Abstract
Multisubunit protein assemblies offer integrated functionalities for efficient cell signal transduction control. One example of such protein assemblies, the BRCA1-A macromolecular complex, couples ubiquitin recognition and metabolism and promotes cellular responses to DNA damage. Specifically, the BRCA1-A complex not only recognizes Lys63-linked ubiquitin (K63-Ub) adducts at the damaged chromatin but is endowed with K63-Ub deubiquitylase (DUB) activity. To explore how the BRCA1-A DUB activity contributes to its function at DNA double strand breaks (DSBs), we used RNAi and genome editing approaches to target BRCC36, the protein subunit that confers the BRCA1-A complex its DUB activity. Intriguingly, we found that the K63-Ub DUB activity, although dispensable for maintaining the integrity of the macromolecular protein assembly, is important in enforcing the accumulation of the BRCA1-A complex onto DSBs. Inactivating BRCC36 DUB attenuated BRCA1-A functions at DSBs and led to unrestrained DSB end resection and hyperactive DNA repair. Together, our findings uncover a pivotal role of BRCC36 DUB in limiting DSB processing and repair and illustrate how cells may physically couple ubiquitin recognition and metabolizing activities for fine tuning of DNA repair processes.
Keywords: BRCA1, DNA damage, DNA damage response, DNA repair, ubiquitin, ubiquitin ligase
Introduction
Active hydrolysis of ubiquitin polymers by deubiquitylases (DUBs)2 has emerged as important biochemical processes that underlie diverse signal transduction pathways, including cell responses to DNA damage (1). To date, around 94 human DUBs have been identified and are classified into five families: ubiquitin C-terminal hydrolases, ubiquitin-specific proteases, ovarian tumor proteases, Josephins, and JAB1/MPN/MOV4 metalloproteases. Notably, the JAB1/MPN/MOV4 metalloprotease family members are zinc-dependent metalloproteases, and the other families are cysteine proteases (1).
BRCC36, originally reported as the BRCA1/BRCA2-containing complex subunit 36 (2), is endowed with DUB activity for lysine 63-linked ubiquitin polymers (K63-Ub). BRCC36 DUB relies on its zinc-dependent JAB1/MPN/MOV4 metalloprotease domain (3) and its interaction with Abraxas/KIAA0157 (3–5). Although BRCC36 forms distinct nuclear and cytoplasmic complexes (5, 6) and plays pleiotropic roles during cell proliferation, the K63-Ub DUB is arguably best known for its strong links with the breast and ovarian susceptible gene product BRCA1 in DNA damage responses (DDRs). Indeed, BRCC36 resides within the BRCA1-A macromolecular protein complex, which comprises the ubiquitin-binding protein RAP80, BRCC45/BRE, Abraxas/CCDC98, Merit40/NBA1, and BRCA1. The BRCA1-A complex is targeted to chromatin domains surrounding DNA double strand breaks (DSBs) by the ubiquitin-binding protein RAP80, which preferentially binds to K63-Ub adducts generated by the ubiquitin-conjugating enzyme UBC13 (3, 7–12). Abraxas plays a major scaffolding role to support the integrity of the BRCA1-A assembly and directly anchors the tumor suppressor BRCA1 via a phosphorylation-dependent interaction (13–15). Indeed, BRCA1, via its phosphopeptide-binding BRCT domain, directly engages Abraxas at pSPXF (where pS represents phosphoserine). A more recent study also revealed that an additional phosphorylation event in the vicinity of pSPXF (serine 404) further stabilizes the Abraxas-BRCA1 interaction by formation of a stable dimer (16). Other components of the BRCA1-A complex, including Merit40 and BRE, are also key in maintaining the integrity of the macromolecular protein complex (6, 9, 17, 18) and for BRCA1 accumulation at DSB sites. Together, these data underscore critically important roles of each of the subunits that make up the protein assembly.
BRCC36 has established roles in promoting BRCA1 function in the DDR. Depletion of BRCC36 compromised the G2/M cell cycle checkpoint, impaired BRCA1 recruitment onto DSBs, resulted in hyperactive homologous recombination (HR)-based gene conversion events and hypersensitized the cell to genotoxic stress (7–12). Consistent with the idea that dysregulated DNA repair promotes tumorigenesis, aberrant expression of BRCC36 has been associated with breast carcinomas (19) and nasopharyngeal carcinomas (20) and represents a promising prognostic marker and a potential target in the therapies for these cancer types. Although BRCC36 has emerged as an essential enzymatic activity in the mammalian DDR, the metabolism of K63-Ub chains and its contribution to DDRs remain obscure. In this study, we report our findings that support an instrumental role of the BRCC36 DUB activity in limiting DNA end resection and in fine tuning HR repair.
Results
BRCC36 DUB Activity Suppresses HR Repair
DSBs are predominantly repaired by one of two major DSB repair pathways: non-homologous end joining or HR. Intriguingly, emerging evidence highlights a key role of the tumor suppressor BRCA1 in maintaining the balance between HR- and non-homologous end joining-mediated DSB repair (21). This is accomplished, in part, by its ability to form distinct macromolecular protein assemblies (22). Indeed, whereas BRCA1 plays established roles in promoting HR repair, the BRCA1-A complex, composed of RAP80, BRCC36, BRCC45/BRE, Abraxas/CCDC98, and Merit40/NBA1, has recently been reported to counter HR repair by inhibiting DSB end resection (23, 24).
We are particularly interested in the BRCA1-A complex not only because it is a highly stable macromolecular complex but also because it is endowed with both ubiquitin binding and ubiquitin hydrolyzing activities specific for K63-Ub polymers. Although K63-Ub binding has been associated with targeting of the BRCA1-A complex onto DSBs (8, 11, 13), the functionality and requirement of the BRCC36-encoded K63-Ub DUB activity remains unexplored. This prompted us to experimentally test whether the BRCC36 enzymatic activity is required for the BRCA1-A complex function in DDRs. To this end, we employed the HR-based gene conversion assay to measure DSB repair activity (25). The reporter was integrated into U2OS cells (DR-U2OS), and HR repair can be monitored by assaying for GFP positivity upon introduction of the I-SceI endonuclease (Fig. 1A). We first established that BRCC36 suppresses DSB repair via HR. Consistent with previous reports (23, 24), siRNA-mediated depletion of BRCC36 led to increased rates of HR repair (Fig. 1B), whereas inactivation of the HR repair factor PALB2 led to a marked reduction in DSB repair events (26). To directly examine how the BRCC36 DUB contributes to DSB repair, we generated cells that stably express siRNA-resistant versions of BRCC36 WT or its DUB enzymatic mutant BRCC36 H122Q/H124Q (QSQ). The QSQ mutation abolishes the BRCC36 DUB activity (10). Notably, following the siRNA-mediated silencing of endogenous BRCC36, reintroduction of BRCC36 WT reversed the hyperactive HR repair phenotype, whereas BRCC36 QSQ did not (Fig. 1, B and C). These data suggest that BRCC36 restricts HR repair in a manner that requires its DUB activity.
FIGURE 1.
BRCC36 DUB activity negatively regulates HR-based repair. A, schematic diagram showing the direct repeated GFP assay to measure HR-mediated DSB repair. Expression of the I-SceI endonuclease leads to a gene conversion event involving the two GFP fragments and restoration of functional GFP proteins. B, BRCC36 limits HR repair. BRCC36-depleted direct repeated GFP cells that stably express either siRNA-resistant HF-BRCC36-WT or its enzymatic inactive mutant H122Q/H124Q (QSQ) were electroporated with an I-SceI expression construct. Cells were incubated for 48 h, and GFP-positive cells arising from gene conversion events were quantified by BD Canto II analyzer. Knockdown of the HR factor PALB2 was used as a control in this assay. Data are representative of three independent experiments (right). *, p < 0.05 versus control (HF). Representative flow cytometric profiles showing percentages of GFP-positive direct repeated GFP cells are shown. C, immunoblotting was performed to assess knockdown efficiency of BRCC36 by use of siRNA and the expression of HF-BRCC36 WT and QSQ in stable cells. Error bars, S.D.
BRCC36 DUB Enforces Its Accumulation at DSBs
To complement the siRNA-mediated knockdown studies, we generated BRCC36 KO cells by use of the CRISPR/Cas9 genome editing approach (Fig. 2A). We selected BRCC36 KO clones by analyzing BRCC36 expression by Western blotting and indirect immunofluorescence experiments (Fig. 2, B and C). Following the isolation of BRCC36 KO cells, we reconstituted these cells with either BRCC36 WT or its DUB mutant (QSQ; Fig. 2D). Notably, in sharp contrast to those observed previously in RNAi studies (see Fig. 2E), expression of the various BRCA1-A subunits, including RAP80, Merit40, and BRE, did not noticeably differ in BRCC36 KO or reconstituted cells (Fig. 2F). Given a documented role of BRCC36 in maintaining the integrity of the BRCA1-A complex, we also performed co-immunoprecipitation experiments to compare the interaction of WT BRCC36 and its QSQ mutant with various subunits of the protein complex. Importantly, assembly of the core complex did not depend on the BRCC36 DUB, indicating that the K63-Ub deubiquitylating activity is dispensable for the stable interactions of the subunits (Fig. 2G). Intriguingly, although expression of ectopic BRCC36 did not differ in the reconstituted cells (WT versus QSQ; Fig. 2F), and there was minimal difference in cell proliferative capacity as indicated by BrdU incorporation (Fig. 2H), ionizing radiation (IR)-induced focus formation of BRCC36 QSQ was reproducibly weaker than that of its WT counterpart (Fig. 2D). Although this was unexpected, we reasoned that BRCC36 DUB may be important in promoting the stable accumulation of the BRCA1-A complex at DSBs and hence may explain why cells with inactivated BRCC36 DUB were hyperactive in HR repair (Fig. 1B). To corroborate the observation made from indirect immunofluorescence studies, we fused BRCC36 with GFP to more quantitatively analyze its accumulation in live cells. Consistently, laser microirradiation triggered robust accumulation of BRCC36 onto DNA damage tracks (Fig. 2I), and this was partially attenuated in cells expressing the BRCC36 DUB mutant. We concluded that K63-Ub DUB activity plays an accessory role to facilitate the stable accumulation of BRCC36 at DSBs.
FIGURE 2.
Characterization of BRCC36 knock-out cells. A, BRCC36 KO cells were generated using the CRISPR/CAS9 genome editing approach. Two independent guide RNAs (gRNA) were designed to target BRCC36 on chromosome X. B and C, analysis of BRCC36 KO cells. Western blotting experiments were performed to screen for BRCC36 KO clones (B), and selected clones were further verified by indirect immunofluorescence studies and Western blotting experiments (C). BRCC36−/− cells or their SFB-BRCC36 or SFB-QSQ reconstituted counterparts were examined by cell labeling using anti-FLAG antibodies (D), by Western blotting experiments (F), and by a BrdU incorporation assay (H). Western blotting experiments were also performed to examine expression of the BRCA1-A complex subunits following RNAi-mediated depletion of BRCC36 (E). G, BRCC36 DUB is dispensable for its interaction with components of the BRCA1-A complex. Co-immunoprecipitation experiments were performed using cell lysates derived from BRCC36 null cells (clone 20) or their reconstituted (wild type or QSQ) counterparts. Lysates from cells expressing SFB-BRCC36 or QSQ were incubated with streptavidin-agarose beads. Expression and the presence of co-purified proteins in the BRCC36 WT complex or BRCC36 QSQ complex were analyzed by Western blotting using the indicated antibodies (RAP80, Abraxas, Merit40, BRE, and FLAG). I, accumulation of BRCC36 and its QSQ mutant at laser microirradiation-induced damage tracks in U2OS cells. U2OS cells were transiently transfected with expression constructs encoding GFP-fused BRCC36 or its QSQ mutant. Cells were irradiated by a 405-nm laser. Images were taken before and after treatment at the indicated time. Intensity of BRCC36 accumulation was analyzed by use of the ImageJ software and was plotted (right), n > 10. Co-immunoprecipitation and Western blotting experiments were also performed to evaluate the integration of GFP-BRCC36 (and QSQ) into the BRCA-A complex (right). U2OS cells transfected with GFP-BRCC36 (or QSQ) expression constructs were processed as in G. Error bars, S.D.
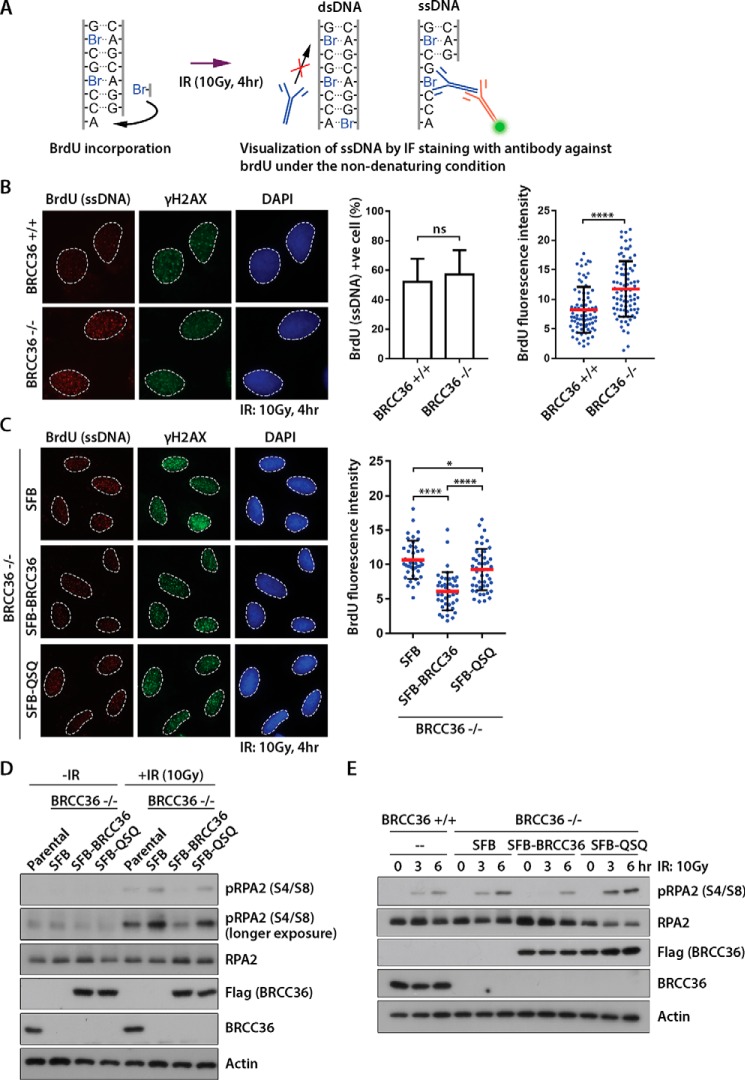
BRCC36 DUB Activity Limits DNA End Resection
DNA end resection generates 3′ single-stranded DNA (ssDNA) overhangs and is an early and decisive process for committing DSB repair via the HR pathway (27–29). Given the requirement of BRCC36 DUB in restraining HR repair (Fig. 1) and that the BRCA1-A complex suppresses HR by modulating DNA end resection (23, 24, 30), we hypothesized that BRCC36 DUB may be important in blocking end resection events. To explore whether BRCC36 DUB modulates these early steps in HR repair, we examined the requirement of BRCC36 DUB in DSB end resection following cell irradiation. We performed a non-denaturing BrdU staining assay in BRCC36 KO and reconstituted cells to assess DNA end resection (Fig. 3A). Because epitopes of the thymidine analogues are buried when they are in a dsDNA configuration, we can specifically and directly analyze ssDNAs. Accordingly, cells were preincubated with culture medium supplemented with BrdU. Following an ionizing radiation treatment, cells were processed for anti-BrdU labeling under native conditions. In line with the requirement of BRCC36 in blocking DSB end resection (23, 24), BRCC36 null cells displayed BrdU foci of higher intensities, which colocalize with the DNA damage marker γH2AX (Fig. 3B). We then directly examined whether the BRCC36 DUB is also required for blocking DSB end resection in BRCC36-reconstituted cells. In support of a role of K63-Ub deubiquitylating activity in the early steps of HR, cells expressing BRCC36 QSQ fueled much more robust IR-induced ssDNA foci, which reflected higher rates of DSB end resection (Fig. 3C). Previous work has established that ssDNA overhangs at DSBs are coated by RPA and that phosphorylation of RPA closely correlates with activation of the ATR-CHK1 pathway (31). Therefore, to complement the findings from the above immunofluorescence experiments, we biochemically analyzed RPA2 phosphorylation in BRCC36 KO cells as well as in their reconstituted counterparts. Consistently, BRCC36-deficient cells mounted a hyperactive response, as judged by increased levels of phosphorylated RPA2 when compared with wild type parental cells (Fig. 3, D and E). RPA phosphorylation following cell irradiation increased in a time-dependent manner, which correlates with DSB end processing (Fig. 3E). Importantly, reintroduction of wild type BRCC36, but not its QSQ mutant, suppressed RPA2 phosphorylation to levels observed in isogenic BRCC36-proficient cells. Taken together, these data implied that BRCC36 negatively regulates DNA end resection in ways that require its DUB activity.
FIGURE 3.
BRCC36 DUB limits DNA end resection. A, schematic illustration describing the non-denaturing BrdU staining assay to detect ssDNA. Note that BrdU (thymidine analogues) are buried from anti-BrdU antibodies upon incorporation into dsDNA. B, BRCC36 null cells (clone 20) exhibited increased BrdU-labeled ssDNAs following cell recovery from ionizing radiation treatment. The percentage of BrdU-positive cells as well as relative intensity of BrdU fluorescence were plotted. ns, non-significant; ****, p < 0.0001. C, reconstitution of BRCC36 limited ssDNA foci at DSBs. BRCC36 null cells (clone 20) or their reconstituted counterparts were labeled with the indicated antibodies as in B. *, p < 0.05; ****, p < 0.0001. D and E, BRCC36 limits RPA2 phosphorylation (serine 4 and serine 8; S4/S8) in a DUB-dependent manner. Cell lysates derived from BRCC36 null cells (clone 20) or their reconstituted counterparts (wild type or its QSQ mutant) were processed for Western blotting experiments using the indicated antibodies. Error bars, S.D.
BRCC36 Promotes Ionizing Radiation-induced Focus (IRIF) Formation of RAP80 Complex via Its DUB Activity
In response to IR, a plethora of DDR proteins are recruited to DSBs to form focal structures that are commonly referred to as IRIFs. γH2AX and 53BP1 are established DSB markers and concentrate at chromatin domains surrounding DSBs. Multiple groups have reported that the components of the RAP80 complex (including RAP80, BRCC36, Abraxas, Merit40, and BRE), the scaffolding core complex that anchors BRCA1 onto DSB domains, play interdependent roles in their recruitment to DSBs. Indeed, RNAi-mediated depletion of each of the individual components of the RAP80 complex compromised the integrity of the complex and resulted in defective IRIF formation. Given that BRCC36 is essential for the IRIF formation of the BRCA1-A complex (9, 12, 32), we examined whether BRCC36 DUB activity may be important in the RAP80-BRCA1 pathway. We first analyzed IRIF formation of three key components of the BRCA1-A complex, namely RAP80, Abraxas, and BRCA1, in cells depleted of BRCC36. Consistently, we found that the numbers of IRIFs of these components were significantly reduced in BRCC36 siRNA-treated cells (Fig. 4, A and D–F). To directly test whether the BRCC36 DUB activity is important in supporting the stable accumulation of RAP80, Abraxas, and BRCA1 at DSB-flanking chromatin domains, we monitored their focus formation in BRCC36-depleted cells that stably express siRNA-resistant versions of either wild type BRCC36 or its DUB-inactive QSQ mutant (Fig. 4, B–F). Notably, formation of IRIFs in BRCC36-depleted cells was restored by reintroducing wild type BRCC36, which excluded off-target effects from the use of siRNA. Notably, the observation that BRCC36 QSQ did not restore IRIF formation of the BRCA1-A complex is clearly indicative of a role of BRCC36 DUB in enforcing the stable accumulation of the complex at DSBs (Fig. 4, D–F).
FIGURE 4.
BRCC36 DUB is required for IRIF formation of the BRCA1-A complex. A–C, U2OS cells or derivatives that stably express SFB-BRCC36 or its QSQ were labeled with anti-FLAG antibodies or processed for Western blotting experiments following siRNA-mediated depletion of BRCC36; D–F, BRCC36 promotes IRIF formation of RAP80 (D), Abraxas (E), and BRCA1 (F) in a DUB-dependent manner. BRCC36-proficient or -depleted cells and their reconstituted counterparts were processed for indirect immunofluorescence experiments using the indicated antibodies. γH2AX was used as a DSB marker. The percentage of IRIF-positive cells was plotted. Data are representative of three independent experiments; *, p < 0.05. Error bars, S.D.
We further our analyses in isogenic wild type and BRCC36 KO cells (Fig. 5A) and in BRCC36-reconstituted counterparts (Fig. 5B). Similar to results generated from RNAi studies, we found that BRCC36 deficiency resulted in marked reduction of IR-induced focal accumulation of RAP80, Abraxas, and BRCA1 at γH2AX-marked DSBs (Fig. 5, C–F). When comparing BRCC36-reconstituted cells (wild type and QSQ) to directly assess the importance of BRCC36 DUB in the RAP80-BRCA1 pathway, we found, interestingly, that both wild type BRCC36 and its QSQ mutant, to a large extent, restored IRIFs of RAP80, Abraxas, and BRCA1. Most notable, however, was our observation that RAP80 and Abraxas foci were reproducibly weaker in QSQ-expressing cells (Fig. 5, C–F). Similar deficits were seen in independent BRCC36 KO clones that stably express either wild type BRCC36 or its QSQ, which argues against clonal effects (Fig. 5F). Perhaps because BRCA1 can be recruited to DSBs via multiple pathways (22, 33), we did not detect a clear difference in BRCA1 IRIFs. Anyhow, the fact that the sizes of each of the RAP80 and Abraxas IRIFs were reduced mirrored our early observation where QSQ does not fully support the stable accumulation of BRCC36 at DSBs (Fig. 2I) and highlights the idea that not only is BRCC36 important as a scaffolding component of the RAP80 complex in the DDRs, but its enzymatic activity as a DUB also contributes to the functionality of the BRCA1-A complex.
FIGURE 5.
BRCC36 DUB is required for RAP80 complex IRIF formation. A and B, BRCC36-proficient or -null cells and their reconstituted counterparts were processed for indirect immunofluorescence experiments; C and D, BRCC36 promotes IRIF formation of RAP80 (C) and Abraxas (D) in a DUB-dependent manner. BRCC36 null cells or their derivatives stably expressing SFB-BRCC36 or its QSQ mutant were treated with IR and processed for cell labeling using the indicated antibodies. Nuclei were counterstained using DAPI. γH2AX was used as a DSB marker. E, BRCA1 IRIF did not noticeably differ in BRCC36 null cells reconstituted with wild type BRCC36 or its DUB mutant. Cells were processed as above. The number of IRIF-positive cells was counted, and relative fluorescence intensity of IRIFs was measured by the Metamorph software and quantified (F). Data are representative of three independent experiments and are of two independent BRCC36 KO clones that have been reconstituted with either wild type BRCC36 or its QSQ mutant. *, p < 0.05; ****, p < 0.0001. Error bars, S.D.
Analysis of K63-Ub Foci in BRCC36-deficient Cells
Our observation that BRCC36, via its DUB, facilitates the stable accumulation of the BRCA1-A complex at DSBs prompted us to examine in more detail the contribution of BRCC36 DUB activity at DSBs. Consistent with documented roles of BRCC36 in hydrolyzing Lys63-linked ubiquitin polymers, a substantial increase in K63-Ub foci was detected in independent BRCC36 KO cells (Fig. 6A). In addition, reconstituting BRCC36 KO cells with the DUB-inactive QSQ, unlike wild type BRCC36, did not suppress the IR-induced K63-Ub foci. These data consolidate a role of BRCC36 as K63-Ub-hydrolyzing activity at the DSB-flanking chromatin domains and suggest that the ability to trim K63-Ub polymers may underlie a positive feedback mechanism that drives the stable accumulation of the ubiquitin-binding RAP80 at DSBs (Fig. 6B).
FIGURE 6.
Key role of BRCC36 DUB in regulating DSB-associated K63-Ub. A, BRCC36 promotes turnover of DSB-associated K63-Ub. BRCC36-proficient and -deficient cells and their reconstituted counterparts (SFB-BRCC36 or SFB-QSQ) were irradiated and processed for indirect immunofluorescence experiments using anti-K63-Ub and anti-γH2AX antibodies. Nuclei were counterstained with DAPI. Percentages of cells positive for K63-Ub IRIF were plotted (right). *, p < 0.05 versus BRCC36+/+ cells. B, working model depicting how BRCC36 DUB, via regulating K63-Ub homeostasis in the DSB microenvironment, enforces the stable accumulation of the BRCA1-A complex at the damaged chromatin. RAP80 interacts with certain K63-Ub adducts at DSBs. BRCC36 may promote recycling of ubiquitin at DSBs to facilitate forming of K63-Ub adducts that target RAP80. Alternatively, unrestrained K63-Ub adduct formation may physically impede anchoring of RAP80. Error bars, S.D.
Discussion
BRCC36 encodes a K63-Ub DUB and participates in cell responses to DSBs by promoting the functionality of the BRCA1-A complex. Although the regulatory basis of its DUB activity is beginning to be understood (3–6, 34), it has remained unknown whether the BRCC36 ubiquitin hydrolyzing activity plays a role in mammalian DNA damage responses. In this study, we have systematically examined, by adopting both RNAi and genome editing approaches, whether BRCC36 and its DUB may be functionally important in driving cellular response to DSBs. We showed that BRCC36, in a DUB-dependent manner, promotes stable accumulation of the BRCA1-A complex at DSBs and modulates DSB repair by limiting DNA end resection, one of the early steps of an HR reaction. Taken together, our study establishes a role of the BRCC36-encoded K63-Ub deubiquitylating activity in DSB signal transduction and repair processes.
Our observations that BRCC36, via its DUB, facilitates IRIF formation of the BRCA1-A complex suggest that ubiquitin processing at DSBs plays a role in the stable accumulation of the protein complex (35). Given that RAP80 binds to Lys63-linked ubiquitin adducts and plays a direct role in anchoring the BRCA1-A complex at DSBs (8, 11, 13), it may first seem counterintuitive that inactivating the BRCC36 DUB, which led to marked increase in K63-Ub foci at DSBs (Fig. 6A), impaired IRIF formation of RAP80 (Figs. 4D and 5C). Notably, the apparent disconnect may be reconciled if K63-Ub foci represent a collection of ubiquitin-modified DSB-occupying factors and if RAP80 is targeted to DSBs via only a subset of these ubiquitin adducts. Addressing this possibility will require further work to identify the ubiquitylated factor(s) that interacts with the RAP80 tandem ubiquitin-interacting motifs at DSBs.
By interacting with certain K63-Ub adducts, RAP80 is envisaged to anchor the BRCA1-A complex in the vicinity of DSBs (36). Intriguingly, we found that genetic inactivation of BRCC36 attenuated RAP80 IRIF, suggesting that the DUB and its ubiquitin hydrolyzing activity may play positive roles in amplifying DNA damage signals that emanate from the damaged chromatin. This idea is not unprecedented because a number of positive feedback mechanisms have previously been shown to drive the amplification of DSB signals (37, 38). Our data thus suggest that BRCC36, in both DUB-dependent and -independent ways, may also play a similar role to facilitate DSB signal propagation via the RAP80-BRCA1 axis. Although it remains unclear exactly what BRCC36 acts on to facilitate the productive accumulation of the BRCA1-A complex at DSBs, the K63-Ub DUB has been reported to regulate the ubiquitylation status of a panel of DDR factors, including γH2AX (10), RAP80 (34), and FANCG (39), raising the possibility that BRCC36 may perform its stabilizing function of the BRCA1-A complex by multiple means. Future studies will be required to further delineate how BRCC36 enforces the BRCA1-A complex at DSB-flanking domains via its DUB activity.
Excessive DNA end resection fuels unrestrained HR repair. In this regard, although the RAP80-BRCA1 axis has been proposed to play a critical role in genome maintenance by limiting DNA end resection and HR-based DSB repair (23, 24), exactly how BRCC36, as a subunit of the BRCA1-A complex, contributes to these functions has remained elusive. Although it remains enigmatic why the BRCC36-encoded DUB activity seems to be more important in the stable accumulation of the BRCA1-A complex at DSBs in cells transiently depleted of BRCC36 (Fig. 4) as opposed to BRCC36 null cells (Fig. 5), given the diverse roles of BRCC36, we speculate that siRNA-mediated depletion of BRCC36 may perturb the homeostatic balance of the ubiquitin pool and may, directly and indirectly, lead to more severe deficits in the ubiquitin-mediated DSB signal transduction cascade. Regardless, our results clearly support the idea that BRCC36 DUB activity represents a critical component that underlies the cell intrinsic ability to restrain HR repair. Our findings strongly suggest that BRCC36 antagonizes HR repair, at least in part, by its ability to promote the stable accumulation of the BRCA1-A complex at DSBs, which in turn may physically impede the DSB end resection machinery. In summary, we have characterized and have uncovered an important role of the BRCC36 DUB in DSB signal transduction and repair. We propose that functional coupling of ubiquitin recognition and processing may underlie the functionality of the BRCA1-A complex in mammalian DDRs.
Materials and Methods
Cell Culture and Transfection
293T and U2OS cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS at 37 °C in 5% CO2. Transient transfection was performed with polyethyleneimine.
Generation of BRCC36 Stable Cells and Knock-out Cells
To generate cells that stably express BRCC36 (wild type or mutant), BOSC23 packaging cells were co-transfected with pEF1A-HA-FLAG (HF)-BRCC36 or pCMV-streptavidin binding peptide-FLAG (SFB)-BRCC36 expression constructs with the retrovirus packaging vector pCL-Ampho. 48 h post-transfection, supernatant containing viral particles was harvested and applied to U2OS cells in the presence of Polybrene (8 μg/ml). Positive clones were selected in DMEM supplemented with puromycin (1 μg/ml). BRCC36 knock-out cells were generated by the CRISPR-CAS9 approach using guide RNAs that target either human chromosome X (155071638–155071656) or human chromosome X (155072321–155072338), respectively. BRCC36 knock-out clones were verified by immunoblotting and immunofluorescence staining experiments using anti-BRCC36 antibodies.
Plasmids and siRNAs
HA-FLAG-BRCC36 or SFB-BRCC36 expression constructs were generated using Gateway technology. Plasmids encoding siRNA-resistant BRCC36 or its mutant were gifts from Roger Greenberg (University of Pennsylvania). For RNAi-mediated depletion experiments, cells were transfected with either non-targeting control or target siRNAs (Dharmacon) using Oligofectamine according to the manufacturer's instructions (Invitrogen). Sequences for siRNAs targeting BRCC36 and PALB2 are as follows: siBRCC36, 5′-GAGGAAGGACCGAGUAGAAdTdT-3′; siPALB2, 5′-GCAUAAACAUUCCGUCGAAdTdT-3′.
Antibodies
BRCC36, Abraxas, and RAP80 polyclonal antibodies were raised by immunizing rabbits with purified GST-BRCC36, GST-Abraxas, and GST-RAP80 proteins, respectively. FLAG (M2) and actin monoclonal antibodies were purchased from Sigma. Myc (9E10) and BRCA1 (D9) monoclonal antibodies were from Santa Cruz Biotechnology. RAP80, phospho-RPA2, and RPA2 antibodies were from Bethyl Laboratories. Mouse and rabbit anti-γH2AX antibodies were described previously (40). Anti-BrdU (clone B44) antibodies were from BD Biosciences. Anti-K63-Ub (Apu3) antibodies were from Millipore. Merit40 and BRE antibodies were gifts from Junjie Chen (MD Anderson Cancer Center).
HR DNA Repair Assay
U2OS cells stably expressing direct repeated GFP and BRCC36 (wild type or mutant) were transfected with either control or BRCC36-targeting siRNAs twice at 24-h intervals. 24 h after siRNA transfection, cells were electroporated with 5 μg of pCBASceI plasmid using a Bio-Rad Gene Pulser Xcell at 250 V and 950 microfarads. GFP-positive cells were analyzed using a BD LSRFortessa flow cytometer. Data quantification was performed using FlowJo software.
Immunofluorescence Staining
U2OS cells grown on coverslips were exposed to IR (10 grays). After 4- or 6-h recovery, cells were pre-extracted with 0.5% Triton X-100 solution followed by fixation with 3% paraformaldehyde at room temperature for 15 min. Cells were blocked with 5% FBS in PBS and were incubated with the indicated primary and secondary antibodies for 30 min, respectively. Images were acquired using an Olympus BX51 fluorescence microscope or Carl Zeiss confocal 710 microscope. For analysis of DNA end resection, non-denaturing BrdU labeling was performed. U2OS cells were preincubated with 10 μm BrdU for 24 h and then irradiated (10 grays). After a 4-h recovery, cells were fixed under non-denaturing conditions for indirect immunofluorescence experiments.
Co-immunoprecipitation Experiments
Cells were harvested and lysed with NETN buffer (20 mm Tris-HCl (pH 8.0), 100 mm NaCl, 1 mm EDTA, 0.5% Nonidet P-40) on ice. The cell lysates were centrifuged at 14,000 rpm for 10 min, and the supernatant was collected and incubated with streptavidin-conjugated beads for 4 h with agitation. The precipitated proteins in BRCC36 WT complex or in BRCC36 QSQ complex were loaded for SDS-PAGE and immunoblotting.
Author Contributions
H. M. N. and M. S. Y. H. designed the study and wrote the paper. H. M. N. performed most experiments, with L. W. and L. L. contributing to the laser microirradiation experiment. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank Dr. Penny Jeggo and all members of the Huen Laboratory for discussion. We are also grateful to Drs. Lin Feng and Junjie Chen for providing anti-BRE and anti-Merit40 antibodies, to Dr. Maria Jasin for providing the direct repeated GFP reporter, and to Dr. Roger Greenberg for providing the BRCC36 expression constructs. We also acknowledge Dr. Jingsong Yuan for sharing expertise on use of the CRISPR/CAS9 system and acknowledge the Faculty Core Facility at the University of Hong Kong for flow cytometric and confocal imaging analyses.
This work is supported by funds from Research Grants Council Hong Kong Project 17104215 and the Outstanding Young Researcher Award from the University of Hong Kong (to M. S. Y. H.). The authors declare that they have no conflicts of interest with the contents of this article.
- DUB
- deubiquitylase
- IRIF
- ionizing radiation-induced focus
- K63-Ub
- Lys63-linked ubiquitin(s)
- DDR
- DNA damage response
- DSB
- DNA double strand break
- HR
- homologous recombination
- QSQ
- mutant H122Q/H124Q
- IR
- ionizing radiation
- IRIF
- ionizing radiation-induced focus
- ssDNA
- single-stranded DNA
- HF
- HA-FLAG
- SFB
- streptavidin binding peptide-FLAG.
References
- 1. Komander D., Clague M. J., and Urbé S. (2009) Breaking the chains: structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol. 10, 550–563 [DOI] [PubMed] [Google Scholar]
- 2. Dong Y., Hakimi M. A., Chen X., Kumaraswamy E., Cooch N. S., Godwin A. K., and Shiekhattar R. (2003) Regulation of BRCC, a holoenzyme complex containing BRCA1 and BRCA2, by a signalosome-like subunit and its role in DNA repair. Mol. Cell 12, 1087–1099 [DOI] [PubMed] [Google Scholar]
- 3. Cooper E. M., Cutcliffe C., Kristiansen T. Z., Pandey A., Pickart C. M., and Cohen R. E. (2009) K63-specific deubiquitination by two JAMM/MPN+ complexes: BRISC-associated Brcc36 and proteasomal Poh1. EMBO J. 28, 621–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zeqiraj E., Tian L., Piggott C. A., Pillon M. C., Duffy N. M., Ceccarelli D. F., Keszei A. F., Lorenzen K., Kurinov I., Orlicky S., Gish G. D., Heck A. J., Guarné A., Greenberg R. A., and Sicheri F. (2015) Higher-order assembly of BRCC36-KIAA0157 is required for DUB activity and biological function. Mol. Cell 59, 970–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feng L., Wang J., and Chen J. (2010) The Lys63-specific deubiquitinating enzyme BRCC36 is regulated by two scaffold proteins localizing in different subcellular compartments. J. Biol. Chem. 285, 30982–30988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu X., Kim J. A., Castillo A., Huang M., Liu J., and Wang B. (2011) NBA1/MERIT40 and BRE interaction is required for the integrity of two distinct deubiquitinating enzyme BRCC36-containing complexes. J. Biol. Chem. 286, 11734–11745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang B., and Elledge S. J. (2007) Ubc13/Rnf8 ubiquitin ligases control foci formation of the Rap80/Abraxas/Brca1/Brcc36 complex in response to DNA damage. Proc. Natl. Acad. Sci. U.S.A. 104, 20759–20763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sobhian B., Shao G., Lilli D. R., Culhane A. C., Moreau L. A., Xia B., Livingston D. M., and Greenberg R. A. (2007) RAP80 targets BRCA1 to specific ubiquitin structures at DNA damage sites. Science 316, 1198–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shao G., Patterson-Fortin J., Messick T. E., Feng D., Shanbhag N., Wang Y., and Greenberg R. A. (2009) MERIT40 controls BRCA1-Rap80 complex integrity and recruitment to DNA double-strand breaks. Genes Dev. 23, 740–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shao G., Lilli D. R., Patterson-Fortin J., Coleman K. A., Morrissey D. E., and Greenberg R. A. (2009) The Rap80-BRCC36 de-ubiquitinating enzyme complex antagonizes RNF8-Ubc13-dependent ubiquitination events at DNA double strand breaks. Proc. Natl. Acad. Sci. U.S.A. 106, 3166–3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim H., Chen J., and Yu X. (2007) Ubiquitin-binding protein RAP80 mediates BRCA1-dependent DNA damage response. Science 316, 1202–1205 [DOI] [PubMed] [Google Scholar]
- 12. Feng L., Huang J., and Chen J. (2009) MERIT40 facilitates BRCA1 localization and DNA damage repair. Genes Dev. 23, 719–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang B., Matsuoka S., Ballif B. A., Zhang D., Smogorzewska A., Gygi S. P., and Elledge S. J. (2007) Abraxas and RAP80 form a BRCA1 protein complex required for the DNA damage response. Science 316, 1194–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim H., Huang J., and Chen J. (2007) CCDC98 is a BRCA1-BRCT domain-binding protein involved in the DNA damage response. Nat. Struct. Mol. Biol. 14, 710–715 [DOI] [PubMed] [Google Scholar]
- 15. Liu Z., Wu J., and Yu X. (2007) CCDC98 targets BRCA1 to DNA damage sites. Nat. Struct. Mol. Biol. 14, 716–720 [DOI] [PubMed] [Google Scholar]
- 16. Wu Q., Paul A., Su D., Mehmood S., Foo T. K., Ochi T., Bunting E. L., Xia B., Robinson C. V., Wang B., and Blundell T. L. (2016) Structure of BRCA1-BRCT/Abraxas complex reveals phosphorylation-dependent BRCT dimerization at DNA damage sites. Mol. Cell 61, 434–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vikrant, Sawant U. U., and Varma A. K. (2014) Role of MERIT40 in stabilization of BRCA1 complex: a protein-protein interaction study. Biochem. Biophys. Res. Commun. 446, 1139–1144 [DOI] [PubMed] [Google Scholar]
- 18. Vikrant, Nakhwa P., Badgujar D. C., Kumar R., Rathore K. K., and Varma A. K. (2014) Structural and functional characterization of the MERIT40 to understand its role in DNA repair. J. Biomol. Struct. Dyn. 32, 2017–2032 [DOI] [PubMed] [Google Scholar]
- 19. Chen X., Arciero C. A., Wang C., Broccoli D., and Godwin A. K. (2006) BRCC36 is essential for ionizing radiation-induced BRCA1 phosphorylation and nuclear foci formation. Cancer Res. 66, 5039–5046 [DOI] [PubMed] [Google Scholar]
- 20. Tu Z., Xu B., Qu C., Tao Y., Chen C., Hua W., Feng G., Chang H., Liu Z., Li G., Jiang C., Yi W., Zeng M., and Xia Y. (2015) BRCC3 acts as a prognostic marker in nasopharyngeal carcinoma patients treated with radiotherapy and mediates radiation resistance in vitro. Radiat. Oncol. 10, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daley J. M., and Sung P. (2014) 53BP1, BRCA1, and the choice between recombination and end joining at DNA double-strand breaks. Mol. Cell. Biol. 34, 1380–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huen M. S., Sy S. M., and Chen J. (2010) BRCA1 and its toolbox for the maintenance of genome integrity. Nat. Rev. Mol. Cell Biol. 11, 138–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coleman K. A., and Greenberg R. A. (2011) The BRCA1-RAP80 complex regulates DNA repair mechanism utilization by restricting end resection. J. Biol. Chem. 286, 13669–13680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu Y., Scully R., Sobhian B., Xie A., Shestakova E., and Livingston D. M. (2011) RAP80-directed tuning of BRCA1 homologous recombination function at ionizing radiation-induced nuclear foci. Genes Dev. 25, 685–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pierce A. J., Johnson R. D., Thompson L. H., and Jasin M. (1999) XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 13, 2633–2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo Y., Feng W., Sy S. M., and Huen M. S. (2015) ATM-dependent Phosphorylation of the Fanconi anemia protein PALB2 promotes the DNA damage response. J. Biol. Chem. 290, 27545–27556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Daley J. M., Niu H., Miller A. S., and Sung P. (2015) Biochemical mechanism of DSB end resection and its regulation. DNA Repair 32, 66–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Symington L. S., and Gautier J. (2011) Double-strand break end resection and repair pathway choice. Annu. Rev. Genet. 45, 247–271 [DOI] [PubMed] [Google Scholar]
- 29. Huertas P. (2010) DNA resection in eukaryotes: deciding how to fix the break. Nat. Struct. Mol. Biol. 17, 11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Castillo A., Paul A., Sun B., Huang T. H., Wang Y., Yazinski S. A., Tyler J., Li L., You M. J., Zou L., Yao J., and Wang B. (2014) The BRCA1-interacting protein Abraxas is required for genomic stability and tumor suppression. Cell Rep. 8, 807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maréchal A., and Zou L. (2015) RPA-coated single-stranded DNA as a platform for post-translational modifications in the DNA damage response. Cell Res. 25, 9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang B., Hurov K., Hofmann K., and Elledge S. J. (2009) NBA1, a new player in the Brca1 A complex, is required for DNA damage resistance and checkpoint control. Genes Dev. 23, 729–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiang Q., and Greenberg R. A. (2015) Deciphering the BRCA1 tumor suppressor network. J. Biol. Chem. 290, 17724–17732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patterson-Fortin J., Shao G., Bretscher H., Messick T. E., and Greenberg R. A. (2010) Differential regulation of JAMM domain deubiquitinating enzyme activity within the RAP80 complex. J. Biol. Chem. 285, 30971–30981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huen M. S., and Chen J. (2010) Assembly of checkpoint and repair machineries at DNA damage sites. Trends Biochem. Sci. 35, 101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aressy B., and Greenberg R. A. (2012) DNA damage: placing BRCA1 in the proper context. Curr. Biol. 22, R806–R808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lou Z., Minter-Dykhouse K., Franco S., Gostissa M., Rivera M. A., Celeste A., Manis J. P., van Deursen J., Nussenzweig A., Paull T. T., Alt F. W., and Chen J. (2006) MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol. Cell 21, 187–200 [DOI] [PubMed] [Google Scholar]
- 38. Panier S., Ichijima Y., Fradet-Turcotte A., Leung C. C., Kaustov L., Arrowsmith C. H., and Durocher D. (2012) Tandem protein interaction modules organize the ubiquitin-dependent response to DNA double-strand breaks. Mol. Cell 47, 383–395 [DOI] [PubMed] [Google Scholar]
- 39. Zhu B., Yan K., Li L., Lin M., Zhang S., He Q., Zheng D., Yang H., and Shao G. (2015) K63-linked ubiquitination of FANCG is required for its association with the Rap80-BRCA1 complex to modulate homologous recombination repair of DNA interstand crosslinks. Oncogene 34, 2867–2878 [DOI] [PubMed] [Google Scholar]
- 40. Huen M. S., Grant R., Manke I., Minn K., Yu X., Yaffe M. B., and Chen J. (2007) RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell 131, 901–914 [DOI] [PMC free article] [PubMed] [Google Scholar]