Abstract
Within the past few decades, the concept of endocrine-disrupting chemicals (EDCs) has risen from a position of total obscurity to become a focus of dialogue, debate, and concern among scientists, physicians, regulators, and the public. The emergence and development of this field of study has not always followed a smooth path, and researchers continue to wrestle with questions about the low-dose effects and nonmonotonic dose responses seen with EDCs, their biological mechanisms of action, the true pervasiveness of these chemicals in our environment and in our bodies, and the extent of their effects on human and wildlife health. This review chronicles the development of the unique, multidisciplinary field of endocrine disruption, highlighting what we have learned about the threat of EDCs and lessons that could be relevant to other fields. It also offers perspectives on the future of the field and opportunities to better protect human health.
Over the past half-century, the concept of endocrine-disrupting chemicals (EDCs) has risen from total obscurity to become nearly a household term. A 2012 Endocrine Society statement defined endocrine disruptors as “an exogenous chemical, or mixture of chemicals, that can interfere with any aspect of hormone action” (1). These chemicals can bind to the body's endocrine receptors to activate, block, or alter natural hormone synthesis and degradation by a plethora of mechanisms resulting in “false” or abnormal hormonal signals that can increase or inhibit normal endocrine functioning (Figure 1).
Figure 1.
Other EDC mechanisms of action may include disruption of hormone synthesis, impairment of cell signaling, and other effects.
Scientific understanding of EDCs has undergone a remarkable evolution. In 1958, Roy Hertz presaged the idea that certain chemicals, then used in livestock feedlots, could find their way into people's bodies and mimic the activity of hormones (2). Little came of the idea until the 1970s, when physicians and researchers began to link certain chemicals with rare cancers and reproductive effects in humans and wildlife. Unfortunately, as our awareness of EDCs rose, so did the ubiquity of anthropogenic EDCs in our environment. Today, there are nearly 1000 chemicals reported to have endocrine effects (3). The aforementioned number will almost certainly rise as thousands of new chemicals enter the marketplace each year and the vast majority are developed with little to no toxicological testing that would enable the detection of potential endocrine disruption. Studies conducted to examine EDC load have found EDCs in every individual tested and in ecosystems at the far corners of the Earth (4, 5).
The prevalence of EDCs in our environment and our bodies represents a significant global public health challenge. The endocrine system plays a central role in all vertebrates and regulates such critical biological functions as metabolism, development, reproduction, and behavior. Epidemiological studies link EDCs with reproductive effects, neurobehavioral and neurodevelopmental changes, metabolic syndrome, bone disorders, immune disorders, and cancers in humans (6). Animal studies show associations with many additional health effects, including asthma, learning and behavioral problems, early puberty, infertility, breast and prostate cancer, Parkinson's disease, obesity, and other diseases (4, 7, 8). The most well-studied EDCs include diethylstilbestrol (DES), dioxins, and other chlorinated hydrocarbons such as dichlorodiphenyltrichloroethane (DDT) and polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), phthalates, and bisphenol A (BPA) (see table 1 below). These and other known EDCs are, or have been, abundant in consumer products and are used frequently in industrial, agricultural, and pharmaceutical applications. Even chemicals that are no longer manufactured can persist in the environment or be created as a byproduct when other materials are burned (9).
The study of EDCs is perhaps best described not as a single field but as an interdisciplinary approach to determining how factors influence the biology of living organisms through endocrine-related effects. As an area of scientific focus, it is, and has always been, multidisciplinary to its core. As such, a historical review of the field's development offers a case study of the incredible advances that can emerge when scientists reach beyond traditional disciplinary boundaries to spark new insights, approaches, and discoveries.
In this article, we trace the key events that propelled the field forward, the challenges that at times slowed its development, and the adaptations and innovations made along the way. We also look toward the future to present a vision for EDC research, for the development and testing of chemicals to support a healthier and more sustainable world, and for evolving remediation strategies to address those EDCs that society will likely continue to use because of their associated benefits.
Laying the Groundwork: The Early Days
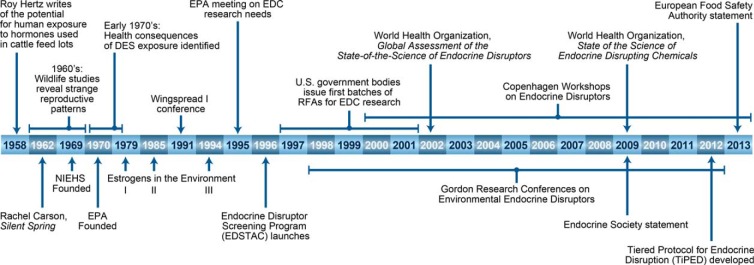
The field of endocrine disruption is in part a scientific offshoot within the larger context of the environmental movement that swept across the United States in the 1960s and 1970s. Rachel Carson's 1962 book Silent Spring was instrumental in awakening the scientific community and the public to the idea that the chemicals manufactured, used, and discarded as part of the inexorable march of human civilization could, and were, causing harm to ecosystems and human health (10). A few years later, the United States government established the National Institute of Environmental Health Sciences (NIEHS) (1966) to study how the environment affects human health and the Environmental Protection Agency (EPA) (1970) to implement regulations to protect human health and the environment. Figure 2 outlines key milestones and seminal studies in EDC research.
Figure 2.
Milestones in the development of the EDC field.
During the same period, ecologists began noticing unexpected patterns in animals, providing the first clues that certain chemicals were causing damage through endocrine disruption. In the Great Lakes, domesticated mink virtually stopped producing pups (11, 12) and herring gull chicks were dying in their eggs (13). In Florida's Lake Apopka, alligators began dying off, with many males afflicted with physically disabled genitalia (14, 15). In England, scientists found fish with severe reproductive abnormalities; some had testes containing eggs, and some males were found to express the estrogen-induced yolk protein vitellogenin (16–18). These cases, and the larger pattern they formed, were significant because the health effects were related to reproduction, not cancer, a counterpoint to the prevailing focus that environmental contaminants were of concern because of their potential carcinogenicity.
Alarm over these observations began spreading beyond the field of ecology. If chemicals could have such effects in wildlife, what might they do in humans? In the early 1970s a series of medical tragedies involving DES provided clues. Designed as an artificial estrogen and prescribed from 1940–1971 to millions of women during pregnancy to reduce miscarriage, DES was later shown to have serious health consequences, such as unusual cancers and reproductive system malformations, for those exposed in utero. Animal studies confirmed DES's activity as a transplacental carcinogen (19). Many of the changes seen in prenatally treated mice were also observed in women who had been exposed to DES in utero (20); in addition to the signal lesion of vaginal clear cell adenocarcinoma seen in women, humans and mice exposed prenatally to DES also had oviduct malformations, ovarian cysts, and histopathological changes in the fallopian tubes (20).
The discovery of DES's tragic legacy was the first time doctors and scientists appreciated the potential for chemicals to cause not only physical deformities that are obvious at birth but also more subtle health effects that emerge many years later (21–23). Given its mission to study environmental factors affecting human health, in 1979 the NIEHS held its first Estrogens in the Environment meeting to begin piecing together the larger picture of hormone-mimics and their health effects. At the time, there were few documented reports of human health effects from xenoestrogens, although researchers had raised concerns about environmental contamination from oral contraceptives, the use of which had grown tremendously in the years preceding the meeting. Moreover, the use of DES in the livestock industry raised concern that people could be exposed to it environmentally as well as pharmaceutically. The meeting focused on identifying the properties and diversity of environmental estrogens (24). One particularly valuable body of work was a series of experiments by Robert L. Metcalf that evaluated the fate of environmental contaminates under controlled conditions in model ecosystems. Using these model ecosystems to test the environmental effects of several chemicals now recognized as EDCs, Metcalf's work demonstrated how chemicals bioaccumulate and biodegrade in living organisms (24). A second Estrogens in the Environment meeting, held in 1985, highlighted the effect of environmental estrogens on puberty in young children, focusing on a mysterious pattern of precocious breast development in girls in Puerto Rico. The meeting examined the biological actions of estrogen exposure, exploring potential links with hypospadias and cryptorchidism, reduced sperm counts, testicular cancer, and other conditions (25).
Throughout the 1970s and 1980s, all of these threads, the growing awareness of harmful environmental chemicals, the abnormal ecological patterns, the DES experience, and the increasing focus on hormone-like chemicals, began to converge. Scientists from a variety of fields convened at the Wingspread Conference Center in Racine, Wisconsin in July 1991. Wingspread, as the meeting came to be called, proved to be a key turning point in the development of the field of endocrine disruption; indeed, it was there that the terms “endocrine disruption” and “endocrine disruptors” were coined. A consensus statement by the meeting participants began with an unequivocal claim: “We are certain of the following: A large number of man-made chemicals that have been released into the environment, as well as a few natural ones, have the potential to disrupt the endocrine system of animals, including humans” (26).
Wingspread attendees foresaw that some areas of commonly held scientific doctrine would need to be revisited to account for the patterns they were seeing in EDCs. No longer could hormone receptors be considered specific to natural hormones, contrary to the view of hormones as a single “key” for the receptor “lock,” it became apparent that a plethora of environmental hormone mimics could activate (or block) the same lock (27). No longer could the “cancer paradigm” of toxicity testing, which assumed carcinogenicity as the primary threat of concern, go unquestioned. And no longer could physicians assume that a baby apparently healthy at birth was indeed unharmed by substances it was exposed to during gestation. In short, the meeting provided the inspiration and framework for the remarkable research that followed.
Mounting Evidence Fuels Concern and Debate
During the 1990s and into the early 2000s, a series of provocative studies drew into greater focus the unique and troubling properties of EDCs. In turtles, exposure to estrogen during a specific gestational window was shown to influence the sex of the offspring (28–30); later, certain PCBs were shown to have similar effects (31). In Florida's lakes, environmental exposures continued to cause deformations in alligators' genitalia (32, 33). With the development of in vitro screens (34), the list of EDCs increased rapidly from a few pesticides (such as DDT, chlordecone, and methoxychlor) and industrial chemicals (such as PCB congeners), as new compounds with estrogenic activity were found among plastics (35–37), disinfectants, and personal care products (38). Studies began to suggest that EDCs could cause health effects even at extremely low doses and show nonmonotonic dose response (NMDR) curves; in an examination of the effects of DES, low doses were shown to stimulate prostate growth, whereas high doses had the opposite effect (22, 39).
As the evidence accumulated, researchers across the spectrum of endocrinology, wildlife ecology, toxicology, and other fields continued to gather frequently to puzzle over their findings and work toward consensus on the larger picture of EDCs in the environment. The third Estrogens in the Environment meeting, held in 1994, explored effects in wildlife and drew linkages between estrogen exposures and human diseases (40). The human connection began attracting attention when Niels Skakkebaek's studies associated falling sperm counts with environmental exposures in Scandinavian men over a 50-year period (41), leading to a major international review of environmental influences on male health (42). In 1995 and 1996 the EPA held 2 international meetings to assess what was known about EDCs and identify research needs (43). A series of workshops coorganized by the EPA, the Chemical Manufacturer's Association, and the World Wildlife Fund generated deeper discussions of the pervasiveness of the EDC challenge and resulted in several publications on EDC effects in fish, mammals, and other animals. In 1996, the United States Congress included EDC-related research mandates in the Food Quality Protection Act (44) and an amendment to the Safe Water Drinking Act (45).
In the mid-1990s, Congress charged EPA with assessing the hormonal activity of more than 70 000 compounds; in response, a federal advisory committee, the Endocrine Disruptor Screening and Testing Advisory Committee, was convened in 1996 and charged with making recommendations to the EPA on how to develop an endocrine disruptor screening and testing program (48). Based on the Endocrine Disruptor Screening and Testing Advisory Committee recommendations and the results of a much-delayed and controversial 1999 National Research Council report (49), the EPA developed a 3-tiered Endocrine Disruptor Screening Program geared at testing the estrogen, androgen, and thyroid axis. The screening program continues to evolve today (50) and has led some of the leading researchers in EDCs and green chemistry to develop their own schemes to assist chemists developing new and replacement chemicals (51).
EDCs were also attracting interest from researchers and policy makers abroad. In Japan, a batch of rice oil contaminated with PCBs in 1968 sickened thousands, and a similar event occurred in Taiwan in 1979. Research later revealed that women exposed to PCBs in these episodes were more likely to have babies with low birth weight and delays in neurological development (52). In the wake of these events, Japan became one of the first nations to address the issue of EDCs on a national level. In 1997 Japan initiated an “Exogenous Endocrine Disrupting Chemical Task Force” to collect, review, and organize scientific information on EDCs. In 1998 Japan's Environment Agency started the Strategic Program on Environmental Endocrine Disruptors (SPEED '98) initiative, which focused on environmental monitoring of suspected EDCs in river water, sediment, air, foods, and wildlife (53). The Japanese Ministry of the Environment sponsored a series of 10 International EDC Symposia sponsored from 1998 to 2007. Throughout this period and to the present day, Japan and Japanese scientists have continued to play an active role internationally in identifying research needs, establishing EDC testing methods, conducting research in animal models, and advancing risk assessment.
EDCs were also attracting attention in Europe during this period. In 1996, researchers convened at the first European Workshop on the Impact of Endocrine Disrupters on Human Health and Wildlife in Weybridge, United Kingdom. A follow-up workshop, Weybridge+10, was held in 2006. In the intervening decade, the European Union allocated substantial funding toward research on the effects of EDCs on aquatic wildlife, birds, mammals, and humans, as well as on mechanisms of action and a variety of specific health endpoints (54). A 2012 European Environment Agency report summarizes EDC research progress from 1996 to 2011 (55). The report reinforces the assertion that endocrine disruption is “a real phenomenon likely affecting both human and wildlife populations globally,” identifies areas for further research, and invokes the precautionary principle to suggest limiting exposure to EDCs even before obtaining full scientific knowledge of them.
By 2000, the research had demonstrated that EDCs can interfere with biological processes by mimicking hormones, activating or blocking the body's hormone receptors, disrupting the synthesis of hormones, or altering their degradation. EDCs had been linked with a plethora of cancers and reproductive, metabolic, and other health effects. Researchers were also finding evidence that many human diseases can have origins stretching back to early development; the theoretical underpinnings of this concept were elaborated by the ecological developmental biology discipline, a field founded on the fact that the environment codetermines the phenotype (56). Although EDCs are not the only exposure of concern during early development, the endocrine disruption field and the field now known as Developmental Origins of Health and Disease (DOHaD) matured in tandem and benefitted from their areas of synergy. Similarly, connections emerged between the EDC field and the budding field of epigenetics.
Throughout this period, studies began to demonstrate that EDCs such as DES, DDT, and BPA could have effects even at very low doses (57–61). Hormones operate in the body at extremely low levels; it seemed logical that chemicals mimicking hormones can potentially interfere with the body's natural systems even at low doses. Still, such findings in EDC research were often met with skepticism, generating substantial debate about whether the results were real and what they could mean for chemical risk assessment. As researchers began testing the effects of EDCs at lower doses than had been studied before, they found unusual dose response curves, in which chemicals showed health effects at extremely low doses, as well as high doses, while showing little effect at midlevel doses (62). Indeed, nonmonotonicity is a common finding among natural hormones (63, 64), but toxicologists are trained to view dose responses in a linear fashion, from low dose to high, and such NMDR curves were widely ignored by toxicologists. Conversely, experiments using radiation, vitamins, and chemicals helped build the case for the phenomenon known as hormesis, pioneered by Edward Calabrese, which postulates that some exposures are harmful at high doses, yet beneficial at low doses (65). Altogether, these results dealt a blow to the notion that the health effects of chemicals at low doses can be extrapolated linearly from effects seen at high doses (66).
EDC research fueled considerable controversy in the scientific, medical, and policy communities, particularly in areas where basic EDC research intersected with toxicology and risk assessment. Because they have multiple mechanisms of action, EDCs can act simultaneously at the level of the receptor, hormone synthesis, and hormone degradation. This can lead, for example, to estrogenic or antiandrogenic effects, sometimes creating integrated estrogenic signals not predicted by studying each action alone. Further complicating research, compounds that alter thyroid signaling can affect the actions of other hormones or EDCs. Although this level of complexity is common to the endocrine and neurological systems, it fits poorly into the frameworks of risk assessment and hazard management, which largely rely on calculating a threshold exposure level below which can be considered “safe.” If EDCs interact like hormones, the most sensitive endpoint can change depending on the endocrine active compounds present and even their pattern of exposure due to EDCs' low-dose effects and NMDR curves. Toxicological research focusing on high doses, such as occupational exposures, is not particularly relevant to typical (low) EDC exposure levels. Finally, the long time period between early exposures and the development of disease later in life makes it challenging to trace morbidity due to EDC exposure; this pattern is further complicated by the potential effects of developmental “windows of susceptibility,” when any endocrine perturbation can have important effects.
A Fundamentally Multidisciplinary Endeavor
The multidisciplinary nature of endocrine disruptor research, which has been a core element of the field from its earliest inception, is at once its greatest asset and its greatest weakness. The field has benefitted enormously from serendipitous connections and synergies among disparate fields. Despite its focus on the impacts of chemicals on living systems, EDC research did not grow from toxicology as might have been expected but rather emerged at the intersections of many other fields in which researchers were noticing EDC effects. For example, in the late 1980s, while investigating the estrogen sensitivity of human breast cells in culture conditions, the Soto-Sonnenschein laboratory accidentally found that estrogenic activity leached out of plastic centrifuge tubes from the compound p-nonylphenol (35). The laboratory work of Lou Guillette and Earl Gray on reproductive abnormalities in wildlife (67) translated to Shanna Swan's approach to assessing antiandrogens in children (68). Similarly, Lou Guillette drew upon the work and advice of Niels Skakkebaek, Howard Bern, and Michael J. Mac to identify target genes to shed light on reproductive abnormalities in alligators. These are only a few examples of how cross-disciplinary connections have advanced EDC research.
On the other hand, the cross-disciplinary interaction has at times led to miscommunication and misunderstanding. In particular, toxicologists and endocrinologists, both central to the investigation and interpretation of endocrine disruption, have frequently fallen into misunderstanding, often because they simply do not speak each other's language or fully grasp the other field's framework for decision making. In toxicology, generally the goal is to establish a threshold dose demarking safe vs unsafe levels of exposure. From an endocrinologist's perspective, the health effects of a certain dose might vary depending on the hormones affected, age, stage of development, and other factors; during certain windows of susceptibility; any dose could have an effect, whereas even a high dose at other times during a lifespan might have little or no effect. In addition to the different approaches of toxicology and endocrinology, EDC researchers have also run into clashes with chemical manufacturers over principles for chemical development and toxicity testing. Translating EDC findings into the frameworks of medicine and policy has also proven difficult throughout the field's history (69).
EDC Spotlight: BPA
Perhaps no EDC has been more widely used or drawn more attention than BPA. British medical researcher Edward Charles Dodds, while in pursuit of a synthetic estrogen, first identified BPA's estrogenic properties in the 1930s. Although BPA never found use as a drug, it did find its way into virtually every food and water container by the late 1970s, and today is one of the most common industrial chemicals produced worldwide (70).
In the early 1990s, Stanford endocrinologists determined that BPA leaching from plastic flasks was capable of activating estrogen-responsive breast cancer cells (36). Struck by the idea that chemicals in the environment could disrupt the endocrine system of humans and wildlife, researchers began investigating synthetic hormones in developing organisms. At the University of Missouri, Fred vom Saal discovered that mice exposed to low levels of BPA displayed estrogenic responses, including increased prostate weight (59). Researchers at Tufts University reported low-dose effects of BPA on mammary (60) reproductive glands (61), and the hypothalamus (71).
This research fueled debates about low-dose effects of EDCs, and BPA became both a poster child and a lightning rod of EDC research as the field emerged. Studies in animals pointed to an increasing number of potential health effects of BPA exposure, and the 2003–2004 National Health and Nutrition Examination Survey reported that 93% of people in the United States had measureable amounts of BPA in their urine (72). However, debates over data collection methodology and BPA's activity within the human body have made it difficult to achieve consensus about the current levels of human exposure to BPA and the health risks of those exposures.
Although BPA's use has not been formally limited by United States regulatory bodies, public attention to the chemical's endocrine activity has shifted consumer demands over the past decade, leading manufacturers to phase BPA out of products such as water bottles and children's products. In 2012, the Food and Drug Administration amended its food additive regulations to no longer provide for the use of polycarbonate resins in baby bottles and “sippy” cups because manufacturers no longer use polycarbonate resins in these products (73).
A Goal Coalesces
By the early 2000s, concern over endocrine disruptors had spread within the scientific community to chemistry and beyond to physicians, regulators, chemical manufacturers, and members of the public. During this period, a confluence of research findings, consensus documents, and attention to EDCs on the part of governments and the public transformed endocrine disruption from a somewhat controversial fringe science into a widely respected and influential field. Although the scientific and political debates surrounding EDCs were by no means settled, there emerged among the various stakeholders a shared goal to identify and address those endocrine disruptors that were posing a danger to human health.
Calls for more effective ways to identify EDCs, determine levels of exposure, and keep new EDCs from entering the marketplace gained traction. A seminal 2002 World Health Organization review outlined the state of the science in endocrine disruption, highlighting the mechanisms of action and health effects in animals and humans (74). The report identified exposure as the least understood aspect of EDCs, a knowledge gap reiterated ten years later in a follow-on 2012 report (6), which also concluded that EDCs had by then become so pervasive that there was no longer any pristine place left on the globe.
Several regular meeting series have provided a backbone for exchange and collaboration around EDCs for more than a decade. The Gordon Research Conferences on Environmental Endocrine Disruptors have brought researchers together every 2 years since 1998. These meetings provide researchers opportunities to regularly share results, exchange ideas, and identify future needs and directions. Another conference series, the Copenhagen Workshops on Endocrine Disrupters, has been an important scientific forum through 7 meetings held since its inception in 2000. The Environment and Hormones meeting series, held annually at Tulane University from 1999 to 2010, provided a forum for stimulating new ideas such as epigenetics, quorum sensing, environmental signaling, structural biology and systems science, and its accompanying website provides a clearinghouse of EDC information as well as teaching materials on the topic.
The first professional society to issue a policy statement on EDCs was the American Chemical Society, which released its first such statement in 2006. This action by American Chemical Society is a testament to the efforts on the part of the chemistry community to recognize the importance of facing and addressing the problem of EDCs (75). In 2009, The Endocrine Society issued a statement identifying EDCs as a top area of concern in the field of endocrinology; that statement proved to be a key milestone in lending legitimacy to the EDC field in the eyes of physicians and other scientists (7). Recent reviews and consensus documents, including a 2012 World Health Organization report and a 2013 statement by the European Food Safety Authority, have reinforced these messages (76).
As EDCs gained recognition, the research investments that ramped up in the late 1990s and early 2000s began to yield intriguing results and spark new areas of inquiry. Discussions surrounding the low-dose effects and NMDRs associated with EDCs that had been swirling since the National Toxicology Program's report on low dose effects of EDCs (77) came to the forefront in 2012. In March 2012, Vandenberg et al (64) published a summary of the weight of the evidence on these aspects of EDCs, concluding that “when nonmonotonic dose-response curves occur, the effects of low doses cannot be predicted by the effects observed at high doses. Thus, fundamental changes in chemical testing and safety determination are needed to protect human health.”
In September 2012, the Joint Research Centre of the European Union and NIEHS convened risk assessors, toxicologists, endocrinologists, chemists, and epidemiologists in Berlin, Germany, to consider whether the current state of knowledge about low-dose effects and NMDRs for EDCs was sufficient to warrant a reexamination of the ways in which chemicals were tested for endocrine disrupting properties and how risk to human health was managed. Although participants did not reach consensus on these issues, the meeting generated lists of experimental design issues related to low-dose effects and NMDRs, data gaps and needs, and suggestions for improving risk assessment (78). In June 2013, the EPA released a draft state-of-the-science evaluation on NMDRs, reaching the conclusion that although NMDRs do occur, the EPA's current testing approaches meet their goals and are “highly unlikely to mischaracterize a chemical that has the potential to adversely perturb the endocrine system due to an NMDR” (79). A National Research Council panel consisting of academic, government, and industry scientists reviewed the EPA report and raised several concerns about the approaches and methods of analysis used to evaluate low dose and NMDRs (80).
In parallel with these scientific developments, the early to mid-2000s saw a significant increase in attention to EDCs on the part of the public, increasing pressure on chemical manufacturers and regulators to respond to and address consumer concerns over EDCs in products. Advocacy and educational organizations have emerged with the goal of increasing public awareness of EDCs and other environmental contaminants; 2 such organizations are the Collaborative on Health and the Environment and the Endocrine Disruption Exchange. The nonprofit organization Environmental Health Sciences and its publication Environmental Health News are also important contributors to the public dialogue on EDCs.
The Current Outlook
It is now clear that some environmental substances contribute to the burden of disease by interfering with the human endocrine system and that they are powerful components of epigenetic modification of the genome (81, 82). For some chemicals, such as DES, DDT, PCBs, and dioxin, documented health effects have prompted regulatory action to restrict human exposure. Many other potential EDCs, including mixtures of chemicals and chemical exposures in combination with changing diet and stress, are still being studied and still being used in consumer products. Table 1 highlights several EDCs of concern.
Table 1.
Legacy Endocrine Dispurting Chemicals
| Compound | Use/Source | Disease Links | References |
|---|---|---|---|
| BPA | Plastics, thermal receipts | Breast and other cancers, metabolism, puberty, neurobehavioral | 83–86 |
| Phthalates | Plastics, fragrances | Low sperm count, metabolism, birth defects, asthma, neurobehavioral | 87, 88 |
| PCBs | Electrical coolant and other uses | Cancer, developmental issues | 89 |
| PBDEs | Flame retardants | Thyroid disruption, neurological issues | 90, 91 |
| Lead | Drinking water, paint, gasoline | Neurological issues, premature birth, kidney disorders | 92, 93 |
| Mercury | Burning coal, seafood | Neurological issues, diabetes | 94 |
| Dioxin | Formed in industrial processing | Cancers, sperm quality, fertility, neurobehavioral | 95, 96 |
| DDT/DDE/DDD | Pesticides | Cancers, developmental toxicity | 96 |
| Arsenic | Drinking water, animal feed, herbicides, fertilizers | Cancers, diabetes, immune suppression, neurodevelopment, cardiovascular disease | 97, 98 |
| Cadmium | Tobacco smoke, fertilizers | Cancers, reproductive issues | 99 |
| Atrazine | Herbicide | Alterations in pubertal development | 100 |
| Alkylphenols and p-Nonyl-phenol | Detergents, additives | Breast cancer | 101, 102 |
A movement has grown across the United States, Europe, Japan, and other countries, including developing countries, to identify EDCs and determine the degree of concern warranted by human exposure at current levels. Today's investigations are shedding light on chemicals, endpoints, and life stages not previously examined. The fields of epigenetics and DOHaD are providing potential framework for transgenerational effects, cumulative and combined effects, and windows of susceptibility. All of these areas of inquiry are beginning to answer important questions and raise new ones.
Intended EDCs and Unintended Consequences
Many EDCs have been produced and released into the environment by “accident,” that is, their developers and manufacturers did not intend or even know they were producing chemicals with endocrine-disrupting properties. Some chemicals, however, have been intentionally designed to disrupt the endocrine system. Two examples of such chemicals offer salient lessons about the nature of EDCs and unintended consequences.
One carefully designed EDC is 17β-ethinylestradiol (EE2), the active ingredient in most birth-control pills. By disrupting the natural hormonal fluctuations that lead to ovulation, EE2 is used to deliberately impair a woman's fertility, at least temporarily. EE2 does its job at exceptionally low concentrations, 10–50 μg per pill depending on the brand, a striking demonstration of the ability for EDCs to cause significant effects even in low quantities. Although its widespread use has had benefits for public health, EE2 also raises a troubling issue: the ability for EDCs designed to benefit humans to cause unintended harm to wildlife. A portion of the EE2 entering a woman's body through birth control pills is excreted in her urine and then carried through sewage treatment plants into bodies of water. Although only a tiny fraction of the EE2 in birth control pills ends up in bodies of water, the sweeping use of such pills allows EE2 to accumulate in concentrations capable of feminizing male fish, making EE2 a top EDC of concern for the public and a significant threat from an ecological perspective (103, 104).
Conversely, some EDCs have been developed as pesticides intended to harm wildlife for the benefit of crop producers. In a disturbing ricochet, some of these chemicals in turn cause endocrine disruption and adverse health effects in people. The pesticide DDT, for example, contributes to early onset of puberty and menopause in humans as well as a number of critical effects in pregnant and nursing mothers (105–107). Atrazine, one of the most widely used herbicides in the United States, has been linked to longer cycles, missed periods, and abnormal bleeding in women (108). Such cases offer a striking reminder that even purposefully engineered chemicals aimed at benefiting humans can also harbor potential for harm. This unpredictability is inherent to biology; evolution is not an engineer but a tinkerer, as F. Jacob remarked (109). It is not surprising that a chemical designed with a well-defined purpose will have unexpected effects, for we do not know the evolutionary history of every protein, cell or structure, and thus, cannot imagine these secondary effects until they materialize.
Transgenerational Effects and Epigenetics
Some chemicals, including some EDCs, have the potential to cause health effects in the offspring of exposed individuals through environmentally induced epigenetic modifications. Experiments by Michael Skinner and coworkers demonstrate that male rats whose ancestors were exposed to the fungicide (and EDC) Vinclozolin are less attractive to females and show accelerated onset of cancer, prostate disease, kidney disease, and immune defects (110, 111). Other studies have shown that high doses of a variety of EDCs could also elicit transgenerational effects in third-generation offspring (81, 112, 113).
The mechanisms by which environmental exposures cause transgenerational effects are unclear. One hypothesis leans toward epigenetic inheritance patterns, which involve chemical modifications to the DNA (DNA methylation), histone modifications, and noncoding RNAs, rather than mutations of the DNA sequence itself. Epigenetic marks carried over from parents are typically wiped clean during events that happen early in embryonic development. Researchers have found that transgenerational effects can result from chemical dosing at precise windows in fetal development, specifically, at the time of sex determination, which occurs around embryonic days 41–44 in humans (111).
Together, this emerging body of research suggests that exposure to EDCs could have consequences not only for our own health and for that of our children, but also for the health of generations to come.
Key Scientific Questions for EDC Researchers
As we delve ever deeper into the nature and behavior of EDCs, new questions have arisen while old ones persist. Top-priority areas of inquiry for future EDC research include the following:
Basic biology and chemistry of EDCs. What are the properties that allow a chemical to mimic hormones in the body? What are their mechanisms of action, particularly regarding latent effects? Can known EDCs currently considered “estrogens” or “antiandrogens” also affect other pathways in different ways?
Exposure and biomonitoring. To what extent are humans and wildlife exposed to EDCs, and how persistent are these chemicals in organisms' bodies? Serial measurements and improved sampling methods are needed to expand on what has been learned about human exposures through past studies, which have primarily relied on intermittent urine sampling (114, 115). There is also need to examine exposures across the lifespan, in different geographic locations, and across socioeconomic and ethnic status, as well as to continue examining levels and impacts of exposure to those EDCs (such as EE2) that humans purposefully create and release into the environment.
Endpoints. Given the importance of the endocrine system during development, are there potential health effects of EDCs that have yet to be investigated? For example, the relationships between EDCs and the nervous system, cardiovascular system, bone development and disease, obesity, diabetes, and metabolic syndrome warrant further exploration (116). In addition, there is a need for more research focusing on disease syndromes, or the contributions of EDCs to multiple diseases at once.
Windows of susceptibility. What are the key periods when exposure to EDCs might cause the most damage? Studies have suggested preconception, gestation, infancy, puberty, and menopause are critical periods, but there remains much to learn about other potential sensitive windows, as well as how exposures during these windows contribute to health effects.
DOHaD. Can predictive biomarkers be identified that would allow the tracing of EDC health effects in a shorter period of time than would be required for epidemiological studies (117)?
Developmental effects. How do environmental factors influence phenotypes? Direct effects on gene expression, epigenetic effects, and mechanisms that bypass genes, such as the effect of alcohol on cell adhesion (56) or physical forces, may all play a role. Epigenetics has attracted the most scientific attention with only minimal attention given to cell-cell interactions, although cell-cell interactions are crucial to determining the phenotypic changes observed during fetal exposure to certain EDCs.
Mixtures. How do EDCs interact with other toxins to influence health and disease? Are there chemicals that would have no effect unless low-dose exposures are combined with exposures to other substances?
Resilience. Why are some organisms, tissues, and time periods more resilient to EDC exposure than others?
Translation of animal research. Understanding of how EDC effects in animal models and wildlife translate to human exposure to EDCs is limited. Despite comparable mechanisms, and important differences, new studies are needed to aid in translating laboratory animals and wildlife data to benefit humans. We are bolstered by the incredible similarities seen in DES-exposed laboratory animals and humans.
Systems biology. A systemic approach would be useful to understand how exposure to an EDC results in an altered phenotype, a process that implies complex interactions across multiple levels of biological organization, as well as to illuminate how EDC exposures, acting across multiple levels of biological organization (cellular, tissue, organs) interact with drugs, nutrition, stress, and infection to contribute to dysfunction or disease.
EDC identification and detection. Underlying all of these questions is the need to continue improving methods for identifying EDCs and developing and testing new chemicals to preventively reduce their release into the environment. A potential strategy for identifying new EDCs is to test for chemicals and metabolites in the urine, cord blood, and other tissues of animals with known EDC-associated abnormalities, such as reduced anogenital distance.
Toxicity Testing and Research and Development in the Context of EDCs
Ultimately, the overarching goal of EDC research is to protect living things from the adverse effects of anthropogenic chemicals and especially to reduce their burden of disease. In the process, fundamental lessons about biology can be incorporated in order to deepen our understanding of how organisms operate and change.
Nearly 1000 chemicals have been classified as EDCs; this is a small fraction of the 80 000 known chemicals in our environment. The need to account for low-dose effects and NMDRs in toxicity testing has gained traction in recent years. For example, the CLARITY-BPA study, a collaboration that brings academic researchers and regulators together to generate research protocols for a multidose guideline-compliant 2-year chronic toxicity study of BPA in rodents (118), offers a potential new model for filling knowledge gaps, enhancing quality control, informing chemical risk assessment, and identifying new methods or endpoints for regulatory hazard assessments. In addition, the Organisation for Economic Co-operation and Development has substantially revised existing test guidelines (and created new ones) for the screening and testing for endocrine disruption in its Conceptual Frameworks issued in 2002 and 2012. Although not prescriptive, the framework provides guidance for detecting endocrine activity of chemicals using a 5-tier process (119). Nonchemical stressors such as infectious agents, diet, and psychosocial stress should be examined for their contribution to health effects associated with EDC exposures. Furthermore, new approaches are needed to examine the effects of mixtures of endocrine disruptors on disease susceptibility and etiology, because examination of 1 endocrine disruptor at a time is likely to underestimate the combined risk from simultaneous exposure to multiple endocrine disruptors (4, 5, 120).
Basic researchers and risk assessors operate from different frameworks but at heart share a common purpose. Greater dialogue and collaboration would enhance and increase the impact of those conducting basic research on EDCs and the risk assessors who interpret scientific evidence to inform decision making. Researchers, typically in laboratories in academic institutions, should be incentivized to design studies in such a way that their findings and study protocols will be translatable and valid for (typically government-based) risk assessors and regulators (121). At the same time, it is important that academic researchers remain independent and continue to investigate the biological underpinnings of health and disease. Each field has a critical role to play in advancing knowledge and protecting human health, and cultivating a more synergistic relationship would move both in a more productive direction.
One of the most exciting and promising recent developments in the EDC field is the movement to “design endocrine disruption out of the next generation of chemicals” through green chemistry. Taking steps to avoid creating new EDCs and releasing them into the environment can save money, time, and even lives. A 2012 article presented the Tiered Protocol for Endocrine Disruption (TiPED), which offers 5 levels of testing that can be employed during chemical development to screen for potential endocrine disruption (51). Although TiPED deployment is currently limited, the framework is a significant step forward and efforts are underway to scale TiPED up for broader application.
Ultimately, the success of TiPED, or alternative strategies to prevent new EDCs from being released into the environment, will require interdisciplinary communication, education, and public discourse. It is essential to recruit chemical manufacturers and the broader public to a shared goal of preventing new EDCs from entering the marketplace and the environment. With the public on board, there could be great economic advantages for chemical manufacturers who employ preventive measures such as those outlined in TiPED, because it would allow companies to offer assurances to consumers that their products are designed with health and safety in mind, potentially giving them a competitive advantage. In this way, chemical manufacturers, regulators, researchers, and the public can work together to keep new EDCs from entering our environment.
Case Study in Green Chemistry: TAML Activators
The removal of EDCs from water effluents is a paramount sustainability challenge. Currently, the best available technologies are ozone and granulated activated carbon; but both are costly and energy intensive. In order to remedy this obstacle, “TAML activators,” developed by Terry Collins and colleagues at Carnegie Mellon University's Institute for Green Science, offers a case example of green chemistry in action.
The TAML activator program was launched in 1980 initially to produce a safe peroxide-based water disinfection technology. Seeking a disinfection approach that could replace chlorine (which produces carcinogenic byproducts), researchers turned to nature for solutions that could kill bacteria by oxidizing vital biochemical components sustainably at low cost, and without producing harmful byproducts. For more than 30 years, the research program has developed and improved TAML activators, essentially by miniaturizing replicas of naturally occurring peroxidase enzymes (which activate hydrogen peroxide to oxidize chemicals throughout the natural world).
Starting in the late 1990s, endocrine disruption became a major focus of TAML activator design and development. A primary goal was to develop technologies that could remove EDCs from water while ensuring that no new EDCs were introduced in the process. This requirement helped inform the development of the TiPED, and the first TiPED-inspired EDCs assays were performed through Institute for Green Science collaborations with Bruce Blumberg (122) and Robert Tanguay (103). TAML activator/peroxide processes are highly efficient, effective against pathogens, and have been shown to effectively degrade numerous pollutants including natural and synthetic estrogens, active pharmaceutical ingredients, polychlorophenols, and many of the other leading contaminants of concern in the water treatment industry. Current development efforts are focused on lowering costs and translating TAML technologies into real-world applications.
Lessons From the EDC Experience
The development of the endocrine disruptor field offers lessons that can be informative to other fields of science, as well as to future challenges in toxicology and environmental health.
A primary lesson is that multidisciplinarity is key. From the beginning, EDC researchers have been extremely collaborative, affording attention and respect to not only what the findings show, but also what is unknown and what can be learned from other fields. Most researchers in the EDC field have found that the enormous challenge posed by endocrine disruption driven health scientists to focus on being part of a solution that is bigger than our individual selves. Multidisciplinarity also gives the EDC field the opportunity to take a true systems biology approach while integrating solutions from the field of chemistry as it moves forward. There is a strong need for creative, cross-platform work to address complex issues and determine how biological systems interact to influence health and disease and also to reduce and eliminate extant EDC exposures. This crosscutting work does not come easily in the context of the current system of scientific training that requires young scientists to burrow ever deeper into a single area of focus as they move from undergraduate work to master's level work to doctoral level work. A new generation of scientists with the skills and awareness to work across disciplines will enhance the EDC field and will accrue additional societal benefits that have been instrumental in making endocrine disruption the strong and diverse field it is today (123).
We also draw from the EDC experience a note of caution. There is no question that some key chemicals, notably DES in the case of endocrine disruption, have played a large role in bringing attention to previously unrecognized phenomena and sparked important research advances. But there lurks a danger that a few chemicals maintain prominence for the very reason that these substances have been well researched in the past. This effect, known as the Matthews principle (124), may represent a self-serving bias in science, which thrives upon high funding, citation rates and attention within specialized scientific groups. As the endocrine disruption field grows and new challenges emerge, researchers and funders may profit from promoting a balance between delving ever further into the effects of known agents and diversifying their goals by casting a wider net that includes less well-known environmental hazards (46). Both approaches could offer the chance for innovation and discovery. Unwisely limiting our investigations to only a handful of prominent chemicals may inadvertently undermine the overall goal of addressing the greatest threats to human health.
Another worthy lesson lays in the roles the public and the medical community have played in the development and acceptance of the EDC field. For instance, BPA was phased out of baby bottles in response to shifting consumer preferences and pressure from advocacy groups. As basic research reveals new potential hazards for human health, it is important to continue to translate that research into language and actions that are relevant to society and to medical practice. The dialogue between the scientific and medical communities should lead to greater clarity on the practical implications of basic research for doctors; conversely, having potential practical solutions in sight can help generate support for basic research as well as save on health care costs associated with diseases attributed to EDC exposers (47).
Finally, the story of the emergence and evolution of the endocrine disruption field has valuable lessons to offer for researchers, regulators, health care providers, and the public. Public policy and regulatory decision making have critical roles to play by drawing insight from research findings to advance the ultimate goal of improving health. Rarely is there such a thing as safe, rather, decisions about allowable levels of chemicals must be made based on the level of risk that is deemed acceptable vs that which requires action. To facilitate science-based decision making, there is a need for a broad-spectrum approach to support more effective communication among scientists, business leaders, regulators, and politicians. This communication should begin in the nation's graduate and business schools and extend throughout the culture and practice of decision making in these diverse fields.
Acknowledgments
We thank numerous colleagues for their help in preparing this manuscript. In particular we thank Heather Patisaul for her keen insights and Katherine Burns and Sylvia Hewitt for careful review of the manuscript. T.Coll. thanks Teresa Heinz-Kerry and the Heinz Endowments for support and for stalwart engagement in developing public understanding of endocrine disruption.
This work was supported by National Institutes of Health (NIH) Grants ES023254 and ES020662 and the National Science Foundation (NSF) Grant IOS-1051623 (to D.C.) and by the NIH Grant ES08314, and the Avon Foundation (to A.M.S. and C.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BPA
- bisphenol A
- DDT
- dichlorodiphenyltrichloroethane
- DES
- diethylstilbestrol
- DOHaD
- Developmental Origins of Health and Disease
- EDC
- endocrine-disrupting chemical
- EE2
- 17β-ethinylestradiol
- EPA
- Environmental Protection Agency
- NIEHS
- National Institute of Environmental Health Sciences
- NMDR
- nonmonotonic dose response
- PBDE
- polybrominated diphenyl ether
- PCB
- polychlorinated biphenyl
- TiPED
- Tiered Protocol for Endocrine Disruption.
References
- 1. Zoeller RT, Brown TR, Doan LL, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology. 2012;153(9):4097–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gassner FX, Reifenstein EC, Jr, Algeo JW, Mattox WE. Effects of hormones on growth, fattening, and meat production potential of livestock. Recent Prog Horm Res. 1958;14. [PubMed] [Google Scholar]
- 3. The Endocrine Disruptor Exchange. TEDX List of Potential Endocrine Disruptors. 2013. Available form http://www.endocrine-disruption.org/ Accessed July 22, 2016.
- 4. Bergman A, Heindel JJ, Kasten T, et al. The impact of endocrine disruption: a consensus statement on the state of the science. Environ Health Perspect. 2013;121(4):104–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kortenkamp A, Martin O, Faust M, et al. State of the Art Assessment of Endocrine Disrupters. European Commission: DG Environment; 2011. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization. State of the Science of Endocrine Disrupting Chemicals. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 7. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 2011;127(3–5):204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan JK, Wong MH. A review of environmental fate, body burdens, and human health risk assessment of PCDD/Fs at two typical electronic waste recycling sites in China. Sci Total Environ. 2013;463–464:1111–1123. [DOI] [PubMed] [Google Scholar]
- 10. Carson RL. Silent Spring. Anniversary Edition. New York, NY: Houghton Miffin Co; 1962. [Google Scholar]
- 11. Aulerich RJ, Ringer RK, Iwamoto S. Reproductive failure and mortality in mink fed on Great Lakes fish. J Reprod Fertil Suppl. 1973;19:365–376. [PubMed] [Google Scholar]
- 12. Aulerich RJ, Ringer RK. Current status of PCB toxicity to mink, and effect on their reproduction. Arch Environ Contam Toxicol. 1977;6(2–3):279–292. [DOI] [PubMed] [Google Scholar]
- 13. Gilbertson M, Reynolds LM. Hexachlorobenzene (HCB) in the eggs of common terns in Hamilton Harbour, ON. Bull Environ Contam Toxicol. 1972;7(6):371–373. [DOI] [PubMed] [Google Scholar]
- 14. Myers JP. Our Stolen Future: Are We Threatening Our Fertility, Intelligence and Survival? Penguin Books, New York, New York; 1997. [Google Scholar]
- 15. Semenza JC, Tolbert PE, Rubin CH, Guillette LJ, Jr, Jackson RJ. Reproductive toxins and alligator abnormalities at Lake Apopka, FL. Environ Health Perspect. 1997;105(10):1030–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jobling S, Beresford N, Nolan M, et al. Altered sexual maturation and gamete production in wild roach (Rutilus rutilus) living in rivers that receive treated sewage effluents. Biol Reprod. 2002;66(2):272–281. [DOI] [PubMed] [Google Scholar]
- 17. Tyler CR, Jobling S, Sumpter JP. Endocrine disruption in wildlife: a critical review of the evidence. Crit Rev Toxicol. 1998;28(4):319–361. [DOI] [PubMed] [Google Scholar]
- 18. Iguchi T, Ostrander PL, Mills KT, Bern HA. Vaginal abnormalities in ovariectomized BALB/cCrgl mice after neonatal exposure to different doses of diethylstilbestrol. Cancer Lett. 1988;43(3):207–214. [DOI] [PubMed] [Google Scholar]
- 19. McLachlan JA, Newbold RR, Bullock BC. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 1980;40(11):3988–3999. [PubMed] [Google Scholar]
- 20. Newbold RR, Bullock BC, McLachlan JA. Progressive proliferative changes in the oviduct of mice following developmental exposure to diethylstilbestrol. Teratog Carcinog Mutagen. 1985;5(6):473–480. [DOI] [PubMed] [Google Scholar]
- 21. McLachlan JA. Environmental signaling: what embryos and evolution teach us about endocrine disrupting chemicals. Endocr Rev. 2001;22(3):319–341. [DOI] [PubMed] [Google Scholar]
- 22. Bern HA. The fragile fetus. In: Chemically-Induced Alterations in Sexual and Functional Development: The Human/Wildlife Connection. Princeton, NJ: Princeton Scientific Pub Co; 1992. [Google Scholar]
- 23. McClachlan JA, Dixon RL. Transplacental toxicity of diethylstilbestrol: a special problem in safety evaluation. In: Mehlman MA, Shapiro RE, Blumenthal H, eds. Advances in Modern Toxicology. Vol Part 1 Washington, DC: Hemisphere Publishing Corp; 1976:423–448. [Google Scholar]
- 24. McLachlan JA. Estrogens and the Environment. New York, NY: Elsevier; 1980. [Google Scholar]
- 25. McLachlan JA. Estrogens and the Environment II. New York, NY: Elsevier; 1985. [Google Scholar]
- 26. Colborn T, Clement C. Chemically-Induced Alterations in Sexual and Functional Development: The Wildlife/Human Connection. Princeton, NJ: Princeton Scientific Publishing Co; 1992. [Google Scholar]
- 27. McLachlan JA. Functional toxicology: a new approach to detect biologically active xenobiotics. Environ Health Perspect. 1993;101(5):386–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bull JJ, Gutzke WH, Crews D. Sex reversal by estradiol in three reptilian orders. Gen Comp Endocrinol. 1988;70(3):425–428. [DOI] [PubMed] [Google Scholar]
- 29. Bergeron JM, Crews D, McLachlan JA. PCBs as environmental estrogens: turtle sex determination as a biomarker of environmental contamination. Environ Health Perspect. 1994;102(9):780–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crews D, Wibbels T, Gutzke WH. Action of sex steroid hormones on temperature-induced sex determination in the snapping turtle (Chelydra serpentina). Gen Comp Endocrinol. 1989;76(1):159–166. [DOI] [PubMed] [Google Scholar]
- 31. Gale RW, Bergeron JM, Willingham EJ, Crews D. Turtle sex determination assay: mass balance and responses to 2,3,7,8-tetrachlorodibenzo-p-dioxin and 3,3′,4,4′,5-pentachlorobiphenyl. Environ Toxicol Chem. 2002;21(11):2477–2482. [PubMed] [Google Scholar]
- 32. Guillette LJ, Jr, Gross TS, Masson GR, Matter JM, Percival HF, Woodward AR. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect. 1994;102(8):680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guillette LJ, Jr, Pickford DB, Crain DA, Rooney AA, Percival HF. Reduction in penis size and plasma testosterone concentrations in juvenile alligators living in a contaminated environment. Gen Comp Endocrinol. 1996;101(1):32–42. [DOI] [PubMed] [Google Scholar]
- 34. Soto AM, Maffini MV, Schaeberle CM, Sonnenschein C. Strengths and weaknesses of in vitro assays for estrogenic and androgenic activity. Best Pract Res Clin Endocrinol Metab. 2006;20(1):15–33. [DOI] [PubMed] [Google Scholar]
- 35. Soto AM, Justicia H, Wray JW, Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from “modified” polystyrene. Environ Health Perspect. 1991;92:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132(6):2279–2286. [DOI] [PubMed] [Google Scholar]
- 37. Jobling S, Reynolds T, White R, Parker MG, Sumpter JP. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect. 1995;103(6):582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schlumpf M, Cotton B, Conscience M, Haller V, Steinmann B, Lichtensteiger W. In vitro and in vivo estrogenicity of UV screens. Environ Health Perspect. 2001;109(3):239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. vom Saal FS, Timms BG, Montano MM, et al. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci USA. 1997;94(5):2056–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McLachlan JA, Korach KS. Symposium on estrogens in the environment, III. Environ Health Perspect. 1995;103(suppl 7):3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. Bmj. 1992;305(6854):609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toppari J, Larsen JC, Christiansen P, et al. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996;104(suppl 4):741–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kavlock RJ, Daston GP, DeRosa C, et al. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ Health Perspect. 1996;104(suppl 4):715–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Environmental Protection Agency. Food Quality Protection Act (FQPA) of 1996. 1996. Available from https://www.epa.gov/laws-regulations/summary-food-quality-protection-act Accessed July 22, 2016.
- 45. Environmental Protection Agency. Safe Drinking Water Act (SDWA). 1974. Available from http://water.epa.gov/lawsregs/rulesregs/sdwa/index.cfm Accessed September 9, 2013.
- 46. Guillette LJ., Jr Endocrine disrupting contaminants–beyond the dogma. Environ Health Perspect. 2006;114(suppl 1):9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Trasande L. Further limiting bisphenol a in food uses could provide health and economic benefits. Health Aff (Millwood). 2014;33(2):316–323. [DOI] [PubMed] [Google Scholar]
- 48. Environmental Protection Agency. Endocrine Disruptor Screening Program (EDSP). 1996. Available from https://www.epa.gov/endocrine-disruption/endocrine-disruptor-screening-program-edsp-overview Accessed July 22, 2016.
- 49. Committee on Hormonally Active Agents in the Environment; Commission on Life Sciences; Division on Earth and Life Studies; National Research Council. Hormonally Active Agents in the Environment. Washington, DC: The National Academies Press; 1999. [Google Scholar]
- 50. Juberg DR, Borghoff SJ, Becker RA, et al. t4 workshop report–lessons learned, challenges, and opportunities: the U.S. Endocrine Disruptor Screening Program. Altex. 2014;31(1):63–78. [DOI] [PubMed] [Google Scholar]
- 51. Schug TT, Abagyan R, Blumberg B, et al. Designing endocrine disruption out of the next generation of chemicals. Green Chem. 2013;15(1):181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aoki Y. Polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans as endocrine disrupters–what we have learned from Yusho disease. Environ Res. 2001;86(1):2–11. [DOI] [PubMed] [Google Scholar]
- 53. Japan Environment Agency. Strategic Programs on Environmental Endocrine Disruptors '98. Tokyo, Japan: Environmental Health Department, Ministry of Environment, Government of Japan; 1998. [Google Scholar]
- 54. Hecker M, Hollert H. Endocrine disruptor screening: regulatory perspectives and needs. Environ Sci Eur. 2011;23(1):1–14. [Google Scholar]
- 55. European Environment Agency. The Impacts of Endocrine Disrupters on Wildlife, People and Their Environments: The Weybridge+15 (1996–2011) Report. Luxembourg: Publications Office of the European Union; 2012. [Google Scholar]
- 56. Gilbert SF, Epel D. Ecological Developmental Biology: Integrating Epigenetics, Medicine, and Evolution. Underland, MA: Sinauer Associates; 2008. [Google Scholar]
- 57. Palanza P, Parmigiani S, Liu H, vom Saal FS. Prenatal exposure to low doses of the estrogenic chemicals diethylstilbestrol and o,p'-DDT alters aggressive behavior of male and female house mice. Pharmacol Biochem Behav. 1999;64(4):665–672. [DOI] [PubMed] [Google Scholar]
- 58. Palanza PL, Howdeshell KL, Parmigiani S, vom Saal FS. Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ Health Perspect. 2002;110(suppl 3):415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nagel SC, vom Saal FS, Thayer KA, Dhar MG, Boechler M, Welshons WV. Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ Health Perspect. 1997;105(1):70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Markey CM, Luque EH, Munoz De Toro M, Sonnenschein C, Soto AM. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol Reprod. 2001;65(4):1215–1223. [DOI] [PubMed] [Google Scholar]
- 61. Markey CM, Coombs MA, Sonnenschein C, Soto AM. Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evol Dev. 2003;5(1):67–75. [DOI] [PubMed] [Google Scholar]
- 62. Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect. 2003;111(8):994–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sonnenschein C, Olea N, Pasanen ME, Soto AM. Negative controls of cell proliferation: human prostate cancer cells and androgens. Cancer Res. 1989;49(13):3474–3481. [PubMed] [Google Scholar]
- 64. Vandenberg LN, Colborn T, Hayes TB, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33(3):378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Calabrese EJ, Baldwin LA. Defining hormesis. Hum Exp Toxicol. 2002;21(2):91–97. [DOI] [PubMed] [Google Scholar]
- 66. Melnick R, Lucier G, Wolfe M, et al. Summary of the National Toxicology Program's report of the endocrine disruptors low-dose peer review. Environ Health Perspect. 2002;110(4):427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gray LE, Ostby J, Furr J, et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7(3):248–264. [DOI] [PubMed] [Google Scholar]
- 68. Swan SH. Prenatal phthalate exposure and anogenital distance in male infants. Environ Health Perspect. 2006;114(2):A88–A89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Marty MS, Carney EW, Rowlands JC. Endocrine disruption: historical perspectives and its impact on the future of toxicology testing. Toxicol Sci. 2011;120(suppl 1):S93–S108. [DOI] [PubMed] [Google Scholar]
- 70. Vogel SA. The politics of plastics: the making and unmaking of bisphenol a “safety.” Am J Public Health. 2009;99(suppl 3):S559–S566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147(8):3681–3691. [DOI] [PubMed] [Google Scholar]
- 72. Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. U.S. Food and Drug Administration. Indirect food additives: polymers. In: CFR - Code of Federal Regulations. Title 21. Part 177 Silver Spring, MD: U.S. Food and Drug Administration; 2012. [Google Scholar]
- 74. International Programme on Chemical Safety. Global Assessment of the State-of-the-Science of Endocrine Disruptors. WHO/PCS/EDC/02.2 Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- 75. American Chemical Society. Statement on Testing for Endocrine Disruption. 2012. Available from http://www.acs.org/content/acs/en/policy/publicpolicies/promote/endocrinedisruptors.html Accessed July 22, 2016.
- 76. World Health Organization. State of the Science of Endocrine Disrupting Chemicals - 2012. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 77. National Toxicology Program. National Toxicology Program's Report of the Endocrine Disruptors Peer Review. Research Triangle Park, NC: National Toxicology Program, Department of Health and Human Services; 2001. [Google Scholar]
- 78. Beausoleil C, Ormsby JN, Gies A, et al. Low dose effects and non-monotonic dose responses for endocrine active chemicals: science to practice workshop: workshop summary. Chemosphere. 2013;93(6):847–856. [DOI] [PubMed] [Google Scholar]
- 79. Environmental Protection Agency. State-of-the-Science Non-Monotonic Dose Response Curve Report. 2013. Available from https://www.epa.gov/chemical-research/endocrine-disruption-research-testing-potential-low-dose-effects Accessed July 22, 2016.
- 80. Committee to Review EPA's State of the Science Paper on Nonmonotonic Dose Reponse; Board on Environmental Studies and Toxicology; Division on Earth and Life Studies; National Research Council. Review of the Environmental Protection Agency's State-of-the-Science Evaluation of Nonmonotonic Dose-Response Relationships as they Apply to Endocrine Disrupters. Washington, DC: The National Academies Press; 2014. [Google Scholar]
- 81. Manikkam M, Guerrero-Bosagna C, Tracey R, Haque MM, Skinner MK. Transgenerational actions of environmental compounds on reproductive disease and identification of epigenetic biomarkers of ancestral exposures. PLoS One. 2012;7(2):e31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Walker DM, Gore AC. Transgenerational neuroendocrine disruption of reproduction. Nat Rev Endocrinol. 2011;7(4):197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Prins GS, Hu WY, Shi GB, et al. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology. 2014;155(3):805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Valvi D, Casas M, Mendez MA, et al. Prenatal bisphenol a urine concentrations and early rapid growth and overweight risk in the offspring. Epidemiology. 2013;24(6):791–799. [DOI] [PubMed] [Google Scholar]
- 85. Acevedo N, Davis B, Schaeberle CM, Sonnenschein C, Soto AM. Perinatally administered bisphenol a as a potential mammary gland carcinogen in rats. Environ Health Perspect. 2013;121(9):1040–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol. 2013;42:132–155. [DOI] [PubMed] [Google Scholar]
- 87. Martino-Andrade AJ, Chahoud I. Reproductive toxicity of phthalate esters. Mol Nutr Food Res. 2010;54(1):148–157. [DOI] [PubMed] [Google Scholar]
- 88. Meeker JD, Sathyanarayana S, Swan SH. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos Trans R Soc Lond B Biol Sci. 2009;364(1526):2097–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ludewig G, Robertson LW. Polychlorinated biphenyls (PCBs) as initiating agents in hepatocellular carcinoma. Cancer Lett. 2012;2(12):00703–00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rice DC, Reeve EA, Herlihy A, Zoeller RT, Thompson WD, Markowski VP. Developmental delays and locomotor activity in the C57BL6/J mouse following neonatal exposure to the fully-brominated PBDE, decabromodiphenyl ether. Neurotoxicol Teratol. 2007;29(4):511–520. [DOI] [PubMed] [Google Scholar]
- 91. Czerska M, Zieliski M, Kamiska J, Ligocka D. Effects of polybrominated diphenyl ethers on thyroid hormone, neurodevelopment and fertility in rodents and humans. Int J Occup Med Environ Health. 2013;26(4):498–510. [DOI] [PubMed] [Google Scholar]
- 92. Lin CC, Chen YC, Su FC, et al. In utero exposure to environmental lead and manganese and neurodevelopment at 2 years of age. Environ Res. 2013;123:52–57. [DOI] [PubMed] [Google Scholar]
- 93. Polaska K, Jurewicz J, Hanke W. Review of current evidence on the impact of pesticides, polychlorinated biphenyls and selected metals on attention deficit / hyperactivity disorder in children. Int J Occup Med Environ Health. 2013;26(1):16–38. [DOI] [PubMed] [Google Scholar]
- 94. Cace IB, Milardovic A, Prpic I, et al. Relationship between the prenatal exposure to low-level of mercury and the size of a newborn's cerebellum. Med Hypotheses. 2011;76(4):514–516. [DOI] [PubMed] [Google Scholar]
- 95. Lin YS, Caffrey JL, Hsu PC, Chang MH, Faramawi MF, Lin JW. Environmental exposure to dioxin-like compounds and the mortality risk in the U.S. population. Int J Hyg Environ Health. 2012;215(6):541–546. [DOI] [PubMed] [Google Scholar]
- 96. Mrema EJ, Rubino FM, Brambilla G, Moretto A, Tsatsakis AM, Colosio C. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology. 2013;307:74–88. [DOI] [PubMed] [Google Scholar]
- 97. Rahman MM, Ng JC, Naidu R. Chronic exposure of arsenic via drinking water and its adverse health impacts on humans. Environ Geochem Health. 2009;31(suppl 1):189–200. [DOI] [PubMed] [Google Scholar]
- 98. Andrew AS, Jewell DA, Mason RA, Whitfield ML, Moore JH, Karagas MR. Drinking-water arsenic exposure modulates gene expression in human lymphocytes from a U.S. population. Environ Health Perspect. 2008;116(4):524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Satarug S. Long-term exposure to cadmium in food and cigarette smoke, liver effects and hepatocellular carcinoma. Curr Drug Metab. 2012;13(3):257–271. [DOI] [PubMed] [Google Scholar]
- 100. Davis LK, Murr AS, Best DS, et al. The effects of prenatal exposure to atrazine on pubertal and postnatal reproductive indices in the female rat. Reprod Toxicol. 2011;32(1):43–51. [DOI] [PubMed] [Google Scholar]
- 101. Isidori M, Cangiano M, Palermo FA, Parrella A. E-screen and vitellogenin assay for the detection of the estrogenic activity of alkylphenols and trace elements. Comp Biochem Physiol C Toxicol Pharmacol. 2010;152(1):51–56. [DOI] [PubMed] [Google Scholar]
- 102. Balabanič D, Rupnik M, Klemenčič AK. Negative impact of endocrine-disrupting compounds on human reproductive health. Reprod Fertil Dev. 2011;23(3):403–416. [DOI] [PubMed] [Google Scholar]
- 103. Truong L, Denardo MA, Kundu S, Collins TJ, Tanguay RL. Zebrafish assays as developmental toxicity indicators in the green design of TAML oxidation catalysts. Green Chem. 2013;15(9):2339–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Jobling S, Williams R, Johnson A, et al. Predicted exposures to steroid estrogens in U.K. rivers correlate with widespread sexual disruption in wild fish populations. Environ Health Perspect. 2006;114(suppl 1):32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Crain DA, Janssen SJ, Edwards TM, et al. Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril. 2008;90(4):911–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Longnecker MP, Klebanoff MA, Zhou H, Brock JW. Association between maternal serum concentration of the DDT metabolite DDE and preterm and small-for-gestational-age babies at birth. Lancet. 2001;358(9276):110–114. [DOI] [PubMed] [Google Scholar]
- 107. Rogan WJ, Chen A. Health risks and benefits of bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT). Lancet. 2005;366(9487):763–773. [DOI] [PubMed] [Google Scholar]
- 108. Sutton P, Perron JG, Giudice LC, Woodruff TJ. Pesticides Matter. A Primer for Reproductive Health Physicians. San Francisco, CA: University of California; 2011. [Google Scholar]
- 109. Jacob F. Evolution and tinkering. Science. 1977;196(4295):1161–1166. [DOI] [PubMed] [Google Scholar]
- 110. Crews D, Gore AC, Hsu TS, et al. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci USA. 2007;104(14):5942–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308(5727):1466–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Crews D, Gillette R, Scarpino SV, Manikkam M, Savenkova MI, Skinner MK. Epigenetic transgenerational inheritance of altered stress responses. Proc Natl Acad Sci USA. 2012;109(23):9143–9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wolstenholme JT, Goldsby JA, Rissman EF. Transgenerational effects of prenatal bisphenol A on social recognition. Horm Behav. 2013;64(5):833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Calafat AM. The U.S. National Health and Nutrition Examination Survey and human exposure to environmental chemicals. Int J Hyg Environ Health. 2012;215(2):99–101. [DOI] [PubMed] [Google Scholar]
- 115. Braun JM, Kalkbrenner AE, Calafat AM, et al. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect. 2011;119(1):131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Miller MD, Crofton KM, Rice DC, Zoeller RT. Thyroid-disrupting chemicals: interpreting upstream biomarkers of adverse outcomes. Environ Health Perspect. 2009;117(7):1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Guillette LJ, Jr, Iguchi T. Ecology. Life in a contaminated world. Science. 2012;337(6102):1614–1615. [DOI] [PubMed] [Google Scholar]
- 118. Schug TT, Heindel JJ, Camacho L, et al. A new approach to synergize academic and guideline-compliant research: the CLARITY-BPA research program. Reprod Toxicol. 2013;40:35–40. [DOI] [PubMed] [Google Scholar]
- 119. Organisation for Economic Co-operation and Development. OECD Conceptual Framework for Testing and Assessment of Endocrine Disrupters. Paris, France: The Organisation for Economic Co-operation and Development; 2012. [Google Scholar]
- 120. Bertin MJ, Moeller P, Guillette LJ, Jr, Chapman RW. Using machine learning tools to model complex toxic interactions with limited sampling regimes. Environ Sci Technol. 2013;47(6):2728–2736. [DOI] [PubMed] [Google Scholar]
- 121. Landis SC, Amara SG, Asadullah K, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490(7419):187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ellis WC, Tran CT, Roy R, et al. Designing green oxidation catalysts for purifying environmental waters. J Am Chem Soc. 2010;132(28):9774–9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Collins T. Essays on science and society. Toward sustainable chemistry. Science. 2001;291(5501):48–49. [DOI] [PubMed] [Google Scholar]
- 124. Grandjean P, Eriksen ML, Ellegaard O, Wallin JA. The Matthew effect in environmental science publication: a bibliometric analysis of chemical substances in journal articles. Environ Health. 2011;10:96. [DOI] [PMC free article] [PubMed] [Google Scholar]