Abstract
Androgen receptor (AR) plays pivotal roles in prostate cancer. Upon androgen stimulation, AR recruits the Protein kinase N1 (PKN1), which phosphorylates histone H3 at threonine 11, with subsequent recruitment of tryptophan, aspartic acid (WD) repeat-containing protein 5 (WDR5) and the su(var)3–9, enhancer of zeste, trithorax/mixed-lineage leukemia (SET1/MLL) histone methyltransferase complex to promote AR target gene activation and prostate cancer cell growth. However, the underlying mechanisms of target gene activation and cell growth subsequent to WDR5 recruitment are not well understood. Here, we demonstrate an epigenetic cross talk between histone modifications and AR target gene regulation. We discovered that K(lysine) acetyltransferase 8 (KAT8), a member of the MOZ, YBF2/SAS2, and TIP 60 protein 1 (MYST) family of histone acetyltransferases that catalyzes histone H4 lysine 16 acetylation, colocalized with WDR5 at AR target genes, resulting in hormone-dependent gene activation in prostate cancer cells. PKN1 or WDR5 knockdown severely inhibited KAT8 association with AR target genes and histone H4 lysine 16 acetylation upon androgen treatment. Knockdown of KAT8 significantly decreased AR target gene expression and prostate cancer cell proliferation. Collectively, these data describe a trans-histone modification pathway involving PKN1/histone H3 threonine 11 phosphorylation followed by WDR5/MLL histone methyltransferase and KAT8/histone acetyltransferase recruitment to effect androgen-dependent gene activation and prostate cancer cell proliferation.
Prostate cancer is the major diagnosed nonskin cancer in men and androgen receptor (AR) plays a critical role in prostate cancer progression (1–4). AR acts as a transcription factor to regulate expression of target genes, which in turn mediate cellular outcomes including proliferation and differentiation (5). Many cofactors associating with AR in a hormone-sensitive manner have been identified, and display chromatin modifying and remodeling activities (6–8). Previous studies have shown that the protein kinase N1 (PKN1, previously known as protein kinase C-related kinase 1) is recruited to AR target genes, leading to hyperphosphorylation of histone H3 at threonine 11 (H3T11P) upon stimulation of cells with androgen to activate gene expression (9). Additionally, we have shown that H3T11P associates with the tryptophan, aspartic acid (WD) repeat-containing protein 5 (WDR5), leading to recruitment of su(var)3–9, enhancer of zeste, trithorax/mixed-lineage leukemia (SET1/MLL) histone methyltransferase complex to AR target genes in androgen-stimulated LNCaP cells and an integrated PKN1-H3T11P-WDR5/MLL pathway is important for AR target gene activation and regulation of prostate cancer cell growth (10).
Human K(lysine) acetyltransferase 8 (KAT8, also called males absent on the first or MYST1) is a member of the MYST (MOZ, YBF2/SAS2, and TIP 60 protein 1) family of histone acetyltransferases, and is the human ortholog of the Drosophila males absent on the first protein (11, 12). MYST family members have a highly conserved MYST domain comprised of an acetyl-coenzyme A-binding motif, a zinc finger motif and a chromo domain, which bind to acetylated histones or participate in protein-protein interactions (11). Previous studies demonstrated that KAT8 acetylates chromatin specifically at histone H4 lysine 16 (H4K16) and depletion of KAT8 in human cells led to decreased acetylation at H4K16 (H4K16Ac), suggesting a role for this key epigenetic modifier in the regulation of gene transcription (13–16). In addition, biochemical purifications have shown that KAT8 associates with multiprotein, male-specific lethal (MSL) and KAT8 regulatory nonspecific lethal (KANSL). Both MSL and KANSL complexes are responsible for histone H4K16Ac. Moreover, KANSL complex can acetylate other histone H4 lysines, including H4K5 and H4K8 (17, 18). Recent studies have shown that KAT8 is also associated with the SET1/MLL histone methyltransferase containing WDR5 and several other proteins in a multiprotein complex that catalyzes both histone acetylation and methylation (16, 18). Additionally, KAT8-containing KANSL complex-mediated histone H4K16Ac promotes dimethylation at histone H3K4 by interacting with SET/MLL complexes (19).
KAT8 and histone H4K16Ac regulate gene activation by cooperating with or affecting other histone modifications. Phosphorylation of histone H3S10 and H4K16Ac are involved in the release of HP1 from chromatin, resulting in activation of transcription (20, 21). Additionally, histone H3K36 methylation and H4K16Ac show antagonistic cross talk, which influences packaging of high-order chromatin (22). Interestingly, methylation of H3K4 by SET1/MLL complex coincides with H4K16Ac at certain genes and facilitates transcription activation (17–19). These studies suggest that H3K4me3 cross talk with histone H4K16Ac may contribute to gene transcriptional regulation in prostate cancer cells. However, the complete mechanisms of the cross talk between trans-histone phosphorylation, methylation and acetylation remain to be elucidated.
In this study, we demonstrate that the epigenetic cross talk between different histone modifications plays an important role in gene regulation and in prostate cancer cell proliferation. We discovered that KAT8 colocalized with WDR5 on chromatin, resulting in transcriptional activation of AR target genes in hormone-stimulated manner. Consequently, we determined that WDR5 and H3K4me3 are necessary for recruitment of KAT8, and subsequently for increase in the acetylation of H4K16 at AR target genes upon androgen treatment. Finally, using cell-based assays, we found that KAT8 is important for cell growth and colony formation of prostate cancer cells. Collectively, these data provide a mechanism involving H3T11P, H3K4me3, and H4K16Ac cross talk mediated at least in part by PKN1-WDR5-KAT8 to mediate transcriptional activation of AR target genes, and prostate cancer cell proliferation.
Materials and Methods
Cell culture and treatment
Human prostate cancer LNCaP cells (CRL-1740), and RWPE-1 cells (CRL-1160) were purchased from the American Type Culture Collection. LAPC4 cells were provided by Dr. Jindan Yu. LNCaP cells, were maintained in RPMI 1640 (Invitrogen), LAPC4 cells in Iscove's MEM (Invitrogen); and RWPE-1 in keratinocyte serum-free medium (Invitrogen) with 0.05-mg/mL bovine pituitary extract and 5-ng/mL human recombinant epidermal growth factor. All culture media except for RWPE-1 cells were supplemented with 10% fetal bovine serum (FBS). For androgen treatment, cells were hormone starved for 2 days in media containing 10% charcoal-stripped FBS and treated with androgen as previously described (10). LNCaP cells were hormone starved for 2 days and treated with 10nM R1881 (methyltrienolone) or 1nM dihydrotestosterone (DHT) for 16 hours (for expression assays) or for 3 hours (for chromatin immunoprecipitation [ChIP] assays). LAPC4 cells were hormone starved for 2 days and treated with 1nM R1881 for 24 hours (for expression assays and ChIP assays).
Antibodies
The antibodies used for ChIP assays were against KAT8 (3 μg, ABE479; Millipore), WDR5 (2 μg, A302–429A; Bethyl Laboratories), AR (1 μg, 39781; Active Motif), PKN1 (2 μg, 610687; BD Transduction), KANSL1 (3 μg, sc-160469; Santa Cruz Biotechnology, Inc), MSL1 (3 μg, ABE469; Millipore), histone H3 phospho-T11 (3 μg, ab5168; Abcam), histone H3 trimethyl K4 (1 μg, 07–473; Millipore), acetyl-histone H4 (Lys16) (1 μg, 07–329; Millipore).
Short hairpin RNA (shRNA) transduction
For knockdown of KAT8, KAT8 lentiviral shRNAs were cloned into EcoRI/BamHI sites of pLVX vector using oligonucleotides containing the following 2 shRNAs target sequences: shKAT8–1, CAAGATCACTCGCAACCAA and shKAT8–2, GAAATTGATGCCTGGTATT. Vesicular stomatitis virus G glycoprotein-pseudotyped lentivirus was produced in 293T/17 cells (6-well plate) by cotransfection with psPAX2 (0.5 μg), pMD2.G (1 μg), and lentiviral construct (1.5 μg) using Lipofectamine 2000 (Invitrogen) according to manufacturer's instructions. After overnight transfection, fresh DMEM was added to cells. WDR5 and PKN1 pSuper.Retro.Puro retroviral shRNA expression vectors have been described previously (10). Vesicular stomatitis virus G glycoprotein-pseudotyped lentivirus or retrovirus was used to spin-infect cells as previously described (23).
Immunoblots and quantitative RT-PCR
Whole-cell extracts were prepared from cells expressing indicated shRNA(s) 3 days after transduction and immunoblotted as previously described (10). Total RNA was prepared from shRNA-expressing cells 6 days after transduction and analyzed by quantitative RT-PCR as previously described (10). Total RNA (500 ng) was reverse transcribed using qScript cDNA synthesis mix (Quanta Biosciences). Quantitative PCR (qPCR) was performed with diluted cDNA using SYBR green PCR master mix (Applied Biosystems) and gene-specific primers. Fold changes in mRNA levels were determined using the ΔΔ threshold cycle method normalized to β-actin.
ChIP assays and sequential ChIP
ChIP assays were performed essentially as previously described, with minor modifications (10). Briefly, LAPC4 cells were stimulated with 1nM R1881 for 24 hours, and LNCaP cells were cultured for 3 hours in the presence of 10nM R1881, 1nM DHT, or vehicle control as indicated and cross-linked by 1% paraformaldehyde in the medium at 37°C for 10 minutes, followed by the addition of 125mM glycine at room temperature for 5 minutes. Samples were further sonicated and diluted for immunoprecipitation with antibodies against KAT8, WDR5, PKN1, KANSL1, MSL1, H4K16Ac, H3T11P, H3K4me3, and IgG. Protein G Dynabeads (30 μL) were added, and immunoprecipitations were continued for an additional 3 hours. Beads were washed 4 times with 1 mL of ChIP-radioimmunoprecipitation assay (RIPA) wash buffer (50mM HEPES-KOH [pH 7.6], 500mM LiCl, 1mM EDTA, 1.0% IGEPAL-CA630, and 0.7% sodium deoxycholate) and once with Tris-EDTA containing 50mM NaCl. After the final wash, Dynabeads and precipitated chromatin DNA were resuspended in 100 μL of 10% Chelex resin (Bio-Rad) and incubated for 10 minutes at 100°C. Samples were centrifuged at 20 000g for 2 minutes to pellet the Chelex-Dynabeads mixture. Supernatants (70 μL) containing the recovered DNA were transferred to clean 1.5-mL tubes, and the Chelex-Dynabeads resins were resuspended in an additional 130 μL of water, vortexed, and centrifuged as before. Supernatants were combined, yielding 200 μL of immunoprecipitated DNA.
For sequential ChIP (ChIP-reChIP), first-round ChIPs were performed as described above except that after the final wash, beads were resuspended in elution buffer (10mM Tris-HCl [pH 7.6], 1mM EDTA, 2% sodium dodecyl sulfate [SDS], and 20mM dithiothreitol [DTT]) and incubated at 37°C for 30 minutes. Eluates were diluted 20-fold with dilution buffer (10mM Tris-HCl [pH 7.6], 100mM NaCl, 1mM EDTA, and 1% Triton X-100) and adjusted to 1-mg/mL BSA. KAT8 and WDR5 ChIP eluates were again subjected to ChIP (reChIP) with 3 μg of anti-KAT8 or anti-WDR5 antibodies, respectively, and appropriate IgG isotype controls overnight at 4°C with gentle inversion. The resulting reChIP products were collected using protein G Dynabeads, washed, and eluted as described above for conventional ChIP. ChIP-PCR was performed using primers specific to AREs (androgen response elements) in the promoter/enhancer regions of AR target genes as described before (10). KIAA0066 gene, which has no apparent ARE and little or no WDR5 occupancy, was used as a negative control (10, 24). For ChIP-PCR of KIAA0066 gene, the following primer sequences were used: primer sequence, 5′-CTAGGAGGGTGGAGGTAGGG-3′ (forward) and 5′-GCCCCAAACAGGAGTAATGA-3′ (reverse). Threshold cycle values of ChIP-enriched DNA were exponentiated and expressed as percent recovery relative to the input DNA analyzed in parallel.
ChIP-immunoblot (ChIP-IB) analysis
ChIP-IB assays to detect protein complex formation on chromatin were performed identically as conventional ChIP assays except immunoprecipitations were performed with 1 mg of cross-linked soluble chromatin fraction and 2 μg of anti-KAT8, anti-WDR5, or anti-IgG antibodies overnight at 4°C with inversion in binding buffer (20mM HEPES-KOH [pH 7.6], 150mM NaCl, 1.5mM MgCl2, 0.2mM EDTA, and 0.5% IGEPAL CA-630). Protein G Dynabeads (Invitrogen) were added, and immunoprecipitation was continued for an additional 2 hours. Beads were then washed 4 times with binding buffer and bound proteins were eluted by boiling in 2× Laemmli buffer. Immunoprecipitated proteins were resolved by SDS-PAGE and immunoblotted with anti-WDR5, AR, and KAT8 antibodies as indicated.
AlamarBlue cell proliferation assay
Proliferation of LNCaP control and KAT8, WDR5, and KAT8/WDR5 double knockdown cells was performed as described previously (10) with minor modifications. KAT8, WDR5, and KAT8/WDR5 double or nonspecific knockdown cells were seeded at 5000 per well (8 wells per condition) in 48-well tissue culture plates. Twenty-four hours after 1nM R1881 treatment, 10% alamarBlue reagent was added per well and incubated for 2 hours at 37°C in a cell culture incubator. Fluorescence measurements were then performed with a BioTek Synergy HT multidetection microplate reader equipped with 540-nm excitation and 590-nm emission filters. Average background fluorescence was calculated from wells (n = 8) containing medium alone and subtracted from the fluorescence of wells containing cells. This represents day 1 of the assays. This process was repeated every 24 hours for 4 consecutive days.
Soft agar colony formation assay
A soft agar assay for colony formation was performed as previously described with a few modifications (25). In brief, a bottom base layer (0.7% agar) was prepared in 6-well dishes with 2× RPMI 1640 media containing 20% FBS. LNCaP cells were infected with single or double knockdown of KAT8/WDR5, and plated into 2 mL of soft agar (Sigma) containing 2× RPMI 1640 with 20% FBS on top of 0.5% agar. The plates were incubated in a humidified incubator at 37°C in 5% CO2 for 3 weeks. Cultures were observed daily for 3 weeks and then fixed with 4% paraformaldehyde and stained with 1 mL of 0.005% crystal violet for more than 1 hour. The colonies were counted using a dissecting microscope. This assay was carried out with 2 replicates.
Statistical analysis
Data are expressed as the means and SDs of 2 or 3 independent experiments. Statistically significant effects (P < .05) were evaluated with the Student's 2-tailed t test. Statistical differences between KAT8/WDR5 double knockdown and single knockdown experimental groups in quantitative RT-PCR, alamarBlue assays (at 4 d) and colony formation assays (at 3 wk) were determined by one-way ANOVA using Prism software (GraphPad Software, Inc).
Results
KAT8 binds AR target genes and regulates hormone-dependent gene expression
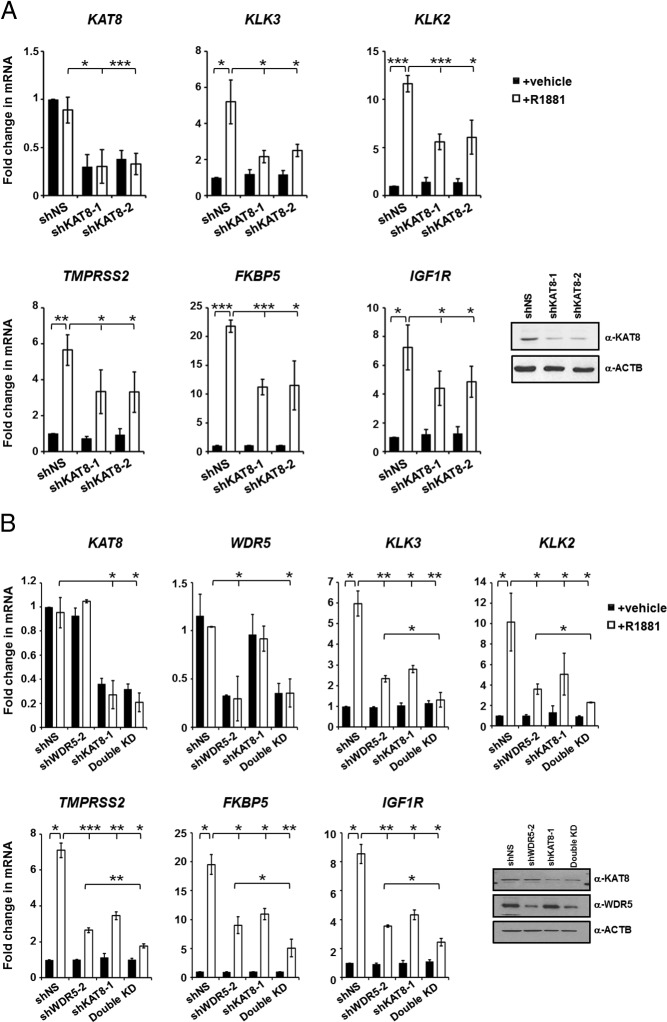
We previously showed that H3T11P facilitates KAT8 and WDR5 recruitment to AR target genes in a hormone-sensitive manner (10). However, the exact roles of KAT8 and its recruitment steps in AR gene regulation and prostate cancer cell proliferation are not fully established. To initiate our study, we analyzed the effects of KAT8 expression on androgen-dependent gene expression in LNCaP cells using KAT8 knockdown. Transduction of 2 different lentiviral short hairpin (shKAT8) constructs led to efficient down-regulation of endogenous KAT8 mRNA and protein when compared with nonspecific shRNA (shNS) (Figure 1A). Importantly, knockdown of endogenous KAT8 significantly decreased the expression of AR target genes such as KLK3, KLK2, TMPRSS2, FKBP5, and IGF1R, in R1881-treated LNCaP cells (Figure 1A). Furthermore, treatment of cells with the endogenous AR ligand, DHT, showed similar pattern of mRNA expression changes (Supplemental Figure 1). Previous studies showed that WDR5 knockdown inhibits AR target gene expression and that WDR5 forms a complex with KAT8, suggesting that WDR5 and KAT8 may cooperate in AR target gene regulation (10). Therefore, to test this possibility, we performed KAT8/WDR5 double knockdown experiments for analysis of AR target gene expression in R1881-treated LNCaP cells. Importantly, and consistent with the cooperative hypothesis, we found that KAT8/WDR5 double knockdown strongly impaired ligand induced AR target gene expression when compared with KAT8 or WDR5 knockdown alone (Figure 1B) (10). Such inhibition of AR target gene expression was also observed in another androgen-sensitive cells, LAPC4, but not in the nonmalignant human prostate epithelial cells (RWPE-1) (Supplemental Figure 2, A and B, respectively).
Figure 1.
KAT8 regulates expression of AR target genes. A and B, Quantitative RT-PCR analysis of mRNA levels of KAT8, WDR5, and AR target genes in LNCaP cells expressing either control (shNS), KAT8 shRNAs (A) or KAT8/WDR5 single and double knockdown (B) and treated with vehicle or 10nM R1881 for 16 hours. RNA or protein expression of KAT8 or WDR5 in knockdown LNCaP cells using respective antibodies is also shown at right (A and B). Values represent the mean ± SD of 3 independent experiments (n = 3); *, P < .05; **, P < .01; ***, P < .001. Statistical differences between KAT8/WDR5 double knockdown and KAT8 or WDR5 single knockdown were determined using one-way ANOVA followed by the Tukey's post hoc test. Also, see Supplemental Figures 1 and 2.
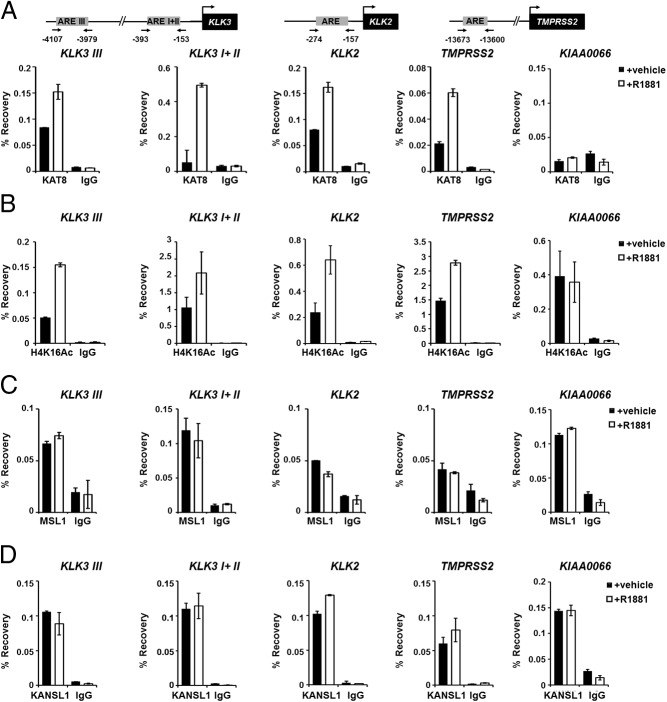
Because KAT8 is critical for target gene activation and has histone acetylase activity, we next determined whether KAT8 is recruited to AR target genes and whether hyperacetylated histone H4K16 is enriched at these genes using ChIP assays. Consistent with previous reports (10), we observed that the recruitment of KAT8 was indeed enriched at AR target genes in R1881- or DHT-treated LNCaP cells (Figure 2A and Supplemental Figure 3A). Also, we found that histone H4K16Ac levels were elevated at AR target genes in a hormone-dependent manner (Figure 2B and Supplemental Figure 3B). As a control, the intronic region of the KIAA0066 was used where KAT8 occupancy or H4K16Ac levels did not change significantly after R1881 or DHT treatment (Figure 2, A and B, and Supplemental Figure 3, A and B). To determine whether subunits of other KAT8-containing complexes are recruited to AR-regulated genes, we examined recruitment of MSL1 and KANSL1 upon R1881 treatment in LNCaP cells. Interestingly, MSL1 and KANSL1 binding to AR target genes were independent of ligand (Figure 2, C and D). Consistent with the results from LNCaP cells, KAT8, WDR5, and AR occupancy, and histone marks, including H3K4me3, and H4K16Ac were increased in hormone stimulated-LAPC4 cells when compared with control cells (Supplemental Figure 4). These findings show that KAT8 is recruited to and plays a specific role as an AR target gene regulator in an androgen-dependent manner in hormone-responsive prostate cancer cells.
Figure 2.
KAT8 recruitment and histone H4K16Ac on AR target genes in LNCaP cells. A–D, ChIP assays were conducted in LNCaP cells treated with vehicle or 10nM R1881 for 3 hours using anti-KAT8 (A), anti-H4K16Ac (B), anti-MSL1 (C), anti-KANSL1 (D), or control IgG antibodies (A–D) at AREs in the promoter/enhancer regions of AR target genes, and KIAA0066 as a negative control and examined by qPCR. Values represent mean ± SD of technical duplicates from a representative experiment. All experiments were performed 3 times with similar results. Also, see Supplemental Figures 3 and 4.
KAT8 forms complex and colocalizes with WDR5 on chromatin
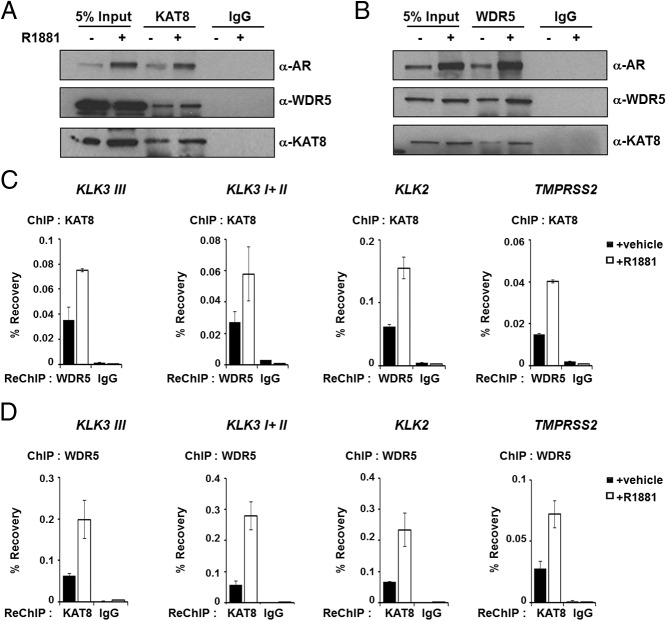
Having established the chromatin recruitment of and regulation of AR target genes by KAT8; we next investigated whether KAT8 interacts with WDR5 on chromatin. We performed ChIP-IB in LNCaP cells treated with AR agonist R1881 or vehicle control. We prepared nuclear lysates from formaldehyde cross-linked and sonicated cells containing soluble nuclear and chromatin/nucleosomal fraction, and immunoprecipitated endogenous KAT8 using anti-KAT8 antibodies. Importantly, the assays showed that KAT8 Immunoprecipitates significantly recovered endogenous WDR5 and AR in hormone-stimulated LNCaP cells compared with vehicle-treated LNCaP cells (Figure 3A). Similar results were obtained when we performed IP assays with anti-WDR5 antibodies and immunoblotting with anti-KAT8 antibodies (Figure 3B). Because a clear interaction between WDR5 and KAT8 was observed biochemically, we performed sequential ChIP (ChIP-reChIP) assays to determine whether KAT8 and WDR5 cooccupy the same chromatin region of AR target genes. Cross-linked chromatin from LNCaP cells treated with or without R1881 was subjected to the first ChIP using anti-KAT8 antibody. Eluted chromatin was then precipitated using anti-WDR5 antibody. As expected, and consistent with biochemical analysis, we observed that KAT8 and WDR5 colocalized on these AR target genes in androgen-sensitive manner (Figure 3C). Consistently, similar results were obtained when the order of antibodies was reversed (ChIP with anti-WDR5, reChIP with anti-KAT8 antibodies), demonstrating enrichment of cooccupancy of KAT8 and WDR5 on AR target genes upon R1881 treatment (Figure 3D).
Figure 3.
KAT8 and WDR5 chromatin occupancy on AR target genes. A and B, KAT8 and WDR5 were immunoprecipitated using soluble nuclear and chromatin/nucleosomal fraction from vehicle-treated or R1881-stimulated LNCaP cells cross-linked with formaldehyde. Immunoprecipitated proteins were detected by immunoblot with AR, WDR5, and KAT8 antibodies. C and D, Sequential ChIP assays were performed on vehicle- or 10nM R1881-treated LNCaP cells at AR target genes. C, KAT8 ChIP followed by WDR5 or control IgG reChIP. D, WDR5 ChIP followed by KAT8 or control IgG reChIP. Values represent mean ± SD of technical duplicates from a representative experiment. All experiments were performed 3 times with similar results.
PKN1 and WDR5 are important for KAT8 recruitment and histone H4K16Ac at AR target genes
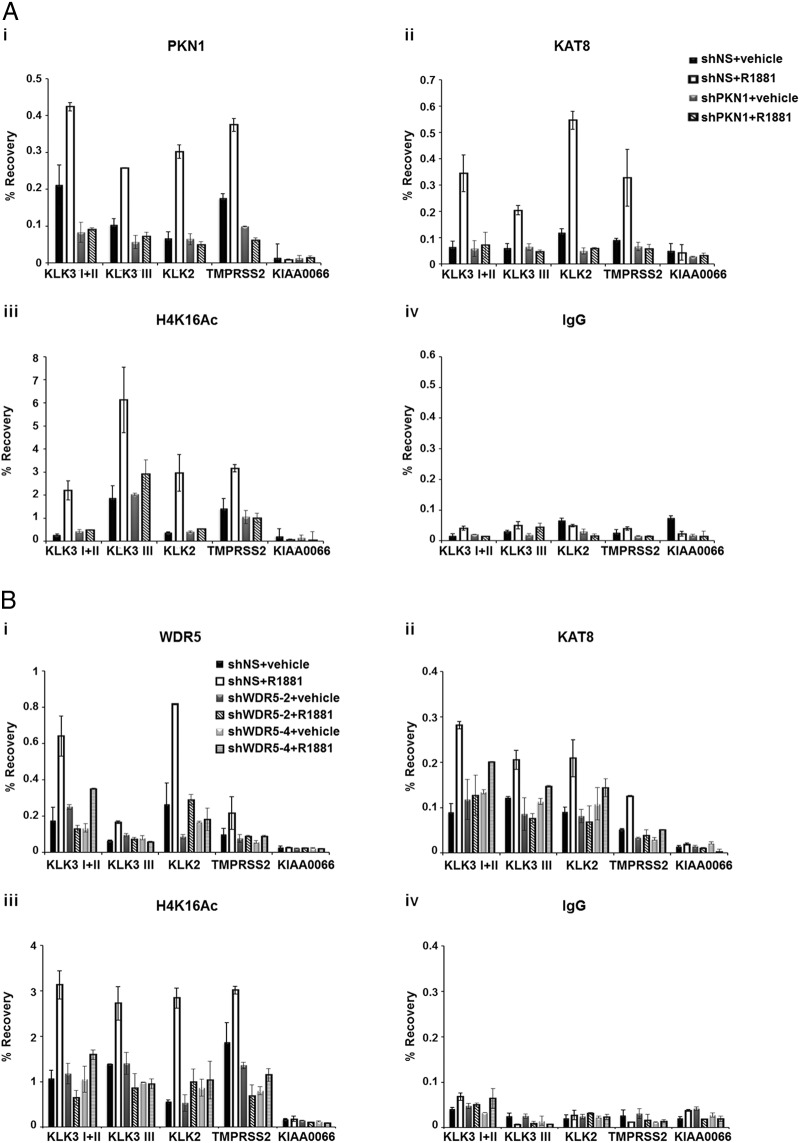
PKN1 and its catalytic activity play a key role in the recruitment of WDR5 and SET1/MLL complex to androgen-responsive genes (10). Previously we also showed that KAT8 recruitment and H4K16Ac were blocked by a PKN1 inhibitor (10). To further determine whether the mechanism underlying KAT8 recruitment is dependent on PKN1, we performed ChIP assays to analyze the status of KAT8 and histone H4K16Ac at AR target genes in LNCaP-PKN1 knockdown cells that were treated with or without R1881. We observed increased PKN1 recruitment at AR target genes in the presence of R1881; however, its recruitment was inhibited by PKN1 knockdown, which was consistent with the previous data (Figure 4Ai) (9, 10). Importantly, KAT8 recruitment was severely dampened on AR target genes in PKN1 knockdown cells (Figure 4Aii). Consequently, PKN1 depletion significantly decreased acetylation of histone H4K16 induced by R1881 at AR target genes (Figure 4A, iii and iv). From these data, we concluded that PKN1 is a prerequisite for KAT8 recruitment and histone H4K16Ac of AR target genes upon ligand stimulation.
Figure 4.
PKN1 and WDR5 are important for KAT8 recruitment and H4K16Ac at AR target genes. A, Control (shNS) or PKN1-knockdown LNCaP cells were grown in the presence or absence of 10nM R1881 for 3 hours. PKN1 (i), KAT8 (ii), H4K16Ac (iii), and IgG (iv) occupancy/level at AR target genes was determined by ChIP assays. Values represent mean ± SD of technical duplicates from a representative experiment. All experiments were performed 3 times with similar results. B, Control (shNS) or WDR5-knockdown LNCaP cells were cultivated in the presence or absence of 10nM R1881 for 3 hours and analyzed by ChIP-qPCR using anti-WDR5 (i), KAT8 (ii), H4K16Ac (iii), or IgG (iv) antibodies at AR target genes. KIAA0066 gene was used as a negative control. Values represent mean ± SD of technical duplicates from a representative experiment. All experiments were performed twice with similar results.
Furthermore, to determine whether KAT8 is recruited at AR target genes in WDR5-dependent manner, we performed ChIP assays in WDR5 knockdown cells. As expected, recruitment of WDR5 increased on AR target genes in a ligand-sensitive manner and decreased in WDR5 knockdown LNCaP cells (Figure 4Bi). Importantly, KAT8 recruitment and histone H4K16Ac were severely blocked on AR target genes in WDR5 knockdown cells treated with R1881 (Figure 4B, ii–iv). These results demonstrated that androgen-induced binding of KAT8 and histone H4K16Ac at AR target genes is dependent on WDR5 and PKN1.
KAT8 recruitment does not affect WDR5 binding to chromatin
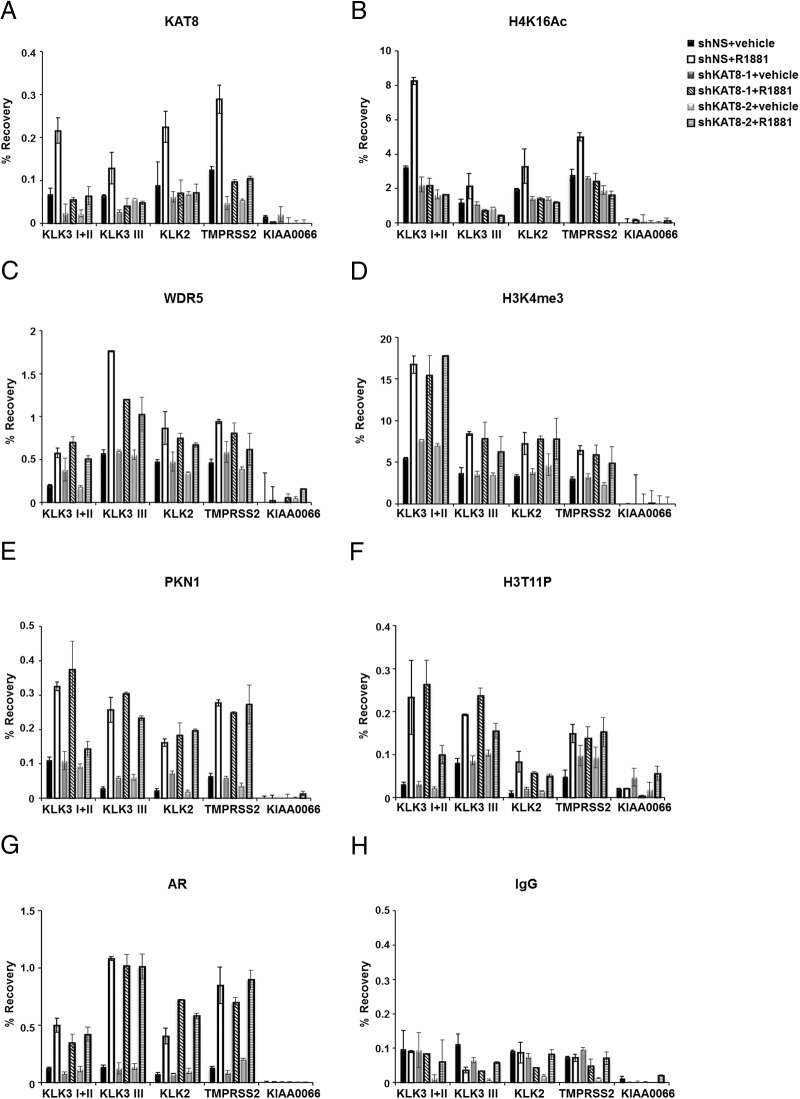
To determine the hierarchical relationship between KAT8 and WDR5 recruitment if any, we wished to determine whether KAT8 was necessary for the recruitment of WDR5 and H3K4me3 at AR target genes. Therefore, we performed ChIP assays in KAT8 depleted LNCaP cells by 2 different shRNA constructs (shKAT8–1 and shKAT8–2) (Figure 1A). KAT8 binding was attenuated in KAT8 knockdown cells treated with R1881 on AR target genes (Figure 5A). Consistent with the fact that the major substrate of KAT8 acetyltransferase activity is the H4K16 residue (11, 13, 16), level of H4K16Ac was also reduced in KAT8 knockdown cells (Figure 5B). Notably, recruitment of WDR5 and the level of H3K4me3 were not significantly impaired by KAT8 knockdown in R1881-stimulated cells (Figure 5, C and D). Moreover, KAT8 knockdown also did not significantly affect the recruitment of PKN1 (Figure 5E), the levels of H3T11P (Figure 5F), or AR (Figure 5G), suggesting that KAT8 possibly does not play a major role in ligand-dependent PKN1 recruitment, phosphorylation of histone H3T11, and AR recruitment (Figure 5, E–H) in LNCaP cells. Together, these results establish a stepwise mechanism in which PKN1 recruitment by androgen leads to histone H3T11 phosphorylation, which is then recognized by WDR5/MLL leading to H3K4 methylation, finally followed by KAT8 recruitment and histone H4K16Ac and gene regulation.
Figure 5.
WDR5 and PKN1 association on AR target genes in KAT8 knockdown LNCaP cells. A–H, ChIP assays were performed using the KAT8 (A) or H4K16Ac (B), WDR5 (C), H3K4me3 (D), PKN1 (E), H3T11P (F), AR (G), or IgG (H) antibodies using control (shNS) or KAT8-knockdown LNCaP cells grown with or without 10nM R1881 for 3 hours. KIAA0066 gene was used as a negative control. Values represent mean ± SD of technical duplicates from a representative experiment. All experiments were performed 3 times with similar results.
KAT8 induces prostate cancer cell proliferation and colony formation
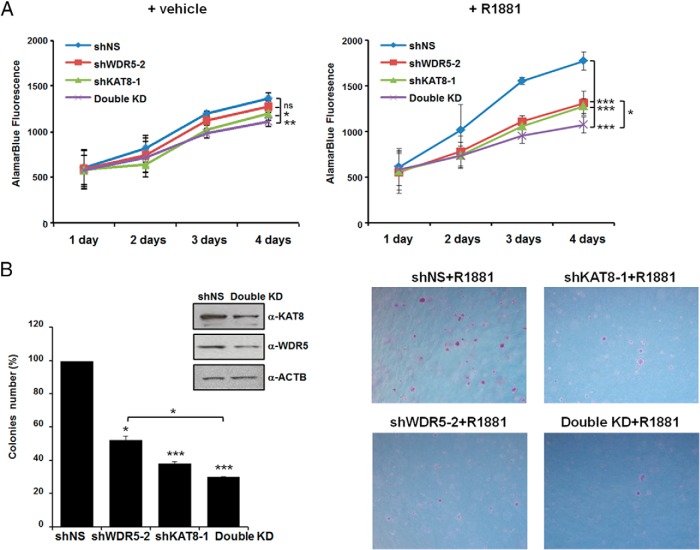
Having determined that KAT8 is important for transcriptional activation of AR target genes in R1881-treated cells, we were interested to investigate whether KAT8 regulates proliferation of prostate cancer cells. We therefore measured the effects of depletion of endogenous KAT8 on cell proliferation using alamarBlue assays in LNCaP and LAPC4 cells expressing either shNS or KAT8 shRNA and treated with R1881 or vehicle control. As expected, the growth of LNCaP cells was significantly stimulated upon R1881 treatment, however, KAT8 depletion decreased prostate cancer cell proliferation when compared with control knockdown in R1881 treatment (Figure 6A). Because double knockdown of KAT8/WDR5 severely inhibited AR target gene expression upon hormone stimulation (Figure 1B), we next performed alamarBlue assays in KAT8/WDR5 double knockdown LNCaP cells. Although individual knockdown of WDR5 or KAT8 has significant effect on ligand-induced proliferation of cells, the KAT8/WDR5 double knockdown showed a dramatic decrease in proliferation (Figure 6A, right panel). Moreover, inhibition of cell growth by KAT8/WDR5 double knockdown was also observed in another AR-positive prostate cancer cell line (LAPC4) (Supplemental Figure 5A). No significant growth inhibition by KAT8/WDR5 double knockdown was seen in the nonmalignant human epithelial prostate cell line, RWPE-1 (Supplemental Figure 5B). To examine relative oncogenic contributions of KAT8 and WDR5, we performed a colony formation assay in WDR5- or KAT8-knockdown or KAT8/WDR5 double knockdown LNCaP cells that were treated with R1881. We observed that knockdown of KAT8 resulted in a reduction of colonies formed; suggesting that expression of KAT8 contributes to LNCaP cell growth (Figure 6B). In addition, we found that WDR5 knockdown or KAT8/WDR5 double knockdown also affected cell size and colony numbers of R1881-treated LNCaP cells (Figure 6B). These results demonstrate that KAT8 and WDR5 have individual and combined effects on the stimulation of growth of androgen-dependent prostate cancer cells.
Figure 6.
Roles of KAT8 and WDR5 in prostate cancer cell proliferation and colony formation. A, Control (shNS) or KAT8, WDR5 alone, or KAT8/WDR5 double knockdown LNCaP cells were grown with (right panel) or without 1nM R1881 (left panel). After indicated number of days, viable cells were detected using the alamarBlue assay. Values are represented as mean ± SD of 3 independent experiments. ns, nonsignificant; *, P < .05; **, P < .01; ***, P < .001. Statistical differences between KAT8/WDR5 double knockdown and KAT8 or WDR5 single knockdown were tested using one-way ANOVA followed by the Tukey's post hoc test. B, LNCaP cells were transduced with shNS, KAT8, WDR5 alone, or KAT8/WDR5 shRNAs and tested for their abilities to promote colony formation in soft agar. After 3 weeks, colonies were counted and colony numbers represented relative to shNS (100%). Values are represented as mean ± SD of 2 independent experiments; *, P < .05; ***, P < .001. Statistical differences between KAT8/WDR5 double knockdown and KAT8 or WDR5 single knockdown were tested using one-way ANOVA followed by the Tukey's post hoc test (left panel). Photomicrograph of representative colonies from soft-agar assays. ×5 magnifications are shown (right panel). Also, see Supplemental Figure 5.
Discussion
In this report, we investigated the roles of KAT8 as a coactivator of AR and a regulator of proliferation in androgen-stimulated prostate cancer cells. We determined that WDR5-mediated H3K4me3 stimulates the association of KAT8 and H4K16Ac at AR target genes. We also defined the biological significance of the KAT8-WDR5 complex in prostate cancer cell proliferation and colony formation upon androgen-agonist treatment.
Our studies uncovered several new discoveries. First, we show that at least on several selected AR target genes KAT8 and WDR5 have a cooperative effect on gene expression. As a consequence, double knockdown of both proteins severely blocks AR dependent gene expression and prostate cancer cell proliferation. Second, histone modifications such as acetylation, methylation, and phosphorylation, have been found to play crucial roles in gene expression (26). However, the interaction network of those key histone modifications in the context of androgen dependent gene regulation is not clear. At the AR target genes studied here we find that a stepwise mechanism involving PKN1, WDR5, and KAT8 established H3T11P-H3K4me3-H4K16Ac signatures of the target genes. Our results indicate that KAT8 recruitment and its associated H4K16 histone acetyltransferase activity at AR target genes are regulated by phosphorylation of H3T11 and H3K4 methylation. This sequential process thus ensures that signal-dependent PKN1 recruitment is integrated into a functional and positive transcriptional outcome. Although we have analyzed a handful of AR target genes, whether this signature and the overall mechanism described here also holds true to other AR target genes or at a global level remains to be determined. In this context, we note, that in contrast to our observation, previous studies in a different cellular system found that the KAT8/KANSL complex promoted histone H3K4 methylation via SET/MLL complexes thereby establishing a reciprocal relationship where H4K16Ac was shown to be important for H3K4me3 (19). Thus, it is possible that sequential recruitment and establishment of epigenomic marks will vary both at gene-specific and genome-wide scale and could be based on the nature of target genes and cellular signaling pathways. Nonetheless, our studies provide evidence to the ever growing concept that a combinatorial epigenomic signature may drive transcriptional outcome. We were also surprised that although KAT8 recruitment was hormone sensitive, association of KANSL and MSL on AR target genes was constitutive in nature. More experiments will be necessary to determine whether KAT8 is recruited to AR target genes as a part of the WDR5 complex. However, our results are consistent with previous observations demonstrating that KAT8 associates with WDR5 and activates embryonic stem cell core transcription factors, such as Nanog, octamer-binding transcription factor 4, and SRY (sex determining region Y)-box 2 in pluripotent embryonic stem cells (27).
Recent studies, although conflicting, suggest that KAT8 plays important roles in various human cancers. KAT8 was underexpressed in renal cell carcinoma, medulloblastoma and primary breast cancer (28–30). In contrast, KAT8 was highly expressed in nonsmall cell lung carcinoma tissues (31). As KAT8 is differentially expressed in cancer, it suggests that KAT8 plays a critical role in gene transcription, cell proliferation, and cell differentiation in various cancers. We found that KAT8 knockdown LNCaP cells only slightly decreased cell proliferation in absence of hormone. A previous report showed that KAT8 interacts with AR and nuclear factor-κB to regulate the expression of key cell cycle proteins, resulting in progression of prostate cancer (32). It is possible that KAT8 might contribute to apoptosis or cell cycle arrest independent of AR pathway in the absence hormones. Nonetheless, our studies, distinct from Jaganathan et al (32), are focused on KAT8 regulation of AR target genes, cell proliferation, and colony formation.
The SET1/MLL complex is present as part of a large protein complex containing WDR5, absent, small or homeotic 2 like (ASH2L), retinoblastoma binding protein 5 (RBBP5), and Menin (33, 34). A recent study showed that Menin can directly interact with AR and regulate AR target gene expression and prostate cancer cell proliferation (35). Consistent with this study, we found that KAT8-WDR5 complex is required for AR-mediated gene expression and stimulated hormone-sensitive prostate cancer cell proliferation (10). Thus, it appears that SET1/MLL complex subunits may interact with different components of AR-transcriptional signaling complex to promote androgen-stimulated genes activation (10, 34). Additionally, published results show that the SET/1MLL complex subunits such as WDR5 and Menin are up-regulated in human prostate cancer samples (35). In this context, it would be interesting to investigate using knockdown-based studies whether Menin also plays a role in KAT8 recruitment to AR target genes and androgen dependent AR target gene regulation and H4K16Ac. It will also be of interest to determine whether WDR5, Menin, and KAT8 play key additional roles in castration resistant prostate cancers and other hormone-sensitive or hormone-resistant human cancers.
In summary, our results demonstrate that KAT8 plays an important role in prostate cancer cell proliferation and colony formation. We show a stepwise mechanism of PKN1-WDR5-KAT8 recruitment establishing transhistone epigenomic signature for transcriptional activation of AR target genes upon androgen stimulation. Finally, our studies provide the basis for identification of key signaling intermediates that allow establishment of downstream histone modifications critical for gene regulation. Our studies suggest that disruption of cross talk of H3T11P-H3K4me3-H4K16Ac by small molecules may provide a therapeutic option for treatment of prostate cancer.
Acknowledgments
Author contributions: D.C. devised researched plan; J.-Y.K. performed all experiments and analyzed data with D.C., S.A.A., and J.Y.; and J.-Y.K. and D.C. wrote the manuscript with help from S.A.A. and J.Y.. All authors reviewed the final manuscript.
This work was supported in part by National Institutes of Health Grants RO1 CA 196270, RO1 CA 123484, and P50 CA 180995 and by the H-foundation multi PI Basic science synergy grant of the Robert H. Lurie Comprehensive Cancer Center.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- androgen receptor
- ARE
- androgen response element
- ChIP
- chromatin immunoprecipitation
- ChIP-IB
- ChIP-immunoblot
- DHT
- dihydrotestosterone
- FBS
- fetal bovine serum
- H4K16
- histone H4 lysine 16
- H4K16Ac
- acetylation at H4K16
- H3T11P
- phosphorylation of histone H3 at threonine 11
- KANSL
- KAT8 regulatory nonspecific lethal
- KAT8
- K(Lysine) acetyltransferase 8
- MSL
- male-specific lethal
- MOZ
- monocytic leukemia zinc-finger protein
- MYST
- MOZ, YBF2/SAS2, and TIP 60 protein 1
- PKN1
- protein kinase N1
- qPCR
- quantitative PCR
- R1881
- methyltrienolone
- reChIP
- sequential ChIP
- SET/MLL
- su(var)3–9, enhancer of zeste, trithorax/mixed-lineage leukemia
- shRNA
- short hairpin RNA
- TIP 60
- tat-interactive protein 60
- WD
- tryptophan, aspartic acid
- WDR5
- WD repeat-containing protein 5.
References
- 1. Izumi K, Mizokami A, Lin WJ, Lai KP, Chang C. Androgen receptor roles in the development of benign prostate hyperplasia. Am J Pathol. 2013;182:1942–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schrecengost R, Knudsen KE. Molecular pathogenesis and progression of prostate cancer. Semin Oncol. 2013;40:244–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Niu Y, Altuwaijri S, Lai KP, et al. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proc Natl Acad Sci USA. 2008;105:12182–12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shafi AA, Yen AE, Weigel NL. Androgen receptors in hormone-dependent and castration-resistant prostate cancer. Pharmacol Ther. 2013;140:223–238. [DOI] [PubMed] [Google Scholar]
- 5. Takayama K, Inoue S. Transcriptional network of androgen receptor in prostate cancer progression. Int J Urol. 2013;20:756–768. [DOI] [PubMed] [Google Scholar]
- 6. Dasgupta S, Lonard DM, O'Malley BW. Nuclear receptor coactivators: master regulators of human health and disease. Annu Rev Med. 2014;65:279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9:601–610. [DOI] [PubMed] [Google Scholar]
- 8. Rosenfeld MG, Glass CK. Coregulator codes of transcriptional regulation by nuclear receptors. J Biol Chem. 2001;276:36865–36868. [DOI] [PubMed] [Google Scholar]
- 9. Metzger E, Yin N, Wissmann M, et al. Phosphorylation of histone H3 at threonine 11 establishes a novel chromatin mark for transcriptional regulation. Nat Cell Biol. 2008;10:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim JY, Banerjee T, Vinckevicius A, et al. A role for WDR5 in integrating threonine 11 phosphorylation to lysine 4 methylation on histone H3 during androgen signaling and in prostate cancer. Mol Cell. 2014;54:613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mellert HS, McMahon SB. hMOF, a KAT(8) with many lives. Mol Cell. 2009;36:174–175. [DOI] [PubMed] [Google Scholar]
- 12. Pillus L. MYSTs mark chromatin for chromosomal functions. Curr Opin Cell Biol. 2008;20:326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taipale M, Rea S, Richter K, et al. hMOF histone acetyltransferase is required for histone H4 lysine 16 acetylation in mammalian cells. Mol Cell Biol. 2005;25:6798–6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith ER, Cayrou C, Huang R, Lane WS, Côté J, Lucchesi JC. A human protein complex homologous to the Drosophila MSL complex is responsible for the majority of histone H4 acetylation at lysine 16. Mol Cell Biol. 2005;25:9175–9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akhtar A, Becker PB. Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol Cell. 2000;5:367–375. [DOI] [PubMed] [Google Scholar]
- 16. Dou Y, Milne TA, Tackett AJ, et al. Physical association and coordinate function of the H3 K4 methyltransferase MLL1 and the H4 K16 acetyltransferase MOF. Cell. 2005;121:873–885. [DOI] [PubMed] [Google Scholar]
- 17. Li X, Wu L, Corsa CA, Kunkel S, Dou Y. Two mammalian MOF complexes regulate transcription activation by distinct mechanisms. Mol Cell. 2009;36:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cai Y, Jin J, Swanson SK, et al. Subunit composition and substrate specificity of a MOF-containing histone acetyltransferase distinct from the male-specific lethal (MSL) complex. J Biol Chem. 2010;285:4268–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao X, Su J, Wang F, et al. Crosstalk between NSL histone acetyltransferase and MLL/SET complexes: NSL complex functions in promoting histone H3K4 di-methylation activity by MLL/SET complexes. PLoS Genet. 2013;9:e1003940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zippo A, Serafini R, Rocchigiani M, Pennacchini S, Krepelova A, Oliviero S. Histone crosstalk between H3S10ph and H4K16ac generates a histone code that mediates transcription elongation. Cell. 2009;138:1122–1136. [DOI] [PubMed] [Google Scholar]
- 21. Loomis RJ, Naoe Y, Parker JB, et al. Chromatin binding of SRp20 and ASF/SF2 and dissociation from mitotic chromosomes is modulated by histone H3 serine 10 phosphorylation. Mol Cell. 2009;33:450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bell O, Wirbelauer C, Hild M, et al. Localized H3K36 methylation states define histone H4K16 acetylation during transcriptional elongation in Drosophila. EMBO J. 2007;26:4974–4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parker JB, Palchaudhuri S, Yin H, Wei J, Chakravarti D. A transcriptional regulatory role of the THAP11-HCF-1 complex in colon cancer cell function. Mol Cell Biol. 2012;32:1654–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim J, Wu L, Zhao JC, Jin HJ, Yu J. TMPRSS2-ERG gene fusions induce prostate tumorigenesis by modulating microRNA miR-200c. Oncogene. 2014;33:5183–5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liao CH, Pan SL, Guh JH, et al. Antitumor mechanism of evodiamine, a constituent from Chinese herb Evodiae fructus, in human multiple-drug resistant breast cancer NCI/ADR-RES cells in vitro and in vivo. Carcinogenesis. 2005;26:968–975. [DOI] [PubMed] [Google Scholar]
- 26. Zhang Y, Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 2001;15:2343–2360. [DOI] [PubMed] [Google Scholar]
- 27. Li X, Li L, Pandey R, et al. The histone acetyltransferase MOF is a key regulator of the embryonic stem cell core transcriptional network. Cell Stem Cell. 2012;11:163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pfister S, Rea S, Taipale M, et al. The histone acetyltransferase hMOF is frequently downregulated in primary breast carcinoma and medulloblastoma and constitutes a biomarker for clinical outcome in medulloblastoma. Int J Cancer. 2008;122:1207–1213. [DOI] [PubMed] [Google Scholar]
- 29. Wang Y, Zhang R, Wu D, et al. Epigenetic change in kidney tumor: downregulation of histone acetyltransferase MYST1 in human renal cell carcinoma. J Exp Clin Cancer Res. 2013;32:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kapoor-Vazirani P, Kagey JD, Vertino PM. SUV420H2-mediated H4K20 trimethylation enforces RNA polymerase II promoter-proximal pausing by blocking hMOF-dependent H4K16 acetylation. Mol Cell Biol. 2011;31:1594–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao L, Wang DL, Liu Y, Chen S, Sun FL. Histone acetyltransferase hMOF promotes S phase entry and tumorigenesis in lung cancer. Cell Signal. 2013;25:1689–1698. [DOI] [PubMed] [Google Scholar]
- 32. Jaganathan A, Chaurasia P, Xiao GQ, et al. Coactivator MYST1 regulates nuclear factor-κB and androgen receptor functions during proliferation of prostate cancer cells. Mol Endocrinol. 2014;28:872–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murai MJ, Chruszcz M, Reddy G, Grembecka J, Cierpicki T. Crystal structure of menin reveals binding site for mixed lineage leukemia (MLL) protein. J Biol Chem. 2011;286:31742–31748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shilatifard A. The COMPASS family of histone H3K4 methylases: mechanisms of regulation in development and disease pathogenesis. Annu Rev Biochem. 2012;81:65–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Malik R, Khan AP, Asangani IA, et al. Targeting the MLL complex in castration-resistant prostate cancer. Nat Med. 2015;21:344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]