed] [Google Scholar]
- 20. Chan D. C. (2006) Mitochondria: dynamic organelles in disease, aging, and development. Cell 125, 1241–1252 [DOI] [PubMed] [Google Scholar]
- 21. Guedes-Dias P., Pinho B. R., Soares T. R., de Proença J., Duchen M. R., and Oliveira J. M. (2016) Mitochondrial dynamics and quality control in Huntington's disease. Neurobiol. Dis. 90, 51–57 [DOI] [PubMed] [Google Scholar]
- 22. Losón O. C., Song Z., Chen H., and Chan D. C. (2013) Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol. Biol. Cell 24, 659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Detmer S. A., and Chan D. C. (2007) Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 8, 870–879 [DOI] [PubMed] [Google Scholar]
- 24. Chen H., Chomyn A., and Chan D. C. (2005) Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. 280, 26185–26192 [DOI] [PubMed] [Google Scholar]
- 25. Frank S., Gaume B., Bergmann-Leitner E. S., Leitner W. W., Robert E. G., Catez F., Smith C. L., and Youle R. J. (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell 1, 515–525 [DOI] [PubMed] [Google Scholar]
- 26. Breckenridge D. G., Stojanovic M., Marcellus R. C., and Shore G. C. (2003) Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J. Cell Biol. 160, 1115–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee Y. J., Jeong S. Y., Karbowski M., Smith C. L., and Youle R. J. (2004) Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol. Biol. Cell 15, 5001–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quirós P. M., Langer T., and López-Otín C. (2015) New roles for mitochondrial proteases in health, ageing and disease. Nat. Rev. Mol. Cell Biol. 16, 345–359 [DOI] [PubMed] [Google Scholar]
- 29. Carelli V., Musumeci O., Caporali L., Zanna C., La Morgia C., Del Dotto V., Porcelli A. M., Rugolo M., Valentino M. L., Iommarini L., Maresca A., Barboni P., Carbonelli M., Trombetta C., Valente E. M., et al. (2015) Syndromic parkinsonism and dementia associated with OPA1 missense mutations. Ann. Neurol. 78, 21–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mourier A., Motori E., Brandt T., Lagouge M., Atanassov I., Galinier A., Rappl G., Brodesser S., Hultenby K., Dieterich C., and Larsson N. G. (2015) Mitofusin 2 is required to maintain mitochondrial coenzyme Q levels. J. Cell Biol. 208, 429–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hroudová J., Singh N., and Fisar Z. (2014) Mitochondrial dysfunctions in neurodegenerative diseases: relevance to Alzheimer's disease. BioMed Res. Int. 2014, 175062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang W., Li L., Lin W. L., Dickson D. W., Petrucelli L., Zhang T., and Wang X. (2013) The ALS disease-associated mutant TDP-43 impairs mitochondrial dynamics and function in motor neurons. Hum. Mol. Genet. 22, 4706–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pla-Martín D., Rueda C. B., Estela A., Sánchez-Piris M., González-Sánchez P., Traba J., de la Fuente S., Scorrano L., Renau-Piqueras J., Alvarez J., Satrústegui J., and Palau F. (2013) Silencing of the Charcot-Marie-Tooth disease-associated gene GDAP1 induces abnormal mitochondrial distribution and affects Ca2+ homeostasis by reducing store-operated Ca2+ entry. Neurobiol. Dis. 55, 140–151 [DOI] [PubMed] [Google Scholar]
- 34. Scorrano L., Ashiya M., Buttle K., Weiler S., Oakes S. A., Mannella C. A., and Korsmeyer S. J. (2002) A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev. Cell 2, 55–67 [DOI] [PubMed] [Google Scholar]
- 35. Purnell P. R., and Fox H. S. (2013) Autophagy-mediated turnover of dynamin-related protein 1. BMC Neurosci. 14, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cavallucci V., Bisicchia E., Cencioni M. T., Ferri A., Latini L., Nobili A., Biamonte F., Nazio F., Fanelli F., Moreno S., Molinari M., Viscomi M. T., and D'Amelio M. (2014) Acute focal brain damage alters mitochondrial dynamics and autophagy in axotomized neurons. Cell Death Dis. 5, e1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chan D. C. (2012) Fusion and fission: interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 46, 265–287 [DOI] [PubMed] [Google Scholar]
- 38. Frezza C., Cipolat S., Martins de Brito O., Micaroni M., Beznoussenko G. V., Rudka T., Bartoli D., Polishuck R. S., Danial N. N., De Strooper B., and Scorrano L. (2006) OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126, 177–189 [DOI] [PubMed] [Google Scholar]
- 39. Jagasia R., Grote P., Westermann B., and Conradt B. (2005) DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature 433, 754–760 [DOI] [PubMed] [Google Scholar]
- 40. Mao C., Zhang J., Lin S., Jing L., Xiang J., Wang M., Wang B., Xu P., Liu W., Song X., and Lv C. (2014) MiRNA-30a inhibits AECs-II apoptosis by blocking mitochondrial fission dependent on Drp-1. J. Cell. Mol. Med. 18, 2404–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alaimo A., Gorojod R. M., Beauquis J., Muñoz M. J., Saravia F., and Kotler M. L. (2014) Deregulation of mitochondria-shaping proteins Opa-1 and Drp-1 in manganese-induced apoptosis. PLoS One 9, e91848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cereghetti G. M., Costa V., and Scorrano L. (2010) Inhibition of Drp1-dependent mitochondrial fragmentation and apoptosis by a polypeptide antagonist of calcineurin. Cell Death Differ. 17, 1785–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cereghetti G. M., Stangherlin A., Martins de Brito O., Chang C. R., Blackstone C., Bernardi P., and Scorrano L. (2008) Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc. Natl. Acad. Sci. U.S.A. 105, 15803–15808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pi H., Xu S., Zhang L., Guo P., Li Y., Xie J., Tian L., He M., Lu Y., Li M., Zhang Y., Zhong M., Xiang Y., Deng L., Zhou Z., and Yu Z. (2013) Dynamin 1-like-dependent mitochondrial fission initiates overactive mitophagy in the hepatotoxicity of cadmium. Autophagy 9, 1780–1800 [DOI] [PubMed] [Google Scholar]
- 45. Burté F., Carelli V., Chinnery P. F., and Yu-Wai-Man P. (2015) Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat. Rev. Neurol. 11, 11–24 [DOI] [PubMed] [Google Scholar]
- 46. Reddy P. H. (2014) Inhibitors of mitochondrial fission as a therapeutic strategy for diseases with oxidative stress and mitochondrial dysfunction. J. Alzheimers Dis. 40, 245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carvalho C., Correia S. C., Cardoso S., Plácido A. I., Candeias E., Duarte A. I., and Moreira P. I. (2015) The role of mitochondrial disturbances in Alzheimer, Parkinson and Huntington diseases. Expert Rev. Neurother. 15, 867–884 [DOI] [PubMed] [Google Scholar]
- 48. Tiwari S. K., Agarwal S., Chauhan L. K., Mishra V. N., and Chaturvedi R. K. (2015) Bisphenol-A impairs myelination potential during development in the hippocampus of the rat brain. Mol. Neurobiol. 51, 1395–1416 [DOI] [PubMed] [Google Scholar]
- 49. Sheridan C., and Martin S. J. (2010) Mitochondrial fission/fusion dynamics and apoptosis. Mitochondrion 10, 640–648 [DOI] [PubMed] [Google Scholar]
- 50. Mizushima N. (2011) Autophagy in protein and organelle turnover. Cold Spring Harbor Symp. Quant. Biol. 76, 397–402 [DOI] [PubMed] [Google Scholar]
- 51. Yoshii S. R., and Mizushima N. (2015) Autophagy machinery in the context of mammalian mitophagy. Biochim. Biophys. Acta 1853, 2797–2801 [DOI] [PubMed] [Google Scholar]
- 52. Watanabe Y., and Tanaka M. (2011) p62/SQSTM1 in autophagic clearance of a non-ubiquitylated substrate. J. Cell Sci. 124, 2692–2701 [DOI] [PubMed] [Google Scholar]
- 53. Komatsu M., and Ichimura Y. (2010) Physiological significance of selective degradation of p62 by autophagy. FEBS Lett. 584, 1374–1378 [DOI] [PubMed] [Google Scholar]
- 54. Bjørkøy G., Lamark T., Brech A., Outzen H., Perander M., Overvatn A., Stenmark H., and Johansen T. (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 171, 603–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Qi X., Qvit N., Su Y. C., and Mochly-Rosen D. (2013) A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J. Cell Sci. 126, 789–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bonet-Ponce L., Saez-Atienzar S., da Casa C., Flores-Bellver M., Barcia J. M., Sancho-Pelluz J., Romero F. J., Jordan J., and Galindo M. F. (2015) On the mechanism underlying ethanol-induced mitochondrial dynamic disruption and autophagy response. Biochim. Biophys. Acta 1852, 1400–1409 [DOI] [PubMed] [Google Scholar]
- 57. Alaimo A., Gorojod R. M., Miglietta E. A., Villarreal A., Ramos A. J., and Kotler M. L. (2013) Manganese induces mitochondrial dynamics impairment and apoptotic cell death: a study in human Gli36 cells. Neurosci. Lett. 554, 76–81 [DOI] [PubMed] [Google Scholar]
- 58. Su Y. C., and Qi X. (2013) Inhibition of excessive mitochondrial fission reduced aberrant autophagy and neuronal damage caused by LRRK2 G2019S mutation. Hum. Mol. Genet. 22, 4545–4561 [DOI] [PubMed] [Google Scholar]
- 59. Arrázola M. S., Silva-Alvarez C., and Inestrosa N. C. (2015) How the Wnt signaling pathway protects from neurodegeneration: the mitochondrial scenario. Front. Cell. Neurosci. 9, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Godoy J. A., Arrázola M. S., Ordenes D., Silva-Alvarez C., Braidy N., and Inestrosa N. C. (2014) Wnt-5a ligand modulates mitochondrial fission-fusion in rat hippocampal neurons. J. Biol. Chem. 289, 36179–36193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cui Y., Han J., Xiao Z., Chen T., Wang B., Chen B., Liu S., Han S., Fang Y., Wei J., Wang X., Ma X., and Dai J. (2016) The miR-20-Rest-Wnt signaling axis regulates neural progenitor cell differentiation. Sci. Rep. 6, 23300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tiwari S. K., Agarwal S., Seth B., Yadav A., Nair S., Bhatnagar P., Karmakar M., Kumari M., Chauhan L. K., Patel D. K., Srivastava V., Singh D., Gupta S. K., Tripathi A., Chaturvedi R. K., et al. (2014) Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer's disease model via canonical Wnt/β-catenin pathway. ACS Nano 8, 76–103 [DOI] [PubMed] [Google Scholar]
- 63. Brewer G. J., and Torricelli J. R. (2007) Isolation and culture of adult neurons and neurospheres. Nat. Protoc. 2, 1490–1498 [DOI] [PubMed] [Google Scholar]
- 64. Mistry S. K., Keefer E. W., Cunningham B. A., Edelman G. M., and Crossin K. L. (2002) Cultured rat hippocampal neural progenitors generate spontaneously active neural networks. Proc. Natl. Acad. Sci. U.S.A. 99, 1621–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tiwari M. N., Agarwal S., Bhatnagar P., Singhal N. K., Tiwari S. K., Kumar P., Chauhan L. K., Patel D. K., Chaturvedi R. K., Singh M. P., and Gupta K. C. (2013) Nicotine-encapsulated poly(lactic-co-glycolic) acid nanoparticles improve neuroprotective efficacy against MPTP-induced parkinsonism. Free Rad. Biol. Med. 65, 704–718 [DOI] [PubMed] [Google Scholar]
- 66. Singhal N. K., Agarwal S., Bhatnagar P., Tiwari M. N., Tiwari S. K., Srivastava G., Kumar P., Brashket S., Patel D. K., Chaturvedi R. K., Singh M. P., and Gupta K. C. (2015) Mechanism of nanotization-mediated improvement in the efficacy of caffeine against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism. J. Biomed. Nanotechnol. 11, 2211–2222 [DOI] [PubMed] [Google Scholar]
- 67. Chaturvedi R. K., Hennessey T., Johri A., Tiwari S. K., Mishra D., Agarwal S., Kim Y. S., and Beal M. F. (2012) Transducer of regulated CREB-binding proteins (TORCs) transcription and function is impaired in Huntington's disease. Hum. Mol. Genet. 21, 3474–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mishra D., Tiwari S. K., Agarwal S., Sharma V. P., and Chaturvedi R. K. (2012) Prenatal carbofuran exposure inhibits hippocampal neurogenesis and causes learning and memory deficits in offspring. Toxicol. Sci. 127, 84–100 [DOI] [PubMed] [Google Scholar]
- 69. Pahuja R., Seth K., Shukla A., Shukla R. K., Bhatnagar P., Chauhan L. K., Saxena P. N., Arun J., Chaudhari B. P., Patel D. K., Singh S. P., Shukla R., Khanna V. K., Kumar P., Chaturvedi R. K., et al. (2015) Trans-blood brain barrier delivery of dopamine-loaded nanoparticles reverses functional deficits in parkinsonian rats. ACS Nano 9, 4850–4871 [DOI] [PubMed] [Google Scholar]
- 70. Brooks C., Wei Q., Cho S. G., and Dong Z. (2009) Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Invest. 119, 1275–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]