Abstract
Nephrogenic syndrome of inappropriate antidiuresis (NSIAD) is a genetic disease first described in 2 unrelated male infants with severe symptomatic hyponatremia. Despite undetectable arginine vasopressin levels, patients have inappropriately concentrated urine resulting in hyponatremia, hypoosmolality, and natriuresis. Here, we describe and functionally characterize a novel vasopressin type 2 receptor (V2R) gain-of-function mutation. An L312S substitution in the seventh transmembrane domain was identified in a boy presenting with water-induced hyponatremic seizures at the age of 5.8 years. We show that, compared with wild-type V2R, the L312S mutation results in the constitutive production of cAMP, indicative of the gain-of-function NSIAD profile. Interestingly, like the previously described F229V and I130N NSIAD-causing mutants, this appears to both occur in the absence of notable constitutive β-arrestin2 recruitment and can be reduced by the inverse agonist Tolvaptan. In addition, to understand the effect of various V2R substitutions on the full receptor “life-cycle,” we have used and further developed a bioluminescence resonance energy transfer intracellular localization assay using multiple localization markers validated with confocal microscopy. This allowed us to characterize differences in the constitutive and ligand-induced localization and trafficking profiles of the novel L312S mutation as well as for previously described V2R gain-of-function mutants (NSIAD; R137C and R137L), loss-of-function mutants (nephrogenic diabetes insipidus; R137H, R181C, and M311V), and a putative silent V266A V2R polymorphism. In doing so, we describe differences in trafficking between unique V2R substitutions, even at the same amino acid position, therefore highlighting the value of full and thorough characterization of receptor function beyond simple signaling pathway analysis.
Arginine vasopressin (AVP) plays the main role in the regulation of water balance homeostasis (1, 2). Binding of this peptide to its vasopressin type 2 receptor (V2R), expressed on the basolateral membrane of kidney collecting duct epithelial cells, triggers production of cAMP after activation of Gαs. This in turn activates adenylyl cyclase, leading to phosphorylation, translocation, and insertion of aquaporin-2 water channels into the apical plasma membrane, ultimately resulting in increased water permeability and antidiuresis (1, 2).
V2R is a G protein-coupled receptor (GPCR) encoded by the AVPR2 gene located on chromosome Xq28 (3, 4). Both loss- and gain-of function variants of V2R are associated with human disease. The most prevalent loss-of-function mutations, of which over 250 have been reported to date (the Human Gene Mutation Database at the Institute of Medical Genetics in Cardiff [5], accessed in December 2015), cause X-linked nephrogenic diabetes insipidus (NDI), characterized by AVP insensitivity and presenting clinically with excessive urine production, dehydration, and thirst (2). Gain-of-function mutations of V2R cause nephrogenic syndrome of inappropriate antidiuresis (NSIAD). This rare condition clinically resembles the syndrome of inappropriate antidiuretic hormone secretion (SIADH), as both cause inappropriately concentrated urine and resultant hyponatremia, hypoosmolality, and natriuresis. However, NSIAD differs from SIADH because it is associated with undetectable plasma AVP levels (6, 7).
Until recently, only 3 gain-of-function mutations in the AVPR2 gene had been described (6, 8). Two of these (R137C and R137L) (6, 9) affect a conserved DRY/H motif in the second intracellular loop, whereas the third mutation, F229V (8), is located in the third intracellular loop of V2R. All 3 mutations were shown to result in constitutive activation and cAMP production (6, 8), although not all of their effects on receptor function were the same. We and others have previously shown that the 2 R137 mutants recruit β-arrestin constitutively and are constitutively internalized (10–13), whereas another group has shown that the F229V mutation does not result in constitutive β-arrestin interaction (8). Recently a fourth gain-of-function mutation was described at I130N in the third transmembrane domain of V2R, which also results in constitutive production of cAMP (14). Interestingly, the I130N mutant has reduced cell surface expression under basal conditions and appears to internalize constitutively via a dynamin-dependent process without constitutively recruiting β-arrestin2. Furthermore, after AVP-induced activation of the I130N V2R mutant, cAMP production is similar to the wild-type receptor, whereas β-arrestin recruitment is reduced, indicating that the I130N variation results in a G protein-biased constitutively active V2R (14).
Importantly with respect to potentially guiding clinical care, inverse agonist Tolvaptan does not reduce the constitutive cAMP production observed with the R137C and R137L mutant receptors (8), whereas it does for the F229V (8) and I130N (14) mutant receptors. Therefore, despite all known NSIAD-causing V2R mutations having consistent etiology, that is, constitutive Gs protein activation and cAMP production resulting in aquaporin-2 insertion and antidiuresis, differences in other aspects of their function, such as β-arrestin interaction and localization/trafficking profiles, may correlate with differences in clinical effectiveness of an inverse agonist like Tolvaptan.
Correct folding and trafficking to the plasma membrane is vital for proper V2R function. The loss-of-function associated with many NDI V2R mutations is due to intracellular retention caused by incorrect folding during synthesis (15). However, beyond observing interactions with β-arrestin2, internalization, or general changes in cell surface expression, neither intracellular trafficking after receptor activation nor basal receptor localization of disease-causing V2R mutations is well understood.
Receptor trafficking and localization can be monitored by fluorescence microscopy, where colocalization of fluorescently labeled receptors with labeled plasma membrane markers or intracellular markers allows for subcellular detection. Such methods are generally limited by the possible spatial resolution of nonsuper-resolution microscopic set ups and can be technically challenging, particularly over extended time courses. Recently, a novel bioluminescence resonance energy transfer (BRET) technique has been developed that allows for the observation of the basal distribution of membrane proteins in multiple subcellular compartments, and of changes in agonist-induced subcellular localization and trafficking of receptors to be monitored. This can be done in live cells over extended time periods with spatial resolution of less than 100 Å (16, 17).
Here, we describe and functionally characterize a novel V2R gain-of-function mutation. An L312S substitution in the seventh transmembrane domain of V2R was identified in a boy presenting with water-induced hyponatremic seizures despite normal adrenal function at the age of 5.8 years. Consequently, NSIAD was suspected and the AVPR2 gene was sequenced. We show that, compared with wild-type V2R, the L312S mutation results in the constitutive production of cAMP, indicative of the gain-of-function NSIAD profile. This is reduced by Tolvaptan, unlike the previously described R137C and R137L mutants (8) but consistent with the more recently described F229V (8) and I130N (14) mutants. Furthermore, L312S V2R does not appear to interact constitutively with β-arrestin2 to any notable degree, again in contrast to the R137 mutants (10), but in keeping with the F229V (8) and I130N (14) mutants. Differences between L312S V2R compared with both R137C and R137L V2R are also clear from confocal microscopy. In addition, using the BRET intracellular localization assay, we describe functional differences in the localization and trafficking profiles of this novel mutant compared with profiles for V2R gain-of-function (NSIAD: R137C and R137L) and loss-of-function (NDI: R137H and R181C) mutants. Indeed interestingly, despite constitutive activity with respect to cAMP production, the L312S V2R exhibits a profile similar to that observed for wild-type V2R and the NDI-associated M311V V2R (18), particularly with respect to ligand-induced receptor trafficking.
Materials and Methods
DNA sequencing and analysis
The study of the novel AVPR2 variant was approved by the institutional review board of the Endocrinology Research Centre, and the patient's parents gave informed consent for DNA analysis. AVPR2 gene sequencing for the patient and his mother with the novel AVPR2 variant was performed as a diagnostic test in order to guide clinical care and was performed in the Laboratory of Inherited Endocrine Disorders of the Endocrinology Research Centre, with the sequence results reported from the same laboratory. Patient samples were not used in any of the research experiments presented in this manuscript. All other V2R mutants have been described previously.
Genomic DNA was extracted from peripheral leukocytes by standard procedure. The coding sequence of the AVPR2 gene was amplified by PCR in one fragment and the amplification products were purified and directly sequenced using an automated DNA sequencer (3130 Genetic Analyzer; Applied Biosystems). The following oligonucleotides were used for PCR and subsequent sequencing: 1F, 5′-GACCCTGGGCCATTGAACTTG-3′; 2F, 5′-CTCTCCATAGTCTTTGTG-3′; 3R, 5′-CACAGGCTCTGGCCAATTCTC-3′. GenBank cDNA entry with accession number Z11687.1 was used as a reference sequence (19).
Materials
V2R/Rluc8 cDNA was generated as described previously from V2R cDNA kindly provided by Brian Feldman (Stanford University, Stanford, CA) (10). V2R/NanoLuc (Nluc) cDNA was provided by Promega. V2R L312S cDNA was synthesized without a stop codon and subcloned into pcDNA3-Rluc8 and pcDNA3-Nluc by GeneArt (Thermo Fisher Scientific). V2Rs containing mutations R137C, R137L, R137H, R181C, V266A, and M311V tagged with Rluc8 or Venus have been described previously (10, 18). To generate R137C V2R/Nluc, R137L V2R/Nluc, and L312S V2R/Venus, pcDNA3 containing R137C V2R/Rluc8, R137L V2R/Rluc8, or L312S V2R/Rluc8 was digested with KpnI and XhoI, purified by gel electrophoresis and QIAquick Gel Extraction kit (QIAGEN), and cloned into pcDNA3 containing Nluc or Venus cut with the same restriction enzymes. The resulting plasmids containing R137C or R137L fused in-frame with Nluc or L312S fused in-frame with Venus were subsequently sequenced by the Australian Genome Research Facility.
The β-arrestin2-Venus cDNA construct was prepared previously from pcC2-Venus kindly provided by Atsushi Miyawaki (RIKEN Brain Science Institute) (20). The β-arrestin2/HaloTag (HT) cDNA was provided by Promega, as was the HT nonchloro TOM (NCT) ligand (21). The plasma membrane marker Venus/K-ras, as well as the subcellular compartment marker RabGTPases (Rabs) Venus/Rab5a (early endosomes), Venus/Rab7a (late endosomes/lysosomes), and Venus/Rab11 (recycling endosomes), were kindly provided by Professor Nevin Lambert (Georgia Regents University). Rab1 (endoplasmic reticulum trafficking to the cis-Golgi), Rab4 (early endosome recycling), Rab6 (Golgi and trans-Golgi network), Rab8 (trans-Golgi network to the plasma membrane), and Rab9 (late endosome trafficking to the trans-Golgi network) (22, 23) were synthesized and subcloned by GeneArt into a pcDNA3 expression vector containing Venus without a stop codon and with a 20 residue linker of GGGAGGGAGGGAGGGAGGGA, to generate the resulting fusion proteins with the Venus tag on the N terminus of the Rab protein.
AVP, carbachol, and forskolin were from Sigma-Aldrich. Tolvaptan was from Shanghai DND Pharm-Technology. EnduRen (Rluc8 substrate) and caged furimazine (Nluc substrate) were from Promega.
Cell culture and transfection for functional characterization
HEK293FT cells were maintained at 37°C in 5% CO2 and complete media (DMEM containing 0.3-mg/mL glutamine, 100-IU/mL penicillin, and 100-μg/mL streptomycin; Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS) (Bovogen Biologicals) and 400-μg/mL Geneticin (Thermo Fisher Scientific). Transient transfections were carried out 24 hours after seeding 550 000 cells/well of a 6-well plate. FuGENE 6 (Promega) transfection reagent was used according to the manufacturer's instructions. Cells were harvested with 0.05% trypsin-EDTA (Thermo Fisher Scientific).
BRET assays profiling mutant V2R interactions with β-arrestin2
HEK293FT cells were transiently transfected with cDNA (10 ng/well of a 6-well plate) encoding wild-type, R137C, R137L, or L312S V2R fused to Rluc8 (for the extended BRET (eBRET) assay with EnduRen) (24) or Nluc (for the new NanoBRET assay with caged furimazine) and cDNA (30 ng/well of a 6-well plate) encoding β-arrestin2 fused to Venus (β-arrestin2/Venus) or β-arrestin2 fused to HT (β-arrestin2/HT). Twenty-four hours after transfection, cells were harvested into white 96-well plates (Greiner) at 50 000 cells/well in phenol-red-free DMEM containing 25mM HEPES, 0.3-mg/mL glutamine, 100-IU/mL penicillin, and 100-μg/mL streptomycin (Thermo Fisher Scientific) supplemented with 5% FBS. Forty-eight hours after transfection, DMEM was removed and cells were incubated with 10μM caged furimazine for Nluc or 30μM EnduRen for Rluc8 (both from Promega) in calcium- and magnesium-free Hanks' balanced salt solution (HBSS) (Thermo Fisher Scientific) for 1.5 hours at 37°C in 5% CO2. Cells were then incubated for a further 30 minutes, with HT NCT ligand (0.25μM) added to cells transfected with HT cDNA constructs. BRET measurements were subsequently taken at 37°C using a CLARIOstar multilabel plate reader (BMG Labtech). Kinetic analysis of β-arrestin2 recruitment was carried out in duplicate with multiple concentrations of AVP (5 independent experiments). Log concentration-response curves were constructed with the data obtained about 30 minutes after AVP addition. Filtered light emissions were sequentially measured at 420–480 nm for Nluc or Rluc8 and 520–620 nm for Venus or more than 610 nm for HT with its NCT ligand. The “BRET ratio (above wild-type baseline)” was calculated as the ratio of 520- to 620-nm emission over 420- to 480-nm emission (for Nluc or Rluc8 and Venus) or more than 610-nm emission over 420- to 480-nm emission (for Nluc and HT) for each cell sample minus the same ratio for the 1pM AVP-treated wild-type V2R cell sample at the respective time point. In this calculation, the 1pM AVP-treated wild-type V2R cell sample represents the background, eliminating the requirement for measuring a donor-only control sample (10).
Assessment of the effect of the L312S mutation on cellular expression
To determine whether the L312S mutation resulted in changes in relative expression levels, luminescence was measured in cells transiently transfected with Nluc-tagged wild-type or L312S V2R as above after a 2-hour pretreatment with 10μM caged furimazine.
BRET assays comparing intracellular distribution of wild-type and various V2R mutants
HEK293FT cells were transiently transfected with cDNA encoding wild-type or mutant V2R fused to Rluc8 (100 ng/well of a 6-well plate), as well as Venus/K-ras (100 ng/well of a 6-well plate) or Venus/Rab proteins or empty vector (200 ng/well of a 6-well plate). Twenty-four hours after transfection, cells were harvested into white 96-well plates at 80 000 cells/well in phenol-red-free DMEM containing 25mM HEPES, 0.3-mg/mL glutamine, 100-IU/mL penicillin, and 100-μg/mL streptomycin supplemented with 5% FBS. Forty-eight hours after transfection, the media were removed, and cells were incubated with 30μM EnduRen (Promega) (24) in HBSS for 2 hours at 37°C in 5% CO2. Cells were treated with HBSS with or without 1μM AVP. Real-time eBRET measurements were taken at 37°C using a CLARIOstar multilabel plate reader. Filtered light emissions were sequentially measured at 420–480 nm for Rluc8 and 520–620 nm for Venus. The “BRET ratio (ligand-vehicle)” was calculated by subtracting the ratio of 520- to 620-nm emission over 420- to 480-nm emission for a vehicle-treated cell sample from the same ratio for a sample treated with agonist. In this calculation, the vehicle-treated cell sample represents the background, eliminating the requirement for measuring a donor-only control sample (24, 25).
BRET signals for assays where basal/constitutive localization of Rluc8-tagged wild-type V2R or V2R mutants proximal to Venus-tagged subcellular markers were calculated as described previously (26) by subtracting the ratio of 520- to 620-nm emission over the 420- to 480-nm emission for a cell sample containing only the Rluc8 fusion protein from the same ratio of a second aliquot of cells containing both the Rluc8 and Venus fusion proteins. The calculated BRET ratios were then divided by the BRET ratio for the wild-type V2R and graphed on a web-plot in order to visualize BRET signals relative to wild-type.
Measurement of cAMP accumulation using time-resolved fluorescence resonance energy transfer
Agonist-induced cAMP accumulation was measured using a homogeneous time-resolved fluorescence (HTRF) cAMP dynamic 2 assay kit (CisBio Bioassays) as described previously (18). Briefly, HEK293FT cells were transfected as described above with plasmid DNA containing wild-type V2R/Nluc or L312S V2R/Nluc (500 ng/well of a 6-well plate) and seeded into white 384-well microplates (Greiner) 24 hours before assay. AVP concentration-response curves were constructed by aspirating and replacing the cell media with stimulation buffer (0.5mM 3-isobutyl-1-methylxanthine (IBMX), 5mM HEPES, and 0.1% BSA in HBSS) with or without AVP (10fM–0.1μM) or forskolin (100μM). The cells were incubated for 30 minutes at 37°C and then lysed by addition of the supplied conjugate-lysis buffer containing d2-labeled cAMP, followed by conjugate-lysis buffer containing terbium cryptate-labeled anti-cAMP antibody, both reconstituted according to the manufacturer's instructions. Plates were incubated for 1 hour at room temperature and fluorescence signals were measured at 620 and 665 nm, 50 microseconds after excitation at 337 nm using a CLARIOstar multilabel plate reader. In a subset of experiments, to investigate the ability of an inverse agonist to reduce constitutive activity, HEK293FT cells were transfected with wild-type V2R/Nluc or L312S V2R/Nluc (250 ng/well of a 6-well plate) and seeded into a white 96-well plate 24 hours before assay. The cells were treated with AVP (0.1nM) or Tolvaptan (1μM), or a combination of both, before being processed as described above.
Measurement of inositol-1-phosphate (IP1) production using time-resolved fluorescence resonance energy transfer
The determination of IP1 accumulation was performed in a 96-well microplate seeded with transfected HEK293FT cells (500 ng/well of a 6-well plate) using the IP-One HTRF assay (CisBio Bioassays) (18, 27). To construct concentration-response curves, cells expressing either wild-type V2R/Nluc or L312S V2R/Nluc were incubated for 30 minutes at 37°C in the stimulation buffer (10mM HEPES [pH 7.4], 1mM CaCl2, 0.5mM MgCl2, 4mM KCl, 146mM NaCl, 5.5mM glucose, and 50mM LiCl) with or without 1pM–10μM AVP or carbachol (2mM) as an internal control. Cells were then lysed using the conjugate-lysis buffer mixed with the d2-labeled IP1 analog and the terbium cryptate-labeled anti-IP1 antibody according to the manufacturer's instructions. The assay was incubated for 1 hour at room temperature before fluorescence signals were measured at 620 and 665 nm, 50 microseconds after excitation at 337 nm using a CLARIOstar multilabel plate reader.
Confocal microscopy
Confocal microscopy studies were carried out by transiently transfecting HEK293 cells using Lipofectamine LTX (Thermo Fisher Scientific), then seeding onto poly-L-lysine-coated glass coverslips. After 48 hours, cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, stained with the indicated primary and secondary antibodies followed by Hoechst 33256 nuclear stain (Thermo Fisher Scientific) and then mounted in ProLong Diamond Antifade (Thermo Fisher Scientific). Samples were viewed using a Nikon A1Si Confocal Microscope equipped with a ×60 (oil) lens (Nikon). Validation of the plasma membrane K-ras marker to evaluate V2R internalization was carried out by transfecting cells with cDNA coding for FLAG-tagged V2R (600 ng/well of a 12-well plate) along with Venus/K-ras (600 ng/well of a 12-well plate). Membrane distribution was assessed either in the absence (naive) or presence of AVP (0.1μM, 15 min), using anti-FLAG M2 antibody (Sigma-Aldrich). To validate the expression and subcellular targeting of Venus/Rab fusion proteins, individual Venus/Rab constructs (green) (1 μg/well of a 12-well plate) were transiently transfected in HEK293 cells and the subcellular distribution assessed against a panel of well-established subcellular organelle markers (red) for cis-Golgi (anti-GM130; BD Biosciences), early endosomes (anti-early endosome antigen 1 [EEA-1]; BD Biosciences), late endosomes/lysosomes (LysoTracker Red, 1μM, 30-min uptake; Thermo Fisher Scientific), and recycling endosomes (Transferrin Alexa Fluor 647 conjugate, 10 μg/mL, 30-min uptake; Thermo Fisher Scientific). Finally, visualization of NSIAD-causing V2R mutants entailed transfecting cells with cDNA coding for the indicated Venus-tagged receptors (1 μg/well of a 12-well plate) and imaging as described above.
Data presentation and statistical analysis
Data were analyzed using GraphPad Prism 6 (GraphPad Software, Inc) with α designated as 0.05. For BRET kinetic assays, where applicable the final pretreatment reading is presented at the zero time point (time of ligand/vehicle addition). Statistical analysis was determined using a one- or two-way ANOVA with an appropriate post hoc test for multiple comparison as indicated in the text. Where statistical analysis was performed on paired observations, repeated measures one- or two-way ANOVA with a post hoc test for multiple comparison was performed.
Results
Case report
A boy (5.9 y old) was referred to the Endocrinology Research Centre, due to persistent hyponatremia. The patient was born at 40 weeks gestation to nonconsanguineous parents. At birth, he was 50 cm in height and weighed 2800 g. His developmental milestones were unremarkable. The drinking behavior of the proband was characterized by the parents as being “almost never thirsty.” The urine-specific gravity values measured in the first 5 years of life in the course of routine medical examinations were in the range of 1018–1028 g/L. The younger child in the family (a 1.3 y old female) was reported to be healthy.
The condition manifested at the age of 5.8 years while the family was on summer vacation. The child became weak and drowsy, which was followed by tonic seizures. The episode was apparently provoked by excessive water intake in the hot weather conditions. The boy was admitted to the local hospital where hyponatremia (116 mmol/L) was documented. After IV infusion of hydrocortisone and saline solution, the condition normalized and the serum sodium levels stabilized in the range of 136–140 mmol/L. An adrenocorticotropic hormone test showed normal adrenal function with basal and stimulated cortisol levels of 127 and 734 nmol/L, respectively. Brain magnetic resonance imaging and electroencephalogram also revealed no abnormalities. After discharge, symptoms of hyponatremia were documented again at home and at this point the boy was referred to the Endocrinology Research Centre. At admission, physical examination revealed no abnormalities. His serum sodium level was 133.2 mmol/L, potassium 4.1 mmol/L, plasma osmolality 274 mOsm/kg, serum aldosterone 1753 pmol/L (94–765), and plasma renin activity 1.8 ng/mL per hour (0.5–6.0). The urine-specific gravity was 1028 g/L. AVP levels were not measured, however, considering the persistent hyponatremia and normal adrenal function, the phenotype strongly pointed to NSIAD, particularly in the absence of any other notable underlying disorder, and so the AVPR2 gene was sequenced.
Sequencing and analysis of AVPR2 gene
By sequencing the AVPR2 gene of the proband, we found a novel hemizygous c.935T>C transition in exon 3, which resulted in a leucine (TTG) to serine (TCG) substitution at position 312 (p.L312S). The heterozygous p.L312S mutation was found in the asymptomatic mother (Figure 1). A search of publicly available genomic databases (including Exome Aggregation Consortium version 0.3) revealed that the p.L312S variant is not present in the general human population and has to our knowledge never previously been implicated in human disease.
Figure 1.
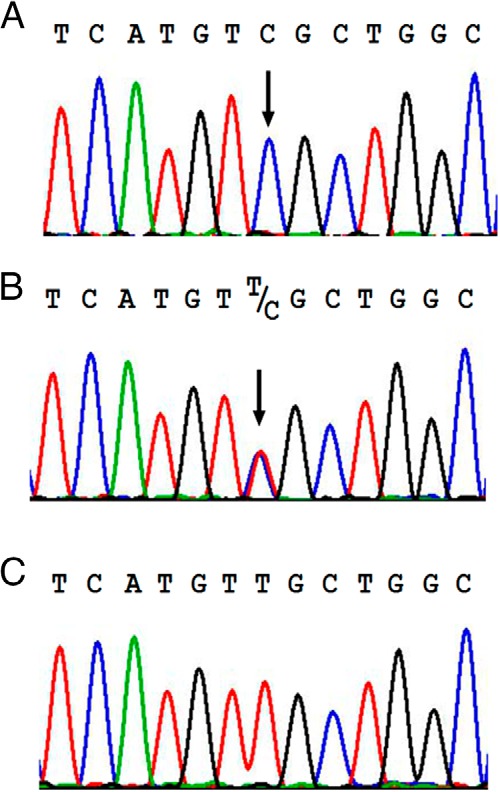
Electropherograms of DNA sequences of the AVPR2 gene. Hemizygous c.935T>C transition (exon 3) changing leucine (TTG) to serine (TCG) at position 312 (L312S) in the proband (A). Heterozygous L312S mutation in the mother (B). Wild-type sequence (C).
Effect of the L312S mutation on cellular expression
No difference was observed in luminescence from cells expressing L312S V2R/Nluc (647 633 ± 59 021; relative light units ± SEM; 4 independent experiments) compared with wild-type V2R/Nluc (630 757 ± 48 122; relative light units ± SEM, 4 independent experiments). Relative cell surface expression was assessed by comparing the proximity between Rluc8-tagged wild-type or L312S mutant V2R and the membrane marker K-ras tagged with Venus (Table 1). We observed no significant difference in cell surface expression of the L312S V2R mutant relative to wild-type V2R (P > .05).
Table 1.
Analysis of Constitutive/Basal Localization of V2R Mutants Relative to Wild-Type V2R
| Wild-Type | NSIAD Mutants |
NDI Mutants |
V2R Polymorphism V266A | |||||
|---|---|---|---|---|---|---|---|---|
| L312S | R137C | R137L | R137H | R181C | M311V | |||
| K-ras | 1.00 ± 0.06 | 1.16 ± 0.06 | 0.73 ± 0.05a | 0.60 ± 0.05c | 0.70 ± 0.05a | 1.05 ± 0.05 | 1.19 ± 0.11 | 1.12 ± 0.05 |
| Rab1 | 1.00 ± 0.11 | 1.16 ± 0.11 | 1.72 ± 0.18b | 1.38 ± 0.20 | 2.57 ± 0.20c,d | 1.14 ± 0.12 | 1.11 ± 0.15 | 1.25 ± 0.19 |
| Rab4 | 1.00 ± 0.03 | 1.38 ± 0.05 | 1.59 ± 0.05b | 1.28 ± 0.06 | 2.54 ± 0.17c,d | 1.11 ± 0.07 | 1.29 ± 0.15 | 1.27 ± 0.04 |
| Rab5 | 1.00 ± 0.03 | 1.23 ± 0.14 | 1.56 ± 0.11b | 1.13 ± 0.10 | 2.04 ± 0.07c,d | 1.06 ± 0.0 | 1.26 ± 0.14 | 1.18 ± 0.04 |
| Rab6 | 1.00 ± 0.02 | 1.47 ± 0.06 | 1.67 ± 0.22a | 1.40 ± 0.18 | 2.77 ± 0.05c,d | 0.99 ± 0.05 | 1.19 ± 0.10 | 1.07 ± 0.05 |
| Rab7 | 1.00 ± 0.01 | 1.19 ± 0.02 | 1.30 ± 0.10b | 1.21 ± 0.08 | 1.62 ± 0.05c,f | 0.98 ± 0.02 | 1.15 ± 0.08 | 1.06 ± 0.02 |
| Rab8 | 1.00 ± 0.19 | 0.97 ± 0.13 | 1.06 ± 0.17 | 0.98 ± 0.16 | 1.52 ± 0.21b,d | 0.97 ± 0.16 | 0.97 ± 0.15 | 0.91 ± 0.15 |
| Rab9 | 1.00 ± 0.06 | 1.39 ± 0.09 | 1.54 ± 0.18a | 1.37 ± 0.17 | 2.44 ± 0.13c,d | 1.03 ± 0.08 | 1.14 ± 0.05 | 1.13 ± 0.09 |
| Rab11 | 1.00 ± 0.06 | 0.99 ± 0.05 | 1.09 ± 0.06 | 0.81 ± 0.04e | 1.68 ± 0.11c,d | 0.96 ± 0.05 | 0.95 ± 0.05 | 1.03 ± 0.06 |
Values were calculated relative to wild-type V2R localization as described in Materials and Methods, with values more than 1 indicating increased basal localization and values less than 1 indicating reduced basal localization. Statistical analysis by one-way ANOVA with Tukey's post hoc test for multiple comparisons. Values are mean ± SEM of 3 independent experiments.
P < .05 vs wild-type.
P < .01 vs wild-type.
P < .001 vs wild-type.
P < at least .05 vs R137C and R137L.
P < .001 vs R137C.
P < .01 vs R137L.
Evaluation of L312S V2R G protein-dependent signaling
GPCRs such as V2R signal via heterotrimeric G proteins, with V2R preferentially coupling to and signaling via Gs proteins, resulting in the activation of adenylate cyclase and a subsequent increase in intracellular cAMP. NSIAD-causing V2R mutations result in constitutive activation and production of cAMP. We therefore examined the effect of the L312S V2R mutation on cAMP accumulation using a HTRF cAMP assay. Cells transiently transfected with L312S V2R tagged with Nluc showed substantial agonist-independent constitutive cAMP accumulation (Figure 2A). Furthermore, stimulation with AVP (10fM–0.1μM) resulted in a robust concentration-dependent increase in cAMP accumulation with a comparable potency (pEC50 ± SEM of 10.71 ± 0.31 and 11.05 ± 0.12 for L312S V2R and wild-type V2R respectively) and maximum (98.1 ± 8.9% of the wild-type maximum) compared with the wild-type V2R.
Figure 2.
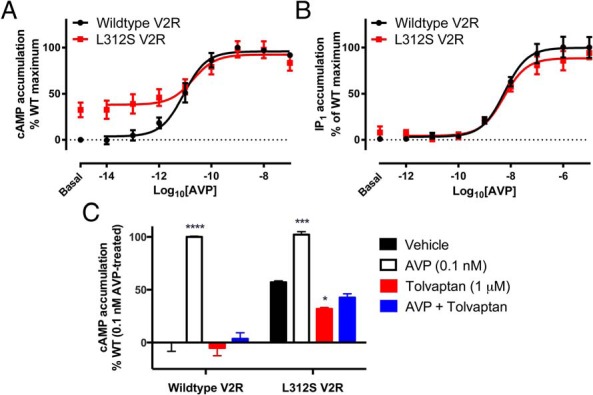
Effect of L312S mutation on AVP mediated G protein signaling. Concentration-response curves to AVP for cAMP (A) and IP1 (B) accumulation were generated using transiently transfected HEK293FT cells expressing either wild-type (black circles) or L312S (red squares) V2R tagged with Nluc. For cAMP accumulation (A), cells were stimulated with AVP (10fM–0.1μM) or forskolin (100μM) for 30 minutes in buffer containing IBMX (500μM). For IP1 accumulation (B), cells were stimulated with AVP (1pM–10μM) or carbachol (2mM) for 30 minutes in buffer containing LiCl (50mM). Cells were then lysed, and cAMP or IP1 accumulation was measured by HTRF. Data shown are normalized to the forskolin or carbachol response for cAMP and IP1 accumulation respectively and expressed as % of the wild-type V2R maximum response to observe constitutive activity. Points represent mean ± SEM of 4 independent experiments (A) and 6 independent experiments (B), and the curve fit is by nonlinear regression. The effect of 1μM inverse agonist Tolvaptan was also assessed (C) in terms of inhibiting constitutive (black and red bars) or ligand-induced (0.1nM AVP; white and blue bars) cAMP accumulation. Observations were made after 30 minutes in a buffer containing IBMX (500μM), in cells expressing either wild-type or L312S V2R tagged with Nluc. Data are expressed as a percentage of the wild-type response to 0.1nM AVP treatment.
Like numerous GPCRs, V2R can couple pleiotropically to various signaling effectors. This includes the Gq protein, which results in the activation of phospholipase C, inositol 1,4,5 trisphosphate production, and a subsequent increase in intracellular calcium. Therefore, to determine the extent of the effect of the L312S mutation on G protein-dependent signaling, Gq-mediated signaling was investigated in addition to Gs-mediated cAMP production, using the HTRF IP1 accumulation assay. Cells transiently transfected with L312S V2R showed a concentration-dependent accumulation of IP1 when stimulated with AVP (1pM–10μM), which was not significantly different from the wild-type V2R response (P > .05) (Figure 2B). Furthermore, constitutive IP1 accumulation relative to the wild-type V2R was not observed. Finally, these results also indicate that the addition of Nluc does not impair the G protein signaling capability of V2R. This finding is consistent with our previous study (18), which showed comparable AVP potency at V2R for cAMP and IP1 accumulation with that observed in this study under the same conditions.
Effect of the inverse agonist Tolvaptan
For wild-type V2R, the cAMP production observed after treatment with a submaximal concentration of AVP (0.1nM) was entirely inhibited by 1μM inverse agonist Tolvaptan (Figure 2C). Constitutive cAMP production mediated by mutant L312S V2R was significantly reduced by treatment with Tolvaptan (P < .05, two-way ANOVA with Tukey's post hoc test for multiple comparisons) (Figure 2C), although it did not result in inhibition to wild-type baseline.
Effect of L312S mutation on β-arrestin2 recruitment
After activation and phosphorylation of V2R, β-arrestin2 is recruited to the receptor resulting in desensitization (with respect to G protein signaling at the plasma membrane) and internalization, as well as scaffolding of a secondary signaling complex. Therefore, the effect of the L312S mutation on β-arrestin2 recruitment was investigated compared with the previously published NSIAD V2R mutants R137C and R137L. BRET assays with live HEK293FT cells were used to measure β-arrestin2 proximity to the receptors in 3 distinct donor-acceptor configurations: V2R/Rluc8 with β-arrestin2/Venus, V2R/Nluc with β-arrestin2/Venus, and V2R/Nluc with β-arrestin2/HT. Significant (P < .05, repeated measures one-way ANOVA with Dunnett's post hoc test for multiple comparisons) constitutive recruitment of β-arrestin2 relative to wild-type was observed for R137C V2R and R137L V2R with all 3 donor-acceptor configurations (Figure 3 and Supplemental Figure 1). In contrast, no such constitutive recruitment of β-arrestin2 relative to wild-type was observed for L312S V2R (Figure 3). Furthermore, the kinetic profiles for L312S V2R were similar to those observed with wild-type V2R with all 3 donor-acceptor configurations, and contrast with those observed for R137C V2R and R137L V2R (Supplemental Figure 1).
Figure 3.
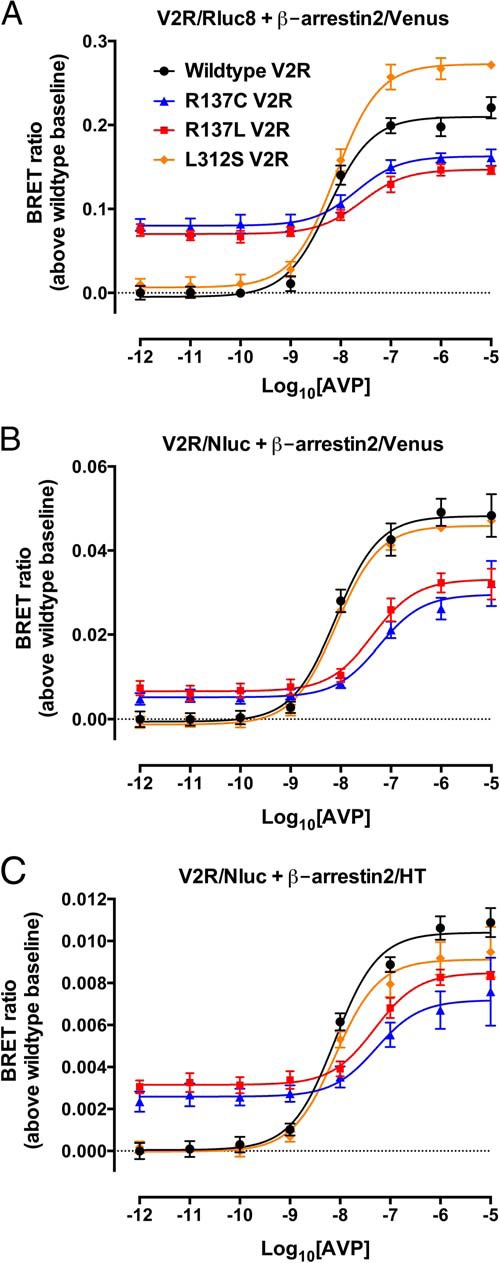
Effect of NSIAD V2R mutations on β-arrestin2 recruitment assessed with multiple BRET configurations. HEK293FT cells transiently transfected with cDNA coding for wild-type V2R (black circles), R137C V2R (blue triangles), R137L V2R (red squares), or L312S V2R (orange diamonds) C-terminally tagged with Rluc8 (A) or Nluc (B and C) and β-arrestin2/Venus (A and B) or β-arrestin2/HT (C) were used to determine concentration-response curves with AVP (1pM–10μM) for β-arrestin2 recruitment to wild-type or mutant V2Rs after about 30 minutes. Data are expressed as BRET ratio (above wild-type baseline) as described in Materials and Methods, and the associated kinetic data are shown in Supplemental Figure 1. Data represent mean ± SEM of 5 independent experiments, with curves fitted by nonlinear regression (GraphPad Prism).
The potency of AVP-mediated β-arrestin2 recruitment to L312S V2R (pEC50 ± SEM: 8.11 ± 0.08, 8.09 ± 0.05, and 8.10 ± 0.10 in the Rluc8/Venus, Nluc/Venus, and Nluc/HT configurations, respectively) was not discernibly different to that of wild-type V2R (pEC50 ± SEM: 8.24 ± 0.09, 8.10 ± 0.10, and 8.11 ± 0.08 in the Rluc8/Venus, Nluc/Venus, and Nluc/HT configurations, respectively). In contrast, R137C V2R and R137L V2R both exhibited a significant (P < .05, repeated measures one-way ANOVA with Dunnett's post hoc test for multiple comparisons) decrease in the potency of AVP-mediated β-arrestin2 recruitment compared with wild-type V2R (R137C V2R pEC50 ± SEM: 7.69 ± 0.21, 7.24 ± 0.16, and 7.28 ± 0.28; and R137L V2R pEC50 ± SEM: 7.59 ± 0.18, 7.36 ± 0.16, and 7.31 ± 0.14 for the Rluc8/Venus, Nluc/Venus, and Nluc/HT configurations, respectively).
BRET localization assay: Validation of Venus-tagged subcellular localization markers
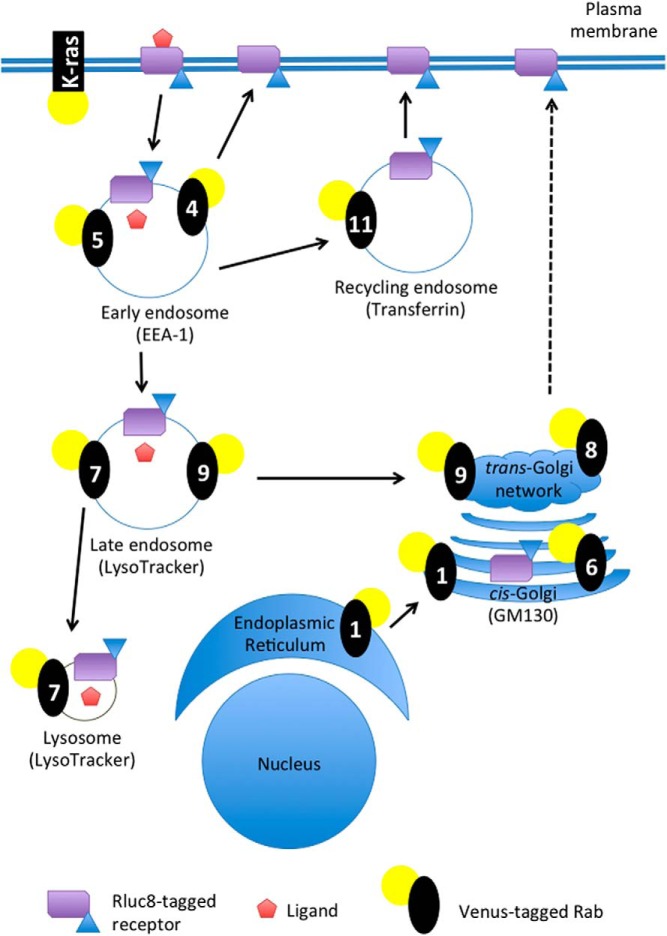
To examine the distribution and trafficking of gain- and loss-of-function V2R mutations, we used a novel BRET localization assay approach (16, 17). We expanded the number of Venus-tagged subcellular localization markers to include Rab1, Rab4, Rab6, Rab8, and Rab9 in addition to the previously described K-ras (16, 26, 28, 29), Rab5 (16, 28, 29), Rab7 (28, 29), and Rab11 (29). Validation of all these markers was carried out by confocal microscopy. Under basal conditions Venus/K-ras was localized to the plasma membrane and colocalized with FLAG-tagged V2R (Figure 4). After AVP stimulation (0.1μM, 15 min), V2R internalized away from the Venus/K-ras, which remained on the plasma membrane. The Venus/Rab constructs were validated by assessing colocalization with other relevant well-established subcellular markers (Figure 5). Consistent with their reported subcellular target membranes, biosynthetic endoplasmic reticulum and Golgi Rabs (ie, Rab1 and Rab6) show prominent perinuclear colocalization with cis-Golgi marker GM130; early endosomal Rabs (ie, Rab4 and Rab5) colocalized with the endosomal resident protein EEA-1; late endosomal/lysomosal Rabs (ie, Rab7 and Rab9) overlapped with LysoTracker and Rab11 was localized to transferrin-bearing recycling endosomes. By comparison, Rab8 showed little to no overlap with cis-Golgi marker GM130 consistent with its reported role in directing trans-Golgi network to plasma membrane trafficking.
Figure 4.
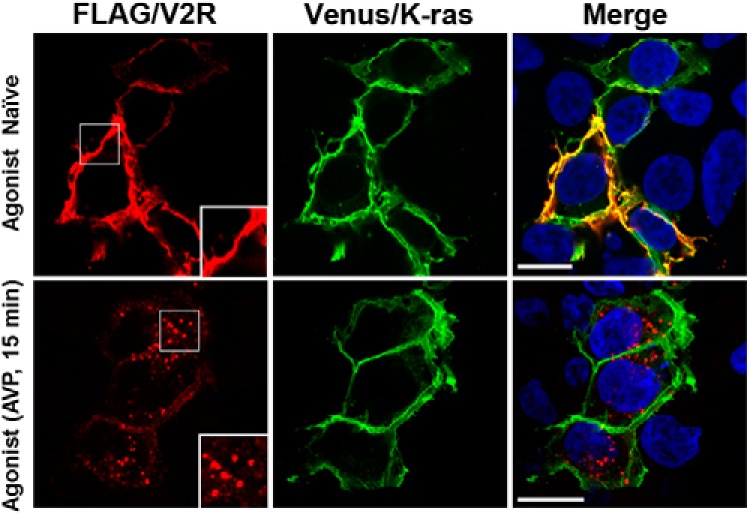
Confocal microscopy validation of Venus/K-ras localization and demonstration of utility to assess V2R internalization. FLAG-tagged wild-type V2R (red) and Venus/K-ras (green) are colocalized on the plasma membrane (yellow) in the absence of agonist. Treatment with AVP for 15 minutes results in V2R internalization, whereas the distribution of Venus/K-ras is unchanged. Insets highlight white boxes. Nuclei are depicted in blue (Hoechst 33256). Scale bar, 10 μm.
Figure 5.
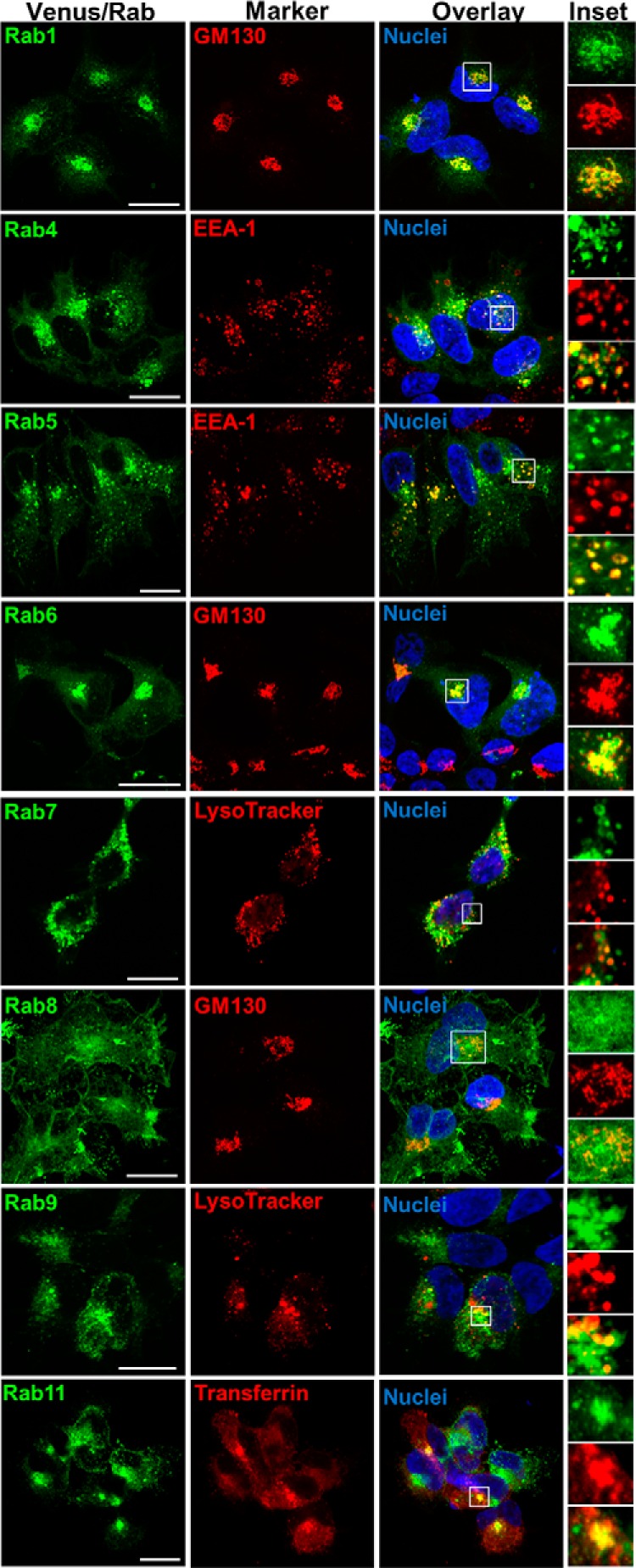
Confocal microscopy validation of Venus/Rab localization. Venus/Rab1, Venus/Rab4, Venus/Rab5, Venus/Rab6, Venus/Rab7, Venus/Rab8, Venus/Rab9, or Venus/Rab11 (green) was imaged with a relevant well-established marker as indicated (red) enabling insights to be obtained regarding subcellular localization (GM130 for cis-Golgi; EEA-1 for early endosomes; LysoTracker for late endosomes/lysosomes; transferrin for recycling endosomes). Representative images from serial confocal z-sections are depicted, with colocalization between Venus/Rabs and markers (yellow) evident upon overlay of individual fluorescence channels. Insets highlight magnifications of boxed regions. Nuclei were visualized with Hoechst 33256 stain (blue). Scale bar, 10 μm.
BRET localization assay: Constitutive/basal subcellular distribution of V2R mutants
Using Rluc8-tagged wild-type and mutant V2Rs in combination with the validated Venus-tagged markers, we were able to monitor constitutive receptor distribution as well as ligand-induced trafficking in real time and in live cells throughout the receptor life-cycle (Figure 6). Relative to wild-type V2R, we observed marked differences in the constitutive localization profile between V2R mutants (Figure 7 and Table 1). We did not observe any significant differences (P > .05, one-way ANOVA with Tukey's post hoc test) in the constitutive localization of the novel NSIAD-causing L312S variant (Table 1 and Figure 7A), the NDI-causing R181C and M311V V2R mutants (Table 1 and Figure 7B), or the polymorphism V266A (Table 1 and Supplemental Figure 2). Interestingly, V2R substitutions at R137 resulted in marked changes relative to wild-type V2R, as well as between mutations. All V2R R137 substitutions resulted in a significant (P < .05) loss of cell surface expression (K-ras proximity) relative to wild-type V2R, with the loss being similar in magnitude between substitutions (Figure 7). In contrast, the NDI-causing R137H V2R substitution displayed severe dysregulation of basal intracellular localization with an increase relative to wild-type found at all compartments tested (P < .01) (Figure 7B), which was greater than that observed for the R137C and R137L V2R NSIAD-causing mutations (P < .05). Moreover, we observed further differences between the constitutive localization profiles of the NSIAD-causing V2R mutants, R137C and R137L. The R137C substitution resulted in a significant increase in basal localization at Rab1, Rab4, Rab5, Rab6, Rab7, and Rab9 relative to wild-type, whereas differences for R137L V2R were not statistically significant.
Figure 6.
A simplified schematic representation of subcellular marker localization and receptor trafficking. Ligand-induced trafficking as well as constitutive localization was monitored using Rluc8-tagged wild-type or mutant V2R by measuring proximity via eBRET with the plasma membrane marker Venus/K-ras, or the subcellular compartment markers Rabs: Venus/Rab5a (5) for early endosomes; Venus/Rab4 (4) for early endosome recycling; Venus/Rab11 (11) for recycling endosomes; Venus/Rab7a (7) for late endosomes/lysosomes; Venus/Rab9 (9) for late endosome trafficking to the trans-Golgi network; Venus/Rab1 (1) for endoplasmic reticulum trafficking to the cis-Golgi; Venus/Rab6 (6) for Golgi apparatus and trans-Golgi network; or Venus/Rab8 (8) for trans-Golgi network to plasma membrane. The subcellular markers used for the confocal microscopy validation are also included for comparison.
Figure 7.
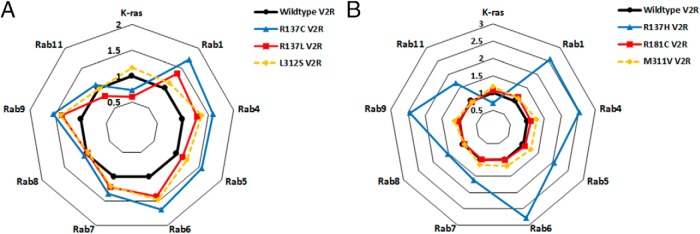
Profiling the constitutive localization of (A) gain and (B) loss-of-function V2R mutants. HEK293FT cells were transiently transfected with wild-type or mutant V2R/Rluc8 with or without the plasma membrane marker Venus/K-ras or Venus/Rab intracellular markers. Constitutive localization is plotted relative to wild-type V2R (black circles and line = 1) calculated as described in Materials and Methods. Values more than 1 indicate increased relative basal localization, and values less than 1 indicate reduced relative basal localization. A, NSIAD V2R mutations R137C (blue triangles and line), R137L (red squares and line), and L312S (yellow diamonds and broken line) relative to wild-type V2R (black circles and line). B, NDI V2R mutations R137H (blue triangles and line), R181C (red squares and line), and M311V (yellow diamonds and broken line) relative to wild-type V2R (black circles and line). Points represent the mean of 3 independent experiments. Note the same wild-type data were used in the generation of A and B as well as Supplemental Figure 2.
It is noteworthy that for both R137C V2R and R137L V2R, the greatest localization relative to wild-type V2R was in proximity to Rab1 and Rab6, but not Rab8 (Table 1 and Figure 7A), implicating particular relative localization to the endoplasmic reticulum and cis-Golgi compartments (Figure 6). This is in contrast to that observed for L312S V2R, at least in terms of Rab1 proximity (Table 1 and Figure 7A). Combined with the significant overall constitutive intracellular localization evidenced by the K-ras marker for R137C and R137L, but not L312S V2R, a pattern emerges from the BRET data of relatively increased perinuclear compartment localization for R137C and R137L mutants, with this being less evident for L312S V2R. This is indeed consistent with what is observed with confocal microscopy (Supplemental Figure 3).
Ligand-induced changes in localization and trafficking of V2R mutants
In addition to observing constitutive V2R distribution, the BRET localization assay was used to analyze the effect of V2R mutations on AVP (1μM)-induced changes in receptor internalization and trafficking. In agreement with observations of basal receptor localization, there were distinct differences in ligand-induced internalization between V2R mutants (Tables 2 and 3, Figures 8 and 9, and Supplemental Figure 4). AVP (1μM) induced internalization and trafficking of the V2R/Rluc8, which we were able to observe as a change in the BRET ratio. However, we did not observe a significant ligand-induced change in the BRET ratio for wild-type V2R or any of the V2R mutants in assays using Rab6 or Rab8 (P > .05) (Tables 2 and 3). The novel L312S V2R mutant underwent AVP-induced internalization and trafficking to a similar extent to wild-type V2R but with a small increase in the magnitude of internalization (K-ras; P < .05) (Figure 8A) and trafficking to the early endosomal recycling compartment (Rab4; P < .05) (Figure 8C). Again there were differences observed between the R137 substitutions, as all 3 were internalized to a small degree (P < .001, P < .01, and P < .05 R137C/H/L, respectively) (Tables 2 and 3); however, relative to wild-type, this was markedly decreased (P < .001). Additionally, the substitutions R137C and R137L resulted in a greater attenuation of internalization relative to R137H, which appears to retain the greatest ability of the R137 substitutions in this regard (P < .01). Largely, we did not observe further ligand-induced trafficking of the R137-substituted V2R mutants to any intracellular markers tested with the exception being a small response seen for R137C at the compartment markers Rab4, Rab5, and Rab7 (P < .05) (Table 2). We did not observe ligand-induced internalization or trafficking of the R181C NDI-causing V2R mutant (P > .05) (Figure 9), whereas for the M311V NDI-causing V2R mutant, ligand-induced internalization and trafficking was unaffected for approximately 60 minutes, at which point a small change in the magnitude of proximity to K-ras (cell membrane) was observed (P < .05) (Figure 9A). The V2R polymorphism V266A (Table 3 and Supplemental Figure 4) internalized and trafficked after stimulation with AVP. The magnitude of internalization (decreased proximity to K-ras) and trafficking proximal to Rab1, Rab4, Rab5, and Rab7 was increased significantly relative to wild-type (Table 3).
Table 2.
Ligand-Induced Change in BRET Ratio of Wild-Type V2R and NSIAD Mutants After Stimulation With AVP
| Wild-Type1 | NSIAD Mutants |
||||||
|---|---|---|---|---|---|---|---|
| L312S1 | Δ From WT2 | R137C1 | Δ From WT | R137L1 | Δ From WT | ||
| K-ras | −0.90 ± 0.02c | −1.01 ± 0.03c | −0.11 ± 0.04d | −0.16 ± 0.02c | 0.74 ± 0.03f | −0.11 ± 0.04b | 0.78 ± 0.04f |
| Rab1 | 0.11 ± 0.01c | 0.12 ± 0.001c | — | 0.04 ± 0.01 | −0.07 ± 0.01f | −0.01 ± 0.01 | −0.12 ± 0.01f |
| Rab4 | 0.12 ± 0.001c | 0.15 ± 0.01c | 0.03 ± 0.01e | 0.03 ± 0.004a | −0.09 ± 0.01f | 0.02 ± 0.01 | −0.10 ± 0.01f |
| Rab5 | 0.18 ± 0.001c | 0.19 ± 0.02c | — | 0.05 ± 0.001a | −0.13 ± 0.001f | 0.02 ± 0.01 | −0.16 ± 0.01f |
| Rab6 | 0.01 ± 0.01 | 0.01 ± 0.001 | — | 0.02 ± 0.002 | — | −0.01 ± 0.01 | — |
| Rab7 | 0.07 ± 0.01c | 0.07 ± 0.01c | — | 0.03 ± 0.01a | — | −0.002 ± 0.01 | −0.06 ± 0.01f |
| Rab8 | 0.02 ± 0.01 | 0.01 ± 0.01 | — | 0.0004 ± 0.003 | — | 0.008 ± 0.01 | — |
| Rab9 | 0.06 ± 0.001c | 0.07 ± 0.003c | — | 0.01 ± 0.01 | −0.05 ± 0.01e | −0.001 ± 0.004 | −0.06 ± 0.01f |
| Rab11 | 0.24 ± 0.03c | 0.19 ± 0.05c | — | 0.06 ± 0.01 | −0.18 ± 0.03f | 0.03 ± 0.01 | −0.20 ± 0.03f |
1. Change in BRET ratio from baseline (at t = 0) after AVP (1μM) addition measured at 95 minutes. Values are mean ± SEM of 3 independent experiments. Statistical analysis by two-way repeated measures ANOVA with Sidak's post hoc test for multiple comparisons. 2. Δ from wild-type (WT) indicates a statistically significant increase or decrease in the response after AVP (1μM) addition to or from the indicated compartment marker compared with wild-type V2R response. Statistical analysis by one-way ANOVA with Dunnett's post hoc test for multiple comparisons. Values are mean ± SEM of 3 independent experiments.
P < .05. Change in BRET ratio from own baseline.
P < .01. Change in BRET ratio from own baseline.
P < .001. Change in BRET ratio from own baseline.
P < .05. Versus wild-type response.
P < .01. Versus wild-type response.
P < .001. Versus wild-type response.
Table 3.
Ligand-Induced Change in the BRET Ratio of Wild-Type V2R and Mutants After Stimulation With AVP
| Wild-Type1 | NDI Mutants |
V2R Polymorphism |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| R137H1 | Δ From WT2 | R181C1 | Δ From WT | M311V1 | Δ From WT | V266A1 | Δ From WT | ||
| K-ras | −0.90 ± 0.02a | −0.26 ± 0.01a | 0.64 ± 0.05d | −0.05 ± 0.003 | 0.84 ± 0.02d | −0.68 ± 0.01a | 0.22 ± 0.02b | −1.16 ± 0.03a | −0.26 ± 0.03b |
| Rab1 | 0.11 ± 0.01a | −0.0002 ± 0.01 | −0.11 ± 0.01d | 0.003 ± 0.01 | −0.11 ± 0.01d | 0.11 ± 0.01a | — | 0.13 ± 0.05a | 0.02 ± 0.05b |
| Rab4 | 0.12 ± 0.001a | 0.02 ± 0.01 | −0.10 ± 0.05d | 0.01 ± 0.005 | −0.11 ± 0.005d | 0.14 ± 0.001a | — | 0.17 ± 0.01a | 0.05 ± 0.01d |
| Rab5 | 0.18 ± 0.001a | 0.02 ± 0.01 | −0.17 ± 0.01d | 0.02 ± 0.005 | −0.16 ± 0.01d | 0.21 ± 0.01a | — | 0.24 ± 0.01a | 0.06 ± 0.01c |
| Rab6 | 0.01 ± 0.01 | 0.001 ± 0.003 | — | 0.004 ± 0.005 | — | −0.01 ± 0.03 | — | 0.02 ± 0.01 | — |
| Rab7 | 0.07 ± 0.01a | −0.002 ± 0.002 | −0.07 ± 0.02d | 0.02 ± 0.003 | −0.05 ± 0.01d | 0.08 ± 0.005a | — | 0.10 ± 0.02a | 0.03 ± 0.01c |
| Rab8 | 0.02 ± 0.01 | 0.001 ± 0.001 | — | −0.005 ± 0.001 | — | 0.01 ± 0.01 | — | 0.02 ± 0.01 | — |
| Rab9 | 0.06 ± 0.001a | 0.01 ± 0.004 | −0.05 ± 0.005d | 0.003 ± 0.01 | −0.06 ± 0.001b | 0.08 ± 0.01a | — | 0.08 ± 0.01a | — |
| Rab11 | 0.24 ± 0.03a | 0.03 ± 0.01 | −0.21 ± 0.03d | 0.003 ± 0.004 | −0.23 ± 0.03d | 0.26 ± 0.02a | — | 0.24 ± 0.05a | — |
1. Change in BRET ratio from baseline (at t = 0) after AVP (1μM) addition measured at 95 minutes. Values are mean ± SEM of 3 independent experiments. Analysis by two-way repeated measures ANOVA with Sidak's post hoc test for multiple comparisons. 2. Δ from wild-type (WT) indicates a statistically significant increase or decrease in the response after AVP (1μM) addition to or from the indicated compartment marker compared with wild-type V2R response. Analysis by a one-way ANOVA with Dunnett's post hoc test for multiple comparisons. Values are mean ± SEM of 3 independent experiments.
P < .001. Change in BRET ratio from own baseline.
P < .05. Versus wild type response.
P < .01. Versus wild type response.
P < .001. Versus wild type response.
Figure 8.
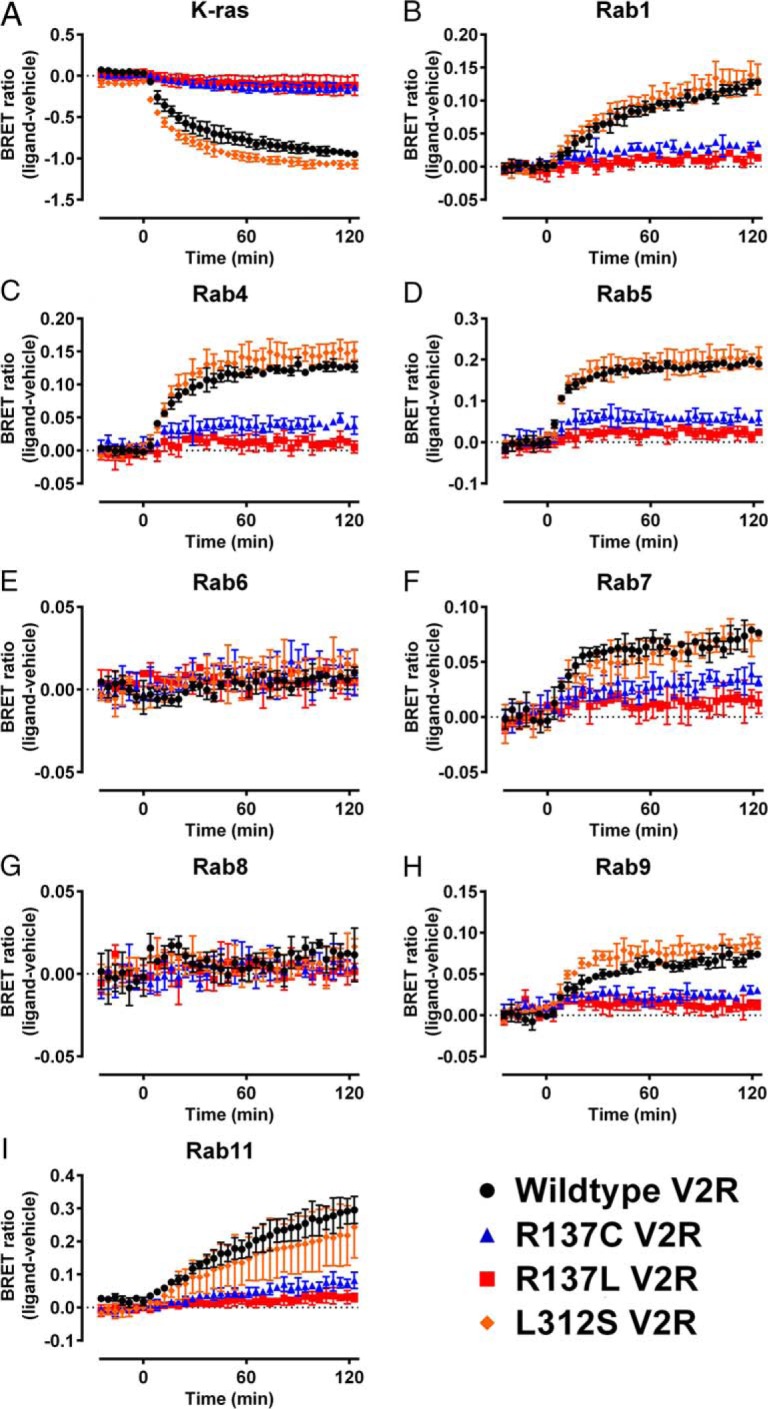
Kinetic profiling of the trafficking properties of gain-of-function V2R NSIAD mutants. HEK293FT cells were transiently transfected with wild-type V2R (black circles), R137C V2R (blue triangles), R137L V2R (red squares), or L312S V2R (orange diamonds) tagged with Rluc8 and (A) the plasma membrane marker K-ras or one of the subcellular markers (B) Rab1, (C) Rab4, (D) Rab5, (E) Rab6, (F) Rab7, (G) Rab8, (H) Rab9, or (I) Rab11 tagged with Venus. BRET ratio (ligand-vehicle) was calculated as described in Materials and Methods. AVP (1μM) or vehicle was added at t = 0 after establishment of the baseline. Points represent mean ± SEM of 3 independent experiments. Note wild-type data are replicated in Figure 9 and Supplemental Figure 4.
Figure 9.
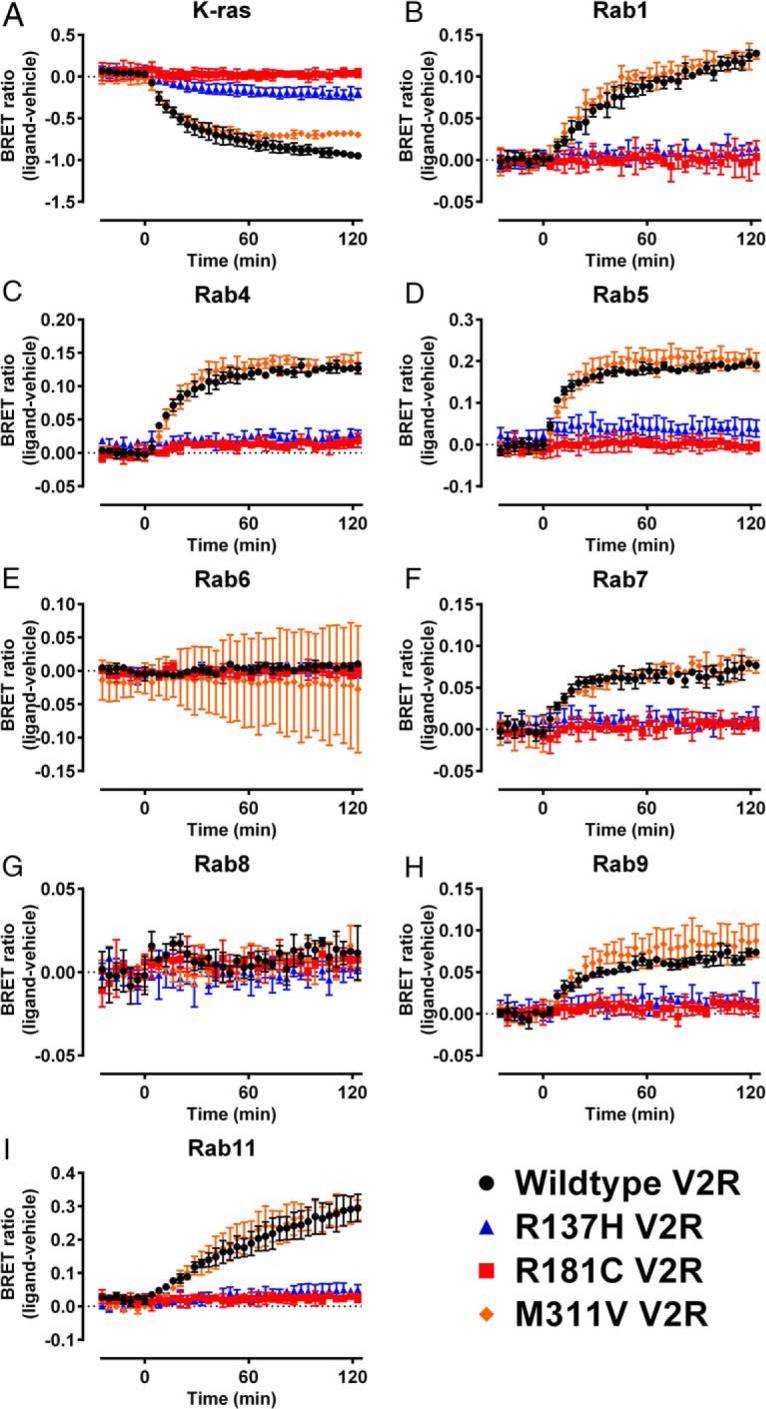
Kinetic profiling of the trafficking properties of loss-of-function V2R NDI mutants. HEK293FT cells were transiently transfected with wild-type V2R (black circles), R137H V2R (blue triangles), R181C V2R (red squares), or M311V V2R (orange diamonds) tagged with Rluc8 and (A) the plasma membrane marker K-ras or one of the subcellular markers (B) Rab1, (C) Rab4, (D) Rab5, (E) Rab6, (F) Rab7, (G) Rab8, (H) Rab9, or (I) Rab11 tagged with Venus. BRET ratio (ligand-vehicle) was calculated as described in Materials and Methods. AVP (1μM) or vehicle was added at t = 0 after establishment of the baseline. Points represent mean ± SEM of 3 independent experiments. Note wild-type data are replicated in Figure 8 and Supplemental Figure 4.
Discussion
In this study, we have identified and characterized a novel V2R mutation found in a boy presenting with persistent hyponatremia, normal adrenal function, and no other notable underlying disorder, a phenotype strongly suggestive of NSIAD rather than the SIADH. We have found that, in vitro, the L312S mutation results in substantially increased basal cAMP levels without a change in AVP potency. This constitutive activity is consistent with previously described V2R NSIAD-causing mutations, R137C, R137L (6, 8, 11), F229V (8), and I130N (14), and supports the current hypothesis that constitutive Gs protein-mediated signaling from the mutant V2R is the major determinant of the NSIAD clinical phenotype. This is further supported by the lack of effect on inositol phosphate signaling.
As distinct NSIAD-causing mutations are discovered, it is becoming clearer that differences in how they affect receptor function may influence the severity of the condition, as well as treatment options. Consistent with the relatively mild effect of the L312S substitution on V2R function, we observed no difference in total cellular expression or expression at the plasma membrane, indicating that this mutant receptor largely undergoes correct posttranslational folding and trafficking to the plasma membrane. In contrast, the results presented here and elsewhere for the R137C and R137L substitutions (8, 11), as well as for the F229V (8) and I130N variants (14), indicate a loss of cell surface expression due to activating V2R mutations. As observed previously with F229V V2R (8) and I130N V2R (14), the constitutive cAMP production seen with L312S V2R was reduced significantly by inverse agonist addition, which appears to be less apparent with the R137C and R137L mutants (8, 11). Furthermore, no discernable constitutive recruitment of β-arrestin to the receptor was observed for L312S V2R, again consistent with that observed for F229V V2R (8) and I130N V2R (14), but not R137C V2R and R137L V2R as seen in this study and previously (10–13).
Because the AVP efficacy and potency relative to that of wild-type V2R for cAMP and inositol phosphate production, as well as β-arrestin2 recruitment, was not compromised by the L312S substitution, this indicates that the orthosteric ligand-binding pocket is not noticeably impaired, nor are the intracellular G protein-binding sites or β-arrestin-binding domain. Interestingly, the L312S mutation is adjacent to the M311V substitution (Figure 10) that results in partial NDI and therefore partial loss-of-function (18) putatively through impairment of AVP binding. In contrast to M311, L312 was not identified as an important residue for AVP binding in a 3D homology model (30), although due to its proximity to M311, L312S may mediate constitutive cAMP signaling by mimicking some of the conformational changes induced by AVP binding involving M311. However, the capacity for further ligand-induced cAMP production also suggests that the L312S substitution only partially stabilizes the active state for Gs coupling. Therefore, our findings are consistent with the hypothesis proposed by Carpentier et al (8) for F229V V2R, namely that the L312S mutation does not “lock” the receptor into an irreversible active conformation. This also appears consistent with findings for I130N V2R (14) and contrasts with R137C and R137L V2R (8).
Figure 10.
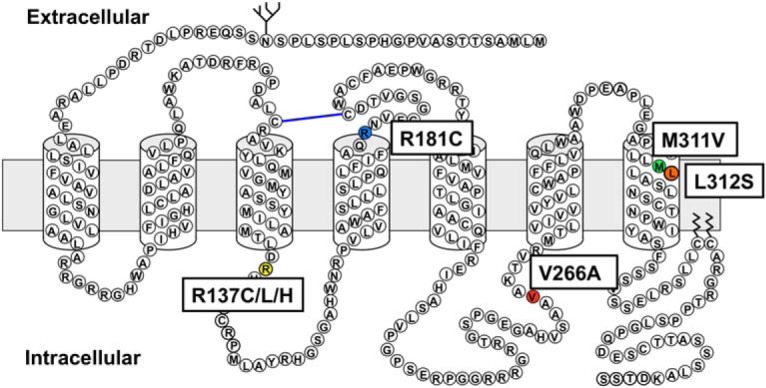
Schematic representation of the vasopressin receptor 2 amino acid sequence. The single amino acid changes under investigation in this study are highlighted, including NSIAD-causing R137C, R137L and L312S, NDI-causing R137H, R181C, and M311V and the putative polymorphism V266A. Note, this figure is purely illustrative and structural details should not be inferred. Actual boundaries between transmembrane helices and loops are likely to differ from that shown here.
Although R137C and R137L were the first mutations observed to cause NSIAD (6), they may turn out to be exceptions to the general rule in terms of overall receptor profile and therapeutic intervention. It is likely that because of the unique importance of the highly conserved R137 residue in V2R in stabilizing active/inactive conformations, certain mutations at this specific residue can result in a “locked active state” (8) resulting in constitutive β-arrestin recruitment as well as constitutive cAMP production, but more importantly, a state that is not clinically amenable to inverse agonist treatment (31). We predict that most NSIAD-causing mutations identified in the fullness of time will not be in positions with such pivotal roles to enable this “locking effect,” and therefore may be amenable to clinical inverse agonist treatment.
The relative lack of effect on receptor internalization conferred by the L312S mutation is also supported by the lack of effect on AVP potency for β-arrestin2 recruitment, in contrast to the significantly decreased potency observed for R137C V2R and R137L V2R. As a technical aside, this potency decrease seen with the R137C and L mutants agrees closely with that observed by Tenenbaum et al (12) using Rluc-tagged receptors and YFP-tagged β-arrestin1, and with similar EC50 values. In contrast, Rochdi et al (11), using EYFP-tagged receptors and Rluc-tagged β-arrestin2, did not observe a significant decrease in potency. Therefore, because the 3 BRET configurations used in the current study and the one used by Tenenbaum et al (12) all used variations of luciferase-tagged receptors with fluorophore-tagged β-arrestin and contrasted with the reciprocal orientation used by Rochdi et al (11), it may be that luciferase-tagging receptors is preferable for discerning the subtleties of receptor function in this case and the potential for configuration having an effect should be considered when designing BRET experiments. On the other hand, obtaining similar kinetic and concentration-response profiles for each V2R mutant in the 3 BRET configurations we have used indicates that similar biology is observed regardless of the luciferase fused to the receptor or fluorescent moiety attached to the β-arrestin2, although it is notable that HT with NCT ligand appears to be a more sensitive acceptor for Nluc than Venus in this instance in terms of detecting the level of constitutive activity.
Building upon the studies by Lan et al (16, 17), the expanded portfolio of subcellular markers we have validated with confocal microscopy enables extensive profiling of protein distribution and trafficking through multiple subcellular compartments using BRET in real-time (Figure 6). This approach has widespread applicability and is particularly suitable for real-time monitoring of trafficking, because ligand-induced changes in BRET were observed with most of the markers. Interestingly, we did not detect ligand-induced changes in proximity to markers of the Golgi (Rab6) and trans-Golgi network to plasma membrane trafficking (Rab8), which may be due to a number of factors: tagging of the Rab proteins may have resulted in an orientation of donor and acceptor unsuitable for BRET to occur, although this seems unlikely as differences in constitutive localization were observed between V2R mutants and the expression patterns were appropriate as seen using confocal microscopy; AVP may not induce increased trafficking to these compartments, at least over the timescale of assessment and in the continued presence of agonist; or AVP addition may change the rates of trafficking both to and from these compartments to a similar degree, resulting in equilibrium and a steady state being observed. The most likely scenario is that, in the presence of constant AVP stimulation, receptor activation results in receptor desensitization through the endosomal/lysosomal pathway and although more receptor is generated in the endoplasmic reticulum in readiness for trafficking to the plasma membrane, it is retained in the endoplasmic reticulum on this timescale and while AVP remains present. This predominantly endosomal receptor distribution is consistent with what we observed with confocal microscopy upon treatment of FLAG-tagged V2R with AVP.
Analysis of the constitutive distribution of V2R mutants confirmed previous reports of misfolding contributing to the reduced plasma membrane localization of the R137 substitutions. All 3 mutations resulted in a comparable reduction in cell surface expression (K-ras proximity). In contrast, R137H resulted in massive dysregulation and constitutive intracellular localization, with large increases seen at all intracellular compartments relative to wild-type as well as both R137 NSIAD-causing substitutions. All 3 R137 substitutions appear to have similar cell surface expression as reported here as well as previously (8, 11, 18, 32), which is in part due to constitutive β-arrestin recruitment and internalization as well as misfolding (11, 18), whereas differences in the constitutive intracellular distribution are likely due to the extent of misfolding and therefore accumulation of immature receptor that does not traffic to the membrane but becomes trapped in intracellular compartments (11). Indeed, comparisons of the levels of immature R137 mutants by western blot have shown that the R137H NDI-causing variant has the highest level of misfolded receptor whereas the R137C and R137L NSIAD-causing substitutions result in similar levels of mature receptor, although still lower than wild-type (8, 11).
The finding that R137C but not R137L resulted in statistically significant increases in constitutive distribution to intracellular compartments relative to wild-type is intriguing, and may contribute to the lower constitutive cAMP production resulting from the R137C compared with R137L mutation (6, 8, 11). Furthermore, we observed internalization of all 3 R137 mutants after AVP treatment, but only R137C V2R was observed to traffic further to intracellular compartments, with small ligand-induced increases observed associated with early endosomes (Rab4 and Rab5) and late endosomes/lysosomes (Rab7). Therefore, it may also be possible that reduced AVP-induced internalization and trafficking may inhibit timely desensitization of R137L V2R more than R137C V2R, thereby further augmenting constitutive cAMP signaling. Indeed, blockade of internalization by overexpression of dominant-negative dynamin K44A results in an increase in constitutive cAMP signaling (11) as well as constitutive localization of receptor and β-arrestin at the plasma membrane (10, 11). This suggests that the greater impairment of intracellular trafficking observed for R137L V2R may contribute to the constitutive G protein activity and thus participate in the differentiation of the R137C and R137L V2R constitutive signaling profiles.
In stark contrast to both R137C and R137L, the novel L312S mutation, despite also causing a clinical phenotype consistent with NSIAD, resulted in no statistically significant change in basal/constitutive localization relative to wild-type. Furthermore, the only significant effects of AVP treatment were slight increases in receptor internalization (decrease in K-ras proximity) and early endosome recycling (Rab4 proximity). This may indicate a slight increase in receptor resensitization compared with wild-type V2R that may in turn augment the constitutive cAMP signal. These findings are consistent with the confocal microscopy showing distinct differences between L312S V2R basal distribution compared with both R137C and R13L V2R. They are also in keeping with L312S V2R's lack of constitutive β-arrestin2 recruitment and similar AVP-induced β-arrestin2 interaction profile compared with wild-type V2R.
The loss of function resulting from the NDI-causing R181C V2R variant is thought to occur due to disruption of the cysteine-cysteine disulphide bond vital for maintaining extracellular structure and the integrity of the AVP-binding pocket (33, 34), whereas the NDI-causing M311V mutation is thought to disrupt ligand binding in the seventh transmembrane domain (30). This is supported by observations that the potency of AVP-induced cAMP, inositol phosphate production and β-arrestin recruitment is reduced for R181C and M311V variants, with the disruption of AVP signaling at R181C V2R being greater than at M311V V2R (18). Despite this and as confirmed in the current study, both of these NDI-causing mutants trafficked normally to the plasma membrane. Additionally, we have shown that R181C and M311V have normal basal intracellular distribution, suggesting that the mutations largely result in properly folded mature protein. We have shown previously that R181C V2R does not recruit β-arrestin even at high AVP concentrations and in agreement with this finding, AVP failed to induce internalization or trafficking. Taken together, these data are consistent with R181C primarily disrupting AVP binding. In contrast, M311V V2R displayed strong internalization and trafficking properties, with the only difference in trafficking, relative to wild-type being a small reduction in the maximum internalization kinetic after prolonged AVP stimulation of approximately 60 minutes. These data indicate that, unlike NDI-causing mutations that result in receptor misfolding (11), pharmacological chaperones that increase cell surface expression are unlikely to relieve NDI symptoms in patients harboring mutations affecting ligand binding.
Finally, the V266A substitution was identified in a patient presenting with symptoms consistent with NSIAD (18). However, functional characterization of this mutant found that this substitution did not result in observable changes in G protein-dependent signaling or β-arrestin recruitment (18, 35). Thus it was suggested to be a silent polymorphism and not the underlying cause of NSIAD. Intriguingly, the current study revealed previously undescribed minor increases in the magnitude of internalization and trafficking of V266A V2R through various subcellular compartments. However, whether these changes are sufficient to induce or contribute to the NSIAD condition remains unclear.
In conclusion, we have shown that a novel hemizygous c.935T>C transition in exon 3 of the AVPR2 gene underlies the NSIAD condition in the presented case study, resulting in a leucine (TTG) to serine (TCG) substitution (L312S) in the V2R protein product. Functional profiling of the L312S mutation showed characteristic NSIAD-associated gain-of-function activity of V2R, including a substantial increase in constitutive Gs protein signaling, in the absence of constitutive β-arrestin recruitment. The implementation of the novel BRET assay for monitoring intracellular protein distribution and trafficking has shown subtle differences in trafficking resulting from distinct V2R mutations, even at the same amino acid position, therefore highlighting the utility of full and thorough characterization of V2R NSIAD and NDI mutations beyond simple signaling pathway analysis. Full understanding of the molecular consequences of V2R mutations may hold the key to the development of variant-specific personalized therapeutics for NDI and NSIAD patients.
Acknowledgments
We thank the facilities and the scientific and technical assistance of the Australian Microscopy and Microanalysis Research Facility at the Centre for Microscopy, Characterisation and Analysis, The University of Western Australia, a facility funded by the University, State, and Commonwealth Governments. Portions of this work were presented at the 54th Annual Meeting of the European Society for Pediatric Endocrinology, Barcelona, Spain, 2015.
This work was supported in part by the Australian Research Council Grant LP130100037 and an Australian Government Researchers in Business grant in partnership with Dimerix Limited. Promega Corp and BMG Labtech Pty Ltd provided funding as partner organizations of Grant LP130100037, of which K.D.G.P. was Chief Investigator. C.W.W. is a National Health and Medical Research Council CJ Martin Research Fellow (1088334). K.D.G.P. was an Australian Research Council Future Fellow (FT100100271) and is currently a National Health and Medical Research Council RD Wright Biomedical Research Fellow (1085842).
Disclosure Summary: A.T., C.W.W., R.S.A., H.B.S., A.S.C., R.M.S., J.I.H., I.D., and N.J.P. have nothing to disclose. K.D.G.P. receives funding from Promega Corp and BMG Labtech as ARC Linkage Grant partner organizations and is Chief Scientific Advisor of Dimerix Limited, of which he is a shareholder.
Footnotes
- β-arrestin2/Venus
- β-arrestin2 fused to Venus
- AVP
- arginine vasopressin
- BRET
- bioluminescence resonance energy transfer
- eBRET
- extended BRET
- EEA-1
- early endosome antigen 1
- FBS
- fetal bovine serum
- GPCR
- G protein-coupled receptor
- HBSS
- Hanks' balanced salt solution
- HT
- HaloTag
- HTRF
- homogeneous time-resolved fluorescence
- IP1
- inositol-1-phosphate
- NCT
- nonchloro TOM IBMX, 3-isobutyl-1-methylxanthine
- NDI
- nephrogenic diabetes insipidus
- Nluc
- NanoLuc
- NSIAD
- nephrogenic syndrome of inappropriate antidiuresis
- Rab
- RabGTPase
- SIADH
- syndrome of inappropriate antidiuretic hormone secretion
- V2R
- vasopressin type 2 receptor.
References
- 1. Ball SG. Vasopressin and disorders of water balance: the physiology and pathophysiology of vasopressin. Ann Clin Biochem. 2007;44(pt 5):417–431. [DOI] [PubMed] [Google Scholar]
- 2. Morello JP, Bichet DG. Nephrogenic diabetes insipidus. Annu Rev Physiol. 2001;63:607–630. [DOI] [PubMed] [Google Scholar]
- 3. Birnbaumer M, Seibold A, Gilbert S, et al. Molecular cloning of the receptor for human antidiuretic hormone. Nature. 1992;357(6376):333–335. [DOI] [PubMed] [Google Scholar]
- 4. Seibold A, Brabet P, Rosenthal W, Birnbaumer M. Structure and chromosomal localization of the human antidiuretic hormone receptor gene. Am J Hum Genet. 1992;51(5):1078–1083. [PMC free article] [PubMed] [Google Scholar]
- 5. Stenson PD, Mort M, Ball EV, Shaw K, Phillips A, Cooper DN. The human gene mutation database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet. 2014;133(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feldman BJ, Rosenthal SM, Vargas GA, et al. Nephrogenic syndrome of inappropriate antidiuresis. N Engl J Med. 2005;352(18):1884–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baylis PH. The syndrome of inappropriate antidiuretic hormone secretion. Int J Biochem Cell Biol. 2003;35(11):1495–1499. [DOI] [PubMed] [Google Scholar]
- 8. Carpentier E, Greenbaum LA, Rochdi D, et al. Identification and characterization of an activating F229V substitution in the V2 vasopressin receptor in an infant with NSIAD. J Am Soc Nephrol. 2012;23(10):1635–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Powlson AS, Challis BG, Halsall DJ, Schoenmakers E, Gurnell M. Nephrogenic syndrome of inappropriate antidiuresis secondary to an activating mutation in the arginine vasopressin receptor AVPR2. [published ahead of print December 30. 2015] Clin Endocrinol (Oxf). 10.1111/cen.13011. [DOI] [PubMed] [Google Scholar]
- 10. Kocan M, See HB, Sampaio NG, Eidne KA, Feldman BJ, Pfleger KD. Agonist-independent interactions between β-arrestins and mutant vasopressin type II receptors associated with nephrogenic syndrome of inappropriate antidiuresis. Mol Endocrinol. 2009;23(4):559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rochdi MD, Vargas GA, Carpentier E, et al. Functional characterization of vasopressin type 2 receptor substitutions (R137H/C/L) leading to nephrogenic diabetes insipidus and nephrogenic syndrome of inappropriate antidiuresis: implications for treatments. Mol Pharmacol. 2010;77(5):836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tenenbaum J, Ayoub MA, Perkovska S, et al. The constitutively active V2 receptor mutants conferring NSIAD are weakly sensitive to agonist and antagonist regulation. PLoS One. 2009;4(12):e8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kocan M, Dalrymple MB, Seeber RM, Feldman BJ, Pfleger KDG. Enhanced BRET technology for the monitoring of agonist-induced and agonist-independent interactions between GPCRs and β-arrestins. Front Endocrinol (Lausanne). 2011;1:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Erdélyi LS, Mann WA, Morris-Rosendahl DJ, et al. Mutation in the V2 vasopressin receptor gene, AVPR2, causes nephrogenic syndrome of inappropriate diuresis. Kidney Int. 2015;88(5):1070–1078. [DOI] [PubMed] [Google Scholar]
- 15. Hermosilla R, Oueslati M, Donalies U, et al. Disease-causing V(2) vasopressin receptors are retained in different compartments of the early secretory pathway. Traffic. 2004;5(12):993–1005. [DOI] [PubMed] [Google Scholar]
- 16. Lan TH, Kuravi S, Lambert NA. Internalization dissociates β2-adrenergic receptors. PLoS One. 2011;6(2):e17361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lan TH, Liu Q, Li C, Wu G, Lambert NA. Sensitive and high resolution localization and tracking of membrane proteins in live cells with BRET. Traffic. 2012;13(11):1450–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Armstrong SP, Seeber RM, Ayoub MA, Feldman BJ, Pfleger KD. Characterization of three vasopressin receptor 2 variants: an apparent polymorphism (V266A) and two loss-of-function mutations (R181C and M311V). PLoS One. 2013;8(6):e65885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benson DA, Cavanaugh M, Clark K, et al. GenBank. Nucleic Acids Res. 2013;41(Database issue):D36–D42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kocan M, See HB, Seeber RM, Eidne KA, Pfleger KD. Demonstration of improvements to the bioluminescence resonance energy transfer (BRET) technology for the monitoring of G protein-coupled receptors in live cells. J Biomol Screen. 2008;13(9):888–898. [DOI] [PubMed] [Google Scholar]
- 21. Machleidt T, Woodroofe CC, Schwinn MK, et al. NanoBRET–a novel BRET platform for the analysis of protein-protein interactions. ACS Chem Biol. 2015;10(8):1797–1804. [DOI] [PubMed] [Google Scholar]
- 22. Sherwood RK, Roy CR. A Rab-centric perspective of bacterial pathogen-occupied vacuoles. Cell Host Microbe. 2013;14(3):256–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wandinger-Ness A, Zerial M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol. 2014;6(11):a022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pfleger KD, Dromey JR, Dalrymple MB, Lim EM, Thomas WG, Eidne KA. Extended bioluminescence resonance energy transfer (eBRET) for monitoring prolonged protein-protein interactions in live cells. Cell Signal. 2006;18(10):1664–1670. [DOI] [PubMed] [Google Scholar]
- 25. Pfleger KD, Seeber RM, Eidne KA. Bioluminescence resonance energy transfer (BRET) for the real-time detection of protein-protein interactions. Nat Protoc. 2006;1(1):337–345. [DOI] [PubMed] [Google Scholar]
- 26. Jaeger WC, Seeber RM, Eidne KA, Pfleger KD. Molecular determinants of orexin receptor-arrestin-ubiquitin complex formation. Br J Pharmacol. 2014;171(2):364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trinquet E, Fink M, Bazin H, et al. D-myo-inositol 1-phosphate as a surrogate of D-myo-inositol 1,4,5-tris phosphate to monitor G protein-coupled receptor activation. Anal Biochem. 2006;358(1):126–135. [DOI] [PubMed] [Google Scholar]
- 28. Jensen DD, Godfrey CB, Niklas C, et al. The bile acid receptor TGR5 does not interact with β-arrestins or traffic to endosomes but transmits sustained signals from plasma membrane rafts. J Biol Chem. 2013;288(32):22942–22960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Szakadáti G, Tóth AD, Oláh I, et al. Investigation of the fate of type I angiotensin receptor after biased activation. Mol Pharmacol. 2015;87(6):972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Slusarz MJ, Giełdoń A, Slusarz R, Ciarkowski J. Analysis of interactions responsible for vasopressin binding to human neurohypophyseal hormone receptors-molecular dynamics study of the activated receptor-vasopressin-G(α) systems. J Pept Sci. 2006;12(3):180–189. [DOI] [PubMed] [Google Scholar]
- 31. Decaux G, Vandergheynst F, Bouko Y, Parma J, Vassart G, Vilain C. Nephrogenic syndrome of inappropriate antidiuresis in adults: high phenotypic variability in men and women from a large pedigree. J Am Soc Nephrol. 2007;18(2):606–612. [DOI] [PubMed] [Google Scholar]
- 32. Barak LS, Oakley RH, Laporte SA, Caron MG. Constitutive arrestin-mediated desensitization of a human vasopressin receptor mutant associated with nephrogenic diabetes insipidus. Proc Natl Acad Sci USA. 2001;98(1):93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schülein R, Zühlke K, Krause G, Rosenthal W. Functional rescue of the nephrogenic diabetes insipidus-causing vasopressin V2 receptor mutants G185C and R202C by a second site suppressor mutation. J Biol Chem. 2001;276(11):8384–8392. [DOI] [PubMed] [Google Scholar]
- 34. Sahakitrungruang T, Tee MK, Rattanachartnarong N, Shotelersuk V, Suphapeetiporn K, Miller WL. Functional characterization of vasopressin receptor 2 mutations causing partial and complete congenital nephrogenic diabetes insipidus in Thai families. Horm Res Paediatr. 2010;73(5):349–354. [DOI] [PubMed] [Google Scholar]
- 35. Schulz A, Sangkuhl K, Lennert T, et al. Aminoglycoside retreatment partially restores the function of truncated V(2) vasopressin receptors found in patients with nephrogenic diabetes insipidus. J Clin Endocrinol Metab. 2002;87(11):5247–5257. [DOI] [PubMed] [Google Scholar]