Abstract
Bevacizumab has shown benefit in the first line setting in combination with interferon; however, data on use as monotherapy are limited. This retrospective analysis of 71 patients assesses the efficacy of bevacizumab monotherapy in patients who progressed on other targeted drugs. Bevacizumab monotherapy resulted in prolonged disease control and few discontinuations for adverse events, including those who were heavily pretreated.
Background
Bevacizumab has shown benefit in 1st-line treatment of metastatic ccRCC in combination with Interferon α. This retrospective analysis assessed the efficacy of bevacizumab monotherapy in patients who have progressed on VEGF receptor tyrosine kinase inhibitors (TKI), and/or mTOR inhibitors.
Methods
A retrospective analysis was performed on metastatic ccRCC patients who received bevacizumab monotherapy after progression on prior targeted therapies. Primary objective was to assess overall survival (OS) and the secondary objectives include progression-free survival (PFS), time on therapy, and incidence of serious adverse events as assessed by visits to the MSKCC urgent care center.
Results
Seventy-one patients were treated with bevacizumab as monotherapy in the salvage setting. Most patients were heavily pretreated with 36 (51%) patients receiving bevacizumab as a 4th line agent or later, and 33 (46%) patients received at least 2 prior VEGF targeted agents. Eighteen (25%) patients had a KPS < 80%, and 20 (28%) patients were poor risk by Memorial Sloan Kettering Cancer (MSKCC) criteria. Median OS was 11.5 months (95% CI 6.4 – 17.4), and median PFS was 1.9 months (95% CI 1.7 – 4.1). Nine (13%) patients had a prolonged time on therapy of >12 months. Four (6%) patients were discontinued on therapy for adverse events. Poor KPS (<80%) and MSKCC poor-risk classification were prognostic for poor OS with hazard ratios of 4.09 (P< 0.001) and 2.84 (P=0.021), respectively.
Conclusions
Bevacizumab monotherapy resulted in prolonged disease control and few discontinuations for adverse events in patients after progression on other targeted therapies, including those who were heavily pretreated.
Keywords: VEGF, TKI, mTOR, kidney cancer
Introduction
Renal cell carcinoma (RCC) is currently the eighth most common malignancy with approximately 65,000 new cases per year and 14,000 deaths1. Clear cell renal cell carcinoma (ccRCC) is the most common histological subtype and comprises approximately 80% of cases2. Over the last 10 years, 7 molecular targeted therapies have gained regulatory approval in the United States for the treatment of metastatic ccRCC. These 7 targeted therapies have broadly been grouped into those that target the VEGF signaling pathway (sunitinib3,4, pazopanib5,6, axitinib7,8, sorafenib9,10, bevacizumab11,12) and those that target the mTOR signaling pathway (everolimus13, temsirolimus14).
VEGF targeted therapies have been approved for the treatment of ccRCC either as single agents (sunitinib, pazopanib, axitinib, sorafenib) or in combination with interferon alfa-2a (bevacizumab). In the first line setting, two phase III clinical trials have demonstrated bevacizumab + interferon alfa-2a (INF) is superior to INF alone, AVOREN and CALGB 90206. Bevacizumab monotherapy was reported to show antitumor activity in two randomized phase 2 trials, as first-line therapy, and following cytokine therapy15,16. Both of these studies were performed before other targeted drugs were available for standard management. At MSKCC, we have utilized bevacizumab monotherapy for patients who have progressed on prior VEGF targeted therapies, such as sunitinib or pazopanib, as well as those patients who were not candidates for TKI therapy.
In this retrospective study, we describe our single institution experience with bevacizumab monotherapy to evaluate both safety and efficacy in ccRCC patients who had been previously treated with targeted agents. The primary end point is overall survival and secondary endpoints include progression-free survival, time on therapy, and toxicity analysis.
Patients and Methods
Cohort Identification
Institutional approval by the Memorial Sloan Kettering Cancer Center (MSKCC) Institutional Review Board was obtained to conduct a retrospective review of patients with RCC treated with bevacizumab as a single agent. Electronic medical records were queried for all patients with RCC who received their first dose of bevacizumab prior to 2/4/2014. The date of drug initiation ranged from 2/6/2009 to 2/4/2014. The cutoff date for follow-up on survival status and toxicity was 5/8/2014. All patients included in the study were > 18 years old, and pathologically confirmed at MSKCC to have clear cell histology and had progressive disease on prior therapy as deemed by the treating physician. Inclusion criteria included receiving bevacizumab as a monotherapy after at least 1 prior systemic targeted therapy for metastatic disease (including sunitinib, pazopanib, sorafenib, axitinib, temsirolimus, or everolimus). Patients who only received cytokine therapy (IFN or IL-2) prior to bevacizumab were excluded from analysis. Prior line of therapy was defined as any systemic therapy for metastatic disease including cytokine therapy and investigational agents, and excluded any treatment in the adjuvant setting. Prior VEGF targeted therapies were defined as sunitinib, pazopanib, axitinib, or sorafenib. Patients who received more than 1 course of bevacizumab were evaluated based on their first course of bevacizumab. Duration of therapy was defined as date of first dose of bevacizumab until the date of and last dose of bevacizumab plus 14 days added to account for the treatment schedule.
Treatment
Patients were treated with bevacizumab monotherapy at 10 mg/kg by intravenous infusion over 20 minutes every 2 weeks until clinician determination of lack of benefit. Doses were modified and delayed as per standard practices including patient tolerability and adverse events. Baseline KPS and lab values were obtained from medical records at the time of bevacizumab initiation.
Response to Therapy
Imaging studies were conducted as per standard of care at MSKCC, which typically consists of radiographic evaluation with a CT chest abdomen and pelvis or CT chest with MRI of the abdomen and pelvis every 8-12 weeks. Radiographic tumor responses were assessed by the treating physician and by board certified radiologists at MSKCC. All radiographs were re-reviewed for the purpose of this trial by a radiologist (AMH or OA) according to Response Evaluation Criteria in Solid Tumors (RECIST 1.1) guidelines. Objective radiographic tumor responses and date of radiographic progression of disease were determined. Patients who discontinued therapy due to decline in performance status or worsening symptoms without radiographic confirmation of progression of disease were considered to have clinical progression of disease.
Adverse Events
At the time of drug discontinuation, MSKCC records from outpatient visits, MSKCC Urgent Care Center visits, and outside hospital records documented in the MSKCC electronic medical record were assessed to determine whether drug discontinuation was associated with adverse events from bevacizumab. Interval severe adverse events were identified by retrospective analysis of referrals to the MSKCC Urgent Care Center documented in the electronic medical records. All referrals to the MSKCC Urgent Care Center occurring after the first dose and within 28 days of the last dose of bevacizumab were evaluated.
Data Analysis
Baseline characteristics, treatments received prior to bevacizumab, and adverse events were summarized descriptively. Kaplan-Meier analysis was used to analyze OS and PFS from the date of first dose of bevacizumab. PFS was defined from this date until date of radiographic or clinical progression, whichever was earlier, or death. Patients who did not experience the event of interest were censored at the date of last follow-up, with a cutoff for analysis of 5/8/2014. The Cox proportional hazards model was used in univariate analysis of risk factors and OS, including number of prior VEGF targeted therapies, prior line of therapy, KPS, and MSKCC risk classification. Percent change in RECIST starting at the time of progression was graphed longitudinally for the subset of patients treated on bevacizumab beyond progression. All statistical tests were two-sided, and a 5% significance level (p<0.05) was used. Statistical analysis was done using R (version 3.1.2; R Development Core Team) with the “survival” package.
Results
Baseline Characteristics
Seventy-one ccRCC patients who were treated with bevacizumab as salvage therapy were identified. Key demographic information is summarized in Table 1. The median age was 65 years old with a range of 37-86 years. 65 (91%) patients were white, 2 (3%) patients were black, and 4 (6%) patients were Asian. Most patients were heavily pretreated, with 36 (51%) patients receiving 3 or more prior lines of systemic therapy, and 33 (46%) receiving at least 2 prior VEGF targeted therapies. Eighteen (25%) patients had a KPS < 80%, 59 (83%) patients had ≥ 3 different sites of organ metastasis, and 20 (28%) patients were poor prognosis by MSKCC risk grouping. Prior therapies are summarized in Table 2. Most patients were previously treated with either TKI (N=64, 90%) or mTOR (N=61, 86%) targeted therapies. The most commonly used TKI prior to bevacizumab in this population was sunitinib (N=51, 72%) followed by sorafenib (N=21, 30%). Use of either everolimus (N=34, 48%) or temsirolimus (N=31, 44%) prior to bevacizumab was similar with some patients receiving both (N=4, 6%). Prior cytokine or immunotherapy was less common with 6 (8%) patients receiving nivolumab, 4 (6%) patients receiving INFa, and 1 (1%) patient receiving high dose IL-2.
Table 1.
Baseline Characteristics of the Study Cohort
| N (%) | N (%) | ||
|---|---|---|---|
| Total Patients | 71 | Prior Nephrectomy | 60 (85) |
| Median Age (Range) in years | 65 (37-86) | Number of Sites of Organ Metastasis | |
| Sex | 1 | 3 (4) | |
| Female | 13 (18) | 2 | 9 (13) |
| Male | 58 (82) | ≥3 | 59 (83) |
| Race | Sites of Metastasis | ||
| White | 65 (92) | Lung | 62 (87) |
| Black | 2 (3) | Lymph Node | 53 (75) |
| Asian | 4 (6) | Bone | 39 (55) |
| Prior lines of systemic therapy | Adrenal | 28 (39) | |
| 1 | 17 (24) | Liver | 23 (32) |
| 2 | 18 (25) | Abdominal soft tissue | 19 (27) |
| 3 | 20 (28) | Nephrectomy Bed | 9 (13) |
| 4 | 10 (14) | Pancreas | 7 (10) |
| ≥5 | 6 (8) | Other | 10 (14) |
| Prior lines of VEGF targeted therapy | KPS | ||
| 0 | 7 (10) | < 80 | 18 (25) |
| 1 | 31 (44) | ≥ 80 | 53 (75) |
| 2 | 24 (34) | MSK risk group | |
| ≥3 | 9 (12) | Favorable | 13 (18) |
| Intermediate | 38 (54) | ||
| Poor | 20 (28) |
Table 2.
Therapies Received Prior to Bevacizumab
| N (%) | |
|---|---|
| TKI Targeted Therapy | |
| Any TKI Targeted Therapy | 64 (90) |
| Sunitinib | 51 (72) |
| Sorafenib | 21 (30) |
| Pazopanib | 17 (24) |
| Axitinib | 11 (15) |
| Tivozanib | 3 (4) |
| Dovitinib | 2 (3) |
| Lenvatinib | 2 (3) |
| mTOR Targeted Therapy | |
| Any mTOR Targeted Therapy | 61 (86) |
| Everolimus | 34 (48) |
| Temsirolimus | 31 (44) |
| Cytokine/Immunotherapy | |
| Nivolumab | 6 (8) |
| INFa | 4 (6) |
| IL-2 | 1 (1) |
| Other | |
| Investigational Agents | 9 (13) |
Bevacizumab Treatment Efficacy
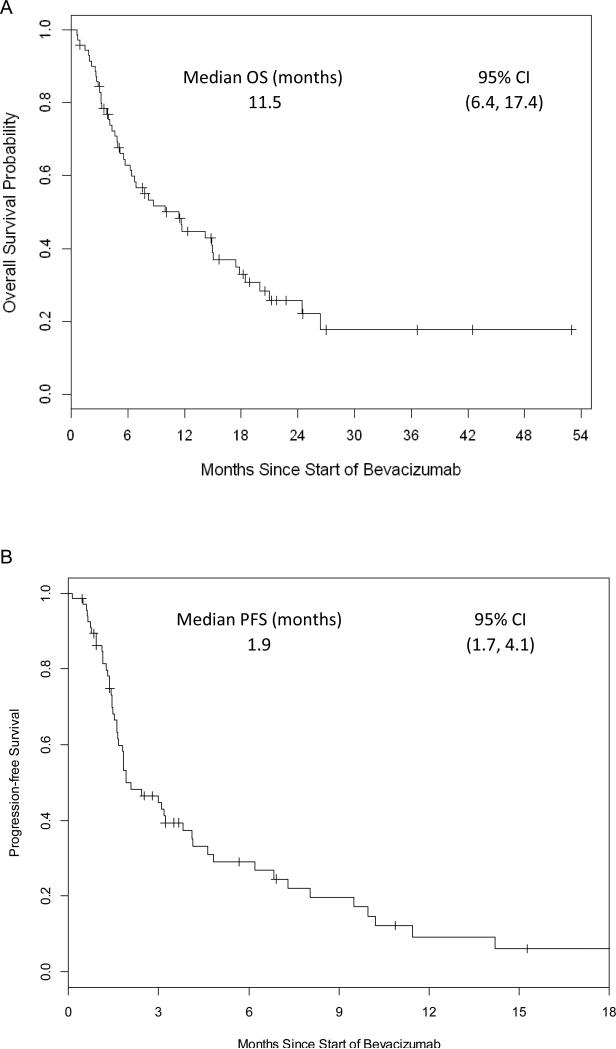
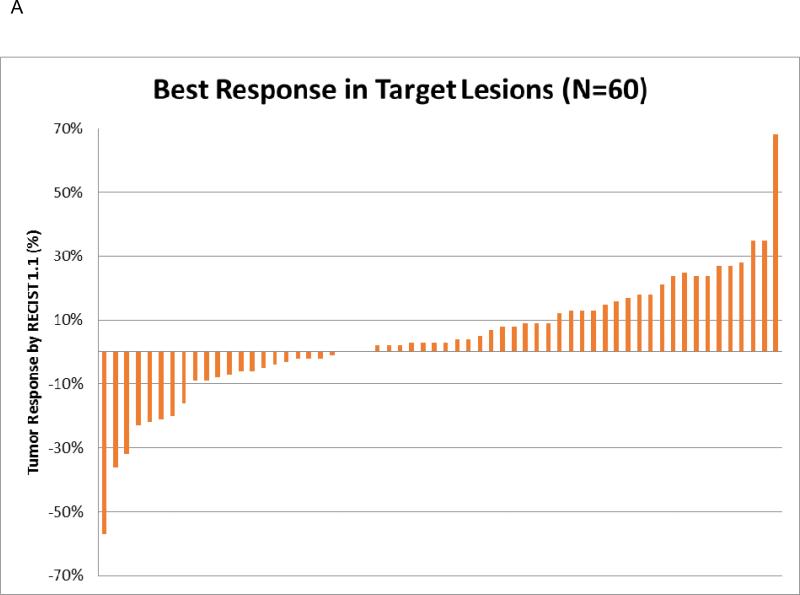
Median OS was 11.5 months (95% CI 6.4 – 17.4 months) and median PFS was 1.9 months (95% CI 1.7 – 4.1 months) (Figure 1A and Figure 1B respectively). Median time on therapy was 2.8 months (range 0.5 – 38.7 months) with 8 (11%) patients remaining on therapy at the time of final analysis (Table 3). 7 (10%) patients received only a single dose of bevacizumab before deemed to have clinical progression; however, 9 (13%) patients had a prolonged time on therapy of > 12 months. Treatment was discontinued predominately for progression of disease (N=56, 89%) with a minority discontinued for adverse events (N=4, 6%) or for unknown/unrelated reasons (N=3, 5%). Sixty-one patients were evaluable by RECIST 1.1 for assessment of objective response rate. When best response was evaluated, 2 patients had a partial response (PR), 28 patients had stable disease (SD), and 31 (30 at target and 1 at non-target lesions) patients had progression of disease (PD) (Table 3 and Figure 2A). In patients with prolonged time on therapy (N=9), 3 patients remain on therapy at time of last analysis, and 8 patients had stable disease as best response, and 1 patient had progression of disease.
Figure 1. Overall Survival and Progression-free Survival.
1A: Overall survival curve of bevacizumab monotherapy patients
1B: Progression-free survival curve of bevacizumab monotherapy patients
Table 3.
Bevacizumab Treatment Response
| N (%) | |
|---|---|
| Patients Remaining on Therapy | 8 (11) |
| Patients Only Receiving a Single Dose | 7 (10) |
| Patients Treated for > 12 Months | 9 (13) |
| Reason For Discontinuation of Therapy | |
| PD | 56 (89) |
| Toxicity | 4 (6) |
| Bleeding | 1 |
| Deep Venous Thrombosis | 1 |
| Failure to Thrive | 1 |
| Myocardial Infarction | 1 |
| Unrelated | 2 |
| Unknown | 1 |
| Best Radiographic Response to Therapy | |
| PR | 2 |
| SD | 28 |
| PD | 31 |
| Deaths | 47 |
| PFS events | 52 |
| Median in Months | |
| Time on Treatment | 2.8 (Range 0.5 – 38.7) |
| Progression Free Survival | 1.9 (95% CI 1.7 – 4.1) |
| Overall Survival | 11.5 (95% CI 6.4 – 17.4) |
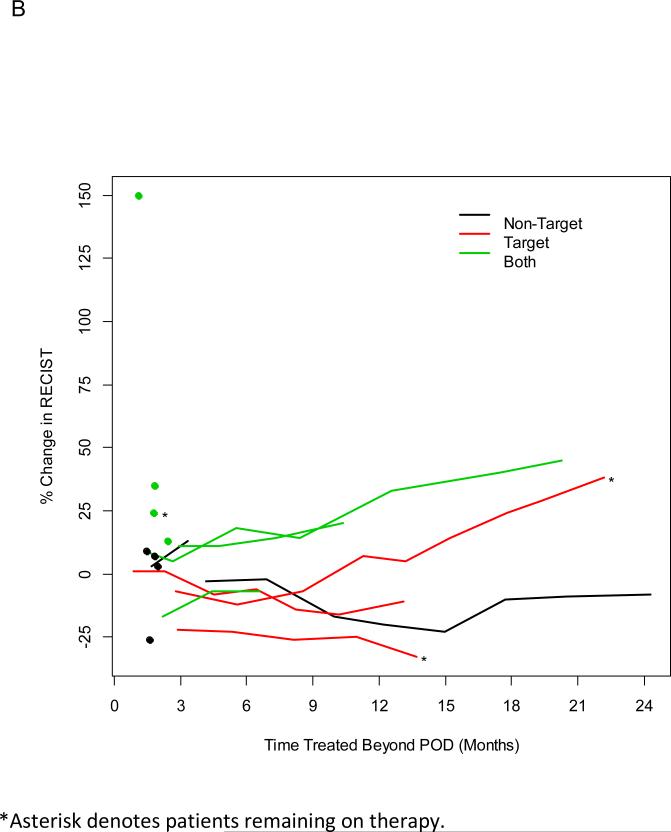
Figure 2.
2A: Radiographic Responses of Target Lesions by RECIST
2B: Patients Treated Beyond POD by site of progression.
Treatment Following Radiographic Progression of Disease
Sixteen patients demonstrated radiographic progression of disease by RECIST, but were continued on bevacizumab due to clinician determined clinical benefit. These patients received follow up imaging, and the change in their target lesions after radiographic progression was determined. At the time of last analysis 3 patients still remain on therapy (Figure 2B). Duration of treatment beyond progression of disease ranged from 1.2 – 24.5 months. Six patients initially progressed at non-target lesions, 3 patients progressed at target lesions, and 7 patients progressed at both target and non-target lesions. Seven patients discontinued therapy after their first follow up imaging. In patients who discontinued therapy after their first follow up imaging, initial progression was seen in either non-target lesions or both target and non-target lesions.
Univariate Risk Factor Analysis for Overall Survival
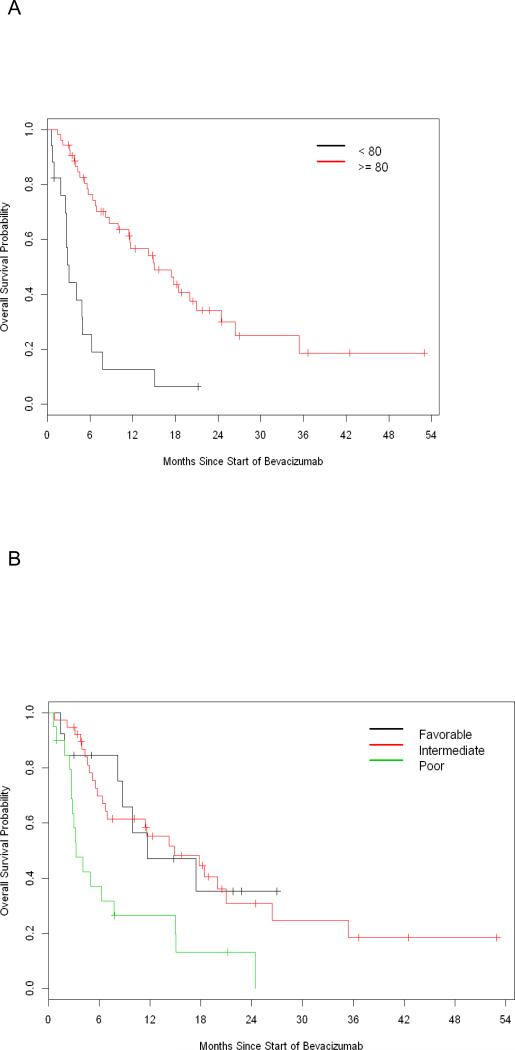
Univariate analysis was used to determine prognostic risk factors associated with a shorter OS, Table 4. Decreased KPS (<80% vs. ≥80%) was associated with a shorter OS with a hazard ratio (HR) of 4.09 (95% CI 2.18 – 7.66, P<0.001), Figure 3A. Using MSKCC risk classification, poor risk patients had worse OS compared to favorable prognosis patients, with a HR of 2.84 (95% CI 1.17 – 6.89, P=0.021), Figure 3B. However, no significant differences in overall survival were seen when comparing the number of prior lines of systemic therapy, or the number of prior VEGF targeted therapies, Table 4.
Table 4.
Univariate Associations Between Clinical Features and Overall Survival
| Risk Factor | HR | 95% CI | P-value |
|---|---|---|---|
| Prior VEGF Targeted Agents | |||
| 1 vs. 0 | 0.43 | (0.17, 1.09) | 0.076 |
| 2 vs. 0 | 0.42 | (0.17, 1.08) | 0.072 |
| 3+ vs. 0 | 0.40 | (0.12, 1.33) | 0.14 |
| Prior lines of therapy (3+ vs. 1-2) | 0.65 | (0.36, 1.17) | 0.15 |
| KPS (< 80 vs. ≥ 80) | 4.09 | (2.18, 7.66) | < 0.001 |
| MSKCC Risk | |||
| Intermediate vs. Favorable | 1.10 | (0.47, 2.56) | 0.83 |
| Poor vs. Favorable | 2.84 | (1.17, 6.89) | 0.021 |
| line 4+ group | 2+ prior VEGF group | |
|---|---|---|
| Median time on therapy | 2.4 | 2.3 |
| Median PFS (95% CI) | 3.0 (1.8, 6.8) | 3.0 (1.6, 6.2) |
| Median OS (95% CI) | 11.5 (6.3, 26.4) | 9.9 (6.3, 26.4) |
Figure 3. Univariate Analysis of Overall Survival by Clinical Features.
3A: Univariate Analysis of KPS and Overall Survival
3B: Univariate Analysis of MSKCC Risk Classification and Overall Survival
Adverse Events on Bevacizumab Monotherapy
While on bevacizumab monotherapy, 35 patients were referred to the MSKCC Urgent Care Center for a total of 78 unique visits. None of the visits not requiring admission were considered serious adverse events (SAE). Twenty seven patients required inpatient admission leading to a total of 51 hospital admissions with an average length of admission of 5.25 days. Most SAEs were due to disease or unrelated reasons and not from bevacizumab therapy. Most common reasons for inpatient hospitalization are hypercalcemia (25%), pain (22%), failure to thrive (14%), bleeding (12%), fever (8%), and shortness of breath (10%). Only 4 (6%) patients were discontinued on therapy for treatment related toxicities. Toxicities leading to discontinuation of bevacizumab include bleeding (N=1), deep venous thrombosis (N=1), myocardial infarction (N=1), and failure to thrive (N=1). 2 patients were discontinued on therapy for non-treatment related events, including pneumonia (N=1) and hip fracture (N=1), and 1 patient was lost to follow up.
Discussion
This single center retrospective study evaluated the use of bevacizumab monotherapy in the salvage setting. This study suggests that even in heavily pretreated populations, some patients derived prolonged disease control on therapy, and few patients experienced adverse events that required drug discontinuation. Many patients were treated beyond radiographic progression of disease due to clinician assessment of clinical benefit, and some patients appear to derive prolonged clinical benefit even after radiographic progression. All these patients received prior targeted agents, and were treated with bevacizumab in the setting of progressive disease. Therefore, the data strongly suggest that this phenomenon is related to drug response rather than indolent disease.
In our population of heavily pretreated patients, patient prognosis was largely guided by KPS and MSK risk grouping, with low KPS and poor risk classification associated with decreased OS. OS after bevacizumab therapy was not correlated to the number of prior therapies or the number of prior VEGF targeted therapies. It remains to be seen whether this effect may relate to differences in targeting the VEGF receptor versus the VEGF ligand. These data are limited by the retrospective nature of this single center study, which include selection, ascertainment and misclassification biases upon retrospective review. Additionally, this study does not fully describe the spectrum of toxicity that may be associated with bevacizumab monotherapy in this patient population. Events leading to discontinuation of the bevacizumab were described; however, interval toxicities during the course of treatment were identified based on the need for hospital admission at MSKCC, but we do not report admissions at other hospitals. In this study, toxicities not leading to inpatient hospitalization were not reported and information regarding dose delays were not available. Despite these limitations, this study represents the largest series of heavily pretreated and poor risk patients with bevacizumab monotherapy.
Our results corroborate the findings of a phase 2 clinical trial investigating high dose bevacizumab (15 mg/kg every 2 weeks or every week) in ccRCC, where median PFS in VEGFR pretreated patients (N=49) was 3.5 months (95% CI 1.9 – 5.5), and median OS was 12.0 months (95% CI 8.3 – 21.8). In this study most patients (76%) had only one prior VEGF targeted therapy, and few patients were poor risk (2%)17. Similarly, in a retrospective study of 21 metastatic RCC patients (17 with ccRCC), who received bevacizumab after prior VEGF targeted therapy, median PFS was 4.4 months (95% CI 2.8 – 9.6), and median OS was 19.4 months (95% CI 9.9 – Not Reached)18. These three studies suggest bevacizumab has activity as monotherapy. Differences in median PFS and OS may reflect differences in patient population, as our patient population receiving bevacizumab monotherapy were often heavily pretreated or not candidates for other TKI therapy. The PFS of 1.9 months seen with bevacizumab in this study is less that what has been reported for 2nd and 3rd line agents; however, the majority of patients in this study received bevacizumab as 4th line therapy or later and 46% receiving at least 2 prior VEGF targeted therapies. In the randomized phase 3 clinical trial for second line axitinib vs. sorafenib which was limited to patients with ECOG 0 or 1, the PFS was 8.3 (95% CI 6.7-9.2) months and 5.7 (95% CI 4.7-6.5) months respectively7. Currently, there are few studies in this heavily pre-treated and poor performance status population.
Conclusion
Currently bevacizumab is approved in combination with interferon for metastatic RCC based on a clinical trial performed in the first-line setting; however, interferon is associated with considerable toxicities, and it remains unclear whether the clinical benefit from the addition of interferon warrant the added toxicities. Even in heavily pretreated populations, few patients discontinue bevacizumab due to toxicities related to therapy. Taken together, bevacizumab monotherapy appears to be to provide clinical benefit in the salvage setting after progression on other targeted agents.
Table 5.
Select Severe Adverse Events on Bevacizumab Monotherapy
| N (%) | |
|---|---|
| Patients Referred to the MSKCC Urgent Care Center | 35 (49%) |
| Total Visits to MSKCC Urgent Care Center | 78 |
| Patients Requiring Admission after Referral to Urgent Care Center | 27 (38%) |
| Total Admissions | 51 |
| Admissions likely related to Bevacizumab | |
| Bleeding | |
| Grade 2 | 3 |
| Grade 3 | 3 |
| Admissions possibly related to Bevacizumab | |
| Failure to Thrive | |
| Grade 3 | 7 |
| Admission unrelated to Bevacizumab | |
| Hypercalcemia | |
| Grade 3 | 10 |
| Grade 4 | 3 |
| Pain | |
| Grade 2 | 1 |
| Grade 3 | 10 |
| Fever/Infection | |
| Grade 3 | 4 |
| Shortness of Breathe | |
| Grade 3 | 4 |
| Grade 4 | 1 |
| Other | |
| Atypical Chest Pain - Grade 2 | 1 |
| Scheduled Surgical Procedure | 1 |
| Post-operative Lower Extremity Edema - Grade 2 | 1 |
| Nausea/Vomiting - Grade 3 | 1 |
| Elevated bilirubin - Grade 4 | 1 |
Clinical Practice Points.
Bevacizumab is approved in combination with interferon in the 1st line setting for RCC
Prior evidence for bevacizumab monotherapy in the targeted therapy salvage setting is limited
Bevacizumab monotherapy is safe even in poor risk patients in the salvage setting
Bevacizumab monotherapy can result in prolonged disease control despite heavy pretreatment in some patients
Bevacizumab monotherapy should be considered in heavily pretreated or poor risk patients.
Acknowledgements
The authors would like to thank the Randall MacDonald Renal Cell Cancer Fund for their generous support of this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Karumanchi SA, Merchan J, Sukhatme VP. Renal cancer: Molecular mechanisms and newer therapeutic options. Curr Opin Nephrol Hypertens. 2002;11(1):37–42. doi: 10.1097/00041552-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295(21):2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 5.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 6.Sternberg CN, Hawkins RE, Wagstaff J, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: Final overall survival results and safety update. Eur J Cancer. 2013;49(6):1287–1296. doi: 10.1016/j.ejca.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet. 2011;378(9807):1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 8.Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: A randomised open-label phase 3 trial. Lancet Oncol. 2013;14(13):1287–1294. doi: 10.1016/S1470-2045(13)70465-0. [DOI] [PubMed] [Google Scholar]
- 9.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27(20):3312–3318. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 10.Escudier B, Szczylik C, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(8):1280–1289. doi: 10.1200/JCO.2008.19.3342. [DOI] [PubMed] [Google Scholar]
- 11.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370(9605):2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 12.Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: Final results of CALGB 90206. J Clin Oncol. 2010;28(13):2137–2143. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 14.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 15.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349(5):427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bukowski RM, Kabbinavar FF, Figlin RA, et al. Randomized phase II study of erlotinib combined with bevacizumab compared with bevacizumab alone in metastatic renal cell cancer. J Clin Oncol. 2007;25(29):4536–4541. doi: 10.1200/JCO.2007.11.5154. [DOI] [PubMed] [Google Scholar]
- 17.Hainsworth JD, Shipley DL, Reeves J, Jr, Arrowsmith ER, Barnes EK, Waterhouse DM. High-dose bevacizumab in the treatment of patients with advanced clear cell renal carcinoma: A phase II trial of the sarah cannon oncology research consortium. Clin Genitourin Cancer. 2013;11(3):283–289.e1. doi: 10.1016/j.clgc.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Turnbull JD, Cobert J, Jaffe T, Harrison MR, George DJ, Armstrong AJ. Activity of single-agent bevacizumab in patients with metastatic renal cell carcinoma previously treated with vascular endothelial growth factor tyrosine kinase inhibitors. Clin Genitourin Cancer. 2013;11(1):45–50. doi: 10.1016/j.clgc.2012.06.001. [DOI] [PubMed] [Google Scholar]