Abstract
In recent years, there have been a number of advances in the pathogenesis and treatment of atherosclerosis and in assessing prognosis in carotid atherosclerosis. Risk stratification to improve vascular prevention by identifying patients most likely to benefit from intensive therapy is much improved by measuring carotid plaque burden. In patients with asymptomatic carotid stenosis, a number of modalities can be used to identify the 10-15% who could benefit from endarterectomy or stenting. Transcranial Doppler embolus detection, echolucency and ulceration on 3D ultrasound, intraplaque hemorrhage on magnetic resonance imaging (MRI), and reduced cerebrovascular reserve are useful already; new approaches including plaque texture on ultrasound and imaging of plaque inflammation and early calcification on positron emission tomography/computed tomography (PET/CT) are in development. The discovery that the intestinal microbiome produces vasculotoxic metabolites from dietary constituents such as carnitine in meat (particularly red meat) and phosphatidylcholine from egg yolk and other sources has revolutionized nutritional aspects of vascular prevention. Because many of these vasculotoxic metabolites are removed by the kidney, it is particularly important in patients with renal failure to limit their intake of red meat and egg yolk. A new approach to lowering low-density lipoprotein (LDL) cholesterol by blocking the action of an enzyme that destroys LDL receptors promises to revolutionize vascular prevention once less costly treatments are developed, and a new approach to vascular prevention—“treating arteries instead of risk factors”—shows promise but requires randomized trials. These advances all promise to help in the quest to prevent strokes in high-risk patients.
Keywords: atherosclerosis, carotid plaque, carnitine, LDL, cholesterol, Transcranial Doppler embolus detection
Introduction
In recent years, there have been important advances in the pathogenesis and treatment of atherosclerosis and in assessing prognosis in carotid atherosclerosis. The effect of the intestinal microbiome on atherosclerosis has revolutionized thinking about diet 1 and about the role of renal failure in increasing cardiovascular risk. In the past, routine treatment with usual therapy for atherosclerosis has reduced cardiovascular risk in most trials by only ~9–30% 2, resulting in a residual risk of 70–80% 3– 6. Recent advances in lipid-lowering therapy, based on a novel mechanism based on blocking the effects of an enzyme that destroys receptors for low-density lipoprotein (LDL) cholesterol, make it possible to lower LDL to a greater degree and in more patients than was previously possible 7. Lifelong reduction of LDL resulting from a hereditary cause of low levels of LDL results in a reduction of coronary risk by ~95% 8, 9, and a new approach to therapy based on “treating arteries instead of treating risk factors” 10 reduced the very high risk in patients with asymptomatic carotid stenosis by more than 80% 11. Most patients (~90%) with asymptomatic carotid stenosis would be better treated by intensive medical therapy than by carotid endarterectomy (CEA) or carotid artery stenting (CAS) 12. Methods to identify the few (10–15%) who could benefit from intervention are being developed 13. In this review, I focus on advances in the understanding of the role of the intestinal microbiome and renal impairment on atherosclerosis, measurement of carotid plaque burden, carotid ulceration and ulcer volume, plaque texture, and detection of microemboli by transcranial Doppler (TCD).
Advances in pathogenesis of atherosclerosis
Atherosclerosis may be thought of as a response to injury 14– 16, related to flow disturbances that injure the endothelium 17, followed by adhesion of platelets, penetration of macrophages into the subendothelium, inflammation 18, oxidative stress, LDL oxidation, and proliferation of smooth muscle cells, similar to a scar in the artery wall. Risk factors identified in the Framingham Heart Study included hypertension, smoking, elevated LDL, diabetes, and left ventricular hypertrophy (essentially reflecting the integral of blood pressure over recent years). The importance of diet has been largely unappreciated, perhaps because lowering of fasting LDL with drugs such as statins has seemingly countered any effect of diet on fasting lipids. However, this misplaced focus on fasting lipids misses the key effects of diet, which occur during the post-prandial state 19. A high-fat/high-cholesterol meal increases arterial inflammation 20, 21 and oxidative stress, and impairs endothelial function 22, for several hours. Most of the day is spent in the post-prandial state, so diet is much more important than would be predicted by fasting LDL.
Several studies have shown that healthy lifestyle choices (not smoking, moderate alcohol intake, regular exercise, consuming a healthy diet, and maintaining a healthy weight) markedly reduce the risk of stroke. In the US Health Professionals Study and the Nurses’ Health study, persons who adopted all of these choices had an 80% reduction of stroke 23; Swedish men with coronary artery disease who did so also had an 80% reduction of recurrent myocardial infarction 24. The Cretan Mediterranean diet reduced cardiovascular events by 70% in secondary prevention 25 and reduced stroke by nearly half in high-risk primary prevention 26. It has been clear for many years that dietary cholesterol increases coronary risk 27. Now there is a completely new window opening on the relationship between diet and atherosclerosis, relating to the intestinal microbiome 1.
Intestinal microbiome and diet
In recent years, it has become clear that an important interaction between diet and the intestinal microbiome brings into play additional metabolic factors that aggravate atherosclerosis beyond dietary cholesterol. This may help to explain the benefits of the Mediterranean diet. Hazen’s group from the Cleveland Clinic reported that phosphatidylcholine from egg yolk 28 and carnitine 29 from animal flesh (four times as much in red meat as in fish or chicken) are converted by intestinal bacteria to trimethylamine (the compound that causes uremic breath to smell fishy). Trimethylamine is oxidized in the liver to trimethylamine N-oxide (TMAO) ( Figure 1), which causes atherosclerosis in animal models. In patients referred for coronary angiography, high levels of TMAO following a test dose of two hard-boiled eggs markedly increased risk. Patients in the top quartile of TMAO had a 2.5-fold increase in the 3-year risk of stroke, death, or myocardial infarction 30.
Figure 1. Pathways linking dietary phosphatidylcholine, intestinal microbiota, and incident adverse cardiovascular events.
Ingested phosphatidylcholine (lecithin), the major dietary source of total choline, is acted on by intestinal lipases to form a variety of metabolic products, including the choline-containing nutrients glycerophosphocholine, phosphocholine, and choline. Choline-containing nutrients that reach the cecum and large bowel may serve as fuel for intestinal microbiota (gut flora), producing trimethylamine (TMA). TMA is rapidly further oxidized to trimethylamine N-oxide (TMAO) by hepatic flavin-containing monooxygenases (FMOs). TMAO enhances the accumulation of cholesterol in macrophages, the accumulation of foam cells in artery walls, and atherosclerosis, all factors that are associated with an increased risk of heart attack, stroke, and death. Choline can also be oxidized to betaine in both the liver and the kidneys. Dietary betaine can serve as a substrate for bacteria to form TMA and presumably TMAO. Reproduced by permission of the Massachusetts Medical Society from: Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL: Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N Engl J Med 2013, 368(17): 1575–1584.
A key issue is that vegans who consumed L-carnitine did not produce TMAO because they did not have the intestinal bacteria that produce TMA from carnitine 29; this means that the intestinal microbiome is modifiable. A novel approach to treating atherosclerosis would be the eradication of harmful bacteria with antibiotics and recolonization by stool transplantation with beneficial bacteria. This is entirely analogous to the treatment of Clostridium difficile infection by “repoopulation” 31. Our group is studying that possibility.
Interaction with renal failure
Patients with renal failure have high levels of total homocysteine (tHcy), but this accounts for only ~20% of the effect of impaired renal function on carotid plaque 32. Elevated levels of thiocyanate and asymmetric dimethylarginine (a nitric oxide antagonist) add further to the very high cardiovascular risk 33, 34 in renal failure; this is not new. What is recently recognized is that metabolic products of the intestinal microbiome are excreted in the urine, so patients with renal failure also have high levels of TMAO, which accelerate the decline of renal function and increase cardiovascular risk 35. Besides TMAO, other metabolic products of the intestinal microbiome that probably contribute to cardiovascular risk in renal failure include indoxyl sulfate, indole-3-acetic acid, p-cresyl sulfate 32, and phenylacetylglutamine 36. Thus patients at risk of cardiovascular disease should limit their intake of meat and egg yolk not only because of the high cholesterol content but also because of the carnitine in meat (particularly red meat) and the phosphatidylcholine in egg yolk. This is particularly important in patients with renal failure.
Advances in treatment of atherosclerosis
Blocking the action of proprotein convertase subtilisin-kexin type 9
Inhibitors of the rate-limiting step in cholesterol synthesis, hydroxymethylglutarate CoA (HMG-CoA) reductase (statins), reduce fasting LDL and reduce cardiovascular events. Those effects are enhanced by combination with ezetimibe, a drug that blocks cholesterol absorption. However, many patients are intolerant of statins. Although there are many myths 37 regarding the adverse effects of statins, such as hepatotoxicity, nephrotoxicity, intracerebral hemorrhage, cataracts, and cognitive decline, true causally related adverse effects include myopathy and a slightly increased risk of diabetes. These probably depend mainly on impairment of mitochondrial function 37 by depletion of ubiquinone (coenzyme Q10), which is needed for mitochondrial function.
An entirely distinct approach to lowering LDL and reducing cardiovascular events that has recently become available is blocking the action of proprotein convertase subtilisin-kexin type 9 (PCSK9), an enzyme that breaks down LDL receptors. By preventing the breakdown of these receptors, and increasing their number and duration of effect, LDL and cardiovascular events are both lowered by ~50% 38, 39. Present approaches to blocking the action of PCSK9—monoclonal antibodies or RNA interference—are very (prohibitively) costly 40, but it is to be hoped that far less costly small molecules to achieve this end will be developed before long.
Treating arteries instead of treating risk factors
A different approach to reducing residual risk is to treat the actual burden of atherosclerosis 41 instead of treating intermediate targets—risk factors such as level of blood pressure or LDL 10. This paradigm was developed in response to the recognition that treating patients according to then current guidelines was failing half our patients: they had plaque progression, and their risk was twice that of patients with stable plaque or plaque regression, after controlling for coronary risk factors 42. This approach, initiated by our group in 2003, was found in 2010 11 to halve the proportion of patients with plaque progression (to a quarter), double the proportion of patients with regression of plaque (to half) 10, reduce microemboli on TCD by three quarters, and reduce the very high risk of patients with asymptomatic carotid stenosis by over 80% 11: the 2-year risk of stroke fell from 8.8% to 1% and the 2-year risk of myocardial infarction from 7.6% to 1%. Efforts are underway to conduct randomized trials of usual care versus “treating arteries” using measurement of 3D plaque volume ( Figure 2), the most sensitive method available to assess the effects of therapies on atherosclerosis 43, 44.
Figure 2. Procedure for determining plaque volumes from 3D ultrasound images.
a) An approximate axis of the vessel is selected in a longitudinal view (colored line) and the internal elastic lamina and lumen boundary are outlined (yellow). b) Using the surfaces generated by the vessel contours and the 3D ultrasound image, the position of the bifurcation (BF; yellow arrow) is determined and marked. The axis of the vessel is selected based on the bifurcation point and marked along the branch as far as the plaque can be measured (colored line). This axis will be used as a reference for distance measurements. c) All plaques within the measurable distance are outlined, different colors being used for each separate plaque to aid in identification. d) Volumes are calculated for each plaque, and surfaces of the vessel wall and plaques are generated to better visualize the plaques in relation to the carotid arteries. Reproduced by permission of Wolters Kluwer from: Ainsworth CD, Blake CC, Tamayo A, Beletsky V, Fenster A, Spence JD: 3D ultrasound measurement of change in carotid plaque volume: a tool for rapid evaluation of new therapies. Stroke 2005, 36(9): 1904–1909.
Assessing prognosis in carotid atherosclerosis
Although it has been reasonably clear that patients with severe symptomatic carotid stenosis benefit from CEA or CAS, the periprocedural risk of stroke or death with CAS is approximately twice that with CEA. However, the risk of asymptomatic carotid stenosis with modern medical therapy has declined markedly in recent years to ~0.5% per year 11, 45, 46, so it has even been suggested 47 that randomized trials in symptomatic stenosis should be repeated comparing intervention with intensive medical therapy. There is a major unwarranted controversy regarding the treatment of asymptomatic carotid stenosis. In the United States, ~90% of carotid intervention is for asymptomatic stenosis, even though 90% of patients would be better treated with intensive medical therapy 12. This is being justified by an invalid extrapolation from the medical risks in randomized trials conducted decades ago to modern risks with CEA and CAS. Although the most recent trials report that the risk of stroke or death after first deducting periprocedural risk is similar to that with modern intensive medical therapy (~0.5% per year), the risk with intervention is still much higher than the risk with medical therapy once the periprocedural risks are taken into account: ~3% with CAS and 1.5% with CEA. Furthermore, there are important caveats regarding the higher risk in real-world practice as opposed to the risks with carefully vetted interventionalists in randomized trials 48, 49. There are huge discrepancies across the world in the proportion of carotid interventions performed for asymptomatic stenosis: 90% in the US, ~60% in Italy and Germany, ~15% in Canada and Australia (about right), and 0% in Denmark. These discrepancies call into question not only the advisability but also the ethics of routine intervention for asymptomatic stenosis, as practiced in the United States. The reasons for this practice do not bear scrutiny 50, 51.
Approximately 10–15% of patients with asymptomatic stenosis could benefit from intervention, and fortunately there are methods available to identify them. Reduced cerebrovascular blood flow reserve is one approach that appears to be promising. In development are a number of other approaches based on imaging of vulnerable plaque, including intraplaque hemorrhage on magnetic resonance imaging (MRI) scans, neovascularity of plaques with contrast ultrasound, and plaque inflammation on positron emission tomography/computed tomography (PET/CT) scans 13. Here, I will focus on the uses of ultrasound in assessing prognosis in patients with carotid atherosclerosis.
Measuring carotid plaque burden
Although widely regarded as “preclinical atherosclerosis”, carotid intima-media thickness (IMT) is a different phenotype. This issue is complicated by two different approaches to the measurement of IMT—with and without plaque thickness—(the latter being a one-dimensional measurement of plaque in those participants with plaque). Then in studies in which plaque thickness is included, participants with and without plaque thickness are combined, conflating the issue 52. It is increasingly clear that measuring carotid plaque burden is superior to measuring IMT, both for risk stratification and for assessment of effects of therapy 53. Plaque burden can be measured as total plaque area (TPA) 42 (the sum of the areas of all plaques seen in the extracranial carotid arteries) or total plaque volume (TPV) 54. In the High Risk Plaque study, 3D plaque burden was highly correlated with coronary calcium, whereas IMT was not 55, and plaque burden predicted risk of cardiovascular events to a similar extent as coronary calcium 56. Often plaque volume is measured not as TPV but as the volume over a defined segment of the carotids, limited to a defined distance above and below the bifurcation. This approach has advantages for such purposes as assessing effects of therapy (simplicity, potential for automatic measurement) but may lose dynamic range, a potential issue with regard to prognostic value. Besides the issue of IMT not truly representing atherosclerosis, a key reason why IMT is a weak predictor of cardiovascular risk 57 is its narrow dynamic range: ~0.5 to 1.5 mm. The dynamic range of TPA is much greater – from 0 to ~1200 mm 2; the range of TPV would be even greater. After adjustment for age, sex, blood pressure, smoking, serum cholesterol, diabetes, homocysteine, and treatment of blood pressure and cholesterol, TPA strongly predicted risk among patients attending a vascular prevention clinic: the 5-year risk of stroke, death, or myocardial infarction, by quartile of TPA, was 5.6%, 10.7%, 13.9%, and 19.5%. Plaque progression also increased risk; patients with plaque progression in the first year of follow up had twice the risk of those with stable plaque or plaque regression. These findings were substantiated in a population-based study in Tromsø, Norway 58, 59. Myocardial infarction and stroke were both strongly predicted by TPA but not by IMT in the common carotid where there was no plaque. The annual change in IMT is too small in relation to the resolution of the method to permit measurement in time frames that are clinically meaningful: the annual change is ~0.15 mm, and the resolution of the method is ~0.2–0.3 mm. A much more useful measurement in persons who do not have plaque would be to measure vessel wall volume ( Figure 3) 60; this parameter amounts to a 3D measurement of the intima-media, has a much greater dynamic range, and unlike IMT, is sensitive to effects of therapy 61, 62. Progression of IMT does not predict risk 63, even in large populations, whereas progression of 3D plaque volume predicted cardiovascular events in a small population of patients in our study 64.
Figure 3. Vessel wall volume.
Vessel wall volume segmentation. ( a) The transverse view of the common carotid artery shows the vessel boundary outlined in red and the lumen boundary outlined in yellow. ( b) The 3D ultrasound image volume is sliced longitudinally to reveal the vessel and lumen boundaries in the common, internal, and external carotid branches. The internal carotid artery vessel and lumen boundaries are shown in blue and pink, respectively. Reproduced by permission of Elsevier from: Egger M, Spence JD, Fenster A, Parraga G: Validation of 3d ultrasound vessel wall volume: an imaging phenotype of carotid atherosclerosis. Ultrasound Med Biol 2007, 33(6): 905–914.
Transcranial Doppler embolus detection
Perhaps the best validated method for identifying high-risk patients with asymptomatic carotid stenosis is TCD embolus detection 12, 65. Figure 4 shows a microembolus in a patient with asymptomatic stenosis. Patients with asymptomatic carotid stenosis who had two or more microemboli in 1 hour of monitoring had a 1-year risk of stroke of 15.6%, indicating that they could benefit from CEA or CAS. The cost of a TCD machine is less than the cost of two CAS, and training and certification in TCD embolus detection can be obtained in a course of 3 or fewer days. Thus TCD embolus detection or some other procedure to identify the patient as being at a higher risk than that with intervention should be considered before patients undergo CAS or CEA for asymptomatic stenosis.
Figure 4. Transcranial Doppler embolus detection.
Microembolus in a patient with asymptomatic carotid stenosis. The upper channel is an M-mode image of an embolus in the middle cerebral artery; the lower panel shows the high-intensity transit signal in the Doppler channel. Besides the visual appearance of the microembolus, a characteristic clicking sound is heard. Reproduced by permission of the Society for Vascular Ultrasound from: Spence JD. Transcranial Doppler: uses in stroke prevention. The Journal for Vascular Ultrasound 2015, 39(4): 183–187.
Plaque characteristics and prediction of risk
In patients with asymptomatic carotid stenosis, the presence of three or more ulcers in either or both carotid arteries carried a similar risk as the presence of microemboli: an 18% 3-year risk of stroke or death. Those with two or more microemboli had a 20% 3-year risk 66. By combining TCD embolus detection and detection of three or more ulcers, the proportion of patients with asymptomatic stenosis who could benefit from intervention was increased from 5% to 10%. In the Asymptomatic Carotid Emboli Study (ACES) 67, plaque echolucency at baseline increased the risk of ipsilateral stroke (hazard ratio [HR] 6.43, 95% confidence interval [CI] 1.36–30.44, P=0.019). A combination of plaque echolucency and presence of TCD microemboli markedly increased the risk of ipsilateral stroke (HR 10.61, 95% CI 2.98–37.82, P=0.0003). This association remained significant after controlling for risk factors, degree of carotid stenosis, and antiplatelet medication. Juxtaluminal black plaque (plaque or thrombus that is so echolucent that it can be seen only by observing a gap between the wall and the Doppler flow signal) 68, plaque echolucency, and plaque texture analysis of ultrasound 69 identified higher risk.
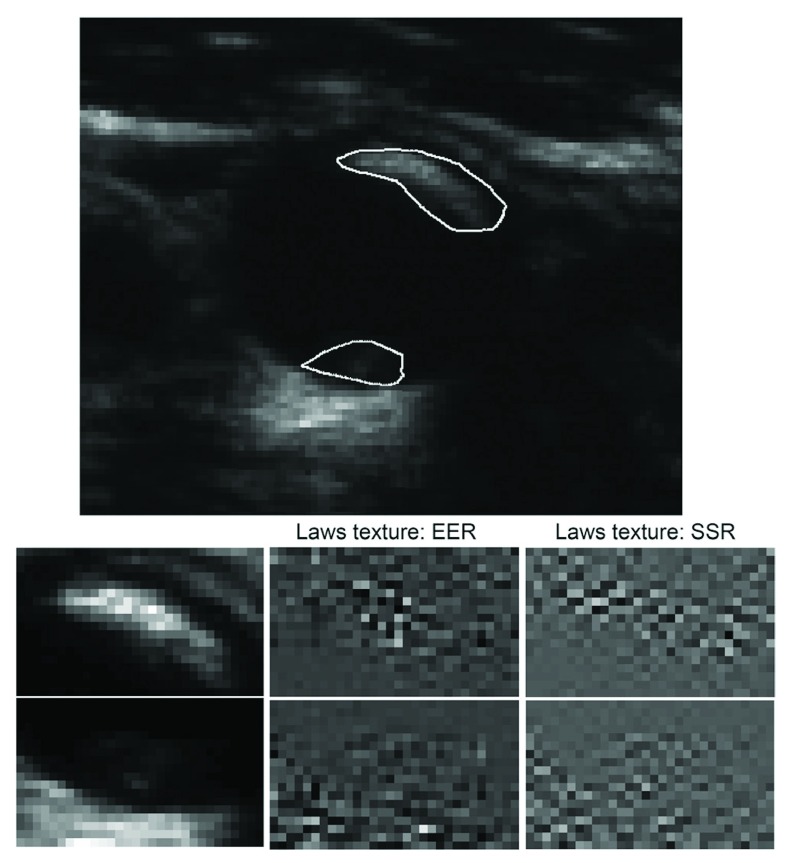
Even in patients without carotid stenosis, plaque characteristics predict cardiovascular risk. In a relatively small sample (349) of patients attending a vascular prevention clinic, followed for 5 years, the volume of ulcers ( Figure 5) predicted risk of cardiovascular events 70. Patients with a total ulcer volume ≥5 mm 3 experienced a significantly higher risk of stroke, transient ischemic attack, or death (P=0.009) and of stroke/transient ischemic attack/death/myocardial infarction/revascularization (P=0.017). In the same patient population, elements of plaque texture identified from radiofrequency signals in ultrasound ( Figure 6) predicted cardiovascular risk 71.
Figure 5. Carotid ulcer volume.
Measurement of ulcer volume and ulcer depth. Contours of ulcers were traced and depth of ulcers measured in cross-sectional views. Each slice had a thickness of 1 mm; ulcer volume was computed from the sum of the volumes of all slices in which ulceration was traced. Reproduced by permission of Wolters Kluwer from: Kuk M, Wannarong T, Beletsky V, Parraga G, Fenster A, Spence JD: Volume of carotid artery ulceration as a predictor of cardiovascular events. Stroke 2014, 45(5): 1437–1441.
Figure 6. Carotid plaque texture.
Texture for two plaques in the same vessel with a different appearance. In a total of 50 runs of sparse Cox regression (5× 10-fold cross-validation) on changes in texture, Laws edge-edge-ripple (EER) was selected in the model 49 times, and Laws spot-spot-ripple (SSR) 48 times. Reproduced by permission of Wolters Kluwer from: van Engelen A, Wannarong T, Parraga G, Niessen WJ, Fenster A, Spence JD, de Bruijne M: Three-dimensional carotid ultrasound plaque texture predicts vascular events. Stroke 2014, 45(9): 2695–2701.
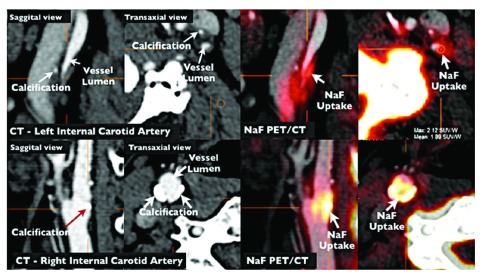
Intraplaque hemorrhage on MRI 72– 74 appears promising as a method for identifying high-risk asymptomatic stenosis, as does imaging of plaque inflammation on PET/CT with fluorodeoxyglucose 75 and imaging of active calcification with sodium fluoride 76 ( Figure 7). It seems likely that by combining several imaging modalities, the proportion of patients with asymptomatic stenosis who could be identified as being at high enough risk to warrant intervention might be increased to ~15%. Just as it is inappropriate to perform CAS or CEA in all patients with asymptomatic stenosis, it is also inappropriate to intervene in none: ~10% of strokes are from carotid stenosis, and most patients were asymptomatic before the event. Modalities to identify the few who could benefit from intervention should be in more widespread use; indeed no patient with asymptomatic stenosis should be subjected to intervention without first being identified as being at a high enough risk of ipsilateral stroke to benefit from intervention.
Figure 7. Imaging of vulnerable plaque by positron emission tomography/computed tomography (PET/CT).
NaF PET/CT imaging of left and right internal carotid arteries of active calcification in a 72-year-old symptomatic patient evaluated at the University of Ottawa Heart Institute. Upper row: evidence of NaF uptake with small foci of calcification on CT in the left internal carotid symptomatic culprit vessel. There is a mismatch between the region of NaF uptake and calcification on CT. Lower row: evidence of calcium nodules with matched NaF uptake at the right internal carotid artery. Reproduced by permission of Springer from: Cocker MS, Mc Ardle AB, Spence JD, Lum C, Hammond RR, Ongaro DC, McDonald MA, Dekemp RA, Tardif JC, Beanlands RS: Imaging atherosclerosis with hybrid [18F]fluorodeoxyglucose positron emission tomography/computed tomography imaging: what Leonardo da Vinci could not see. J Nucl Cardiol 2012, 19(6): 1211–1225.
Conclusion
Atherosclerosis is a complex process. Diet is much more important than most physicians suppose, and the intestinal microbiome has major effects on metabolic products derived from dietary constituents such as carnitine from meat and phosphatidylcholine from egg yolk. Avoidance of red meat and egg yolk is particularly important in patients with impaired renal function. New approaches to lowering LDL by blocking the effect of PCSK9, and a strategy of treating atherosclerosis directly instead of focusing on intermediate targets, show promise of reducing the residual risk that remains after current therapy. Most patients with asymptomatic carotid stenosis would be better treated with intensive medical therapy than with CEA or CAS. Microemboli on TCD, impaired cerebral blood flow reserve, juxtaluminal black plaque, echolucency, intraplaque hemorrhage on MRI, and ulceration on 3D ultrasound are ways to identify the 10–15% of patients with asymptomatic carotid stenosis who are at sufficiently high risk to benefit from intervention. Other approaches such as plaque texture on ultrasound and PET/CT imaging of inflamed plaque and early calcification will in future provide further evidence of their contribution to identifying high-risk asymptomatic carotid stenosis.
Abbreviations
CAS, carotid artery stenting; CEA, carotid endarterectomy; CI, confidence interval; HMG CoA, hydroxymethylglutarate CoA; HR, hazard ratio; IMT, intima-media thickness; LDL, low-density lipoprotein; MRI, magnetic resonance imaging; PCSK9, proprotein convertase subtilisin-kexin type 9; PET/CT, positron emission tomography/computed tomography; TCD, transcranial Doppler; TMAO, trimethylamine N-oxide; TPA, total plaque area; TPV, total plaque volume.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Tanja Rundek, Department of Neurology, Miller School of Medicine, University of Miami, Coral Gables, FL, USA
Tasneem Naqvi, Echocardiography Laboratories, Mayo Clinic, Scottsdale, AZ, USA
Conrado Estol, Heart and Brain Medicine, Buenos Aires, Argentina
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 3 approved]
References
- 1. Spence JD: Effects of the intestinal microbiome on constituents of red meat and egg yolks: a new window opens on nutrition and cardiovascular disease. Can J Cardiol. 2014;30(2):150–1. 10.1016/j.cjca.2013.11.019 [DOI] [PubMed] [Google Scholar]
- 2. Fox KM, EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators: Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet. 2003;362(9386):782–8. 10.1016/S0140-6736(03)14286-9 [DOI] [PubMed] [Google Scholar]
- 3. Heart Protection Study Collaborative Group: MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. 10.1016/S0140-6736(02)09327-3 [DOI] [PubMed] [Google Scholar]
- 4. Chen ZM, Jiang LX, Chen YP, et al. : Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366(9497):1607–21. 10.1016/S0140-6736(05)67660-X [DOI] [PubMed] [Google Scholar]
- 5. Ridker PM, Danielson E, Fonseca FA, et al. : Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207. 10.1056/NEJMoa0807646 [DOI] [PubMed] [Google Scholar]
- 6. Yusuf S, Sleight P, Pogue J, et al. : Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342(3):145–53. 10.1056/NEJM200001203420301 [DOI] [PubMed] [Google Scholar]
- 7. Navarese EP, Kolodziejczak M, Schulze V, et al. : Effects of Proprotein Convertase Subtilisin/Kexin Type 9 Antibodies in Adults With Hypercholesterolemia: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;163(1):40–51. 10.7326/M14-2957 [DOI] [PubMed] [Google Scholar]
- 8. Tall AR: Protease variants, LDL, and coronary heart disease. N Engl J Med. 2006;354(12):1310–2. 10.1056/NEJMe068026 [DOI] [PubMed] [Google Scholar]
- 9. Cohen JC, Boerwinkle E, Mosley TH, Jr, et al. : Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264–72. 10.1056/NEJMoa054013 [DOI] [PubMed] [Google Scholar]
- 10. Spence JD, Hackam DG: Treating arteries instead of risk factors: a paradigm change in management of atherosclerosis. Stroke. 2010;41(6):1193–9. 10.1161/STROKEAHA.110.577973 [DOI] [PubMed] [Google Scholar]
- 11. Spence JD, Coates V, Li H, et al. : Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol. 2010;67(2):180–6. 10.1001/archneurol.2009.289 [DOI] [PubMed] [Google Scholar]
- 12. Spence JD, Tamayo A, Lownie SP, et al. : Absence of microemboli on transcranial Doppler identifies low-risk patients with asymptomatic carotid stenosis. Stroke. 2005;36(11):2373–8. 10.1161/01.STR.0000185922.49809.46 [DOI] [PubMed] [Google Scholar]
- 13. Paraskevas KI, Spence JD, Veith FJ, et al. : Identifying which patients with asymptomatic carotid stenosis could benefit from intervention. Stroke. 2014;45(12):3720–4. 10.1161/STROKEAHA.114.006912 [DOI] [PubMed] [Google Scholar]
- 14. Haust MD, More RH, Bencosme SA, et al. : Electron microscopic studies in human atherosclerosis extracellular elements in aortic dots and streaks. Exp Mol Pathol. 1967;6(3):300–13. 10.1016/0014-4800(67)90013-5 [DOI] [PubMed] [Google Scholar]
- 15. Ross R, Glomset JA: The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976;295(8):420–5. 10.1056/NEJM197608192950805 [DOI] [PubMed] [Google Scholar]
- 16. Ross R, Glomset JA: The pathogenesis of atherosclerosis (first of two parts). N Engl J Med. 1976;295(7):369–77. 10.1056/NEJM197608122950707 [DOI] [PubMed] [Google Scholar]
- 17. Spence JD, Perkins DG, Kline RL, et al. : Hemodynamic modification of aortic atherosclerosis. Effects of propranolol vs hydralazine in hypertensive hyperlipidemic rabbits. Atherosclerosis. 1984;50(3):325–33. 10.1016/0021-9150(84)90079-0 [DOI] [PubMed] [Google Scholar]
- 18. Hansson GK: Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–95. 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- 19. Spence JD: Fasting lipids: the carrot in the snowman. Can J Cardiol. 2003;19(8):890–2. [PubMed] [Google Scholar]
- 20. Ghanim H, Abuaysheh S, Sia CL, et al. : Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 2009;32(12):2281–7. 10.2337/dc09-0979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dandona P, Ghanim H, Chaudhuri A, et al. : Macronutrient intake induces oxidative and inflammatory stress: potential relevance to atherosclerosis and insulin resistance. Exp Mol Med. 2010;42(4):245–53. 10.3858/emm.2010.42.4.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vogel RA, Corretti MC, Plotnick GD: Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol. 1997;79(3):350–4. 10.1016/S0002-9149(96)00760-6 [DOI] [PubMed] [Google Scholar]
- 23. Chiuve SE, McCullough ML, Sacks FM, et al. : Healthy lifestyle factors in the primary prevention of coronary heart disease among men: benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation. 2006;114(2):160–7. 10.1161/CIRCULATIONAHA.106.621417 [DOI] [PubMed] [Google Scholar]
- 24. Akesson A, Larsson SC, Discacciati A, et al. : Low-risk diet and lifestyle habits in the primary prevention of myocardial infarction in men: a population-based prospective cohort study. J Am Coll Cardiol. 2014;64(13):1299–306. 10.1016/j.jacc.2014.06.1190 [DOI] [PubMed] [Google Scholar]
- 25. de Lorgeril M, Salen P, Martin JL, et al. : Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99(6):779–85. 10.1161/01.CIR.99.6.779 [DOI] [PubMed] [Google Scholar]
- 26. Estruch R, Ros E, Salas-Salvadó J, et al. : Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–90. 10.1056/NEJMoa1200303 [DOI] [PubMed] [Google Scholar]
- 27. Spence JD, Jenkins DJ, Davignon J: Dietary cholesterol and egg yolks: not for patients at risk of vascular disease. Can J Cardiol. 2010;26(9):e336–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cohen DJ, Stolker JM, Wang K, et al. : Health-related quality of life after carotid stenting versus carotid endarterectomy: results from CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial). J Am Coll Cardiol. 2011;58(15):1557–65. 10.1016/j.jacc.2011.05.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koeth RA, Wang Z, Levison BS, et al. : Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85. 10.1038/nm.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tang WH, Wang Z, Levison BS, et al. : Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–84. 10.1056/NEJMoa1109400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Petrof EO, Gloor GB, Vanner SJ, et al. : Stool substitute transplant therapy for the eradication of Clostridium difficile infection: 'RePOOPulating' the gut. Microbiome. 2013;1(1):3. 10.1186/2049-2618-1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spence JD, Urquhart BL, Bang H: Effect of renal impairment on atherosclerosis: only partially mediated by homocysteine. Nephrol Dial Transplant. 2016;31(6):937–44. 10.1093/ndt/gfv380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wheeler DC: Cardiovascular disease in patients with chronic renal failure. The Lancet. 1996;348(9043):1673–4. 10.1016/S0140-6736(05)65816-3 [DOI] [PubMed] [Google Scholar]
- 34. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. : Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–52. 10.1016/S0140-6736(13)60595-4 [DOI] [PubMed] [Google Scholar]
- 35. Tang WH, Wang Z, Kennedy DJ, et al. : Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–55. 10.1161/CIRCRESAHA.116.305360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Poesen R, Claes K, Evenepoel P, et al. : Microbiota-Derived Phenylacetylglutamine Associates with Overall Mortality and Cardiovascular Disease in Patients with CKD. J Am Soc Nephrol. 2016. pii: ASN.2015121302. 10.1681/ASN.2015121302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spence JD, Dresser GK: Overcoming Challenges With Statin Therapy. J Am Heart Assoc. 2016;5(1). pii: e002497. 10.1161/JAHA.115.002497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sabatine MS, Giugliano RP, Wiviott SD, et al. : Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500–9. 10.1056/NEJMoa1500858 [DOI] [PubMed] [Google Scholar]
- 39. Robinson JG, Farnier M, Krempf M, et al. : Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–99. 10.1056/NEJMoa1501031 [DOI] [PubMed] [Google Scholar]
- 40. Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, et al. : Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383(9911):60–8. 10.1016/S0140-6736(13)61914-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spence JD: Measurement of carotid plaque burden. JAMA Neurol. 2015;72(4):383–4. 10.1001/jamaneurol.2014.3002 [DOI] [PubMed] [Google Scholar]
- 42. Spence JD, Eliasziw M, DiCicco M, et al. : Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke. 2002;33(12):2916–22. 10.1161/01.STR.0000042207.16156.B9 [DOI] [PubMed] [Google Scholar]
- 43. Spence JD: Time course of atherosclerosis regression. Atherosclerosis. 2014;235(2):347–8. 10.1016/j.atherosclerosis.2014.05.929 [DOI] [PubMed] [Google Scholar]
- 44. Ainsworth CD, Blake CC, Tamayo A, et al. : 3D ultrasound measurement of change in carotid plaque volume: a tool for rapid evaluation of new therapies. Stroke. 2005;36(9):1904–9. 10.1161/01.STR.0000178543.19433.20 [DOI] [PubMed] [Google Scholar]
- 45. Naylor AR: Time to rethink management strategies in asymptomatic carotid artery disease. Nat Rev Cardiol. 2011;9(2):116–24. 10.1038/nrcardio.2011.151 [DOI] [PubMed] [Google Scholar]
- 46. Marquardt L, Geraghty OC, Mehta Z, et al. : Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke. 2010;41(1):e11–7. 10.1161/STROKEAHA.109.561837 [DOI] [PubMed] [Google Scholar]
- 47. Chaturvedi S, Rothwell PM: Stroke risk with symptomatic carotid stenosis: The future is not what it used to be. Neurology. 2016;86(6):494–5. 10.1212/WNL.0000000000002363 [DOI] [PubMed] [Google Scholar]
- 48. Paraskevas KI, Kalmykov EL, Naylor AR: Stroke/Death Rates Following Carotid Artery Stenting and Carotid Endarterectomy in Contemporary Administrative Dataset Registries: A Systematic Review. Eur J Vasc Endovasc Surg. 2016;51(1):3–12. 10.1016/j.ejvs.2015.07.032 [DOI] [PubMed] [Google Scholar]
- 49. Spence JD, Naylor AR: Endarterectomy, Stenting, or Neither for Asymptomatic Carotid-Artery Stenosis. N Engl J Med. 2016;374(11):1087–8. 10.1056/NEJMe1600123 [DOI] [PubMed] [Google Scholar]
- 50. Naylor AR, Gaines PA, Rothwell PM: Who benefits most from intervention for asymptomatic carotid stenosis: patients or professionals? Eur J Vasc Endovasc Surg. 2009;37(6):625–32. 10.1016/j.ejvs.2009.01.026 [DOI] [PubMed] [Google Scholar]
- 51. Spence JD: Management of Patients with an Asymptomatic Carotid Stenosis--Medical Management, Endovascular Treatment, or Carotid Endarterectomy? Curr Neurol Neurosci Rep. 2016;16(1):3. 10.1007/s11910-015-0605-6 [DOI] [PubMed] [Google Scholar]
- 52. Inaba Y, Chen JA, Bergmann SR: Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012;220(1):128–33. 10.1016/j.atherosclerosis.2011.06.044 [DOI] [PubMed] [Google Scholar]
- 53. Spence JD: Carotid plaque measurement is superior to IMT Invited editorial comment on: carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis-Yoichi Inaba, M.D., Jennifer A. Chen M.D., Steven R. Bergmann M.D., Ph.D. Atherosclerosis. 2012;220(1):34–5. 10.1016/j.atherosclerosis.2011.07.006 [DOI] [PubMed] [Google Scholar]
- 54. Spence JD, Parraga G: Three-Dimensional Ultrasound of Carotid Plaque. Neuroimaging Clin N Am. 2016;26(1):69–80. 10.1016/j.nic.2015.09.006 [DOI] [PubMed] [Google Scholar]
- 55. Sillesen H, Muntendam P, Adourian A, et al. : Carotid plaque burden as a measure of subclinical atherosclerosis: comparison with other tests for subclinical arterial disease in the High Risk Plaque BioImage study. JACC Cardiovasc Imaging. 2012;5(7):681–9. 10.1016/j.jcmg.2012.03.013 [DOI] [PubMed] [Google Scholar]
- 56. Baber U, Mehran R, Sartori S, et al. : Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: the BioImage study. J Am Coll Cardiol. 2015;65(11):1065–74. 10.1016/j.jacc.2015.01.017 [DOI] [PubMed] [Google Scholar]
- 57. Den Ruijter HM, Peters SA, Anderson TJ, et al. : Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308(8):796–803. 10.1001/jama.2012.9630 [DOI] [PubMed] [Google Scholar]
- 58. Johnsen SH, Mathiesen EB, Joakimsen O, et al. : Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: a 6-year follow-up study of 6226 persons: the Tromsø Study. Stroke. 2007;38(11):2873–80. 10.1161/STROKEAHA.107.487264 [DOI] [PubMed] [Google Scholar]
- 59. Mathiesen EB, Johnsen SH, Wilsgaard T, et al. : Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke: a 10-year follow-up of 6584 men and women: the Tromsø Study. Stroke. 2011;42(4):972–8. 10.1161/STROKEAHA.110.589754 [DOI] [PubMed] [Google Scholar]
- 60. Egger M, Spence JD, Fenster A, et al. : Validation of 3D ultrasound vessel wall volume: an imaging phenotype of carotid atherosclerosis. Ultrasound Med Biol. 2007;33(6):905–14. 10.1016/j.ultrasmedbio.2007.01.013 [DOI] [PubMed] [Google Scholar]
- 61. Krasinski A, Chiu B, Spence JD, et al. : Three-dimensional ultrasound quantification of intensive statin treatment of carotid atherosclerosis. Ultrasound Med Biol. 2009;35(11):1763–72. 10.1016/j.ultrasmedbio.2009.05.017 [DOI] [PubMed] [Google Scholar]
- 62. Shai I, Spence JD, Schwarzfuchs D, et al. : Dietary intervention to reverse carotid atherosclerosis. Circulation. 2010;121(10):1200–8. 10.1161/CIRCULATIONAHA.109.879254 [DOI] [PubMed] [Google Scholar]
- 63. Lorenz MW, Polak JF, Kavousi M, et al. : Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012;379(9831):2053–62. 10.1016/S0140-6736(12)60441-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wannarong T, Parraga G, Buchanan D, et al. : Progression of carotid plaque volume predicts cardiovascular events. Stroke. 2013;44(7):1859–65. 10.1161/STROKEAHA.113.001461 [DOI] [PubMed] [Google Scholar]
- 65. Markus HS, King A, Shipley M, et al. : Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol. 2010;9(7):663–71. 10.1016/S1474-4422(10)70120-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Madani A, Beletsky V, Tamayo A, et al. : High-risk asymptomatic carotid stenosis: ulceration on 3D ultrasound vs TCD microemboli. Neurology. 2011;77(8):744–50. 10.1212/WNL.0b013e31822b0090 [DOI] [PubMed] [Google Scholar]
- 67. Topakian R, King A, Kwon SU, et al. : Ultrasonic plaque echolucency and emboli signals predict stroke in asymptomatic carotid stenosis. Neurology. 2011;77(8):751–8. 10.1212/WNL.0b013e31822b00a6 [DOI] [PubMed] [Google Scholar]
- 68. Kakkos SK, Griffin MB, Nicolaides AN, et al. : The size of juxtaluminal hypoechoic area in ultrasound images of asymptomatic carotid plaques predicts the occurrence of stroke. J Vasc Surg. 2013;57(3):609–618.e1; discussion 617-8. 10.1016/j.jvs.2012.09.045 [DOI] [PubMed] [Google Scholar]
- 69. Kakkos SK, Nicolaides AN, Kyriacou E, et al. : Computerized texture analysis of carotid plaque ultrasonic images can identify unstable plaques associated with ipsilateral neurological symptoms. Angiology. 2011;62(4):317–28. 10.1177/0003319710384397 [DOI] [PubMed] [Google Scholar]
- 70. Kuk M, Wannarong T, Beletsky V, et al. : Volume of carotid artery ulceration as a predictor of cardiovascular events. Stroke. 2014;45(5):1437–41. 10.1161/STROKEAHA.114.005163 [DOI] [PubMed] [Google Scholar]
- 71. van Engelen A, Wannarong T, Parraga G, et al. : Three-dimensional carotid ultrasound plaque texture predicts vascular events. Stroke. 2014;45(9):2695–701. 10.1161/STROKEAHA.114.005752 [DOI] [PubMed] [Google Scholar]
- 72. Altaf N, Daniels L, Morgan PS, et al. : Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. J Vasc Surg. 2008;47(2):337–42. 10.1016/j.jvs.2007.09.064 [DOI] [PubMed] [Google Scholar]
- 73. Altaf N, Morgan PS, Moody A, et al. : Brain white matter hyperintensities are associated with carotid intraplaque hemorrhage. Radiology. 2008;248(1):202–9. 10.1148/radiol.2481070300 [DOI] [PubMed] [Google Scholar]
- 74. Singh N, Moody AR, Gladstone DJ, et al. : Moderate carotid artery stenosis: MR imaging-depicted intraplaque hemorrhage predicts risk of cerebrovascular ischemic events in asymptomatic men. Radiology. 2009;252(2):502–8. 10.1148/radiol.2522080792 [DOI] [PubMed] [Google Scholar]
- 75. Rudd JH, Warburton EA, Fryer TD, et al. : Imaging atherosclerotic plaque inflammation with [ 18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105(23):2708–11. 10.1161/01.CIR.0000020548.60110.76 [DOI] [PubMed] [Google Scholar]
- 76. Cocker MS, Mc Ardle B, Spence JD, et al. : Imaging atherosclerosis with hybrid [ 18F]fluorodeoxyglucose positron emission tomography/computed tomography imaging: what Leonardo da Vinci could not see. J Nucl Cardiol. 2012;19(6):1211–25. 10.1007/s12350-012-9631-9 [DOI] [PMC free article] [PubMed] [Google Scholar]