Abstract
The nerve growth factor family of growth factors, collectively known as neurotrophins, are evolutionarily ancient regulators with an enormous range of biological functions. Reflecting this long history and functional diversity, mechanisms for cellular responses to neurotrophins are exceptionally complex. Neurotrophins signal through p75 NTR, a member of the TNF receptor superfamily member, and through receptor tyrosine kinases (TrkA, TrkB, TrkC), often with opposite functional outcomes. The two classes of receptors are activated preferentially by proneurotrophins and mature processed neurotrophins, respectively. However, both receptor classes also possess neurotrophin-independent signaling functions. Signaling functions of p75 NTR and Trk receptors are each influenced by the other class of receptors. This review focuses on the mechanisms responsible for the functional interplay between the two neurotrophin receptor signaling systems.
Keywords: 75 kDa neurotrophin receptor, proneurotrophins, mature processed neurotrophins, Spätzle proteins
Nerve growth factor (NGF) and its orthologs are collectively known as neurotrophins. Mammals have four neurotrophins – NGF, brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophin 4 (NT-4, also known as NT-4/5). Neurotrophins, functioning as homodimers, have a wide variety of functions in both neural and non-neural tissues, and they control adult physiology as well as embryonic development.
The 4 neurotrophins signal through three paralogous receptor tyrosine kinases (TrkA, TrkB and TrkC) and the 75 kDa neurotrophin receptor (p75 NTR), which is a member of the death domain-containing receptor subgroup (so-called death receptors) of the TNF receptor superfamily. While p75 NTR is activated by all four neurotrophins, the Trk receptors are more selective, as shown in the table.
Table 1. Ligand preferences of neurotrophin receptors.
Ligands listed in italic type have lower affinity and/or are less commonly important for receptor activation in vivo.
| Receptor | Ligand |
|---|---|
| p75 NTR | NGF, BDNF, NT3, NT4 |
| TrkA | NGF, NT3 |
| TrkB | BDNF, NT4, NT3 |
| TrkC | NT3 |
The neurotrophin system is ancient, as orthologs of neurotrophins, p75 NTR and Trks are found in invertebrates as diverse as sea urchins, mollusks and round worms 1, 2. Consequently, this signaling system has had half a billion years of evolution to develop extraordinary complexity. A goal of this review will be to capture the many levels of complexity of the neurotrophin receptor signaling system. Perversely, the entire signaling system has been lost in Caenorhabditis and Drosophila lineages, depriving investigators of convenient genetic systems to unravel the complexity of neurotrophin signaling.
Although neurotrophic Drosophila proteins have been referred to as neurotrophins, they are only distantly similar to neurotrophins of other invertebrate and vertebrate species, and they signal via toll-like receptors, rather than p75 NTR or Trk-like receptors 3– 5. I prefer to refer to these neurotrophin-like cytokines by their original names, Spätzle and Spätzle-family proteins, rather than as neurotrophins, to avoid confusion.
It has been said that there is a yin and yang relationship between p75 NTR and Trk receptors, because they often are co-expressed and function oppositely 6. For example, TrkA signaling in sympathetic neurons promotes axon growth and neuronal survival, whereas p75 NTR signaling promotes axon degeneration and neuronal cell death 7. BDNF controls hippocampal neuronal synaptic plasticity, learning and memory with TrkB signaling promotes synaptic long term potentiation (LTP) and p75 NTR signaling promoting long term depression (LTD) 6. Functional interactions between the two receptor systems produce multiple levels of complexity, while several different mechanisms control the balance between the yin and the yang of neurotrophin signaling.
Like many biologically active polypeptides, neurotrophins are synthesized as precursors (pro-neurotrophins), which are cleaved to release an N-terminal prodomain peptide and a C-terminal mature neurotrophin. This cleavage event may occur either within the secretory pathway or following secretion, so that receptors may be exposed to both proneurotrophins and mature neurotrophins. Importantly, p75 NTR binds both mature and proneurotrophins, and is more effectively activated by proneurotrophins, while only mature neurotrophins activate Trk receptors 8, 9. The enhanced action of proneurotrophins binding to p75 NTR is dependent on association of p75 NTR with sortilin or SorCS2, Vps10p-domain proteins which bind a conserved motif in proneurotrophin prodomains 10, 11.
The complexity of function that can be generated by these relationships is well illustrated by sympathetic neurons, which express TrkA and p75 NTR, but not TrkB. For these neurons, proNGF, which activates p75 NTR but not TrkA, promotes cell death. Mature NGF, which activates both p75 NTR and TrkA, promotes cell survival. ProBDNF or mature BDNF promotes cell death, because these ligands bind p75 NTR but not TrkA 12– 14.
The canonical mode of signaling by Trk receptors is similar to signaling by other receptor tyrosine kinases. Neurotrophin binding promotes formation of Trk dimers, and induces trans-phosphorylation of Trk cytoplasmic domain tyrosine residues, initiating recruitment of signaling adapter proteins that foster signaling by ras/ERK1/2, PI3 kinase/Akt STAT and phospholipase Cγ pathways 15. However, alternatively spliced forms of TrkB and TrkC (misleadingly known as truncated TrkB and truncated TrkC) lack a tyrosine kinase domain, but possess alternative cytoplasmic domain sequences that signal by less extensively characterized mechanisms 16, 17.
One feature of canonical signaling by Trk receptors that differs from many other receptor tyrosine kinases is the use of so-called signaling endosomes to achieve retrograde axonal signaling. For most receptor tyrosine kinases, ligand-mediated activation of the receptor leads to receptor endocytosis, followed either by lysosomal degradation of the receptor or recycling back to the cell surface. However, in many physiological scenarios, the survival and/or differentiated state of neurons is regulated by neurotrophins secreted by the target tissues those neurons innervate. In this context, endocytosis of the neurotrophin/Trk complex generates signaling endosomes, which undergo retrograde axonal transport, delivering the activated neurotrophin/receptor complex to the somatic compartment in order to permit control of nuclear transactivation of genes 18– 21. The exquisite complexity associated with this mode of signaling is nicely illustrated by sympathetic neurons, where NT3 and NGF differently control axonal TrkA signaling functions because of differences in the pH-dependence for NT3 and NGF binding. Sympathetic axons encounter NT3 on the route to their target. NT3 activates TrkA and achieves local control of axonal growth cone dynamics, but does not engage signaling to the cell soma because NT3 dissociates from TrkA at the acidic pH within endosomes, causing TrkA receptors to recycle to the local plasma membrane without production of axonally transported signaling endosomes. In contrast, NGF/TrkA complexes remain intact as endosomes acidify, allowing TrkA to engage the motor systems that mediate retrograde axonal transport of TrkA-bearing endosomes 22.
p75 NTR signaling shares several features with other death receptors. A juxta-membrane region of the cytoplasmic domain binds TRAF6, which engages signaling pathways leading to activation of NF-κB and JNK 23, 24. The death domain interacts with RhoGDI, which controls RhoA activation, and RIP2 kinase, which contributes to NF-κB and JNK activation 25. Neurotrophin binding to p75 NTR inhibits RhoA activation 26, while enhancing JNK activation 27, 28. However, TRAF6, RhoGDI and RIP2 are only a few of the bewildering array of p75 NTR-binding signaling adapter proteins that have been reported to mediate p75 NTR signaling. Other notable examples include NRIF, which promotes JNK activation 28, 29, MAGE proteins including NRAGE, which promote Rac1 and JNK activation 30, and Bex1, which negatively affects NF-κB signaling 31, 32. Further, p75 NTR has been reported to influence glucose uptake in adipocytes via Glut4 by directly binding the trafficking regulators Rab5 and Rab31 33, to controls energy expenditure in obese mice on a high-fat diet by inhibiting cAMP signaling in adipocytes via direct association of p75 NTR with protein kinase A 34, and to promote fibrinolysis in nerve injury and lung fibrosis by binding and enhancing the cAMP degradative activity of phosphodiesterase PDE4A4/5 35.
One feature of p75 NTR function is unique, so far, among known receptors. A cysteinyl residue in the membrane spanning domain of p75 NTR forms a disulfide bond, within the lipid bilayer, creating a covalently linked-dimeric form of p75 NTR, and this covalent linkage is required for neurotrophin-dependent JNK activation, but not inhibition of RhoA activity 36. Mutation of the single cysteinyl residue required to form this disulfide bond eliminates p75 NTR-dependent death signaling in neurons in vitro and in vivo 37. A physiologically occurring disulfide bond within a lipid bilayer has never been described previously in any membrane protein, and the mechanism by which a disulfide forms in such an unusual environment is unclear. However, our unpublished evidence (Leslayann Schecterson and Mark Bothwell) demonstrates that this linkage forms within 2 minutes when cells are exposed to minute concentrations of hydrogen peroxide, indicating that oxidative stress may control p75 NTR signaling, as we have reported previously 38.
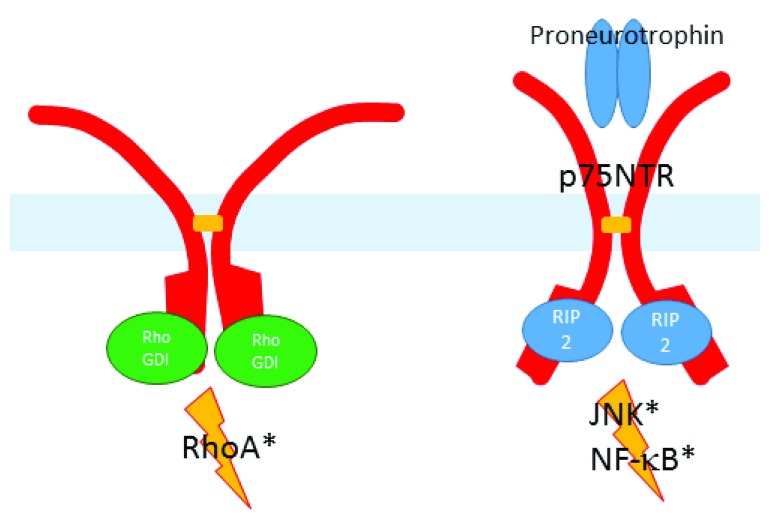
A detailed model, illustrated in Figure 1, has recently been proposed for death domain-mediated p75 NTR signaling 25. In the absence of bound neurotrophin, the death domains of p75 NTR dimers form a homodimeric complex. The RhoGDI binding site is not occluded by this interaction, so non-liganded p75 NTR engages RhoGDI-dependent RhoA activation. Binding of a neurotrophin dimer to the extracellular domain of a disulfide-linked p75 NTR dimer, causes a scissoring action (or more accurately a snail-tong action) around the disulfide pivot-point, separating originally juxtaposed death domains, and allowing access of RIP2 to a binding site that was previously partially occluded by the death domain/death domain interaction. The RIP2 binding site partially overlaps with the RhoGDI binding site, so RIP2 binding displaces RhoGDI, initiating JNK activation and terminating RhoA activation.
Figure 1. p75 NTR Signaling.
In absence of ligand, death domains of disulfide-linked p75 NTR dimer bind RhoGDI, promoting formation of active GTP-bound RhoA. Binding of neurotrophin or proneurotrophin causes a scissoring action of the dimer, displacing the death domains laterally and allowing RIP2 to bind the death domains. RIP2 promotes activation of JNK and NF-κB and by displacing RhoGDI, terminates RhoA activation.
Challenging the elegant simplicity of this model, an element of controversy has been introduced by the suggestion that the p75 NTR oligomer observed on non-reducing SDS gels is a trimer, rather than a dimer 39, 40. This conclusion relies primarily on the ratio of the apparent molecular weights of p75 NTR monomer and oligomer on non-reducing SDS gels. A caveat for such analysis is that the theoretical basis for the proportionality of electrophoretic mobility and protein mass on SDS gels assumes that SDS-induced denaturation fully unfolds the protein and causes the protein to form linear structures with length proportional to mass 41. This assumption does not apply to p75 NTR, which contains multiple intra-chain disulfide linkages if disulfide bonds are not reduced before electrophoresis. p75 NTR function as a trimer is inconsistent with X-ray crystallographic and/or NMR generated three-dimensional structures indicating that the extracellular domain of p75 NTR 42, the death domain region of the intracellular domain of p75 NTR 25, and the membrane spanning domain of p75 NTR 43 each forms dimers, not trimers. Application of emerging technologies such as cryo-EM will be required to provide definitive evidence about the stoichiometry of intact p75 NTR.
Although the reader may think that the preceding account is already quite complicated enough, another mode of p75 NTR signaling, and its manner of influence by Trk receptors, provides substantial additional complexity, as summarized in Figure 2. Soon after the discovery of Trk receptors, it was reported that p75 NTR/Trk heterodimeric complexes could form, enhancing the affinity of NGF binding to TrkA 44, and causing TrkA and TrkB to be less effectively activated by NT3 15. Although the physiological importance of these p75 NTR effects on Trk signaling remain uncertain, recently Trk-dependent effects on p75 NTR signaling have emerged that seem likely to have physiological relevance. p75 NTR has an alternative signaling pathway that resembles the mode of signaling of Notch. ADAM10 or ADAM17-dependent cleavage of the p75 NTR extracellular domain near the membrane, followed by γ-secretase mediated release of the intracellular domain into the cytoplasm, fosters signaling 45– 47. Interestingly, a similar mode of signaling by another TNF receptor superfamily member, TNFR1, has been described recently 48. Differential cleavage of p75 NTR in different types of neurons produced different signaling outcomes 49. Signaling effects attributed to the mobilized p75 NTR intracellular domain include nuclear accumulation of NRIF 47, association with the ubiquitin ligase siah2 controlling degradation of the transcription factor Hif1α 50, and association with nuclear pore complexes promoting nuclear uptake of the SMAD2 transcription factor 51. Neurotrophin binding to p75 NTR does not directly influence the rate of ADAM protease-mediated cleavage of p75 NTR 45, 46, 52 although signaling pathways initiated by neurotrophin binding may enhance p75 NTR cleavage many hours after neurotrophin exposure 47. Interestingly, activation of Trk receptors promotes the p75 NTR cleavage pathway 45, 53.
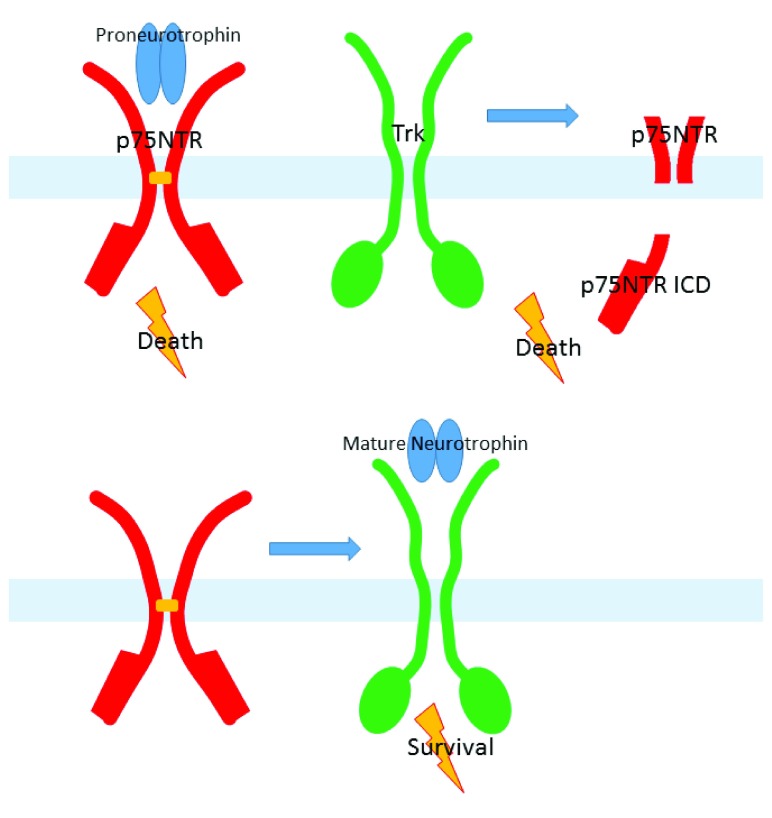
Figure 2. Cell death and cell survival signaling by p75 NTR and Trk receptors.
( Above) Proneurotrophins interact preferentially with p75 NTR, promoting JNK dependent caspase activation and cell death. Sequential cleavage of p75 NTR by ADAM10/17 and γ-secretase, allowing cytoplasmic mobilization of the intracellular domain of p75 NTR, may also promote cell death, by a mechanism that is only indirectly promoted by neurotrophins. Non-liganded Trk A and TrkC promote cell death by a mechanism that implicates the p75 NTR cleavage pathway. ( Below) Mature neurotrophins preferentially activate Trk receptors, in a manner that may be enhanced by p75 NTR, particularly for TrkA. Neurotrophin activation of Trks promotes cell survival.
It remains to be determined definitively whether Trk activity represents a major mode of regulation of the p75 NTR cleavage signaling pathways in vivo. However, the recently reported function of TrkA and TrkC as dependence receptors may reflect a consequence of this mode of interaction. Dependence receptors are receptors that signal constitutively until ligand binding terminates signaling. Although each of the three Trk paralogs was originally found to promote neuronal survival, in some neuronal populations, neurotrophin-independent effects of TrkA and TrkC were reported to promote neuronal cell death, and the cleavage mediated signaling pathway of p75 NTR has been implicated as a mediator of this effect 54, 55.
The foregoing paragraphs have focused on neurotrophin-dependent signaling by neurotrophin receptors, but other ligands importantly engage signaling by both Trk and p75 NTR receptors. For Trk receptors, the most common mechanism for signaling in response to non-neurotrophin ligands involves receptor transactivation, most commonly of TrkB. A variety of G protein-coupled receptors, including PACAP and A2a adenosine receptors, activate TrkB via Gsα-dependent activation of Src family kinases (commonly Fyn in neural tissue) 56, 57. In the context of embryonic cerebral cortex, where developing neurons express abundant TrkB receptors, EGF-dependent activation of EGF receptors engages Src-dependent TrkB activation 58. Src family kinase-mediated Trk transactivation also is induced by ligand-dependent activation of Low-density lipoprotein receptor-related protein 1 (LRP1) 59, and by zinc ion, which is co-released during glutamatergic neurotransmission 60, 61. One interesting feature of transactivation of TrkB is that activation commonly occurs in the ERGIC or Golgi compartments, rather than at the cell surface. Signaling from intracellular sites may not be functionally equivalent to signaling from the plasma membrane. For example, PACAP-dependent transactivation of TrkB in cultured hippocampal neurons, by coupling to pathways that otherwise control Golgi dynamics during cell division, induces fragmentation of the Golgi apparatus and alters Golgi-dependent processing of other membrane proteins 62.
A variety of modes of neurotrophin-independent activation of p75 NTR have been reported. p75 NTR is one of several receptors that bind Aβ peptide and putatively engage in pathogenic signaling in Alzheimer’s disease 40, 63. Other scenarios in which non-neurotrophin ligands control p75 NTR signaling involve association of p75 NTR with co-receptors that bind the activating ligand. Axon--repellant signaling by CNS myelin proteins such as Nogo, MAG, or OMgp, mediated by association of NgR1 with p75 NTR, 64, 65 or the p75 NTR homolog, Troy 66 while ephrin-A/p75 NTR complexes have been implicated as mediators of EPH-dependent reverse signaling 67.
Although great progress has been made in elucidating the signaling pathways employed by neurotrophin receptors, a systems level understanding of how these signaling pathways are selectively engaged in vivo is sadly lacking. It is a daunting task to understand how this extraordinarily rich palette of neurotrophin receptor signaling modalities is controlled physiologically.
Abbreviations
BDNF, brain-derived neurotrophic factor; EGF, epidermal growth factor; ERGIC, endoplasmic reticulum Golgi intermediate compartment; JNK, Jun kinase; MAG, myelin associated glycoprotein; NGF, nerve growth factor; NMR, nuclear magnetic resonance; NT3, neurotrophin 3; NT4, neurotrophin 4; OMgp, oligomyelin glycoprotein; p75 NTR, 75 kDa neurotrophin receptor; RhoGDI, Rho GDP dissociation inhibitor; TNF, tumor necrosis factor; Trk, tropomyosin related kinase.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Bruce Carter, Department of Biochemistry, Vanderbilt University Medical Center, Nashville, TN, USA; Vanderbilt Brain Institute, Vanderbilt University Medical Center, Nashville, TN, USA
Philip Barker, Biology Unit, University of British Columbia, Kelowna, British Columbia, Canada
Funding Statement
This work was supported by a grant from the Tietze Family Foundation.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Wilson KH: The genome sequence of the protostome Daphnia pulex encodes respective orthologues of a neurotrophin, a Trk and a p75 NTR: evolution of neurotrophin signaling components and related proteins in the bilateria. BMC Evol Biol. 2009;9:243. 10.1186/1471-2148-9-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bothwell M: Evolution of the neurotrophin signaling system in invertebrates. Brain Behav Evol. 2006;68(3):124–132. 10.1159/000094082 [DOI] [PubMed] [Google Scholar]
- 3. Ballard SL, Miller DL, Ganetzky B: Retrograde neurotrophin signaling through Tollo regulates synaptic growth in Drosophila. J Cell Biol. 2014;204(7):1157–1172. 10.1083/jcb.201308115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeLotto Y, DeLotto R: Proteolytic processing of the Drosophila Spätzle protein by easter generates a dimeric NGF-like molecule with ventralising activity. Mech Dev. 1998;72(1–2):141–148. 10.1016/S0925-4773(98)00024-0 [DOI] [PubMed] [Google Scholar]
- 5. Zhu B, Pennack JA, McQuilton P, et al. : Drosophila neurotrophins reveal a common mechanism for nervous system formation. PLoS Biol. 2008;6(11):e284. 10.1371/journal.pbio.0060284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu B, Pang PT, Woo NH: The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6(8):603–614. 10.1038/nrn1726 [DOI] [PubMed] [Google Scholar]
- 7. Singh KK, Park KJ, Hong EJ, et al. : Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat Neurosci. 2008;11(6):649–658. 10.1038/nn.2114 [DOI] [PubMed] [Google Scholar]
- 8. Lee R, Kermani P, Teng KK, et al. : Regulation of cell survival by secreted proneurotrophins. Science. 2001;294(5548):1945–1948. 10.1126/science.1065057 [DOI] [PubMed] [Google Scholar]
- 9. Hempstead BL: Deciphering Proneurotrophin Actions.2014;220:17–32. 10.1007/978-3-642-45106-5_2 [DOI] [PubMed] [Google Scholar]
- 10. Nykjaer A, Lee R, Teng KK, et al. : Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427(6977):843–848. 10.1038/nature02319 [DOI] [PubMed] [Google Scholar]
- 11. Glerup S, Olsen D, Vaegter CB, et al. : SorCS2 regulates dopaminergic wiring and is processed into an apoptotic two-chain receptor in peripheral glia. Neuron. 2014;82(5):1074–1087. 10.1016/j.neuron.2014.04.022 [DOI] [PubMed] [Google Scholar]
- 12. Teng HK, Teng KK, Lee R, et al. : ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25(22):5455–5463. 10.1523/JNEUROSCI.5123-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kohn J, Aloyz RS, Toma JG, et al. : Functionally antagonistic interactions between the TrkA and p75 neurotrophin receptors regulate sympathetic neuron growth and target innervation. J Neurosci. 1999;19(13):5393–5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bamji SX, Majdan M, Pozniak CD, et al. : The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J Cell Biol. 1998;140(4):911–923. 10.1083/jcb.140.4.911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang EJ, Reichardt LF: Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. 10.1146/annurev.biochem.72.121801.161629 [DOI] [PubMed] [Google Scholar]
- 16. Fulgenzi G, Tomassoni-Ardori F, Babini L, et al. : BDNF modulates heart contraction force and long-term homeostasis through truncated TrkB.T1 receptor activation. J Cell Biol. 2015;210(6):1003–1012. 10.1083/jcb.201502100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Esteban PF, Yoon HY, Becker J, et al. : A kinase-deficient TrkC receptor isoform activates Arf6-Rac1 signaling through the scaffold protein tamalin. J Cell Biol. 2006;173(2):291–299. 10.1083/jcb.200512013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cosker KE, Segal RA: Neuronal signaling through endocytosis. Cold Spring Harb Perspect Biol. 2014;6(2):pii: a020669. 10.1101/cshperspect.a020669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grimes ML, Zhou J, Beattie EC, et al. : Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes. J Neurosci. 1996;16(24):7950–7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Howe CL, Valletta JS, Rusnak AS, et al. : NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron. 2001;32(5):801–814. [DOI] [PubMed] [Google Scholar]
- 21. Ginty DD, Segal RA: Retrograde neurotrophin signaling: Trk-ing along the axon. Curr Opin Neurobiol. 2002;12(3):268–274. 10.1016/S0959-4388(02)00326-4 [DOI] [PubMed] [Google Scholar]
- 22. Harrington AW, St Hillaire C, Zweifel LS, et al. : Recruitment of actin modifiers to TrkA endosomes governs retrograde NGF signaling and survival. Cell. 2011;146(3):421–434. 10.1016/j.cell.2011.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khursigara G, Orlinick JR, Chao MV: Association of the p75 neurotrophin receptor with TRAF6. J Biol Chem. 1999;274(5):2597–2600. 10.1074/jbc.274.5.2597 [DOI] [PubMed] [Google Scholar]
- 24. Zampieri N, Chao MV: Mechanisms of neurotrophin receptor signalling. Biochem Soc Trans. 2006;34(Pt 4):607–611. 10.1042/BST0340607 [DOI] [PubMed] [Google Scholar]
- 25. Lin Z, Tann JY, Goh ET, et al. : Structural basis of death domain signaling in the p75 neurotrophin receptor. eLife. 2015;4:e11692. 10.7554/eLife.11692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamashita T, Tucker KL, Barde YA: Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron. 1999;24(3):585–593. 10.1016/S0896-6273(00)81114-9 [DOI] [PubMed] [Google Scholar]
- 27. Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, et al. : Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383(6602):716–719. 10.1038/383716a0 [DOI] [PubMed] [Google Scholar]
- 28. Linggi MS, Burke TL, Williams BB, et al. : Neurotrophin receptor interacting factor (NRIF) is an essential mediator of apoptotic signaling by the p75 neurotrophin receptor. J Biol Chem. 2005;280(14):13801–13808. 10.1074/jbc.M410435200 [DOI] [PubMed] [Google Scholar]
- 29. Casademunt E, Carter BD, Benzel I, et al. : The zinc finger protein NRIF interacts with the neurotrophin receptor p75 NTR and participates in programmed cell death. EMBO J. 1999;18(21):6050–6061. 10.1093/emboj/18.21.6050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salehi AH, Roux PP, Kubu CJ, et al. : NRAGE, a novel MAGE protein, interacts with the p75 neurotrophin receptor and facilitates nerve growth factor-dependent apoptosis. Neuron. 2000;27(2):279–288. 10.1016/S0896-6273(00)00036-2 [DOI] [PubMed] [Google Scholar]
- 31. Mukai J, Suvant P, Sato TA: Nerve growth factor-dependent regulation of NADE-induced apoptosis. Vitam Horm. 2003;66:385–402. 10.1016/S0083-6729(03)01011-2 [DOI] [PubMed] [Google Scholar]
- 32. Vilar M, Murillo-Carretero M, Mira H, et al. : Bex1, a novel interactor of the p75 neurotrophin receptor, links neurotrophin signaling to the cell cycle. EMBO J. 2006;25(6):1219–1230. 10.1038/sj.emboj.7601017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baeza-Raja B, Li P, Le Moan N, et al. : p75 neurotrophin receptor regulates glucose homeostasis and insulin sensitivity. Proc Natl Acad Sci U S A. 2012;109(15):5838–5843. 10.1073/pnas.1103638109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baeza-Raja B, Sachs BD, Li P, et al. : p75 Neurotrophin Receptor Regulates Energy Balance in Obesity. Cell Rep. 2016;14(2):255–268. 10.1016/j.celrep.2015.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sachs BD, Baillie GS, McCall JR, et al. : p75 neurotrophin receptor regulates tissue fibrosis through inhibition of plasminogen activation via a PDE4/cAMP/PKA pathway. J Cell Biol. 2007;177(6):1119–1132. 10.1083/jcb.200701040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vilar M, Charalampopoulos I, Kenchappa RS, et al. : Activation of the p75 neurotrophin receptor through conformational rearrangement of disulphide-linked receptor dimers. Neuron. 2009;62(1):72–83. 10.1016/j.neuron.2009.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tanaka K, Kelly CE, Goh KY, et al. : Death Domain Signaling by Disulfide-Linked Dimers of the p75 Neurotrophin Receptor Mediates Neuronal Death in the CNS. Journal of Neuroscience. 2016;36(20):5587–5595. 10.1523/JNEUROSCI.4536-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burke MA, Bothwell M: p75 neurotrophin receptor mediates neurotrophin activation of NF-kappa B and induction of iNOS expression in P19 neurons. J Neurobiol. 2003;55(2):191–203. 10.1002/neu.10174 [DOI] [PubMed] [Google Scholar]
- 39. Anastasia A, Barker PA, Chao MV, et al. : Detection of p75 NTR Trimers: Implications for Receptor Stoichiometry and Activation. J Neurosci. 2015;35(34):11911–11920. 10.1523/JNEUROSCI.0591-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yaar M, Zhai S, Fine RE, et al. : Amyloid beta binds trimers as well as monomers of the 75-kDa neurotrophin receptor and activates receptor signaling. J Biol Chem. 2002;277(10):7720–7725. 10.1074/jbc.M110929200 [DOI] [PubMed] [Google Scholar]
- 41. Reynolds JA, Tanford C: The gross conformation of protein-sodium dodecyl sulfate complexes. J Biol Chem. 1970;245(19):5161–5165. [PubMed] [Google Scholar]
- 42. Gong Y, Cao P, Yu HJ, et al. : Crystal structure of the neurotrophin-3 and p75 NTR symmetrical complex. Nature. 2008;454(7205):789–793. 10.1038/nature07089 [DOI] [PubMed] [Google Scholar]
- 43. Nadezhdin KD, García-Carpio I, Goncharuk SA, et al. : Structural Basis of p75 Transmembrane Domain Dimerization. J Biol Chem. 2016;291(23):12346–57. 10.1074/jbc.M116.723585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hempstead BL, Martin-Zanca D, Kaplan DR, et al. : High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350(6320):678–683. 10.1038/350678a0 [DOI] [PubMed] [Google Scholar]
- 45. Kanning KC, Hudson M, Amieux PS, et al. : Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. J Neurosci. 2003;23(13):5425–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jung KM, Tan S, Landman N, et al. : Regulated intramembrane proteolysis of the p75 neurotrophin receptor modulates its association with the TrkA receptor. J Biol Chem. 2003;278(43):42161–42169. 10.1074/jbc.M306028200 [DOI] [PubMed] [Google Scholar]
- 47. Kenchappa RS, Zampieri N, Chao MV, et al. : Ligand-dependent cleavage of the P75 neurotrophin receptor is necessary for NRIF nuclear translocation and apoptosis in sympathetic neurons. Neuron. 2006;50(2):219–232. 10.1016/j.neuron.2006.03.011 [DOI] [PubMed] [Google Scholar]
- 48. Chhibber-Goel J, Coleman-Vaughan C, Agrawal V, et al. : γ-Secretase Activity Is Required for Regulated Intramembrane Proteolysis of Tumor Necrosis Factor (TNF) Receptor 1 and TNF-mediated Pro-apoptotic Signaling. J Biol Chem. 2016;291(11):5971–85. 10.1074/jbc.M115.679076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vicario A, Kisiswa L, Tann JY, et al. : Neuron-type-specific signaling by the p75 NTR death receptor is regulated by differential proteolytic cleavage. J Cell Sci. 2015;128(8):1507–17. 10.1242/jcs.161745 [DOI] [PubMed] [Google Scholar]
- 50. Le Moan N, Houslay DM, Christian F, et al. : Oxygen-dependent cleavage of the p75 neurotrophin receptor triggers stabilization of HIF-1α. Mol Cell. 2011;44(3):476–490. 10.1016/j.molcel.2011.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schachtrup C, Ryu JK, Mammadzada K, et al. : Nuclear pore complex remodeling by p75(NTR) cleavage controls TGF-β signaling and astrocyte functions. Nat Neurosci. 2015;18(8):1077–1080. 10.1038/nn.4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sykes AM, Palstra N, Abankwa D, et al. : The effects of transmembrane sequence and dimerization on cleavage of the p75 neurotrophin receptor by γ-secretase. J Biol Chem. 2012;287(52):43810–43824. 10.1074/jbc.M112.382903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Urra S, Escudero CA, Ramos P, et al. : TrkA receptor activation by nerve growth factor induces shedding of the p75 neurotrophin receptor followed by endosomal gamma-secretase-mediated release of the p75 intracellular domain. J Biol Chem. 2007;282(10):7606–7615. 10.1074/jbc.M610458200 [DOI] [PubMed] [Google Scholar]
- 54. Nikoletopoulou V, Lickert H, Frade JM, et al. : Neurotrophin receptors TrkA and TrkC cause neuronal death whereas TrkB does not. Nature. 2010;467(7311):59–63. 10.1038/nature09336 [DOI] [PubMed] [Google Scholar]
- 55. Dekkers MP, Nikoletopoulou V, Barde YA: Cell biology in neuroscience: Death of developing neurons: new insights and implications for connectivity. J Cell Biol. 2013;203(3):385–393. 10.1083/jcb.201306136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee FS, Rajagopal R, Kim AH, et al. : Activation of Trk neurotrophin receptor signaling by pituitary adenylate cyclase-activating polypeptides. J Biol Chem. 2002;277(11):9096–9102. 10.1074/jbc.M107421200 [DOI] [PubMed] [Google Scholar]
- 57. Lee FS, Rajagopal R, Chao MV: Distinctive features of Trk neurotrophin receptor transactivation by G protein-coupled receptors. Cytokine Growth Factor Rev. 2002;13(1):11–17. 10.1016/S1359-6101(01)00024-7 [DOI] [PubMed] [Google Scholar]
- 58. Puehringer D, Orel N, Lüningschrör P, et al. : EGF transactivation of Trk receptors regulates the migration of newborn cortical neurons. Nat Neurosci. 2013;16(4):407–415. 10.1038/nn.3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shi Y, Mantuano E, Inoue G, et al. : Ligand binding to LRP1 transactivates Trk receptors by a Src family kinase-dependent pathway. Sci Signal. 2009;2(68):ra18. 10.1126/scisignal.2000188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Huang YZ, Pan E, Xiong ZQ, et al. : Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron. 2008;57(4):546–558. 10.1016/j.neuron.2007.11.026 [DOI] [PubMed] [Google Scholar]
- 61. Huang YZ, McNamara JO: Mutual regulation of Src family kinases and the neurotrophin receptor TrkB. J Biol Chem. 2010;285(11):8207–8217. 10.1074/jbc.M109.091041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schecterson LC, Hudson MP, Ko M, et al. : Trk activation in the secretory pathway promotes Golgi fragmentation. Mol Cell Neurosci. 2010;43(4):403–413. 10.1016/j.mcn.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 63. Sotthibundhu A, Sykes AM, Fox B, et al. : Beta-amyloid 1-42 induces neuronal death through the p75 neurotrophin receptor. J Neurosci. 2008;28(15):3941–3946. 10.1523/JNEUROSCI.0350-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wong ST, Henley JR, Kanning KC, et al. : A p75 NTR and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat Neurosci. 2002;5(12):1302–1308. 10.1038/nn975 [DOI] [PubMed] [Google Scholar]
- 65. Wang KC, Kim JA, Sivasankaran R, et al. : P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420(6911):74–78. 10.1038/nature01176 [DOI] [PubMed] [Google Scholar]
- 66. Park JB, Yiu G, Kaneko S, et al. : A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45(3):345–351. 10.1016/j.neuron.2004.12.040 [DOI] [PubMed] [Google Scholar]
- 67. Lim YS, McLaughlin T, Sung TC, et al. : p75 NTR mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron. 2008;59(5):746–758. 10.1016/j.neuron.2008.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]