Abstract
Cutaneous T cell lymphomas (CTCLs) are a heterogeneous group of extranodal non-Hodgkin’s lymphomas that are characterized by a cutaneous infiltration of malignant monoclonal T lymphocytes. They typically afflict adults with a median age of 55 to 60 years, and the annual incidence is about 0.5 per 100,000. Mycosis fungoides, Sézary syndrome, and primary cutaneous peripheral T cell lymphomas not otherwise specified are the most important subtypes of CTCL. CTCL is a complicated concept in terms of etiopathogenesis, diagnosis, therapy, and prognosis. Herein, we summarize advances which have been achieved in these fields.
Keywords: cutaneous T cell lymphoma, mycosis fungoides, Sézary syndrome
Introduction
In 1806, Alibert initially described mycosis fungoides (MF) as the infiltration of skin by lymphocytes. In 1974, Edelson used the term “cutaneous T cell lymphomas” (CTCLs) for MF and its leukemic variant, Sézary syndrome (SS), which are the major types of CTCL 1. Nowadays, the CTCLs, which are characterized by infiltration of malignant monoclonal T lymphocytes in the skin, are considered a heterogeneous group of extranodal non-Hodgkin’s lymphomas 1– 3. Approximately 25% to 40% of non-Hodgkin’s lymphoma cases involve extranodal sites. The skin is the most common site after the gastrointestinal system 4. The annual incidence of CTCL is about 0.5 per 100,000, and men are more involved than women (1.6:1 to 2.0:1) 1. They typically afflict adults with a median age of 55 to 60 years 1, 5.
In 1975, for the first time, the North American Mycosis Fungoides Cooperative Study Group classified the CTCLs on the basis of a tumor-node-metastasis (TNM) system. Thereafter, the classification was modified and updated by the CTCL workshop to the one used today, known as the Bunn and Lambert system 1. The International Society for Cutaneous Lymphomas/European Organization for Research and Treatment of Cancer classified MF and SS according to clinical, pathological, biological, and immunological features 6.
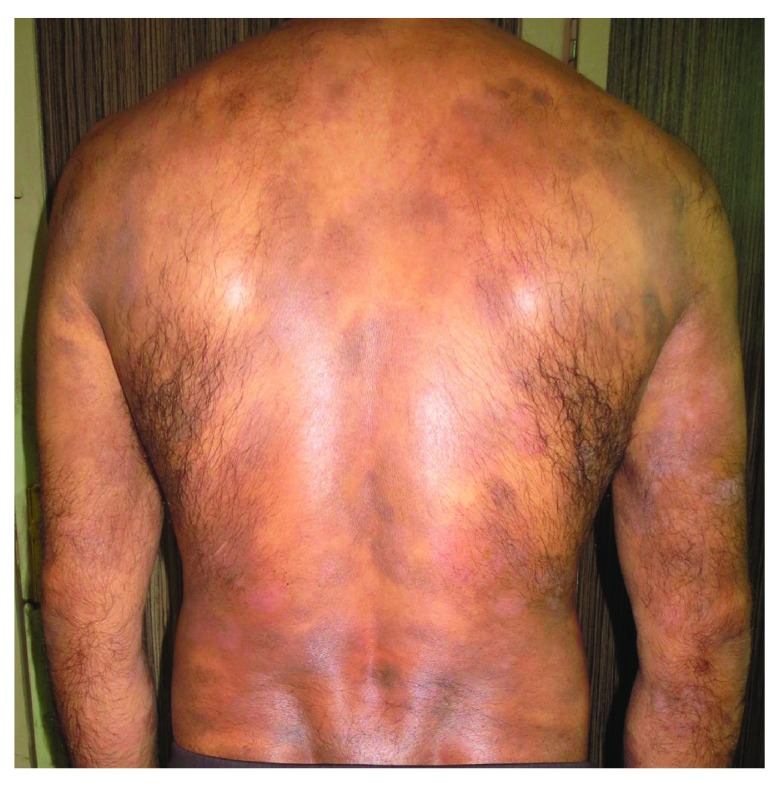
MF, SS, and primary cutaneous peripheral T cell lymphomas not otherwise specified (PCTCL - NOS) are among the most important subtypes of the CTCLs 7, 8. MF is the commonest type of CTCLs, representing 44% to 62% of cases 9. MF restricted to the skin has an indolent progression passing from macule and patch stage to infiltrated plaque and tumor stage ( Figure 1) 5, 10, 11 in sun-protected body sites 12. SS is defined as an aggressive leukemic-phase type of MF 11, clinically characterized by erythroderma and generalized lymphadenopathy 13. PCTCL-NOS can present with a solitary red-violaceous tumor-like nodule or scattered multifocal or diffuse nodules on any part of the body, which mostly become ulcerated and infected. Rapid cutaneous dissemination and systemic involvement are key features of this class of CTCL 8.
Figure 1. Mycosis fungoides in a 40-year-old man manifested as generalized atrophic patches.
At the early stages, CTCLs are often misdiagnosed as benign skin conditions 1, 9, 13– 17. Their most important differential diagnoses have been listed in Table 1.
Table 1. The most important differential diagnoses of the cutaneous T cell lymphomas.
| All kinds of dermatitis and eczema
1,
9,
13–
15
Adverse drug reactions 13 Parapsoriasis 9 Psoriasis 1, 14, 15 Lichen planus 16 Morphea 16 Panniculitis 17 Folliculitis 14 Pityriasis lichenoides chronica 14 Pityriasis lichenoides et varioliformis acuta 14 Pigmented purpuric dermatoses 14 Vitiligo 14 Lymphomatoid papulosis 9 |
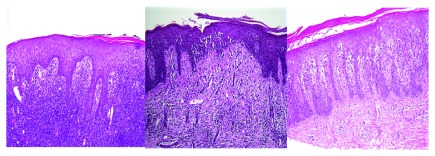
In most patients with CTCL, the histologic features are subtle, so that the differentiation of these disorders from benign inflammatory diseases is difficult 18, 19. Haloed lymphocytes, exocytosis, epidermotropism, Pautrier’s microabscess, large hyperconvoluted, hyperchromatic lymphocytes in the epidermis, and lymphocytes aligned within the basal layer are findings seen in histologic sections of MF ( Figure 2) 16, 20.
Figure 2. In pathological view, the cutaneous T cell lymphomas are characterized by haloed lymphocytes, exocytosis, epidermotropism, Pautrier’s microabscess, large hyperconvoluted, hyperchromatic lymphocytes in the epidermis, and lymphocytes aligned within the basal layer.
Figure 2A (left) A lymphocytic infiltrate is present in the dermis and extending into the overlying epidermis with minimal overlying spongiosis. Figure 2B (center) Lymphocytes with surrounding haloes are present in the epidermis as single cells and small clusters (Pautrier’s microabscesses). There is minimal accompanying spongiosis. Figure 2C (right) Psoriasiform epidermal hyperplasia with epidermotropism of haloed lymphocytes is seen in this case of patch-stage mycosis fungoides.
CTCL is a complicated concept in terms of etiopathogenesis, diagnosis, therapy, and prognosis. Herein, we have summarized advances which have been achieved in these fields.
Etiopathogenesis
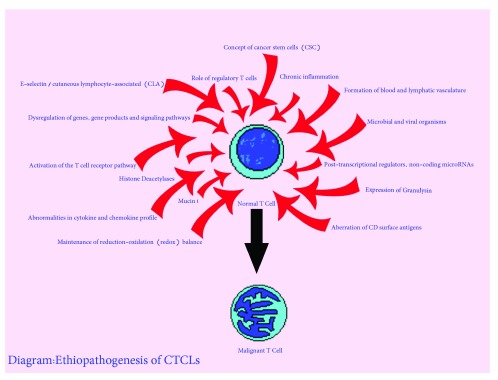
Although different views of CTCL etiopathogenesis have been elucidated in depth over the last few decades, the exact mechanism of initiation and progression of this disorder is not yet known 4, 10, 21 ( Figure 3). Although dysregulation of some genes and signaling pathways has been reported in the CTCLs ( Table 2), their exact role in the pathogenesis of these disorders is unknown 2, 3, 13, 22– 34. Most of these aberrations are seen in chromosome 10 35. Recent studies demonstrate ectopic expression of cancer testis genes in the CTCLs. It appears that this gene works through inhibiting apoptosis, inducing resistance to various forms of therapeutic modalities, and contributing to oncogenesis by targeting tumor suppressor genes such as p53 and p21 2. Deficient expression or function of negative regulators, including SOCS3 and protein tyrosine phosphatases such as SHP1, have been implicated in dysregulation of the Jak-3/STAT pathway and interleukin (IL)-independent proliferation of malignant T cells. The Jak-3/STAT pathway has a role in fighting against CTCLs by promoting production of IL-5, IL-10, IL-17A, and IL-17F; regulating angiogenic factors; and interfering with resistance to histone deacetylase inhibitor (HDACI) therapy 25. An additional report demonstrates pathogenic involvement of the NOTCH1 signaling pathway in the pathogenesis of SS 13. NOTCH includes a family of transmembrane receptors, which play roles in cell differentiation, proliferation, and stemness 31.
Figure 3. Etiopathogenesis of cutaneous T cell lymphoma.
CTCL, cutaneous T cell lymphoma.
Table 2. An alphabetical list of dysregulation of genes and signaling pathways seen in the cutaneous T cell lymphomas.
| AT-rich interactive domain-containing protein 1A (ARID1A)
23
B-Raf proto-oncogene, serine/threonine kinase (BRAF) 24 Bromodomain-9 (BRD-9) 24 Cancer testis (CT) 2 Caspase recruitment domain (CARD11) 23, 24 C-C chemokine receptor-4 (CCR-4) 23 Chromodomain-helicase-DNA-binding protein (CHD)-3 24 CREB-binding protein (CREBBP) 24 Cyclin-dependent kinase-2 (CDKN-2) 23– 26 Cyclooxygenase-2 (COX-2) 25 Dynamin-3 (DNM-3) 22 Embryonic stem cell regulators 3, 25 Eph receptor A4 (EPHA4) 13 Forkhead box P3 (FOXP3) 27 GATA-binding protein-3 (GATA-3) 22, 25 Histone deacetylase-6 (HDAC-6) 25 Histone-lysine N-methyltransferase (KMT)-2D or myeloid/lymphoid or mixed-lineage leukemia protein-2 (MLL-2) 24 Interleukin-2 receptor common gamma chain (IL-2Rgc) 3, 25 Janus kinase-3 (Jak-3)/signal transducers and activators of transcription (STAT) 25, 28 KIRD3DL2 22 Lysine (K)-specific methyltransferase (KMT)-2C or myeloid/lymphoid or mixed-lineage leukemia protein-3 (MLL-3) 24 MYC-associated factor X (MAX) 25 Metallothioneins I and II (MTI/II) 29 Methylthioadenosine-phosphorylase (MTAP) 30 Mitogen-activated protein kinase 1 (MAPK1) 24 MYC-binding protein (MYCBP) 25 NOTCH1 13, 31 Nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) 3, 24, 25 Nuclear factor of activated T cells (NFAT) 24 Phospholipase C gamma 1 (PLCG1) 23, 32 P21 33 P53 28, 32 Phosphatase and tensin homolog (PTEN) 24, 25, 34 Plastin-3 (PLS-3) 22 Protein kinase, CGMP-dependent (PRKG1) 24 Receptor tyrosine kinase (RTK) 13 Retinoblastoma-1 (RB-1) 24 Ribosomal protein S6 kinase-1 (RPS6KA-1) 23 Runt-related transcription factor-3 (RUNX-3) 22 Src homology region 2 domain-containing phosphatase-1 (SHP1) 25 Special AT-rich sequence-binding protein 1 (SATB1) 29 SRC proto-oncogene, non-receptor tyrosine kinase (Src kinases) 25 Suppressors of cytokine signaling-3 (SOCS3) 25 SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin-A4 (SMARC-A4) 24 Tet methylcytosine dioxygenase 2 (TET2) 24 Thymocyte selection-associated high-mobility group box (TOX) 22, 25 Tumor protein p53 (TP53) 23, 24 Tumor necrosis factor receptor superfamily-1B (TNFRSF-1B) 30 Twist 22 Zinc finger E-box-binding homeobox 1 (ZEB1) 23 |
The loss of heterozygosity of phosphatase and tensin homolog (PTEN) in MF has been reported, but the significance of the finding remains unclear. In one study, a statistically significant decrease in the percentage of cells retaining PTEN and altered staining intensity were shown in MF from patch to plaque stage, but this attenuation was not significant in tumor stage in comparison with plaque stage 32. The mRNA expression of a subfamily of receptor tyrosine kinase (RTK) is high in SS but not in other types of CTCLs 13.
TOX is a transcription factor with a role in the development of CD4 + T cells, including downstream effects on the expression of RUNX 3, a well-known tumor suppressor gene. Studies show that the overexpression of TOX and its protein product is associated with thicker lesions of MF, disease progression, and poor prognosis. Furthermore, dysregulation of this gene is reported in lesions and peripheral blood mononuclear cells of cases with SS 22.
Patients with CTCL show a distinct microRNA (miRNA) expression profile. Studies have shown that miR-21 and miR-155 are associated with poor prognosis and aggressive behavior by interfering with resistance to apoptosis and promoting malignant proliferation, respectively. On the other hand, the expression of miR-22, a tumor suppressor, is downregulated in SS. It appears that Jak-3/STAT is responsible for the loss of miR-22 expression 25. miR-16 is another non-coding microRNA that induces cellular senescence and is downregulated in CTCLs 33. Studies have shown that miRNAs inhibit the expression of many oncogenes such as MAX, MYCBP, nuclear receptor coactivator-1 ( NCOA-1), and cyclin-dependent kinase-6 ( CDK-6) 25.
A role for IL-2Rgc-signaling cytokines, including IL-2, IL-4, IL-7, IL-15, and IL-21, has been suggested in the pathogenesis of CTCLs 25. IL-12 plays a role as a potent anti-tumor agent. Its expression is decreased during tumoral-stage MF 36. On the other hand, in lesional skin of CTCLs, increased expression of IL-9 that is regulated by STAT3/5 and silencing of STAT5 has been reported 37. Additionally, upregulation of CC chemokine receptor 6 (CCR6) 38 and CCR7 3 has been reported in CTCLs and alleged to be responsible for spreading malignant T cells to sentinel lymph nodes, the bloodstream, and internal organs. Chemokine (C-X-C motif) ligand 12 (CXCL12) belongs to the superfamily of chemokines that is expressed on endothelial and stromal cells in different organs. Most hematopoietic cells such as CD34 + progenitor cells and CD4 + T cells express CXCR4, the receptor of this chemokine. This receptor plays a role in chemotaxis, invasion, angiogenesis, and proliferation. The role of the CXCR4/CXCL12 axis has been implicated in the pathogenesis of MF 10.
In CTCLs, malignant T cells display activation of the T cell receptor (TCR) pathway, which leads to TCR-dependent T helper 2 (Th2) cytokines such as IL-4 and IL-13 and resistance to natural mechanisms that prevent uncontrolled proliferation, such as Fas cell surface death receptor (FAS)-mediated apoptosis and transforming growth factor-beta-mediated growth suppression 12. Regulatory T cells with a CD4 + CD25 + phenotype comprise about 5% to 10% of peripheral T cells and play a role in tumor immunology. The role of these cells in CTCLs is controversial. Most studies have shown that, in CTCLs, a high count of FOXP3 + regulatory T cells is correlated with improved prognosis. This finding is completely opposite to that of studies on the role of these cells in solid tumors 27.
Studies have shown that CD26 is able to cleave and inactivate CXCL12; hence, its lack of expression in CTCLs results in enhanced CXCL12-dependent chemotaxis 10. On the other hand, CD164 is significantly overexpressed on CD4 + lymphocytes in SS. It seems that this factor can be a diagnostic parameter and a potential target for therapeutic approaches in SS 39. These are zinc-dependent enzymes implicated in gene regulation and in the modulation of numerous cellular pathways, including proliferation, differentiation, apoptosis, and migration. Aberration in the activity of these enzymes and their mutations has been reported in CTCLs 1.
In MF, the malignant cells migrate to the skin by using the ligand E-selectin on endothelial cells by the expression of a marker for skin homing, cutaneous lymphocyte-associated antigen (CLA). The ability of CLA to mediate leukocyte homing to the skin is dependent on specific chemokine receptor-ligand interactions. One of these interactions occurs through chemokine receptor CCR4; overexpression of this receptor has been reported in CTCL cases with peripheral blood involvement 1.
Granulysin is a cytotoxic, proinflammatory, and anti-microbial agent that is expressed along with granzymes and perforin in granules of cytotoxic T cells and natural killer cells. It plays a role in innate immunity, chemotaxis, and tumor immunology and has been shown to be involved in the progression of MF 27. Mucin 1 C-terminal subunit controls important pathways of oncogenesis by governing cell proliferation, self-renewal, tissue invasion, and apoptosis. This heterodimeric protein protects cells against reactive oxygen species-induced death. Overexpression of this protein has been shown in CTCL cell lines 40. It has been suggested that the maintenance of redox balance plays a critical factor in protecting malignant cells in the CTCLs from apoptosis 40.
Cancer stem cells have many similarities to normal stem cells, including infrequent division, high self-renewal capacity, resistance to apoptosis, and ability to maintain an undifferentiated state, overcome cellular senescence, and differentiate to all cell types. Because of their rare cell divisions, they are resistant to chemotherapeutic agents. Additionally, these cells are responsible for relapse and metastasis of tumors. The expression of embryonic stem cell genes such as Nanog homeobox ( NANOG), SRY (sex determining region Y)-box ( SOX)- 2, and OCT4 ( POU class 5 homeobox [ POU5F] -1) and their upstream and downstream signaling members was shown in CTCL lesions 2.
It was proposed that the formation of blood and lymphatic vasculature is involved in the progression of CTCL. Malignant T cells produce several angiogenic factors, such as podoplanin (PDPN), lymphatic vessel hyaluronan receptor-1 (LYVE-1), vascular endothelial growth factor-C (VEGF-C), VEGF-R3, and lymphotoxin alpha (LTα), which play roles in neoangiogenesis and neo-lymphoangiogenesis. The interaction of LTα, IL-6, and VEGF has been shown to induce angiogenesis by promoting endothelial cell sprouting and tube formation 3.
Some studies have reported an association between chronic cutaneous inflammation and subsequent development of CTCL 9. Chronic or professional exposure to topical chemical agents, long-lasting psoriasis, and urticaria have been proposed as risk factors 21. Chronically activated T lymphocytes may eventually result in the creation of an atypical T cell clone 9. For instance, in granulomatous MF, it appears the granulomatous inflammation may precede the lymphoma, resulting in lymphocyte proliferation through macrophage-produced IL-6 41.
A relationship between microbial colonization/infection and MF has been suggested 9, 42. It has been shown that bacterial isolates containing staphylococcal enterotoxin-A (SEA) promote disease progression by inducing STAT3 activation and IL-17 expression in malignant T cells 42. On the other hand, during the evolutionary process of CTCLs, loss of the normal TCR repertoire results in immunosuppression and opportunistic infections leading to death 12.
The role of viral infection in the pathogenesis of the CTCLs remains controversial. Recently, the role of retroviruses such as human T cell leukemia virus type 1 (HTLV-1) and HTLV-2 and human immunodeficiency virus and herpesvirus family members like Epstein-Barr virus, human herpesvirus 8, and cytomegalovirus have been suggested in the pathogenesis of these disorders. Viral infection may promote tumoral infiltration by inducing the production of tumor necrosis factor-alpha (TNF-α), IL-6, and IL-1a in keratinocytes. Additionally, in the skin, these organisms play the role of a stable chronic antigen, which results in a clonal proliferation of T cells, leading to CTCLs 4.
Diagnosis
The diagnosis of CTCLs is difficult at early stages because of the presence of multiple clinical presentations 1, 43, 44 and lack of definitive diagnostic criteria 1, 45. Hence, in most cases, it takes an average of 6 years from disease onset until confirmation of the diagnosis 1, 44.
Recently, there have been advances in the accurate diagnosis of CTCLs. To diagnose the CTCLs, guidelines prepared by the National Comprehensive Cancer Network recommend biopsy of suspicious skin sites and subsequent assessment in terms of dermatopathology, immunohistochemistry, and molecular analysis (TCR gene rearrangement) 14. Observation and palpation of the skin are mainstays in suspecting CTCLs. Palpation of lymph nodes remains the traditional approach for staging of these disorders 14, 45, 46. Frequently, many biopsies are required to make the definitive diagnosis, as morphologic and phenotypic manifestations of CTCLs are variable and information derived from a single biopsy can lead to misdiagnosis 18, 19, 45, 46. Identifying malignant cells in the peripheral blood of patients with CTCL is invaluable for detecting SS in early stages and determining prognosis 47, 48. However, blood analysis is of limited value because there is no precise marker in this analysis to detect the CTCLs in a sensitive way 9, 14. Lactate dehydrogenase (LDH) is a non-specific marker of tumor burden and is related to poor prognosis of CTCLs 9, 14. These studies provide a robust technique for assessing aberration of genes in the CTCLs 3, 35.
Detection of a malignant T cell clone is a critical marker for definite diagnosis of CTCLs. TCRγ polymerase chain reaction (PCR) analysis detects clones of T cells in only a subset of patients, whereas the sensitivity and specificity of high-throughput TCR sequencing to detect T cell clones are higher than in TCRγ PCR. Indeed, the technique of high-throughput TCR sequencing is useful for the accurate diagnosis of all stages of CTCLs, differentiation of these disorders from benign inflammatory disorders, and determination of origin and location of malignant CTCL cells 14, 44. Detection of malignant cells using flow cytometry in patients with SS is an important marker for diagnosing SS 14, 39, 45. In advanced cases, biopsies from bone marrow and lymph nodes are important factors in diagnosis 14.
The loss of cell surface markers such as CD26, CD27, and CD7 on malignant T cells is of value in diagnosing CTCLs. On the other hand, the overexpression of CD164 has been reported on CD4 + T cells of patients with SS. In flow cytometric studies, detection of more than 20% CD164 on CD4 + cells in the blood of erythrodermic cases is highly suspicious for SS. However, the sensitivity and specificity of these assays should be interpreted with caution 48. PCTCL-NOS is characterized by variable loss of almost all T cell antigens, CD56 positivity, and limited or absent CD30 expression 8. Ki67, CD34, and AgNORs are parameters of cell proliferation and angiogenesis that are well-known markers for progression of CTCLs. They are highly expressed in advanced stages of MF and are associated with shorter survival 10.
T-cell-specific soluble IL-2 receptor (sIL-2r) 9 is not specific for diagnosing CTCL but is a potential marker for activity, severity, and prognosis of this disorder. The association between increased sIL-2r and either adnexal disease or advanced-stage MF has been reported. This factor has better specificity as a prognostic factor than does LDH 9.
Recent studies have confirmed the role of the TOX gene as a disease marker of CTCLs. Moreover, this gene is a candidate for therapeutic targeting 22. Studies have suggested that EPHA4 can be a diagnostic and prognostic marker for SS 13. miRNA profiling is a diagnostic marker for CTCLs. Studies have shown that minimal miRNA classifiers can determine malignant dermatoses 25.
HTLV serology should be considered in advanced cases 14. Magnetic resonance imaging (MRI) or computed tomography (CT) scan 9 is used to investigate nodal and systemic involvement 9, 46. Fluorine-18 fluorodeoxyglucose positron emission tomography-CT ( 18F-FDG PET-CT) 46 can determine cutaneous and extracutaneous lesions in CTCLs, response to therapy, and disease recurrence. In comparison with CT scan, this modality is more sensitive and specific in detecting both cutaneous and extracutaneous involvement, particularly in determining lymph node involvement 46.
Management
There is no known cure for MF and SS 11; hence, therapeutic options are mostly palliative 1, 49, and the goals of treatment include relieving symptoms, inducing remission, and postponing progression while decreasing significant side effects caused by therapeutic modalities 12. Multi-drug therapeutic approaches are inappropriate for CTCLs because of the high risk of infection in patients with poor skin barrier 50.
Before choosing the best option for treating this malignant condition, accurate staging is essential 1, 46, 51. Generally, therapeutic options are classified into two groups. Skin-directed therapies 1, 11, 45, 52, 53 are the first choice for treating early stages of disease (IA to IIA) when involving less than 20% of the body surface 1. Systemic therapies 1, 11, 45, 52, 53 are used for refractory cases in the early stages and cases with advanced stages (at least IIB) 1, 6.
Corticosteroids in topical and systemic forms are effective in treating CTCLs 9, 54, 55. Topical corticosteroids can be employed for treating refractory cases of both early stage disease and more advanced cases. One of the problems with corticosteroid therapy is relapse of disease 1.
Retinoids are effective in treating the CTCLs through anti-proliferative and apoptosis-inducing effects 56, 57. Retinoic acid receptor β2 works as a tumor suppressor gene 56. Among topical retinoids, bexarotene, also known as Targretin, is approved by the US Food and Drug Administration (FDA) for treating stage I MF 1, 58, 59 and relapsed refractory CTCLs 58. Severe mixed hyperlipidemia with a significant decrease in high-density lipoprotein cholesterol level and central hypothyroidism are reversible, dose-dependent adverse effects of bexarotene 60. Tazarotene is another topical retinoid whose efficacy as a monotherapy has been shown in treating stages I to IIA CTCL 61. Systemic retinoids such as acitretin, isotretinoin, and bexarotene have been used successfully in the treatment of CTCLs 9.
HDACIs, classified as anti-neoplastic agents, are novel therapeutic options for treating CTCLs 1, 9, 62. Their mechanisms of action are via transcription-dependent and transcription-independent ways 11, including (a) promoting the expression of genes that regulate cell differentiation and apoptosis, (b) inducing changes to the structural integrity of chromatin 1, (c) regulating miR-22 expression 25, and (d) increasing the production of reactive oxygen species and decreasing mitochondrial membrane 63. These agents preferentially destroy transformed cells over normal cells 11.
In this therapeutic group, vorinostat 1, 11, 50, 58 and romidepsin 1, 11, 50, 58, 63 are FDA approved for treating progressive, persistent, or recurrent CTCLs. These agents, when given as single agents, can induce an overall response rate of 30% to 35%, but a complete response rate is seen in only 2% to 6% of cases 11. Entinostat, belinostat, panobinostat 1, AN-7 11, and quisinostat 64 are other HDACIs currently under study. Generally, HDACIs are well tolerated. Fatigue, gastrointestinal discomfort, thrombocytopenia, neutropenia, anemia, and dehydration are insignificant side effects which have been reported with these agents 1.
Imiquimod is a Toll-like receptor 7 (TLR7) agonist that is effective in treating MF. It works through inducing the production of interferon-alpha (IFN-α), TNF-α, IL-1α, IL-6, and IL-8 from plasmacytoid dendritic cells, which are seen in inflamed and malignant skin lesions. The efficacy of topical resiquimod, an imidazoquinoline with TLR7- and TLR8-stimulating activity, was shown in treating early stage CTCLs. Its effectiveness in inducing regression of untreated lesions was reported, probably mediated by enhancing systemic anti-tumor immunity. Resiquimod appears to act by recruiting and expanding benign T cell clones, increasing skin T cell effector and natural killer cell functions 54.
Denileukin diftitox is a recombinant fusion protein 9, 50, composed of diphtheria toxin and IL-2, approved by the FDA for treating CTCLs 51.
The efficacy of zanolimumab and alemtuzumab in treating the CTCLs has been reported. Zanolimumab has a lower risk of infection than does alemtuzumab 50.
Cytokines such as IFN-α are effective in treating cases with MF and SS but can exacerbate PCTCL-NOS 8. IFN-α2b is still the best option as first-line systemic therapy for MF 51. Recombinant IL-12 is beneficial in treating CTCLs by inducing cellular immunity and cytotoxic T cell responses in the host 54.
Chemotherapeutic agents play a role in managing CTCLs, but severe adverse effects are reported 11. For the most part, topical chemotherapeutic agents such as mechlorethamine (nitrogen mustard) and carmustine are successful in managing early stage disorders, but their effectiveness in treating advanced cases is doubtful 1, 11. Mechlorethamine was approved by the FDA for the treatment of stage Ia and Ib MF. It is an alkylating agent, which acts by inhibiting proliferating cells and affecting keratinocyte-Langerhans cell–T cell interactions. Non-melanoma skin cancers have been seen in patients who have received this agent in combination with phototherapy, radiation, and immunosuppressive chemotherapy 11.
Other systemic chemotherapeutic agents that have been used to treat CTCLs include methotrexate, chlorambucil, gemcitabine, and pegylated doxorubicin 9. Pralatrexate is a methotrexate analog approved by the FDA for treating relapsed or refractory CTCLs 50. Studies have shown variable efficacy for cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) in the treatment of advanced cases of CTCLs 9, 50.
Psoralen plus ultraviolet A (PUVA), ultraviolet B (UVB) 9, UVA1, and excimer laser 65 are among the most common treatments used for achieving remission or avoidance of progression in MF. In comparison with PUVA, UVB is less effective at treating infiltrated lesions; additionally, remission duration is shorter with UVB 9.
Radiotherapy is an effective skin-directed therapy for the treatment of CTCLs 66, 67. Lymphocytes are sensitive to radiation therapy. In more advanced cases, radiation therapy to local lesions or to the entire skin can control disease. For cases with a single lesion, this modality can be curative 66.
Electron beam radiation therapy is effective in treating CTCLs 49, 50, 68, 69 in stages I to III 50. Whole-body total skin electron beam is an appropriate modality for more advanced cases 1, 50. Complete response rate is lower in tumor-stage disease in comparison with plaque-stage cases (36% versus 98.3%) 49.
Conventional photodynamic therapy with aminolevulinic acid (ALA-PDT) is effective in a subset of CTCLs because it acts through apoptosis while the expression of death receptors like FAS in malignant T cells is low. The combination of methotrexate with ALA-PDT enhances the efficacy of photodynamic therapy through upregulating FAS by inhibiting the methylation of its promoter 70.
Extracorporeal photopheresis is an immunomodulating method resulting in the expansion of the peripheral blood dendritic cell population and enhancement of the TH1 immune response 71. It is an appropriate modality for treating refractory, early stage MF 71 and SS 50, 71. With this modality, a partial response rate of 30% to 80% and a complete remission rate of 14% to 25% are estimated 50.
Allogeneic hematopoietic stem cell transplantations are used for treating advanced stages of MF, SS 7, 53, 54, 72, 73, and PCTCL-NOS 8. Studies have shown that this therapeutic option is appropriate for young patients 7, 50, 72 with relapsing diseases which progress in spite of several lines of chemotherapy 7.
The efficacy of some agents, including tazarotene, lenalidomide, forodesine (BCX-1777), synthetic oligonucleotides, temozolomide, C-beta kinase inhibitor 50, mucin 1 C inhibitors 40, everolimus 74, PD1/PD-L1 inhibitors 75, brentuximab vedotin, and mogamulizumab, is under investigation 62, 75.
As mucin 1 is overexpressed in cells of CTCLs, therapies targeting mucin 1 are effective in treating CTCLs. Theoretically, mucin 1 C inhibitors (such as GO-203), which increase the level of reactive oxygen species and lead to oxidative-stress-induced late apoptosis/necrosis, can be an effective treatment 40. Everolimus, which targets the mammalian target of rapamycin (mTOR) pathway, appears to be effective in treating T cell lymphoma by inhibiting malignant T cell proliferation 74.
Prognosis
CTCLs are lifelong disorders that recur after discontinuation of therapy, even in cases that do not progress 54. In spite of the introduction of several therapeutic options for CTCLs, as they progress and become refractory to treatment, the malignant cells have the propensity to infiltrate lymph nodes and peripheral blood vessels, resulting in debilitating states. Progression to tumor stage where the neoplastic cells spread to the lymph nodes and internal organs has been reported in less than 5% of cases with CTCL 1. In Table 3, prognostic factors for CTCLs are listed 1, 5, 8, 9, 12, 14, 22, 27, 39, 40, 46, 51, 52, 76, 77.
Table 3. Prognostic factors in cutaneous T cell lymphoma cases.
|
Clinical factors
Age 5, 8, 52, 77 Sex 52 Staging 47, 52, 53 Extent and type of cutaneous involvement 1, 9, 47, 53 Presence of extracutaneous involvement 1, 5, 46, 52 such as blood 1, 5, 52, 53, bone marrow 8, and adnexal 9 involvement Disease progression 1 Old refractoriness to successive treatment protocols 5 |
|
Laboratory factors
Proliferation index 52 Folliculotropism 52 Presence of Sézary cells 5, 40 Large cell transformation in histology 14, 41, 52 White blood cell/lymphocyte count 52 Loss of T cell receptor repertoire 39 Increased level of soluble interleukin-2 receptor at initial diagnosis 9 Normal or high levels of lactate dehydrogenase 8, 52, 77 CD30 expression of less than 10% 52, 77 Overexpression of TOX 22 Expression of proliferation markers Ki-67, MCM-3, and MCM-7 78 MicroRNA profiling 25 Granulysin expression 27 Presence of FOXP3 + regulatory T cells 27 EPHA4 expression 13 |
In CTCL cases with visceral involvement, skin lesions are severe and the risk of skin infection is high. On the other hand, autopsy studies have shown that between 70% and 90% of patients with MF die with visceral involvement 5. Moreover, primary CTCLs have distinctive clinical behavior in comparison with systemic lymphomas with skin involvement 78.
At early stages, there is no significant difference between the life expectancy of patients with CTCL and that of healthy people 1, 5, whereas in more progressed cases, life expectancy decreases to 3.2 to 9.9 years 1.
Patients with MF have a chronic course lasting from years to decades; many of them die from unrelated disorders, whereas about 25% of them die of lymphoma 9. Immunosuppression and opportunistic infections are the most common causes of disease-related death 11. The prognosis of SS is poor. Its median survival rate is from 2 to 4 years 1, and its 5-year survival rate is approximately 18% to 20% 7. In PCTCL-NOSs, the 5-year survival rate is less than 20% 8.
Conclusion
Although there are many studies regarding the pathogenesis and management of the CTCLs, many questions about this complicated group of diseases remain unanswered.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Stefano Pileri, Unit of Haematopathology, European Institute of Oncology, Milan/Bologna University School of Medicine, Bologna, Italy
Mary Jo Lechowicz, Department of Hematology Oncology, Winship Cancer Institute, Emory University, Atlanta, Georgia, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Rodd AL, Ververis K, Karagiannis TC: Current and Emerging Therapeutics for Cutaneous T-Cell Lymphoma: Histone Deacetylase Inhibitors. Lymphoma. 2012;2012:1–10, 290685. 10.1155/2012/290685 [DOI] [Google Scholar]
- 2. Litvinov IV, Netchiporouk E, Cordeiro B, et al. : Ectopic expression of embryonic stem cell and other developmental genes in cutaneous T-cell lymphoma. Oncoimmunology. 2014;3(11):e970025. 10.4161/21624011.2014.970025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lauenborg B, Christensen L, Ralfkiaer U, et al. : Malignant T cells express lymphotoxin α and drive endothelial activation in cutaneous T cell lymphoma. Oncotarget. 2015;6(17):15235–49. 10.18632/oncotarget.3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nahidi Y, Meibodi NT, Ghazvini K, et al. : Evaluation of the Association Between Epstein-Barr Virus and Mycosis Fungoides. Indian J Dermatol. 2015;60(3):321. 10.4103/0019-5154.156423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gómez Venegas ÁA, Vargas Rubio RD: Unusual involvement in mycosis fungoides: Duodenal papilla. Rev Esp Enferm Dig. 2015. 10.17235/reed.2015.3831/2015 [DOI] [PubMed] [Google Scholar]
- 6. Devata S, Wilcox RA: Cutaneous T-Cell Lymphoma: A Review with a Focus on Targeted Agents. Am J Clin Dermatol. 2016;17(3):225–37. 10.1007/s40257-016-0177-5 [DOI] [PubMed] [Google Scholar]
- 7. Väkevä L, Niittyvuopio R, Leppä S, et al. : Allogeneic Haematopoietic Stem Cell Transplantation for Patients with Cutaneous T-cell Lymphoma. Acta Derm Venereol. 2016. 10.2340/00015555-2362 [DOI] [PubMed] [Google Scholar]
- 8. Aderhold K, Carpenter L, Brown K, et al. : Primary Cutaneous Peripheral T-Cell Lymphoma Not Otherwise Specified: A Rapidly Progressive Variant of Cutaneous T-Cell Lymphoma. Case Rep Oncol Med. 2015;2015: 429068. 10.1155/2015/429068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eklund Y, Aronsson A, Schmidtchen A, et al. : Mycosis Fungoides: A Retrospective Study of 44 Swedish Cases. Acta Derm Venereol. 2016;96(5):669–73. 10.2340/00015555-2337 [DOI] [PubMed] [Google Scholar]
- 10. Maj J, Jankowska-Konsur AM, Hałoń A, et al. : Expression of CXCR4 and CXCL12 and their correlations to the cell proliferation and angiogenesis in mycosis fungoides. Postepy Dermatol Alergol. 2015;32(6):437–42. 10.5114/pdia.2015.48034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moyal L, Feldbaum N, Goldfeiz N, et al. : The Therapeutic Potential of AN-7, a Novel Histone Deacetylase Inhibitor, for Treatment of Mycosis Fungoides/Sezary Syndrome Alone or with Doxorubicin. PLoS One. 2016;11(1):e0146115. 10.1371/journal.pone.0146115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choi J, Goh G, Walradt T, et al. : Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47(9):1011–9. 10.1038/ng.3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hameetman L, van der Fits L, Zoutman WH, et al. : EPHA4 is overexpressed but not functionally active in Sézary syndrome. Oncotarget. 2015;6(31):31868–76. 10.18632/oncotarget.5573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benjamin Chase A, Markel K, Tawa MC: Optimizing Care and Compliance for the Treatment of Mycosis Fungoides Cutaneous T-Cell Lymphoma With Mechlorethamine Gel. Clin J Oncol Nurs. 2015;19(6):E131–9. 10.1188/15.CJON.E131-E139 [DOI] [PubMed] [Google Scholar]
- 15. Foo SH, Shah F, Chaganti S, et al. : Unmasking mycosis fungoides/Sézary syndrome from preceding or co-existing benign inflammatory dermatoses requiring systemic therapies: patients frequently present with advanced disease and have an aggressive clinical course. Br J Dermatol. 2016;174(4):901–4. 10.1111/bjd.14238 [DOI] [PubMed] [Google Scholar]
- 16. Pankratov O, Gradova S, Tarasevich S, et al. : Poikilodermatous mycosis fungoides: clinical and histopathological analysis of a case and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2015;24(2):37–41. 10.15570/actaapa.2015.10 [DOI] [PubMed] [Google Scholar]
- 17. LeBlanc RE, Tavallaee M, Kim YH, et al. : Useful Parameters for Distinguishing Subcutaneous Panniculitis-like T-Cell Lymphoma From Lupus Erythematosus Panniculitis. Am J Surg Pathol. 2016;40(6):745–54. 10.1097/PAS.0000000000000596 [DOI] [PubMed] [Google Scholar]
- 18. Smoller BR, Bishop K, Glusac E, et al. : Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol. 1995;19(12):1423–30. [DOI] [PubMed] [Google Scholar]
- 19. Kash N, Massone C, Fink-Puches R, et al. : Phenotypic Variation in Different Lesions of Mycosis Fungoides Biopsied Within a Short Period of Time From the Same Patient. Am J Dermatopathol. 2016;38(7):541–5. 10.1097/DAD.0000000000000493 [DOI] [PubMed] [Google Scholar]
- 20. Smoller BR: Mycosis fungoides: what do/do not we know? J Cutan Pathol. 2008;35(Suppl 2):35–9. 10.1111/j.1600-0560.2008.01120.x [DOI] [PubMed] [Google Scholar]
- 21. Lebas E, Libon F, Nikkels AF: Koebner Phenomenon and Mycosis Fungoides. Case Rep Dermatol. 2015;7(3):287–91. 10.1159/000440856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dulmage BO, Akilov O, Vu JR, et al. : Dysregulation of the TOX-RUNX3 pathway in cutaneous T-cell lymphoma. Oncotarget. 2015. 10.18632/oncotarget.5742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang L, Ni X, Covington KR, et al. : Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47(12):1426–34. 10.1038/ng.3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. da Silva Almeida AC, Abate F, Khiabanian H, et al. : The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47(12):1465–70. 10.1038/ng.3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sibbesen NA, Kopp KL, Litvinov IV, et al. : Jak3, STAT3, and STAT5 inhibit expression of miR-22, a novel tumor suppressor microRNA, in cutaneous T-Cell lymphoma. Oncotarget. 2015;6(24):20555–69. 10.18632/oncotarget.4111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Woollard WJ, Kalaivani NP, Jones CL, et al. : Independent Loss of Methylthioadenosine Phosphorylase (MTAP) in Primary Cutaneous T-Cell Lymphoma. J Invest Dermatol. 2016;136(6):1238–46. 10.1016/j.jid.2016.01.028 [DOI] [PubMed] [Google Scholar]
- 27. Shareef MM, Elgarhy LH, Wasfy Rel-S: Expression of Granulysin and FOXP3 in Cutaneous T Cell Lymphoma and Sézary Syndrome. Asian Pac J Cancer Prev. 2015;16(13):5359–64. 10.7314/APJCP.2015.16.13.5359 [DOI] [PubMed] [Google Scholar]
- 28. McGirt LY, Jia P, Baerenwald DA, et al. : Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126(4):508–19. 10.1182/blood-2014-11-611194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jankowska-Konsur A, Kobierzycki C, Reich A, et al. : Expression of SATB1, MTI/II and Ki-67 in Mycosis Fungoides. Anticancer Res. 2016;36(1):189–97. [PubMed] [Google Scholar]
- 30. Ungewickell A, Bhaduri A, Rios E, et al. : Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47(8):1056–60. 10.1038/ng.3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gallardo F, Sandoval J, Díaz-Lagares A, et al. : Notch1 Pathway Activation Results from the Epigenetic Abrogation of Notch-Related MicroRNAs in Mycosis Fungoides. J Invest Dermatol. 2015;135(12):3144–52. 10.1038/jid.2015.328 [DOI] [PubMed] [Google Scholar]
- 32. Tensen CP: PLCG1 Gene Mutations in Cutaneous T-Cell Lymphomas Revisited. J Invest Dermatol. 2015;135(9):2153–4. 10.1038/jid.2015.221 [DOI] [PubMed] [Google Scholar]
- 33. Kitadate A, Ikeda S, Teshima K, et al. : MicroRNA-16 mediates the regulation of a senescence-apoptosis switch in cutaneous T-cell and other non-Hodgkin lymphomas. Oncogene. 2016;35(28):3692–704. 10.1038/onc.2015.435 [DOI] [PubMed] [Google Scholar]
- 34. Katona TM, Smoller BR, Webb AL, et al. : Expression of PTEN in mycosis fungoides and correlation with loss of heterozygosity. Am J Dermatopathol. 2013;35(5):555–60. 10.1097/DAD.0b013e318276cc68 [DOI] [PubMed] [Google Scholar]
- 35. Katona TM, O'Malley DP, Cheng L, et al. : Loss of heterozygosity analysis identifies genetic abnormalities in mycosis fungoides and specific loci associated with disease progression. Am J Surg Pathol. 2007;31(10):1552–6. 10.1097/PAS.0b013e3180408d76 [DOI] [PubMed] [Google Scholar]
- 36. Kabasawa M, Sugaya M, Oka T, et al. : Decreased interleukin-21 expression in skin and blood in advanced mycosis fungoides. J Dermatol. 2016;43(7):819–22. 10.1111/1346-8138.13278 [DOI] [PubMed] [Google Scholar]
- 37. Vieyra-Garcia PA, Wei T, Naym DG, et al. : STAT3/5-Dependent IL9 Overexpression Contributes to Neoplastic Cell Survival in Mycosis Fungoides. Clin Cancer Res. 2016;22(13):3328–39. 10.1158/1078-0432.CCR-15-1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ikeda S, Kitadate A, Ito M, et al. : Disruption of CCL20-CCR6 interaction inhibits metastasis of advanced cutaneous T-cell lymphoma. Oncotarget. 2016;7(12):13563–74. 10.18632/oncotarget.6916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guenova E, Ignatova D, Chang YT, et al. : Expression of CD164 on Malignant T cells in Sézary Syndrome. Acta Derm Venereol. 2016;96(4):464–7. 10.2340/00015555-2264 [DOI] [PubMed] [Google Scholar]
- 40. Jain S, Stroopinsky D, Yin L, et al. : Mucin 1 is a potential therapeutic target in cutaneous T-cell lymphoma. Blood. 2015;126(3):354–62. 10.1182/blood-2015-02-628149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pousa CM, Nery NS, Mann D, et al. : Granulomatous mycosis fungoides--a diagnostic challenge. An Bras Dermatol. 2015;90(4):554–6. 10.1590/abd1806-4841.20153460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Willerslev-Olsen A, Krejsgaard T, Lindahl LM, et al. : Staphylococcal enterotoxin A (SEA) stimulates STAT3 activation and IL-17 expression in cutaneous T-cell lymphoma. Blood. 2016;127(10):1287–96. 10.1182/blood-2015-08-662353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sidiropoulos KG, Martinez-Escala ME, Yelamos O, et al. : Primary cutaneous T-cell lymphomas: a review. J Clin Pathol. 2015;68(12):1003–10. 10.1136/jclinpath-2015-203133 [DOI] [PubMed] [Google Scholar]
- 44. Kirsch IR, Watanabe R, O'Malley JT, et al. : TCR sequencing facilitates diagnosis and identifies mature T cells as the cell of origin in CTCL. Sci Transl Med. 2015;7(308):308ra158. 10.1126/scitranslmed.aaa9122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hughes CF, Newland K, McCormack C, et al. : Mycosis fungoides and Sézary syndrome: Current challenges in assessment, management and prognostic markers. Australas J Dermatol. 2015. 10.1111/ajd.12349 [DOI] [PubMed] [Google Scholar]
- 46. Alanteri E, Usmani S, Marafi F, et al. : The role of fluorine-18 fluorodeoxyglucose positron emission tomography in patients with mycosis fungoides. Indian J Nucl Med. 2015;30(3):199–203. 10.4103/0972-3919.158527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gibson JF, Huang J, Liu KJ, et al. : Cutaneous T-cell lymphoma (CTCL): Current practices in blood assessment and the utility of T-cell receptor (TCR)-Vβ chain restriction. J Am Acad Dermatol. 2016;74(5):870–7. 10.1016/j.jaad.2015.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Aggarwal S, Topaloglu H, Kumar S: Systematic Review Of Burden Of Cutaneous T-Cell Lymphoma. Value Health. 2015;18(7):A438. 10.1016/j.jval.2015.09.1065 26532464 [DOI] [Google Scholar]
- 49. Ahmed SK, Grams MP, Locher SE, et al. : Adaptation of the Stanford technique for treatment of bulky cutaneous T-cell lymphoma of the head. Pract Radiat Oncol. 2016;6(3):183–6. 10.1016/j.prro.2015.10.021 [DOI] [PubMed] [Google Scholar]
- 50. Chmielowska E, Studziński M, Giebel S, et al. : Follow-up of patients with mycosis fungoides after interferon α2b treatment failure. Postepy Dermatol Alergol. 2015;32(2):67–72. 10.5114/pdia.2014.40941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Scarisbrick JJ, Prince HM, Vermeer MH, et al. : Cutaneous Lymphoma International Consortium Study of Outcome in Advanced Stages of Mycosis Fungoides and Sézary Syndrome: Effect of Specific Prognostic Markers on Survival and Development of a Prognostic Model. J Clin Oncol. 2015;33(32):3766–73. 10.1200/JCO.2015.61.7142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wilcox RA: Cutaneous T-cell lymphoma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91(1):151–65. 10.1002/ajh.24233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Virmani P, Zain J, Rosen ST, et al. : Hematopoietic Stem Cell Transplant for Mycosis Fungoides and Sézary Syndrome. Dermatol Clin. 2015;33(4):807–18. 10.1016/j.det.2015.05.014 [DOI] [PubMed] [Google Scholar]
- 54. Rook AH, Gelfand JM, Wysocka M, et al. : Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126(12):1452–61. 10.1182/blood-2015-02-630335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nguyen CV, Bohjanen KA: Skin-Directed Therapies in Cutaneous T-Cell Lymphoma. Dermatol Clin. 2015;33(4):683–96. 10.1016/j.det.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 56. Kato Y, Egusa C, Maeda T, et al. : Combination of retinoid and histone deacetylase inhibitor produced an anti-tumor effect in cutaneous T-cell lymphoma by restoring tumor suppressor gene, retinoic acid receptorβ2, via histone acetylation. J Dermatol Sci. 2016;81(1):17–25. 10.1016/j.jdermsci.2015.10.016 [DOI] [PubMed] [Google Scholar]
- 57. Huen AO, Kim EJ: The Role of Systemic Retinoids in the Treatment of Cutaneous T-Cell Lymphoma. Dermatol Clin. 2015;33(4):715–29. 10.1016/j.det.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 58. Zinzani PL, Bonthapally V, Huebner D, et al. : Panoptic clinical review of the current and future treatment of relapsed/refractory T-cell lymphomas: Cutaneous T-cell lymphomas. Crit Rev Oncol Hematol. 2016;99:228–40. 10.1016/j.critrevonc.2015.12.018 [DOI] [PubMed] [Google Scholar]
- 59. Marciano DP, Kuruvilla DS, Pascal BD, et al. : Identification of Bexarotene as a PPARγ Antagonist with HDX. PPAR Res. 2015;2015: 254560. 10.1155/2015/254560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rodriguez Suarez S, Pamies Andreu E, Muñiz Grijalvo O, et al. : [Thyroid and lipidic dysfunction associated with bexarotene in cutaneous T-cell lymphoma]. Med Clin (Barc). 2016;146(3):117–20. 10.1016/j.medcli.2015.07.017 [DOI] [PubMed] [Google Scholar]
- 61. Besner Morin C, Roberge D, Turchin I, et al. : Tazarotene 0.1% Cream as Monotherapy for Early-Stage Cutaneous T-Cell Lymphoma. J Cutan Med Surg. 2016;20(3):244–8. 10.1177/1203475415626686 [DOI] [PubMed] [Google Scholar]
- 62. Chung CG, Poligone B: Cutaneous T cell Lymphoma: an Update on Pathogenesis and Systemic Therapy. Curr Hematol Malig Rep. 2015;10(4):468–76. 10.1007/s11899-015-0293-y [DOI] [PubMed] [Google Scholar]
- 63. Valdez BC, Brammer JE, Li Y, et al. : Romidepsin targets multiple survival signaling pathways in malignant T cells. Blood Cancer J. 2015;5:e357. 10.1038/bcj.2015.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Child F, Ortiz-Romero PL, Alvarez R, et al. : Phase II Multicenter Trial of Oral Quisinostat, a Histone Deacetylase Inhibitor, in Patients with Previously Treated Stage IB-IVA Mycosis Fungoides/Sézary Syndrome. Br J Dermatol. 2016. 10.1111/bjd.14427 [DOI] [PubMed] [Google Scholar]
- 65. Olsen EA, Hodak E, Anderson T, et al. : Guidelines for phototherapy of mycosis fungoides and Sézary syndrome: A consensus statement of the United States Cutaneous Lymphoma Consortium. J Am Acad Dermatol. 2016;74(1):27–58. 10.1016/j.jaad.2015.09.033 [DOI] [PubMed] [Google Scholar]
- 66. Tandberg DJ, Craciunescu O, Kelsey CR: Radiation Therapy for Cutaneous T-Cell Lymphomas. Dermatol Clin. 2015;33(4):703–13. 10.1016/j.det.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 67. Goddard AL, Vleugels RA, LeBoeuf NR, et al. : Palliative Therapy for Recalcitrant Cutaneous T-Cell Lymphoma of the Hands and Feet With Low-Dose, High Dose-Rate Brachytherapy. JAMA Dermatol. 2015;151(12):1354–7. 10.1001/jamadermatol.2015.3028 [DOI] [PubMed] [Google Scholar]
- 68. Elsayad K, Kriz J, Moustakis C, et al. : Total Skin Electron Beam for Primary Cutaneous T-cell Lymphoma. Int J Radiat Oncol Biol Phys. 2015;93(5):1077–86. 10.1016/j.ijrobp.2015.08.041 [DOI] [PubMed] [Google Scholar]
- 69. Gamsiz H, Beyzadeoglu M, Sager O, et al. : Evaluation of mycosis fungoides management by total skin electron beam therapy with "translational technique". J BUON. 2015;20(4):1124–31. [PubMed] [Google Scholar]
- 70. Salva KA, Wood GS: Epigenetically Enhanced Photodynamic Therapy (ePDT) is Superior to Conventional Photodynamic Therapy for Inducing Apoptosis in Cutaneous T-Cell Lymphoma. Photochem Photobiol. 2015;91(6):1444–51. 10.1111/php.12521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zic JA: Extracorporeal Photopheresis in the Treatment of Mycosis Fungoides and Sézary Syndrome. Dermatol Clin. 2015;33(4):765–76. 10.1016/j.det.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 72. Oka T, Sugaya M, Cury-Martins J, et al. : Hematopoietic stem cell transplantation for cutaneous T-cell lymphoma: Summary of 11 cases from two facilities in Japan and Brazil. J Dermatol. 2016;43(6):638–42. 10.1111/1346-8138.13199 [DOI] [PubMed] [Google Scholar]
- 73. Hosing C, Bassett R, Dabaja B, et al. : Allogeneic stem-cell transplantation in patients with cutaneous lymphoma: updated results from a single institution. Ann Oncol. 2015;26(12):2490–5. 10.1093/annonc/mdv473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Witzig TE, Reeder C, Han JJ, et al. : The mTORC1 inhibitor everolimus has antitumor activity in vitro and produces tumor responses in patients with relapsed T-cell lymphoma. Blood. 2015;126(3):328–35. 10.1182/blood-2015-02-629543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rozati S, Kim YH: Experimental treatment strategies in primary cutaneous T-cell lymphomas. Curr Opin Oncol. 2016;28(2):166–71. 10.1097/CCO.0000000000000272 [DOI] [PubMed] [Google Scholar]
- 76. Talpur R, Sui D, Gangar P, et al. : Retrospective Analysis of Prognostic Factors in 187 Cases of Transformed Mycosis Fungoides. Clin Lymphoma Myeloma Leuk. 2016;16(1):49–56. 10.1016/j.clml.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 77. Jankowska-Konsur A, Kobierzycki C, Reich A, et al. : Expression of MCM-3 and MCM-7 in Primary Cutaneous T-cell Lymphomas. Anticancer Res. 2015;35(11):6017–26. [PubMed] [Google Scholar]
- 78. Lee HS, Suh KS, Lee DY, et al. : Cutaneous Lymphoma in Korea: A Nationwide Retrospective Study. Acta Derm Venereol. 2016;96(4):535–9. 10.2340/00015555-2283 [DOI] [PubMed] [Google Scholar]