Abstract
The epigenome undergoes significant remodeling during tissue and organ development, which coincides with a period of exquisite sensitivity to environmental exposures. In the case of endocrine-disrupting compounds (EDCs), exposures can reprogram the epigenome of developing tissues to increase susceptibility to diseases later in life, a process termed “developmental reprogramming.” Both DNA methylation and histone modifications have been shown to be vulnerable to disruption by EDC exposures, and several mechanisms have been identified by which EDCs can reprogram the epigenome. These include altered methyl donor availability, loss of imprinting control, changes in dioxygenase activity, altered expression of noncoding RNAs, and activation of cell signaling pathways that can phosphorylate, and alter the activity of, histone methyltransferases. This altered epigenomic programming can persist across the life course, and in some instances generations, to alter gene expression in ways that correlate with increased disease susceptibility. Together, these studies on developmental reprogramming of the epigenome by EDCs are providing new insights into epigenomic plasticity that is vulnerable to disruption by environmental exposures.
During development, organogenesis and tissue differentiation occur through a continuous series of tightly regulated and precisely timed molecular, biochemical, and cellular events. It is now appreciated that adverse environmental exposures during this period can have life-long consequences for exposed individuals. Ravelli et al (1) first noted the link between the early environment and adult disease, specifically, increased rates of obesity in individuals exposed to famine in utero. This and subsequent studies reported maternal starvation during the Dutch “hunger winter” of the Second World War correlated with an increased risk in exposed offspring of cardiovascular disease, and metabolic diseases such as obesity, metabolic syndrome, and diabetes in adulthood (2–4). In the 1980s, using birth weight as a surrogate for poor in utero nutrition, Barker reported a similar link between birth weight and increased coronary heart disease in adulthood (5). This and later confirmatory studies (6) found that low birth weight infants had the highest rates of coronary heart disease, hypertension, and stroke as adults. We now know that birth weight per se is not responsible for increased disease risk but rather is a surrogate for a poor in utero nutritional environment (7, 8).
One of the reasons this developmental period is one of sensitivity to environmental exposures is that it is a critical time when epigenetic programs are being “installed” on the genome. During this time, epigenetic programmers, such as histone methyltransferase (HMT) and DNA methyltransferase (DNMT) “writers,” add methyl groups to lysine and arginine residues on histone tails, and to CpG sites on DNA, respectively. Cytosine methylation at CpG sites plays an important role in several biological processes, including imprinting, regulation of gene expression, and silencing of transposable elements. Similarly, histone modifications and the epigenetic programs that are installed by HMT form a “histone code” that is interpreted by “readers,” the effector molecules that recognize histone modifications (9, 10). Both DNA and histone methylation can be modified by “erasers” that remove methyl marks. The histone demethylases include members of the lysine-specific demethylase and Jumonji domain-containing histone demethylases. Methylated CpG sites can also be remodeled by ten-eleven translocation (Tet) enzymes that function as erasers for DNA methylation, converting 5-methylcytosine to 5-hydroxymethylcytosine (5hmC) and other oxidation products. The activity of Tet enzymes is particularly important during development, where they play an important role in remodeling the epigenome after fertilization and during gametogenesis (11).
Methyl marks on histones H3 and H4, the primary targets for methylation on chromatin, are by convention denoted by the lysine or arginine residues that are mono-, di- or trimethylated. Histone methyl marks can activate or repress gene expression, for example, active histone H3 lysine 4 trimethyl (H3K4me3) or repressive histone H3 lysine 27 trimethyl (H3K27me3) marks. These epigenetic marks in turn generate binding sites for readers such as RNA polymerase II, as well as writers such as DNMTs, which can convert more labile repressive histone marks into more stably repressed methylated DNA (12–17). In certain settings during development, some genes are actually bivalent, containing both active and repressive histone methyl marks. During cell fate determination and differentiation, these genes convert to a univalent state, with repressed genes losing their active mark and active genes losing their repressive mark.
During the early stages of embryogenesis, DNA methylation is dynamic, with methylation patterns erased and reestablished on both the maternal and paternal germline, albeit with different kinetics (18–20). Prenatal exposure to the famine of the Dutch hunger winter and season of conception in areas of rural Gambia that experience dramatic seasonal fluctuations in nutritional status have both been associated with epigenetic changes at specific gene loci in affected individuals (21, 22). Histone methylation is also dynamic. In the case of the collinear Hox genes, for example, where gene expression is under both temporal and spatial control, deposition of both repressive and activating methyl marks by polycomb and trithorax HMT complexes, respectively, during development induces dynamic changes in chromatin modifications that repress or activate the expression of specific Hox genes to regulate patterning in the developing embryo (23). This process of developmental programming of the epigenome continues through organogenesis, which, in some tissues and organs, such as the breast (humans and rodents), reproductive tract (rodents), and kidney (rodents), may extend into the perinatal, early childhood, and peripubertal periods.
Mechanisms of Endocrine-Disrupting Compounds (EDCs) Disruption of the Epigenome
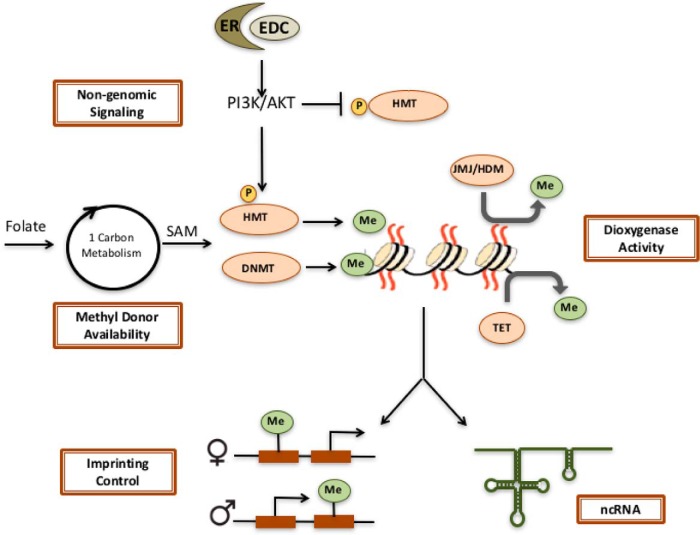
As shown in Figure 1, several mechanisms have been identified by which EDC exposures can induce epigenetic reprogramming, including modulating methyl donor availability, loss of imprinting control, changes in the activity of dioxygenases, altered expression of noncoding RNAs (ncRNAs), and activation of kinases that phosphorylate and modulate the activity of epigenetic readers, writers, and erasers.
Figure 1.
Mechanisms for EDC reprogramming of the epigenome. Several mechanisms have been described, by which EDC exposures can impact the epigenome, including altered methyl donor availability that can modulate SAM levels and the activity of DNMTs and HMTs, loss of imprinting control, inhibition of dioxygenases such as Jumonji family histone demethylases and Tet enzymes, altered expression of ncRNAs and activation of cell signaling pathways that can phosphorylate, and activate/inhibit HMTs.
Altered availability of methyl donors
S-adenosylmethionine (SAM), produced from folate via 1-carbon metabolism, is the methyl donor for both DNMT and HMT, and altering SAM levels can be translated into changes in DNA methylation (24–27). The availability of this methyl donor can be modulated by diet and exposure to EDCs, as shown using the viable yellow Agouti (Avy) mouse model. Avy mice have a retrotransposon insertion upstream of the Agouti locus, with a long terminal repeat (LTR) that drives ectopic expression of this gene and causes them to have a yellow coat color, become obese, and be cancer prone (28, 29). DNA methylation of the LTR element silences ectopic expression, and returns Agouti expression to normal levels. Unexposed Avy mice exhibit variable levels of methylation of this LTR and expression of the Agouti gene, causing coat color variation from yellow (unmethylated), to mottled, to brown (pseudoagouti/methylated). Dietary folate supplementation, which increases SAM levels, increases methylation at this locus, returning Agouti expression to normal, wild-type levels, producing more pseudoagouti colored mice, and decreasing obesity. Exposure to EDCs also effects the methylation of this metastable epiallele. Maternal dietary supplementation with genistein increases LTR methylation, producing pseudoagouti offspring that are resistant to obesity (30). Maternal bisphenol A (BPA) exposure in contrast, reduces methylation at this allele, promoting ectopic expression and shifting offspring towards yellow coat color, an effect that can be reversed by maternal nutritional supplementation with methyl donors such as folate (31). However, some recent studies have suggested that coat color changes may not be tightly linked to other phenotypes modulated by EDC exposures in Avy mice (32, 33).
Loss of imprinting control
Maternal EDC exposure during pregnancy can modify imprinted loci. Imprinted genes are epigenetically regulated, so that only a single parental allele is expressed, whereas the other is silenced (34). Imprinting often occurs at genes that play important roles in placental development, fetal growth, and normal cell and tissue function. Loss of imprinting control underlies several imprinting disorders including Beckwith-Wiedemann, Angelman, and Prader-Willi syndromes. Imprinted genes are usually located in clusters, which contain both a maternally and a paternally expressed gene and a DNA domain known as the imprinting control region, which regulates expression of the imprinted genes in that cluster (34). Although there is still much to learn about mechanisms regulating gene imprinting, cytosine methylation of DNA is to date the best-characterized epigenetic mechanism for imprinting.
In the case of EDCs, maternal exposure to BPA has been shown in the mouse to disrupt DNA methylation at several imprinting control regions. Altered DNA methylation has been linked to aberrant expression of the imprinted Snrpn, Ube3a, Kcnq1ot1, and Cdkn1c genes in midgestation placentas (35). Importantly, improper expression of imprinted genes in the F1 placenta was linked to abnormal development as evidenced in the altered morphology of BPA-exposed placentas. In the midgestation F1 fetus, maternal BPA exposure was associated with increased DNA methylation at the Igf2 differentially methylated region, biallelic Igf2 expression, and increased expression of this growth factor (36). Interestingly, elevated Igf2 in the F1 fetus was linked to obesity and altered glucose homeostasis during adulthood in BPA-exposed male mice. The dioxin tetrachlorodibenzo-p-dioxin has also been shown to disrupt imprinting of the Igf2r locus (37).
Changes in dioxygenase activity
As mentioned above, the Tet DNA demethylases are responsible for converting 5-methylcytosine to 5hmC, and other oxidation products (38). Recently, in the developing uterus, the EDC diethylstilbestrol (DES) was shown to significantly reduce Tet1 expression, which correlated decreased global 5hmC levels in the adult uterus (39). Furthermore, both histone demethylases in the Jumonji family and the Tet family of DNA demethylases are dioxygenases, which means they require oxygen, Fe, ascorbate, and α-ketoglutarate to catalyze the demethylation reaction (40). Displacement of Fe by metals such as nickel, chromium and arsenic is thought to contribute to their toxicity via disruption of demethylase activity and reprogramming of the epigenome (41). In the case of nickel, decreased Jumonji demethylase activity has been shown to increase H3K9me2 at the SPRY promoter and decrease expression of this gene (42, 43).
Altered expression of ncRNAs
Modulation of ncRNAs, with downstream epigenetic consequences, has recently been reported to be induced by EDC exposure (44, 45). In prostaspheres, BPA repressed the expression of small nucleolar RNAs with C/D motif (SNORDs), ncRNAs that regulate ribosomal RNA assembly and function. BPA-induced changes in H3K9me3, H3K4me3, and H3K27me3 histone methyl marks at several SNORDs while having little effect on DNA methylation (44). The long non-coding RNA HOX transcript antisense RNA (HOTAIR) promotes gene silencing via its interaction with polycomb-repressive complex (PRC)2 and lysine-specific demethylase 1, and recruitment to target gene promoters (46). HOTAIR is induced in response to BPA and DES binding to estrogen receptors (ERs), corecruiting the complex proteins associated with Set1 (COMPASS) complex, and increasing the H3K4me3 active histone mark at the HOTAIR promoter in vitro and in vivo (45).
Activation of kinases via nongenomic ER signaling
For EDCs that function as ER ligands, which includes DES, BPA, and genistein, ER binding can activate cytoplasmic signaling cascades, termed nongenomic ER signaling pathways (47, 48). Several cell signaling pathways including phosphoinositide 3-kinase (PI3K)/AKT and MAPK/ERK are activated in the cytoplasm by this rapid nongenomic ER signaling (49), and in mice, extranuclear (nongenomic) ER signaling can be separated from nuclear (genomic) activity of this receptor (50). Kinases in these pathways have been shown to modulate the activity of HMTs in the PRC and the COMPASS complex, which oppose each other to repress or activate gene transcription, respectively (51). The HMT enhancer of zeste homolog 2 in the PRC2 complex responsible for the H3K27me2-repressive histone mark. EDCs that induce PI3K/AKT signaling via nongenomic ER activity cause AKT phosphorylation of EZH2 at serine 21, which inactivates this methyltransferase and reduces levels of the repressive H3K27me3 mark on chromatin (52, 53). Conversely, AKT can induce activation of MLL1 in the COMPASS complex responsible for the active H3K4 methyl mark, significantly increasing H3K4me3 and expression of genes targeted for epigenetic reprogramming by EDCs (54, 55). Importantly, activation of kinases via nongenomic ER signaling represents a direct mechanism by which EDCs can disrupt the cells epigenetic machinery to reprogram the epigenome.
Epigenetic Reprogramming Persists Across the Life Course
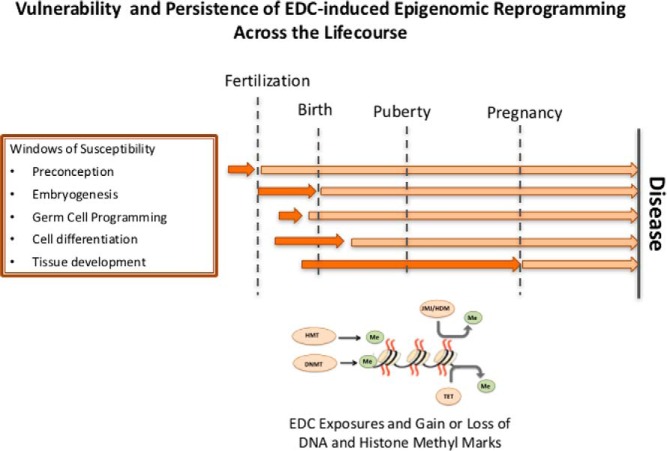
In addition to metastable alleles and imprinted loci, gene-specific changes in DNA and histone methylation have been observed during developmental reprogramming by EDCs that can persist into adulthood (Figure 2). As shown in Table 1, for several EDCs and multiple gene targets, changes in DNA and/or histone methylation induced by developmental exposures have been shown to persist in adult tissues long after the initial early life exposures. Secretoglobin family 2A member 1 (Scgb2a1) is a component of prostatein, a major androgen-binding protein secreted by the rat prostate. Scgb2a1 was significantly up-regulated (>100-fold) in the prostate of adult rats neonatally exposed to BPA, concordant with hypomethylation of a CpG island upstream of the transcription start site of this gene (54). However, because this gene was constitutively overexpressed in the reprogrammed tissues, it was impossible to determine if the decrease in DNA methylation was a cause or consequence of increased expression of Scgb2a1.
Figure 2.
EDC reprogramming of the epigenome across the life course. Key periods for programming the genome associated with embryogenesis and development create vulnerabilities to environmental exposures. Exposures to EDCs at these times can disrupt the epigenome and cause epigenetic changes that may persist across the life course. Windows of vulnerability to EDC-induced reprogramming differ among tissues and can be modulated by later life events such as puberty and pregnancy.
Table 1.
Examples of Genes Targeted by Developmental Reprogramming
| Developmental EDC Exposure | Gene Changes Seen in Adults | Persistent Epigenetic Alterations | Reference |
|---|---|---|---|
| DES | Hoxa10 | DNA hypermethylation | 65 |
| Ltf | DNA hypomethylation | 59 | |
| Six1 | Increased H3K9ac, H4K5ac and H3K4me3 histone methylation | 39 | |
| c-fos | DNA hypomethylation | 66 | |
| Nsbp1 | DNA hypomethylation | 58 | |
| Svs4 | DNA hypermethylation | 67 | |
| Vinclozolin | Imprinted H19, Gtl2, Peg1, Snrpn, and Peg 3 loci | DNA hyper- and hypomethylation | 68 |
| BPA | Scgb2a1 | DNA hypomethylation | 54 |
| Psbpc and Klk1 gene clusters and prostate cancer KEGG pathway genes | Increased H3K4me3 histone methylation | 55 | |
| SNORDs | Altered H3K4me3, H3K9me3, and H3K27me3 | 44 | |
| Methoxychlor | Imprinted Gtl2, Peg1, Snrpn, Peg3 loci | DNA hyper- and hypomethylation | 69 |
| Esr2 | DNA hypermethylation | 70 | |
| Genistein | Nsbp1 | DNA hypomethylation | 58 |
| DEHP | Vmn and Olfr genes, Sfi1 | DNA hypermethylation | 71 |
| miRNAs | DNA hypomethylation | 71 |
More recent studies focused on developmental reprogramming of histone methyl marks have revealed that gene-specific reprogramming events can precede changes in gene expression, making it possible to draw cause and effect inferences. As mentioned above, BPA increases the activity of MLL1 in the COMPASS complex, which writes the H3K4me3 active mark (55). MLL1 is degraded by the S-phase kinase associated protein 2 (SKP2) E3 ligase (56) and activated by the protease taspase (57). MLL1 taspase cleavage and activation is increased by AKT in response to activation by nongenomic ER signaling (55). In the developing prostate, active, cleaved MLL1 is increased by BPA, increasing the H3K4me3 methyl mark on reprogrammed genes. Importantly, elevated H3K4me3 induced by postnatal BPA exposure at reprogrammed genes persists into adulthood, resulting in increased expression of androgen-responsive genes. This was observed for the Psbpc (which includes Scgb2a1) and Klk1 clusters on rat chromosome 1, as well as several genes in the Kyoto Encyclopedia of Genes and Genomes (KEGG) prostate cancer pathway, where BPA exposure resulted in a significant increase in H3K4me3 at transcription start sites. Notably, and in contrast to the constitutively elevated expression seen for the Psbpc and Klk1 gene clusters, in adult animals, the reprogrammed KEGG pathway genes with increased H3K4me3 exhibited no change in basal expression. However, these reprogrammed genes exhibited increased expression in response to testosterone, which correlated with increased risk for PIN lesions in a rat model for prostate carcinogenesis (54, 55). Chromatin immunoprecipitation (ChIP)-sequencing (ChIP-seq) analysis revealed that in BPA-exposed neonates, as well as the adult prostates of reprogrammed animals, H3K4me3 increased at the promoter Psbpc and Klk1 genes, and Ccne1, Erbb2, Grb2, Ikbkb, Map2k2, Nfkbia, and Pdgfb genes in the KEGG prostate cancer pathway. Importantly, although the KEGG prostate cancer pathway genes were expressed at the same level in reprogrammed and vehicle control animals (55), upon hormone stimulation, the reprogrammed genes with elevated H3K4me3 exhibited an exaggerated response to hormone and significantly increased hormone-induced gene expression.
A “priming” effect of developmental reprogramming for increased transcription in response to a later challenge is a recurrent theme. Early life exposure to the phytoestrogen genistein can reprogram high-mobility group nucleosome-binding domain 5 (Hmgn5) (also known as Nsbp1) gene methylation, causing the promoter of this gene to become hypomethylated in the adult uterus, which increases gene expression (58). However, if genistein-exposed animals are ovariectomized, aberrant hypomethylation of the Hmgn5 promoter does not occur. Similarly, the manifestation of the reprogramming event itself may remain hidden until triggered by later- life events. This is illustrated by altered DNA methylation that is observed in the uterus in response to in utero exposure to DES. Adult uteri of animals exposed to this EDC in utero exhibit alterations in the methylation of the lactoferrin (Ltf) gene after puberty (59). However, if ovariectomized, the aberrant DNA methylation seen in intact, reprogrammed animals is not observed in castrated females.
Finally, in addition to persistence across the life course, in some instances, epigenetic reprogramming induced by EDC exposure can be transmitted to subsequent generations. After maternal (F0) EDC exposure, F1 offspring exposed gestationally may exhibit reprogramming as a result of their in utero exposures. If the gametes of the F1 generation also have been exposed during periods of epigenetic programming for germ cells (depending of the exact exposure window), the F2 generation would also have seen EDCs pregestationally. In this setting EDC effects across the F1 and F2 generations are considered to be multior intergenerational. Transgenerational effects of EDC exposures, that is those that persist out to the F3 generation in individuals that had never been exposed gestationally or pregestationally, have also been reported (for reviews, see Refs. 60–63).
Conclusions
Experimental studies on EDCs have obvious relevance for identifying exposures that could potentially adversely affect human health. However, many other factors must be considered to translate data on disruption of the epigenome by EDCs into a better understanding of health risks associated with EDC exposure: dose response, age and duration of exposure, and a better understanding of how epigenetic reprogramming of gene expression translates into increased (or decreased) risk for disease. Just as importantly, but often overlooked, is the potential to use these toxicants as tools to understand basic biological process, including programming of the epigenome. EDC exposures during development have shown us the remarkable plasticity of the epigenome, and revealed that changes in epigenetic programming once induced, can be fixed and maintained through many subsequent cell divisions, and persist in mature tissues long after the exposure occurred. The “raison d'être” for this is not immediately obvious, but could reflect a contribution of epigenetic plasticity to evolutionary drive, where as noted by Arthur, developmental reprogramming “lies logically between mutation and selection” (64). Although epigenomic plasticity may play a key role in evolution, it also creates a vulnerability to environmental exposures, which, if able to disrupt the developing epigenome, can have far reaching consequences across an exposed individual's lifetime and even subsequent generations.
Acknowledgments
The authors would like to thank Dr. Lindsey S. Treviño for her assistance with editorial review.
This work was supported by National Institute of Environmental Health Sciences Grants RC2ES018789, P30ES023512, and ES023206; the Cancer Prevention Research Institute of Texas Grant RP120855; and the Welch Foundation Grant BE-0023 (Houston, TX).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AKT
- protein kinase B
- Avy
- viable yellow Agouti
- BPA
- bisphenol A
- COMPASS
- complex proteins associated with Set1
- DEHP
- di(2-ethylhexyl)phthalate
- DES
- diethylstilbestrol
- DNMT
- DNA methyltransferase
- EDC
- endocrine-disrupting compound
- ER
- estrogen receptor
- F1
- filial 1 hybrid
- H3K4me3
- histone H3 lysine 4 trimethyl
- H3K27me3
- histone H3 lysine 27 trimethyl
- HMT
- histone methyltransferase
- HOTAIR
- HOX transcript antisense RNA
- KEGG
- Kyoto Encyclopedia of Genes and Genomes
- LTR
- long terminal repeat
- 5hmC
- 5-hydroxymethylcytosine
- MLL1
- mixed lineage leukemia 1
- ncRNA
- noncoding RNA
- PRC
- polycomb-repressive complex
- SAM
- S-adenosylmethionine
- Scgb2a1
- secretoglobin family 2A member 1
- SNORD
- small nucleolar RNAs with C/D motif
- Tet
- ten-eleven translocation.
References
- 1. Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–353. [DOI] [PubMed] [Google Scholar]
- 2. Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol. 2001;185:93–98. [DOI] [PubMed] [Google Scholar]
- 3. Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol. 2005;20:345–352. [DOI] [PubMed] [Google Scholar]
- 4. Roseboom TJ. Undernutrition during fetal life and the risk of cardiovascular disease in adulthood. Future Cardiol. 2012;8:5–7. [DOI] [PubMed] [Google Scholar]
- 5. Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Swanson JM, Entringer S, Buss C, Wadhwa PD. Developmental origins of health and disease: environmental exposures. Semin Reprod Med. 2009;27:391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stein AD, Zybert PA, van de Bor M, Lumey LH. Intrauterine famine exposure and body proportions at birth: the Dutch Hunger Winter. Int J Epidemiol. 2004;33:831–836. [DOI] [PubMed] [Google Scholar]
- 8. Morley R, Owens J, Blair E, Dwyer T. Is birthweight a good marker for gestational exposures that increase the risk of adult disease? Paediatr Perinat Epidemiol. 2002;16:194–199. [DOI] [PubMed] [Google Scholar]
- 9. Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. [DOI] [PubMed] [Google Scholar]
- 10. Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. [DOI] [PubMed] [Google Scholar]
- 11. Tan L, Shi YG. Tet family proteins and 5-hydroxymethylcytosine in development and disease. Development. 2012;139:1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao Q, Rank G, Tan YT, et al. PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. Nat Struct Mol Biol. 2009;16:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Viré E, Brenner C, Deplus R, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874. [DOI] [PubMed] [Google Scholar]
- 14. Smallwood A, Estève PO, Pradhan S, Carey M. Functional cooperation between HP1 and DNMT1 mediates gene silencing. Genes Dev. 2007;21:1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bannister AJ, Zegerman P, Partridge JF, et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. [DOI] [PubMed] [Google Scholar]
- 16. Lachner M, O'Carroll D, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–120. [DOI] [PubMed] [Google Scholar]
- 17. Rose NR, Klose RJ. Understanding the relationship between DNA methylation and histone lysine methylation. Biochim Biophys Acta. 2014;1839:1362–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hogg K, Western PS. Refurbishing the germline epigenome: out with the old, in with the new. Semin Cell Dev Biol. 2015;45:104–113. [DOI] [PubMed] [Google Scholar]
- 19. Combes AN, Whitelaw E. Epigenetic reprogramming: enforcer or enabler of developmental fate? Dev Growth Differ. 2010;52:483–491. [DOI] [PubMed] [Google Scholar]
- 20. Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer. 2011;11:726–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tobi EW, Lumey LH, Talens RP, et al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet. 2009;18:4046–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Waterland RA, Kellermayer R, Laritsky E, et al. Season of conception in rural gambia affects DNA methylation at putative human metastable epialleles. PLoS Genet. 2010;6:e1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soshnikova N, Duboule D. Epigenetic regulation of Hox gene activation: the waltz of methyls. Bioessays. 2008;30:199–202. [DOI] [PubMed] [Google Scholar]
- 24. Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate's role. Adv Nutr. 2012;3:21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 2011;13:97–109. [DOI] [PubMed] [Google Scholar]
- 26. Kim YI. Nutritional epigenetics: impact of folate deficiency on DNA methylation and colon cancer susceptibility. J Nutr. 2005;135:2703–2709. [DOI] [PubMed] [Google Scholar]
- 27. Poirier LA, Wise CK, Delongchamp RR, Sinha R. Blood determinations of S-adenosylmethionine, S-adenosylhomocysteine, and homocysteine: correlations with diet. Cancer Epidemiol Biomarkers Prev. 2001;10:649–655. [PubMed] [Google Scholar]
- 28. Morgan HD, Sutherland HG, Martin DI, Whitelaw E. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet. 1999;23:314–318. [DOI] [PubMed] [Google Scholar]
- 29. Dolinoy DC. The agouti mouse model: an epigenetic biosensor for nutritional and environmental alterations on the fetal epigenome. Nutr Rev. 2008;66(suppl 1):S7–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA. 2007;104:13056–13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Badger TM, Ronis MJ, Wolff G, et al. Soy protein isolate reduces hepatosteatosis in yellow Avy/a mice without altering coat color phenotype. Exp Biol Med (Maywood). 2008;233:1242–1254. [DOI] [PubMed] [Google Scholar]
- 33. Rosenfeld CS, Sieli PT, Warzak DA, Ellersieck MR, Pennington KA, Roberts RM. Maternal exposure to bisphenol A and genistein has minimal effect on A(vy)/a offspring coat color but favors birth of agouti over nonagouti mice. Proc Natl Acad Sci USA. 2013;110:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barlow DP, Bartolomei MS. Genomic imprinting in mammals. Cold Spring Harb Perspect Biol. 2014;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Susiarjo M, Sasson I, Mesaros C, Bartolomei MS. Bisphenol a exposure disrupts genomic imprinting in the mouse. PLoS Genet. 2013;9:e1003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Susiarjo M, Xin F, Bansal A, et al. Bisphenol a exposure disrupts metabolic health across multiple generations in the mouse. Endocrinology. 2015;156:2049–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Somm E, Stouder C, Paoloni-Giacobino A. Effect of developmental dioxin exposure on methylation and expression of specific imprinted genes in mice. Reprod Toxicol. 2013;35:150–155. [DOI] [PubMed] [Google Scholar]
- 38. Rasmussen KD, Helin K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016;30:733–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jefferson WN, Chevalier DM, Phelps JY, et al. Persistently altered epigenetic marks in the mouse uterus after neonatal estrogen exposure. Mol Endocrinol. 2013;27:1666–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Klose RJ, Zhang Y. Regulation of histone methylation by demethylimination and demethylation. Nat Rev Mol Cell Biol. 2007;8:307–318. [DOI] [PubMed] [Google Scholar]
- 41. Chervona Y, Arita A, Costa M. Carcinogenic metals and the epigenome: understanding the effect of nickel, arsenic, and chromium. Metallomics. 2012;4:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen H, Kluz T, Zhang R, Costa M. Hypoxia and nickel inhibit histone demethylase JMJD1A and repress Spry2 expression in human bronchial epithelial BEAS-2B cells. Carcinogenesis. 2010;31:2136–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen H, Giri NC, Zhang R, et al. Nickel ions inhibit histone demethylase JMJD1A and DNA repair enzyme ABH2 by replacing the ferrous iron in the catalytic centers. J Biol Chem. 2010;285:7374–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ho SM, Cheong A, Lam HM, et al. Exposure of human prostaspheres to bisphenol A epigenetically regulates SNORD family noncoding RNAs via histone modification. Endocrinology. 2015;156:3984–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bhan A, Hussain I, Ansari KI, Bobzean SA, Perrotti LI, Mandal SS. Bisphenol-A and diethylstilbestrol exposure induces the expression of breast cancer associated long noncoding RNA HOTAIR in vitro and in vivo. J Steroid Biochem Mol Biol. 2014;141:160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsai MC, Manor O, Wan Y, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lösel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4:46–56. [DOI] [PubMed] [Google Scholar]
- 48. Treviño LS, Wang Q, Walker CL. Hypothesis: activation of rapid signaling by environmental estrogens and epigenetic reprogramming in breast cancer. Reprod Toxicol. 2015;54:136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wong RL, Walker CL. Molecular pathways: environmental estrogens activate nongenomic signaling to developmentally reprogram the epigenome. Clin Cancer Res. 2013;19:3732–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Levin ER. Extranuclear estrogen receptor's roles in physiology: lessons from mouse models. Am J Physiol Endocrinol Metab. 2014;307:E133–E140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Piunti A, Shilatifard A. Epigenetic balance of gene expression by Polycomb and COMPASS families. Science. 2016;352:aad9780. [DOI] [PubMed] [Google Scholar]
- 52. Bredfeldt TG, Greathouse KL, Safe SH, Hung MC, Bedford MT, Walker CL. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Mol Endocrinol. 2010;24:993–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Greathouse KL, Bredfeldt T, Everitt JI, et al. Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis. Mol Cancer Res. 2012;10:546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wong RL, Wang Q, Treviño LS, et al. Identification of secretaglobin Scgb2a1 as a target for developmental reprogramming by BPA in the rat prostate. Epigenetics. 2015;10:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang Q, Trevino LS, Lee Yean Wong R, et al. Reprogramming of the epigenome by MLL1 links early-life environmental exposures to prostate cancer risk. Mol Endocrinol. 2016;me20151310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu H, Cheng EH, Hsieh JJ. Bimodal degradation of MLL by SCFSkp2 and APCCdc20 assures cell cycle execution: a critical regulatory circuit lost in leukemogenic MLL fusions. Genes Dev. 2007;21:2385–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hsieh JJ, Cheng EH, Korsmeyer SJ. Taspase1: a threonine aspartase required for cleavage of MLL and proper HOX gene expression. Cell. 2003;115:293–303. [DOI] [PubMed] [Google Scholar]
- 58. Tang WY, Newbold R, Mardilovich K, et al. Persistent hypomethylation in the promoter of nucleosomal binding protein 1 (Nsbp1) correlates with overexpression of Nsbp1 in mouse uteri neonatally exposed to diethylstilbestrol or genistein. Endocrinology. 2008;149:5922–5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li S, Washburn KA, Moore R, et al. Developmental exposure to diethylstilbestrol elicits demethylation of estrogen-responsive lactoferrin gene in mouse uterus. Cancer Res. 1997;57:4356–4359. [PubMed] [Google Scholar]
- 60. Xin F, Susiarjo M, Bartolomei MS. Multigenerational and transgenerational effects of endocrine disrupting chemicals: a role for altered epigenetic regulation? Semin Cell Dev Biol. 2015;43:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van Otterdijk SD, Michels KB. Transgenerational epigenetic inheritance in mammals: how good is the evidence? FASEB J. 2016;30:2457–2465. [DOI] [PubMed] [Google Scholar]
- 62. Skinner MK. Endocrine disruptors in 2015: epigenetic transgenerational inheritance. Nat Rev Endocrinol. 2016;12:68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Daxinger L, Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat Rev Genet. 2012;13:153–162. [DOI] [PubMed] [Google Scholar]
- 64. Arthur W. The concept of developmental reprogramming and the quest for an inclusive theory of evolutionary mechanisms. Evol Dev. 2000;2:49–57. [DOI] [PubMed] [Google Scholar]
- 65. Bromer JG, Wu J, Zhou Y, Taylor HS. Hypermethylation of homeobox A10 by in utero diethylstilbestrol exposure: an epigenetic mechanism for altered developmental programming. Endocrinology. 2009;150:3376–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li S, Hansman R, Newbold R, Davis B, McLachlan JA, Barrett JC. Neonatal diethylstilbestrol exposure induces persistent elevation of c-fos expression and hypomethylation in its exon-4 in mouse uterus. Mol Carcinog. 2003;38:78–84. [DOI] [PubMed] [Google Scholar]
- 67. Li Y, Hamilton KJ, Lai AY, et al. Diethylstilbestrol (DES)-stimulated hormonal toxicity is mediated by ERα alteration of target gene methylation patterns and epigenetic modifiers (DNMT3A, MBD2, and HDAC2) in the mouse seminal vesicle. Environ Health Perspect. 2014;122:262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stouder C, Paoloni-Giacobino A. Transgenerational effects of the endocrine disruptor vinclozolin on the methylation pattern of imprinted genes in the mouse sperm. Reproduction. 2010;139:373–379. [DOI] [PubMed] [Google Scholar]
- 69. Stouder C, Paoloni-Giacobino A. Specific transgenerational imprinting effects of the endocrine disruptor methoxychlor on male gametes. Reproduction. 2011;141:207–216. [DOI] [PubMed] [Google Scholar]
- 70. Zama AM, Uzumcu M. Fetal and neonatal exposure to the endocrine disruptor methoxychlor causes epigenetic alterations in adult ovarian genes. Endocrinology. 2009;150:4681–4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Prados J, Stenz L, Somm E, Stouder C, Dayer A, Paoloni-Giacobino A. Prenatal exposure to DEHP affects spermatogenesis and sperm DNA methylation in a strain-dependent manner. PLoS One. 2015;10:e0132136. [DOI] [PMC free article] [PubMed] [Google Scholar]