Highlights
-
•
Skap1-deficient (skap1-/-) mice are resistant to the induction of collagen induced arthritis (CIA).
-
•
Skap1-/- mice show a reduction in presence of IL-17+ (Th17) T-cells in response to CII peptide.
-
•
No effect was seen on the production of other cytokines such as IL-10.
-
•
Our findings implicate SKAP1 as a novel upstream regulator murine autoimmune arthritis.
Keywords: T-cells, SKAP1, SKAP-55, Adaptor, Murine autoimmune arthritis
Abstract
SKAP1 is an immune cell adaptor that couples the T-cell receptor with the ‘inside-out’ signalling pathway for LFA-1 mediated adhesion in T-cells. A connection of SKAP1 to the regulation of an autoimmune disorder has not previously been reported. In this study, we show that Skap1-deficient (skap1-/-) mice are highly resistant to the induction of collagen-induced arthritis (CIA), both in terms of incidence or severity. Skap1-/- T-cells were characterised by a selective reduction in the presence IL-17+ (Th17) in response to CII peptide and a marked reduction of joint infiltrating T-cells in Skap1-/- mice. SKAP1 therefore represents a novel connection to Th17 producing T-cells and is new potential target in the therapeutic intervention in autoimmune and inflammatory diseases.
1. Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that affects joints, causing cartilage and bone destruction. The disease can be induced by the immunization of susceptible mice with type II collagen (CII) leading to humoral and cell-mediated responses [1], [2]. Arthritic severity in murine arthritis is correlated with autoantibody responses to CII [3]. Further, CD4+ T cell involvement in Rheumatoid arthritis (RA) has been inferred from linkage to HLA-DR alleles and the presence of activated CD4+ T cells in inflamed joints [4]. Despite the success of Tumour necrosis factor-alpha (TNF-α) blocking biologics in treating RA [2], [5], novel therapies are needed to modulate the underlying immune response [2], [6]. Murine models have been used in the pre-clinical testing of drugs such as TNF-α blockade [5], [7].
LFA-1 (lymphocyte function-associated antigen; αLβ2) antagonists also reduce inflammation in murine arthritis [8]. LFA-1 on T cells binds ICAM-1/3 on antigen presenting cells [9], [10], and its activation involves GTP-binding protein Rac-1 [11], [12], [13], [14], [15] and immune cell adaptors in T-cells termed ADAP (adhesion and degranulation-promoting adaptor protein; also FYB: Fyn binding protein) and SKAP1 (Src-kinase-associated phosphoprotein1 or SKAP-55) [16]. SKAP1 has a pleckstrin-homology domain, a C-terminal SH3 domain and tyrosine residues [17], [18] and regulates integrin clustering and adhesion [12], [13], [16], [19], [20] via the Rap1 binding to its partner, RAPL [16], [21], [22].
Despite its importance in T-cell function, a connection of SKAP1 to an autoimmune disorder has not been reported. Here, we show that Skap1-deficient mice are resistant to the induction of CIA, as characterised by reduced pro-inflammatory cytokine IL-17. Our finding indicates for the first time a role for SKAP1 in regulating inflammatory arthritis and suggests that the adaptor might serve as a novel therapeutic target.
2. Results and discussion
2.1. Skap1-/- mice are less susceptible to collagen-induced arthritis (CIA)
To assess the role of SKAP1 in the induction of CIA, Skap1+/+ and Skap1-/- mice were injected intradermally (i.d.) at two sites into the base of the tail with a chicken type II (CII) in an emulsion of complete Freund adjuvant (CFA) and monitored for signs of disease over 100 days (see Section 3). CII collagen can induce arthritis in C57BL/6 mice with incidences of 50–70% [23], [24], [25], [26]. Skap1+/+ and Skap1-/- mice have the same genetic background, and in many cases, were littermates. We found that CII collagen induced arthritis in B6 mice with a maximum cumulative incidence of 70% and mean day of onset between days 30 and 40 after primary immunization, as reported [26] (Fig. 1A). By contrast, Skap1-/- mice showed a much lower cumulative incidence of 10% (n = 18–22). The mean arthritic score was also lower in Skap1-/- mice from day 50–80 with a value of 3.7 for Skap1+/+ mice and 0.4 for Skap1-/- mice (Fig. 1B). A dot plot of different mice at day 40 showed the range of responses for Skap1+/+ vs. −/- mice with a mean that was consistently lower for Skap1-/- mice (p value = 0.0007) (Fig. 1C). No difference was noted in the body weight of Skap1+/+ and Skap1-/- mice (Fig. 1D). These data showed that the loss of SKAP1 conferred relative resistance of mice to the induction of arthritis.
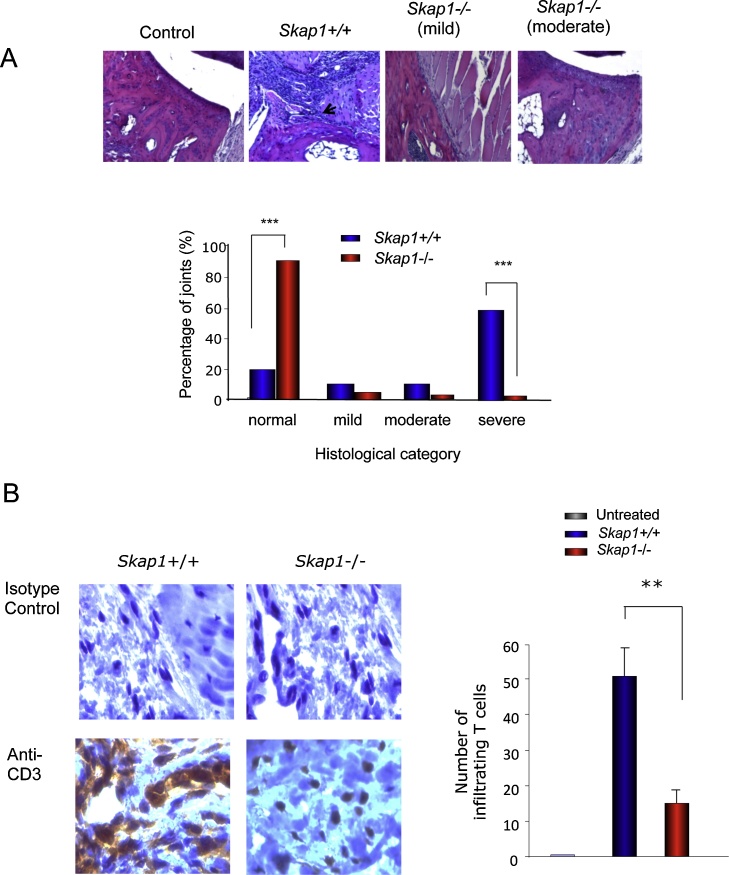
Fig. 1.
Skap1−/− mice are resistant to collagen induced arthritis. Collagen induced arthritis (CIA) was induced in wild type mice and Skap1−/− mice as described in Section 3. Panel A: Skap1−/− mice show a reduced incidence of inflammation. Panel B: Skap1-/- mice show a reduced mean arthritis score. Reduced clinical scores in Skap1−/− mice from day 20–90. The Data shown in Panel A and B are from four pooled experiments. The presented results are the mean ± SEM.* p < 0.05, **p < 0.01. Panel C: Dot plot of individual arthritis scores of mice at day 40. Panel D: The body weight of Skap1−/− and WT mice from week 1–15. No difference in body weights was observed.
A histological assessment of joints showed that 60% of Skap1+/+ mice showed severe pathology, 10% moderate, 10% mild and 20% normal histology (Fig. 2A, upper and lower panels). Severely affected joints showed inflammatory cell infiltration and invasive pannus tissue formation associated with bone and cartilage degradation. By contrast, Skap–/– mice showed 85% in the normal category when compared to the WT mice (P < 0.001). The remaining 5% showed either a mild and moderate pathology pattern. Overall, these findings showed that SKAP1 deficiency provides protection against the induction of CIA. We also examined the presence of T-cells in the joints Skap1-/- and +/+ mice in response to CII. CD3+ T cells were subjected to in situ staining of the joint area and revealed a major reduction in the infiltration of T-cells in Skap1−/− mice (Fig. 2B; T-cells seen in brown color). Mice were immunized with CII and boosted at day 21, the mice were sacrified at day 40, and the joints were sectioned and stained with antibody. In a given area, 52 CD3+ T-cells were detected in skap1+/+ mice, and only 16 in skap1-/- mice (right histogram). There were no detectable T-cells in non-diseased mouse joints.
Fig. 2.
Joint histology of Skap1−/− vs Skap1+/+ (WT) mice. Panel A: Joint histology. Upper panels: Histological features of CIA. Sections of an untreated control (left panel), severely arthritic Skap1+/+ (middle left), mild arthritic CIA-immunized Skap1−/− mouse (middle right) and moderately diseased Skap1−/− mouse (right panel) (×200 magnification). Lower panel: Histogram of percentage of joints. SKAP1-/- (n = 504 joints) and WT (n = 556 joints) mice in each group. Data are from four pooled experiments. ***P < 0.001 compared with the same histological grade in WT mice. (n = 18–22). Panel B: Skap1−/− mice show a reduced numbers of infiltrating T-cells in joints. Immunoperoxidase staining of the tissue sessions with an anti-CD3 Ab. Dark brown cells indicate CD3-positive T cells (×40). Upper panel: images of stained sections; lower panel: histogram showing the number of T-cells in joints on 0–50 scale. At least five areas from each specimen were chosen to determine the numbers of T cell infitrating in each specimen. T cells infiltration of the synovial area of Skap-1-/-mice was significantly reduced, compared with WT mice. P = 0.0005.
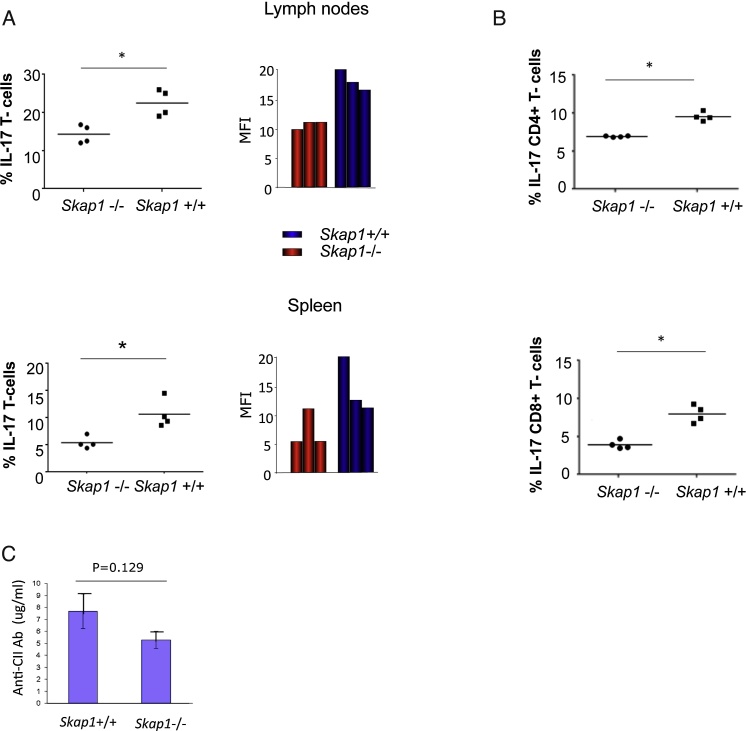
2.2. Reduced expression of IL-17 in T-cells of Skap1-/- mice
To investigate cytokine responses, inguinal LNs and spleen were harvested on day 14 (early arthritis) post primary CII immunization followed by intracellular staining for various cytokines (Fig. 3). When gated on CD3, Skap1-/- LNs showed a significantly lower percent of IL-17 expressing cells with 15.5% relative to 22.6% for Skap1+/+ cells (Fig. 3A, upper left panel). The MFI for Skap1-/- T-cells was also lower (upper right panel). Similarly, only 5.6% of CD3 positive splenic Skap1−/− T-cells were positive relative to 10.7 for Skap1+/+ T-cells (lower left panel). The MFI was 6.2 for Skap1-/- spleen T-cells relative to 11.7 for wild-type cells (lower right panel) (n = 5). Likewise, when gated for CD4, Skap1-/- CD3+ spleen T-cells showed a lower percentage of IL-17 positive cells with 7.2% versus 10.2% for Skap1+/+ T-cells (Fig. 3B, upper panel; Fig. S1). When gated for CD8, 4.3% of Skap1-/- CD3+ T-cells expressed the cytokine relative to 9.0% of CD3+ Skap1+/+ T-cells. These data showed that Skap1-/- T-cells have a lower prevalence of IL-17 expression in response to CII immunization. Surprisingly, the expression of other inflammatory or inhibitory cytokines such as IL-10, TNF-α, IFN-β, and IL-2 was unaffected by the loss of SKAP1 (Fig. S2A). No difference was noted in the expression of surface receptors such as the differentiation marker CD62L or inhibitory co-receptors such as PD-1 and LAG-3 (Fig. S2B). A difference was noted for CTLA-4 expression in T-cells from spleen but not lymph nodes.
Fig. 3.
Cellular and humoral responses to CII in mice. T-cells were removed from mesenteric lymph nodes at day 14 following CII injection and assessed for the various cytokines by intracellular staining and flow cytometry. Panel A: The number of CD3+ IL-17 expressing T-cells is reduced in Skap1-/- mice in response to CII. Upper panels: lymph nodes; lower panels: spleen. Left panels: Percentage of cells; right panels: mean fluorescent intensity (MFI). Panel B: IL-17 expressing CD4 and CD8 cells reduced in Skap1-/- mice in response to CII. Upper panels: IL-17 expression in CD4+ T-cells; lower panels: IL-17 expression in CD8+ T-cells. Panel C: Serum anti-CII IgG levels. Circulating levels of CII-specific IgG were determined in individual sera from SKAP1-/- (n = 18) and WT (n = 22) mice at 100 days after primary immunization with CII. Results show the mean± SEM (n = 3).) *p < 0.05, **p < 0.01.
As an additional control, anti-serum anti-C11 antibody levels were assessed by ELISA (Fig. 3C). Blood was collected from the retro-orbital sinus at day 40 post-primary immunization, while bound anti-collagen IgG was detected with HRP-labelled goat anti-mouse IgG. Skap1+/+ mice (WT) showed an average of 7.5 μg/ml of anti-CII antibodies, while a mean was reduced to 5.4 μg/ml in Skap1-/- mice as averaged over 3 experiments. No significant difference in antibody production was seen between Skap1−/− and WT mice, consistent with the report that Th17 dervived IL-17 is dispensible for antibody production [27].
Overall, our findings identify SKAP1 for the first time as a key regulator of CII induced RA in mice. Skap1-deficient mice were markedly resistant to the induction of CIA as characterised by a reduction in the key pro-inflammatory cytokine IL-17. Both the number of Th17 cells and the production of IL-17 were significantly reduced, an effect that would be expected to reduce the inflammatory response. This is also the first reported connection between SKAP1 and the generation of Th17 cells. The ability of SKAP1 to mediate inflammatory responses is consistent with its role in mediating T-cell binding to antigen presenting cells and ensuring oprtimal responses to antigen [12]. The exact integrin targeted by Skap1 in resistence is not clear since the activation of both β1 and β2 integrins is impaired in skap1−/− T-cells [19], [20]. Leukocyte recruitment to sites of inflammation is a multistep process that involves the multiple adhesion molecules and their ligands on leukocytes and the inflamed tissue. Antibody blocking studies suggest that the LFA-1 I-domain mediates a critical interaction in synovial inflammation, either to counterreceptors ICAM-1 (CD54) or JAM-A in the synovial [28]. Disease onset and initial severity was affected in ICAM-1 null mice, but not ICAM-2 null mice. Another report has shown a marked reduction in acute leukocyte recruitment in CD18- and CD11a-deficient mice in peritoneal inflammation, but not in mice lacking CD18 [29]. Our findings are therefore consistent with a role for SKAP1 in facilitating the activation of LFA-1 adhesion in inflammation. It provides an alternative target to anti-LFA-1 in therapeutic intervention in inflammatory arthritis.
Our findings are also compatible with the finding that SKAP1 is needed primarily in responses to lower affinity or dose antigen [30], [31]. We previously showed that high TCR occupancy with anti-CD3 can over-ride the dependency of LFA-1 activation on the SKAP1 adaptor [19]. At the same time, it was surprising that other cytokines such as IL-10 were not affected. The rationale for this selectivity is unclear, but may related to additional roles of SKAP1 in the inhibition of the p21-ERK pathway [32], [33] and in ‘outside-in’ signalling via integrins [13], [34]. While the full range of mediators affected by these processes are unknown, the balance of positive and negative signals could alter the threshold of signalling favouring the induction of one cytokine vs. another in different conditions. The potency of the effect of the loss of SKAP1 also suggests that its function cannot be substituted by related Skap2 (Hom or related) [35]. Overall, our findings suggest that SKAP1 may be an attractive target for therapeutic intervention in autoimmune and inflammatory diseases.
3. Materials and methods
3.1. Collagen-induced arthritis (CIA)
C57BL/6J mice from Jackson laboratories (Bar Harbor, ME) while skap1-/- mice were derived as decribed in Refs. [19], [36]. Chick type II collagen, CII (Sigma) in Complete Freund adjuvant (CFA) was injected intradermally (i.d.) at two sites into tail base (100 μl emulsion with 100 μg CII and 250 μg Mycobacterium tuberculosis) [7]. Injections were repeated on day 21 and joints were assessed for swelling and restriction. Each paw was scored (0 indicates normal; 1, mild swelling; 2 extensive swelling; and 3, joint distortion and/or rigidity; maximum score per mouse, 12). Joint histology was examined by joints (knee, elbow, ankle and wrist) being harvested, fixed in 10% formaldehyde/PBS forand decalcified using EDTA for 4 weeks. Specimens were cut longitudinally to the midline, and 5 μm sections mounted for staining with haematoxylin and eosin. These sections were graded in severity from 0 (normal) to 5 (severe) for five components, including joint exudate, synovitis, panus formation, cartilage degradation and bone degradation. Based on the histological scores, joints were classified as demonstration inflammatory arthritis if there was an exudate score of 1 or more and a synovitis score of 2 or more. Destructive arthritis was defined for joints that scored 2 or more for pannus or 1 or more for either cartilage degradation or bone degradation.
T cell infiltration in the joint was determined by immunoperoxidase staining of tissue sections with anti-CD3 (DAKO) for 1 h. DAB staining kit (SK4100, Vector Laboratories) was used for color visualization and counterstaining with hematoxylin and eosin. Five fields were randomly selected for each joint, and the average number of infiltrating T cells was determined by adding the total number of T cells, then dividing by five to obtain the number of infiltrating T cells per filed of each joint.
3.2. CII-specific and cytokine responses
Isotype-specific anti-collagen Abs from retro-orbital sinus blood was determined by ELISA on Immulon II plates (Dynex Technologies, Chantilly, VA). Bound anti-collagen IgG was detected with HRP-labeled goat anti-mouse IgG (Southern Biotechnology Associates, Birmingham, AL). Absorbance at 450 nm.
For cytokine production, T-cells from inguinal LNs or spleen were harvested at 14 days post immunization followed by intracellular staining for TNFα, IL-2 and IFN-γ and other cytolines (BD). Differences between mice was analyzed by a Mann-Whitney U test. All data are represented as mean ± SEM or SD and values of p < 0.05 were considered statistically significant. Flow cytometry was also conducted using antibodies to CD3, CD4, CD44, CD62L (BD, Cell Signal) with Alexa conjugated secondary antibodies (Invitrogen).
Conflict of interest
The authors have no conflict of interest or competing interests.
Acknowledgment
This work was supported by Wellcome Trust Program Grant (PG) 092627/Z/10/Z to C.E. Rudd.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.imlet.2016.04.007.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Seki N., Sudo Y., Yoshioka T., Sugihara S., Fujitsu T., Sakuma S. Type II collagen-induced murine arthritis. I. Induction and perpetuation of arthritis require synergy between humoral and cell-mediated immunity. J. Immunol. 1988;140:1477–1484. [PubMed] [Google Scholar]
- 2.Feldmann M., Steinman L. Design of effective immunotherapy for human autoimmunity. Nature. 2005;435:612–619. doi: 10.1038/nature03727. [DOI] [PubMed] [Google Scholar]
- 3.Courtenay J.S., Dallman M.J., Dayan A.D., Martin A., Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980;283:666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- 4.Rosloniec E.F., Brand D.D., Myers L.K., Whittington K.B., Gumanovskaya M., Zaller D.M. An HLA-DR1 transgene confers susceptibility to col- lagen-induced arthritis elicited with human type II collagen. J. Exp. Med. 1997;185:1113–1122. doi: 10.1084/jem.185.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldmann M., Maini R.N. Lasker clinical medical research award: TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat. Med. 2003;9:1245–1250. doi: 10.1038/nm939. [DOI] [PubMed] [Google Scholar]
- 6.Alzabin S., Williams R.O., Effector T. cells in rheumatoid arthritis: lessons from animal models. FEBS Lett. 2011;585:3649–3659. doi: 10.1016/j.febslet.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 7.Williams R.O., Feldmann M., Maini R.N. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. U. S. A. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suchard S.J., Stetsko D.K., Davis P.M., Skala S., Potin D., Launay M. An LFA-1 (αLβ2) small-molecule antagonist reduces inflammation and joint destruction in murine models of arthritis. J. Immunol. 2010;184:3917–3926. doi: 10.4049/jimmunol.0901095. [DOI] [PubMed] [Google Scholar]
- 9.Hogg N., Patzak I., Willenbrock F. The insider’s guide to leukocyte integrin signalling and function. Nat. Rev. Immunol. 2011;11:416–426. doi: 10.1038/nri2986. [DOI] [PubMed] [Google Scholar]
- 10.Dustin M.L., Bivona T.G., Philips M.R. Membranes as messengers in T cell adhesion signaling. Nat. Immunol. 2004;5:363–372. doi: 10.1038/ni1057. [DOI] [PubMed] [Google Scholar]
- 11.Bachmann M.F., McKall-Faienza K., Schmits R., Bouchard D., Beach J., Speiser D.E. Distinct roles for LFA-1 and CD28 during activation of naive T cells: adhesion versus costimulation. Immunity. 1997;7:549–557. doi: 10.1016/s1074-7613(00)80376-3. [DOI] [PubMed] [Google Scholar]
- 12.Wang H., McCann F.E., Gordan J.D., Wu X., Raab M., Malik T.H. ADAP-SLP-76 binding differentially regulates supramolecular activation cluster (SMAC) formation relative to T cell-APC conjugation. J. Exp. Med. 2004;200:1063–1074. doi: 10.1084/jem.20040780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H., Wei B., Bismuth G., Rudd C.E. SLP-76-ADAP adaptor module regulates LFA-1 mediated costimulation and T cell motility. Proc. Natl. Acad. Sci. U. S. A. 2009;106:12436–12441. doi: 10.1073/pnas.0900510106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki J., Yamasaki S., Wu J., Koretzky G.A., Saito T. The actin cloud induced by LFA_1-mediated outsude-in signals lowers the threshold of T-cell acitvation. Blood. 2007;109:168–175. doi: 10.1182/blood-2005-12-020164. [DOI] [PubMed] [Google Scholar]
- 15.Smith A., Carrasco Y.R., Stanley P., Kieffer N., Batista F.D., Hogg N. A talin-dependent LFA-1 focal zone is formed by rapidly migrating T lymphocytes. J. Cell Biol. 2005;170:141–151. doi: 10.1083/jcb.200412032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H., Rudd C.E. SKAP-55, SKAP-55-related and ADAP adaptors modulate integrin-mediated immune-cell adhesion. Trends Cell Biol. 2008;18:486–493. doi: 10.1016/j.tcb.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J., Kang H., Raab M., da Silva A.J., Kraeft S.K., Rudd C.E. FYB (FYN binding protein) serves as a binding partner for lymphoid protein and FYN kinase substrate SKAP55 and a SKAP55-related protein in T cells. Proc. Natl. Acad. Sci. U. S. A. 1998;95:8779–8784. doi: 10.1073/pnas.95.15.8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marie-Cardine A., Bruyns E., Eckerskorn C., Kirchgessner H., Meuer S.C., Schraven B. Molecular cloning of SKAP55, a novel protein that associates with the protein tyrosine kinase p59fyn in human T-lymphocytes. J. Biol. Chem. 1997;272:16077–16080. doi: 10.1074/jbc.272.26.16077. [DOI] [PubMed] [Google Scholar]
- 19.Wang H., Moon E.Y., Azouz A., Wu X., Smith A., Schneider H. SKAP-55 regulates integrin adhesion and formation of T cell-APC conjugates. Nat. Immunol. 2003;4:366–374. doi: 10.1038/ni913. [DOI] [PubMed] [Google Scholar]
- 20.Kliche S., Breitling D., Togni M., Pusch R., Heuer K., Wang X. The ADAP/SKAP55 signaling module regulates T-cell receptor-mediated integrin activation through plasma membrane targeting of Rap1. Mol. Cell. Biol. 2006;26:7130–7144. doi: 10.1128/MCB.00331-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raab M., Wang H., Lu Y., Smith X., Wu Z., Strebhardt K. T cell receptor inside-out pathway via signaling module SKAP1-RapL regulates T cell motility and interactions in lymph nodes. Immunity. 2010;32:541–556. doi: 10.1016/j.immuni.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raab M., Smith X., Matthes Y., Strebhardt K., Rudd C.E. SKAP1 pH domain determines RAPL membrane localization and Rap1 complex formation for TCR activation of LFA-1. J. Biol. Chem. 2011;2011:29663–70286. doi: 10.1074/jbc.M111.222661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell I.K., Rich M.J., Bischof R.J., Dunn A.R., Grail D., Hamilton J.A. Protection from collagen-induced arthritis in granulocyte-macrophage colony-stimulating factor-deficient mice. J. Immunol. 1998;161:3639–3644. [PubMed] [Google Scholar]
- 24.Campbell I.K., Hamilton J.A., Wicks I.P. Collagen-induced arthritis in C57BL/6 (H-2b) mice: new insights into an important disease model of rheumatoid arthritis. Eur. J. Immunol. 2000;30:1568–1575. doi: 10.1002/1521-4141(200006)30:6<1568::AID-IMMU1568>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 25.Kai H., Shibuya K., Wang Y., Kameta H., Kameyama T., Tahara-Hanaoka S. Critical role of M. tuberculosis for dendritic cell maturation to induce collagen-induced arthritis in H-2b background of C57BL/6 mice. Immunology. 2006;118:233–239. doi: 10.1111/j.1365-2567.2006.02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inglis J.J., Criado G., Medghalchi M., Andrews M., Sandison A., Feldmann M. Collagen-induced arthritis in C57BL/6 mice is associated with a robust and sustained T-cell response to type II collagen. Arthritis Res. Ther. 2007;9:R113. doi: 10.1186/ar2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibui A., Shimura E., Nambu A., Yamaguchi S., Leonard W.J., Okumura K. Th17 cell-derived IL-17 is dispensable for B cell antibody production. Cytokine. 2012;59:108–114. doi: 10.1016/j.cyto.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watts G.M., Beurskens F.J., Martin-Padura I., Ballantyne C.M., Klickstein L.B., Brenner M.B. Manifestations of inflammatory arthritis are critically dependent on LFA-1. J. Immunol. 2005;174:3668–3675. doi: 10.4049/jimmunol.174.6.3668. [DOI] [PubMed] [Google Scholar]
- 29.Mizgerd J.P., Kubo H., Kutkoski G.J., Bhagwan S.D., Scharffetter-Kochanek K., Beaudet A.L. Neutrophil emigration in the skin, lungs, and peritoneum: different requirements for CD11/CD18 revealed by CD18-deficient mice. J. Exp. Med. 1997;186:1357–1364. doi: 10.1084/jem.186.8.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller K.L., Thomas M.S., Burbach B.J., Peterson E.J., Shimizu Y. Adhesion and degranulation-promoting adapter protein (ADAP) positively regulates T cell sensitivity to antigen and T cell survival. J. Immunol. 2007;179:3559–3569. doi: 10.4049/jimmunol.179.6.3559. [DOI] [PubMed] [Google Scholar]
- 31.Wang H., Liu H., Lu Y., Lovatt M., Wei B., Rudd C.E. Functional defects of SKAP-55-deficient T cells identify a regulatory role for the adaptor in LFA-1 adhesion. Mol. Cell. Biol. 2007;27:6863–6875. doi: 10.1128/MCB.00556-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider H., Wang H., Raab M., Valk E., Smith X., Lovatt M. Adaptor SKAP-55 binds p21 activating exchange factor RasGRP1 and negatively regulates the p21-ERK pathway in T-cells. PLoS One. 2008;3:e1718. doi: 10.1371/journal.pone.0001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kosco K.A., Cerignoli F., Williams S., Abraham R.T., Mustelin T. SKAP55 modulates T cell antigen receptor-induced activation of the Ras-Erk-AP1 pathway by binding RasGRP1. Mol. Immunol. 2008;45:510–522. doi: 10.1016/j.molimm.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 34.Baker R.G., Hsu C.J., Lee D., Jordan M.S., Maltzman J.S., Hammer D.A. The adapter protein SLP-76 mediates Outside-In integrin signaling and function in T cells. Mol. Cell. Biol. 2009;29:5578–5589. doi: 10.1128/MCB.00283-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jo E.K., Wang H., Rudd C.E. An essential role for SKAP-55 in LFA-1 clustering on T cells that cannot be substituted by SKAP-55R. J. Exp. Med. 2005;201:1733–1739. doi: 10.1084/jem.20042577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H., Lu Y., Rudd C.E. SKAP1 is dispensable for chemokine-induced migration of primary T-cells. Immunol. Lett. 2010;128:148–153. doi: 10.1016/j.imlet.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.