Abstract
The ability to activate drugs only at desired locations avoiding systemic immunosuppression and other dose limiting toxicities is highly desirable. Here we present a new approach, named local drug activation, that uses bioorthogonal chemistry to concentrate and activate systemic small molecules at a location of choice. This method is independent of endogenous cellular or environmental markers and only depends on the presence of a preimplanted biomaterial near a desired site (e.g., tumor). We demonstrate the clear therapeutic benefit with minimal side effects of this approach in mice over systemic therapy using a doxorubicin pro-drug against xenograft tumors of a type of soft tissue sarcoma (HT1080).
Short abstract
Injection of tetrazine-modified hydrogel near soft tissue sarcoma tumors, followed by a short course of systemic doxorubicin pro-drug, allows effective local drug activation leading to tumor remission with minimal side effects.
Soft tissue sarcoma (STS) is an aggressive malignant tumor diagnosed in more than 12,000 people in the United States per year,1,2 and its incidence is increasing.3 About 6000 patients die from this disease every year.3−5 This heterogeneous disease with more than 100 types and subtypes6 disproportionately affects the young, accounting for 15–20% of childhood cancer, and 10% of neoplasms in adolescents and young adults.7 It usually starts as a local mass in a limb (40–60%) or retroperitoneum (15–20%).3,4,8 Current management includes imaging, biopsy for staging purposes, and wide surgical resection with curative intent.1−3,9,10 Microscopic positive margins, local recurrence, unresectable tumors, and metastasis are ominous characteristics that correlate with a major increase in morbidity and mortality.3,11−13 In order to minimize those events, radiation and chemotherapy are used as neoadjuvant (before surgery) or adjuvant therapy (after surgery).1−3,14−18
Radiation therapy improves local control in the pre- or postoperative management of STS by 30%,19 but the limitations and side effects are not trivial, including the size of the field, proximity to vital organs,10,20 20–30% increase in wound complications,11,20,21 and even radiation-induced sarcomas.11,13,22 Despite a response rate of only 16–27%, the chemotherapy of choice against STS is doxorubicin as a single agent or in combination.3,4,9,23−26 The primary mechanism of action is based on inhibition of topoisomerase II leading to cumulative DNA damage.14,27 The dose limiting toxicity of this cytotoxic agent is bone marrow suppression, and the lifetime cumulative dose is limited by anthracycline-induced cardiomyopathy.9,14 These side effects limit dosing options and lead to poor patient compliance, even during clinical trials.28
Historical approaches to overcome these issues include isolated limb perfusion,29,30 hyperthermic intraperitoneal chemotherapy,30,31 high dose chemotherapy with hematopoietic stem-cell transplantation,32 and others with limited improvements in efficacy.22,28,33 Drug delivery technologies based on increased circulation and enhanced permeability and retention (EPR) effect for doxorubicin have provided improvements in the side effect profile, but no improvement in efficacy for STS.33,34 Experimental35 and recently approved treatments36,37 have only shown efficacy in a subset of STS subtypes.36,38 The heterogeneity of this disease makes it an elusive target for molecularly targeted therapies.39
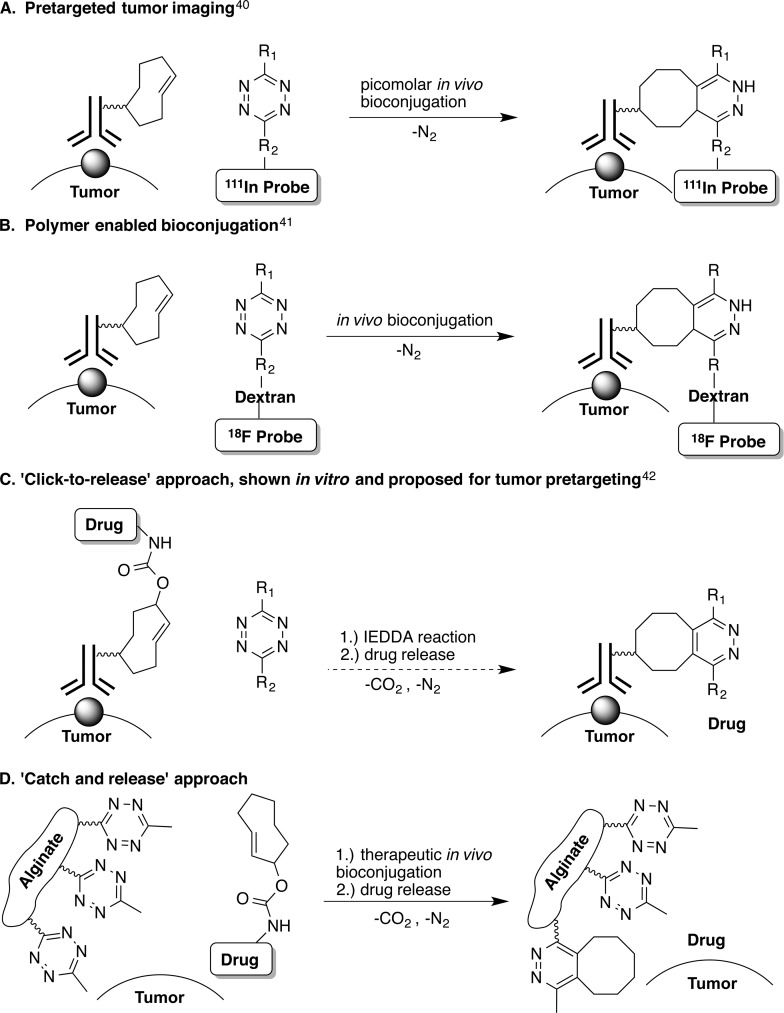
Bioorthogonal chemistry has been explored in recent years to achieve tissue-specific in vivo delivery of active biopharmaceuticals. In particular, the inverse-electron demand Diels–Alder (IEDDA) reaction between trans-cyclooctene (TCO) and tetrazine has been utilized for in vivo bioconjugation, capitalizing on the fast reactivity of the two bioorthogonal groups, their stability and inertness to biological functionalities. A “tumor pretargeting” approach utilized TCO-conjugated antibodies to deliver the bioorthogonal payload to cancerous cells with a specific antigen (Figure 1A).40,41
Figure 1.
In vivo biconjugation using IEDDA chemistry: (A) pretargeted tumor imaging using SPECT/CT achieved picomolar bioconjugation; (B) bioconjugation using tetrazine attached to dextran; (C) proposed approach for in vivo drug release; (D) local drug activation approach for bioconjugation at therapeutically relevant concentrations described in this work.
Recent advances utilizing the “tumor pretargeting” approach are outlined in Figure 1. This approach achieves in vivo bioconjugation at picomolar levels.40 Targeted bioconjugation at therapeutically relevant concentrations has proven to be very challenging in vivo. A widely accepted model41 predicts that IEDDA chemistry can lead to therapeutically positive outcomes only upon optimization of both the reaction kinetics and the pharmacokinetic profiles of the molecules carrying the two bioorthogonal groups, limiting the opportunity for bioconjugation of small molecule drugs. The proposed solution is that both bioorthogonal groups be attached to macromolecular structures, such as proteins or polymers (MW > 10 kDa) increasing the half-life in the circulatory system (Figure 1B).
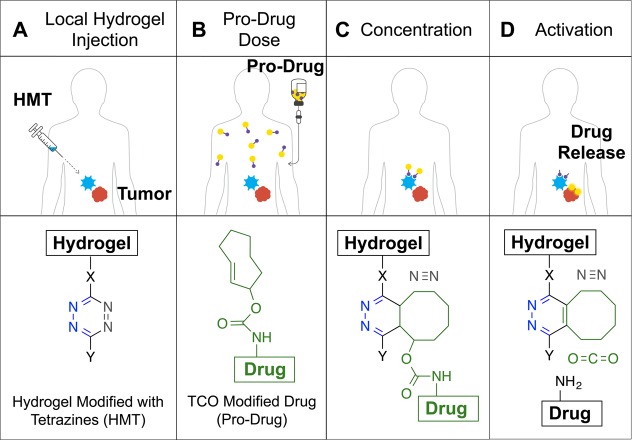
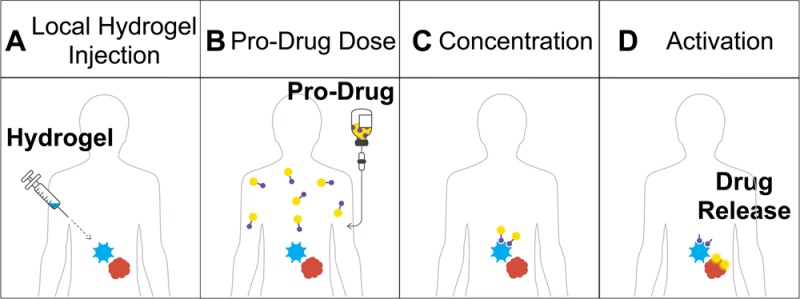
Here, we report a bioorthogonal chemistry-based approach termed local drug activation that is capable of activating small molecule pro-drugs at a location of choice, thereby allowing the in vivo concentration of cytotoxic agents at a tumor site in therapeutically meaningful quantities (Figures 1D). The approach is based on a recent development of the IEDDA chemistry that allows release of a payload attached to the TCO group after the initial cycloaddition step (Figure 1C).42 As illustrated in Figure 2, the approach starts with the injection of the biocompatible hydrogel modified with tetrazine near the mass where the drug is needed (Figure 2A). A drug with attenuated activity containing a releasable TCO moiety (pro-drug) is injected intravenously and travels through the circulatory system (Figure 2B). When the pro-drug and the hydrogel come near, the bioorthogonal agents react with each other through the IEDDA reaction localizing the payload (Figure 2C). The multivalency of the hydrogel’s surface provides a large number of tetrazine groups capable of concentrating the systemically administered small molecule pro-drug, thus compensating for its suboptimal pharmacokinetic properties. Finally, the resulting intermediate isomerizes spontaneously releasing the active drug from the hydrogel to perform its therapeutic function locally (Figure 2D).
Figure 2.
In vivo bioorthogonal chemistry for the concentration and activation of systemic pro-drugs. (A) A hydrogel modified with Tz (HMT) is injected into the area where the drugs are needed. (B) A drug covalently modified with a TCO carbamate (pro-drug) is given to the patient. (C) When the pro-drug and the material come in contact, the rapid cycloaddition reaction enhances the amount of drug present at the desired location with the concomitant release of a molecule of nitrogen. (D) The resulting cycloadduct isomerizes in vivo leading to decomposition of the self-immolable carbamate linker, releasing an equivalent of carbon dioxide and most importantly the drug at the local site to perform its therapeutic function.
In contrast to other targeted therapies, such as ADCs, “tumor pretargeting”, or the original report describing in vitro uncaging of doxorubicin42 (Figure 1C), the local drug activation approach provides a viable in vivo bioconjugation strategy for small molecule pro-drugs. In addition, it does not rely on endogenous molecular markers on the tumor cell surface, or local processes like enzymatic activity or oxygen levels that are characteristic of diseased tissue. As a result, this approach is not encumbered by genetically and phenotypically heterogeneous tumors such as soft tissue sarcoma that have proven elusive targets.38 Additionally, the described system provides an opportunity to modulate the therapeutic agent and dose which can be administered in multiple rounds while there is an adequate number of tetrazine groups on the hydrogel’s surface available for conjugation.
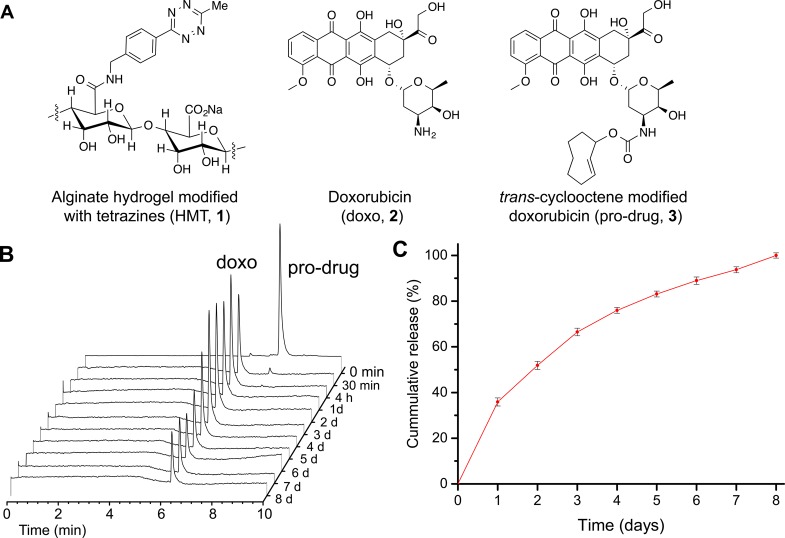
To evaluate the local drug activation strategy, we modified an alginate hydrogel with tetrazine moieties (HMT), as shown in Figure 3A. On the basis of 1H NMR analysis, HMT was determined to contain about 400 nmol of tetrazine per milligram of the material (Figure S6).43 Meanwhile, we also converted doxorubicin (2), a well-known cytotoxic agent, into a pro-drug by covalent modification with a trans-cyclooctene moiety 3 (Figure 2B).42 This modification resulted in an agent that is 57 times less active against HT1080 cells than regular doxorubicin (Figure S1).
Figure 3.
In vitro activation of doxorubicin pro-drug when mixed with HMT. (A) Chemical structures of an alginate monosaccharide modified with tetrazine, doxorubicin, and doxorubicin pro-drug. (B) Sample data from high-pressure liquid chromatography analysis of the supernatant after mixing HTM with doxorubicin pro-drug for 30 min. (C) Cumulative release of doxorubicin after mixing HTM with doxorubicin pro-drug. For HPLC analysis, the concentration of the pro-drug shown at t = 0 min was diluted 10-fold. Data are averages ± SEM, n = 3.
HMT was found to be very effective at capturing 3in vitro. When HMT and the doxorubicin pro-drug were mixed in PBS for 30 min at room temperature, over 99% of the compounds detected in the supernatant were 2 as shown by high performance liquid chromatography analysis (Figure 3B). Subsequent daily measurements during a week detected only the release of 2 (Figure 3B,C). This confirms that the doxorubicin pro-drug is rapidly captured by the HMT and that the product released from the material is unmodified doxorubicin.
To evaluate the antitumor activity of the doxorubicin pro-drug, we performed efficacy studies with athymic nude mice bearing fibrosarcoma (HT-1080) xenografts, a type of human soft tissue sarcoma that is often used to evaluate new therapies. We report greater efficacy of the pro-drug treatment without myelosuppresion and sustained tumor regression compared to the standard of care (the maximum tolerable dose of systemic doxorubicin).
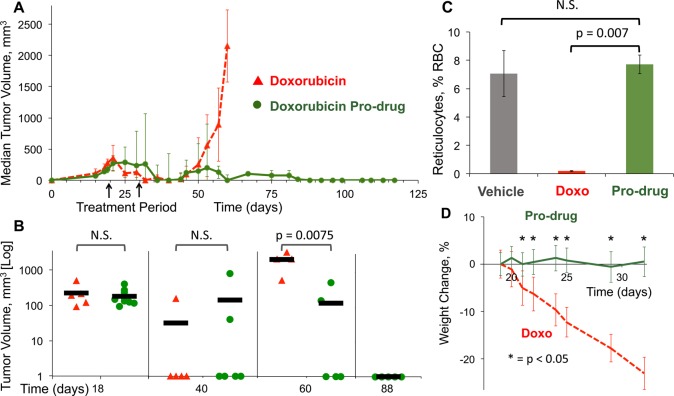
HMT was injected through palpation immediately next to the tumors 18 days after initial implantation when their size reached an average of 195 mm3 (range, 90–500 mm3). The mice were separated into two cohorts: (i) 3 intravenous doses of 14 μmol/kg of standard doxorubicin every 4 days (maximum tolerable dose),26,44,45 or (ii) daily doses of 14 μmol/kg of doxorubicin pro-drug for 10 days (Figure 4B). Tumor volumes were measured twice a week for 13 weeks after initiation of therapy (Figure 4A). No further therapies were given to the subjects 28 days post tumoral implantation (dpi). The animals were humanely euthanized and removed from analysis as they reached a tumor volume of 2000 mm3.
Figure 4.
Therapeutic effect of doxorubicin pro-drug in a xenograft model of soft tissue sarcoma. (A) NCR/nu:nu mice were injected with human HT-1080 fibrosarcoma cells at day 0. Tumors were then injected with HMT and started on intravenous doses of either doxorubicin pro-drug or a maximum tolerable dose of doxorubicin. Tumor sizes were monitored for more than 16 weeks (n = 5–10). (B) The tumor size of the members of each cohort at relevant time points in a logarithmic scale illustrate the differences between standard chemotherapy treatment and the material pro-drug approach. P values were determined by unpaired t test. Solid bars represent the mean for each cohort (n = 5–10). (C) Evaluation of reticulocyte counts as a surrogate for bone marrow suppression in a xenograft model of soft tissue sarcoma. Mice were given vehicle, doxorubicin, or doxorubicin pro-drug after injection of HMT. Samples were collected 3 days after the last treatment. Data are means ± SD (n = 2). (D) Body weight changes in response to therapy. Data are mean body weight changes as a percentage of initial weight ± SD (n = 5–10). P values were determined by unpaired t test.
For both groups the median tumor size was undetectable 2 weeks after the last treatment dose (40 dpi). Thirty days after the last treatment dose (60 dpi) the median tumor size of the systemic doxorubicin cohort was greater than 2000 mm3, and the mice were euthanized shortly therafter. This is consistent with previous studies evaluating systemic doxorubicin on HT1080.26,44,45 In contrast, the median tumor size of the pro-drug cohort remained undetectable (P = 0.021). At 88 dpi, half of the mice with tumors of the pro-drug cohort were euthanized as they reached the end point. The other half of the mice in the cohort did not show any detectable signs of tumors and remained that way until the end of the study (118 dpi, Figure 4B).
In order to exclude issues such as nonspecific in vivo activation of the pro-drug or microenvironment changes due to the placement of an alginate polymer, multiple additional controls were tested (Figure S2). No differences in tumor volume were observed between untreated mice and mice treated with (i) local injection of HMT and i.v. administration of saline, or (ii) local injection of unmodified alginate and i.v. doxorubicin pro-drug administration. This confirms that the pro-drug does not spontaneously turn into the regular doxorubicin without the presence of the gel in clinically meaningful quantities or that an inherent characteristic of the pro-drug independent of the bioorthogonal reaction is responsible for the increase in efficacy.
This bioorthogonal approach resulted in substantially lower side-effects relative to the doxorubicin treatment. Myelosuppression is the main acute dose-limiting toxicity of doxorubicin. A standard measure for this side effect is reticulocyte count, based on short-lived precursors of red blood cells that are easily quantified.26,44 The nadir of reticulocytes after systemic doxorubicin occurs 3 days after the end of therapy.26,44 As expected the systemic doxorobucin-treated cohort showed a dramatic decrease in reticulocytes (P = 0.007) (Figure 4C). In contrast, the cohort treated with doxorubicin pro-drug showed reticulocyte counts similar to mice treated with vehicle. Furthermore, mice treated with doxorubicin pro-drug did not show any overt signs of toxicity, including weight loss or changes in coat texture, while the regular doxorubicin cohort lost on average about 20% of body weight (Figure 4D).
While tumor remission of 50% of the treatment group with fewer side effects than the standard of care is remarkable, we need further studies to evaluate nonrespondents and the variables needed to maximize the response rate. The optimal dosing schedule and placement of the hydrogel also needs to be elucidated. Given the low level of toxicity observed, shorter courses with higher doses, or longer courses with smaller doses may be even more effective as has been recently suggested in the literature.46,47 More studies are also needed to establish the dose limiting toxicities of the pro-drug as well as the HMT and the effect of the native tumor microenvironment on this approach.
In summary, we have shown that the bioorthogonal chemistry-based local drug activation approach is capable of in vivo bioconjugation of small molecule pro-drugs at therapeutically relevant concentrations. The “catch” step is followed by local release of active cytotoxic agents in vivo. The approach increases the efficacy of doxorubicin, by harnessing the benefits of local activation via exogenous chemical factors, minimizing systemic toxicity, and optimizing the local therapeutic effects. Improved delivery of cytotoxic agents to a desired area may increase the number of patients with resectable tumors, as well as the number of resected tumors with clean margins, improving patient outcomes. With regard to distant micrometastasis, the low level of systemic toxicity of the approach would not preclude the concomitant use of systemic doxorubicin or alternative immunotherapies.
In recent years, the bond cleaving capabilities of the IEDDA chemistry have found a number of intriguing applications such as uncaging TCO-modified proteins48,49 or HPLC-free solid phase synthesis of RNA.50 The potential applications of a modified therapeutic agent that is concentrated and activated by a preinjected material extend well beyond soft tissue sarcoma and doxorubicin. In cancers with limited response, this approach could be applied to a number of other therapeutic agents, such as other cytotoxics, immunomodulating drugs, radiomodulating entities as well as peptide- and gene-based therapies. Decreased toxicity may improve patient compliance for cytotoxic agents, enable therapies for people who are too frail to receive them, or allow new pro-drug regimens to be evaluated in combinations that were previously impossible due to dose limiting toxicities. This approach presents a new method for drug delivery orthogonal to endogenous markers and has tremendous potential to improve the outcomes of challenging neoplasms and other disease processes.
Acknowledgments
We are grateful for the assistance of Mayuko Omori, Malavika Ghosh, and the rest of the team at Aragen for their support with animal studies. This work was supported by Shasqi Inc, the startup funds provided to M.R. by the State University of New York at Albany and partially funded by NSF under award number 1549133.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.6b00150.
Experimental details including synthetic procedures, compound characterization, and biochemical assays. Figure S1: Dose–response curve of HT1080 cells treated with different concentrations of doxorubicin and pro-drug. Figure S2: Negative controls for therapeutic effect of doxorubicin pro-drug in a xenograft model of soft tissue sarcoma. Figure S3: Fluorescently labeled TCO compounds. Figure S4: Functional assay to determine stability of HMT in PBS. Figure S5: Functional assay to determine stability of HMT in cell lysate. Figure S6: NMR spectrum of the hydrogel modified tetrazine (HMT). Figure S7: IR spectrum of the hydrogel modified tetrazine (HMT). Figure S8: 1H NMR spectrum of the doxorubicin pro-drug. Figure S9: High resolution ESI-MS spectrum of the doxorubicin pro-drug. Figure S10: Synthesis of TCO-NR-Fl. Figure S11: Synthesis of TCO-R-Rh. Figure S12: NMR spectra of TCO-NR-Fl. Figure S13: NMR spectra of TCO-R-Rh (PDF)
The authors declare the following competing financial interest(s): Jose M. Mejia Oneto is the founder of Shasqi, Inc. Its core technology is based on the research described herein.
Supplementary Material
References
- von Mehren M.; Randall R. L.; Benjamin R. S.; Boles S.; Bui M. M.; Casper E. S.; Conrad E. U. 3rd; Delaney T. F.; Ganjoo K. N.; George S.; Gonzalez R. J.; Heslin M. J.; Kane J. M. 3rd; Mayerson J.; McGarry S. V.; Meyer C.; O’Donnell R. J.; Pappo A. S.; Paz I. B.; Pfeifer J. D.; Riedel R. F.; Schuetze S.; Schupak K. D.; Schwartz H. S.; Van Tine B. A.; Wayne J. D.; Bergman M. A.; Sundar H. National Comprehensive Cancer, N., Soft tissue sarcoma, version 2.2014. J. Natl. Compr. Canc. Netw. 2014, 12, 473–483. [DOI] [PubMed] [Google Scholar]
- Demetri G. D.; Antonia S.; Benjamin R. S.; Bui M. M.; Casper E. S.; Conrad E. U. 3rd; DeLaney T. F.; Ganjoo K. N.; Heslin M. J.; Hutchinson R. J.; Kane J. M. 3rd; Letson G. D.; McGarry S. V.; O’Donnell R. J.; Paz I. B.; Pfeifer J. D.; Pollock R. E.; Randall R. L.; Riedel R. F.; Schupak K. D.; Schwartz H. S.; Thornton K.; von Mehren M.; Wayne J. National Comprehensive Cancer Network Soft Tissue Sarcoma, P., Soft tissue sarcoma. J. Natl. Compr. Canc. Netw. 2010, 8, 630–674. [DOI] [PubMed] [Google Scholar]
- Clark M. A.; Fisher C.; Judson I.; Thomas J. M. Soft-tissue sarcomas in adults. N. Engl. J. Med. 2005, 353, 701–711. 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- Sarcoma Meta-analysis, C., Adjuvant chemotherapy for localised resectable soft tissue sarcoma in adults. Cochrane Database Syst. Rev. 2000, CD001419. [DOI] [PubMed] [Google Scholar]
- Brennan M. F.; Antonescu C. R.; Moraco N.; Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann. Surg. 2014, 260, 416–421. 10.1097/SLA.0000000000000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J. S.; Lee S. Contemporary diagnostics: sarcoma pathology update. J. Surg. Oncol. 2015, 111, 513–519. 10.1002/jso.23853. [DOI] [PubMed] [Google Scholar]
- Aflatoon K.; Aboulafia A. J.; McCarthy E. F. Jr.; Frassica F. J.; Levine A. M. Pediatric soft-tissue tumors. J. Am. Acad. Orthop. Surg. 2003, 11, 332–343. 10.5435/00124635-200309000-00006. [DOI] [PubMed] [Google Scholar]
- Bramwell V. H.; Anderson D.; Charette M. L.; Sarcoma Disease Site, G., Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst. Rev. 2003, CD003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco N. S.; Henshaw R. M. Unplanned Resection of Sarcoma. J. Am. Acad. Orthop. Surg. 2016, 24, 150–159. 10.5435/JAAOS-D-15-00074. [DOI] [PubMed] [Google Scholar]
- Gilbert N. F.; Cannon C. P.; Lin P. P.; Lewis V. O. Soft-tissue sarcoma. J. Am. Acad. Orthop. Surg. 2009, 17, 40–47. 10.5435/00124635-200901000-00006. [DOI] [PubMed] [Google Scholar]
- Wolinsky J. B.; Colson Y. L.; Grinstaff M. W. Local drug delivery strategies for cancer treatment: gels, nanoparticles, polymeric films, rods, and wafers. J. Controlled Release 2012, 159, 14–26. 10.1016/j.jconrel.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt C. A.; Crist W. M. Common musculoskeletal tumors of childhood and adolescence. N. Engl. J. Med. 1999, 341, 342–352. 10.1056/NEJM199907293410507. [DOI] [PubMed] [Google Scholar]
- Liebner D. A. The indications and efficacy of conventional chemotherapy in primary and recurrent sarcoma. J. Surg. Oncol. 2015, 111, 622–631. 10.1002/jso.23866. [DOI] [PubMed] [Google Scholar]
- Wolfson A. H. Preoperative vs postoperative radiation therapy for extremity soft tissue sarcoma: controversy and present management. Curr. Opin. Oncol. 2005, 17, 357–360. 10.1097/01.cco.0000161745.24887.82. [DOI] [PubMed] [Google Scholar]
- Walczak B. E.; Irwin R. B. Sarcoma chemotherapy. J. Am. Acad. Orthop. Surg. 2013, 21, 480–491. 10.5435/JAAOS-21-08-480. [DOI] [PubMed] [Google Scholar]
- Gronchi A.; Frustaci S.; Mercuri M.; Martin J.; Lopez-Pousa A.; Verderio P.; Mariani L.; Valagussa P.; Miceli R.; Stacchiotti S.; Dei Tos A. P.; De Paoli A.; Longhi A.; Poveda A.; Quagliuolo V.; Comandone A.; Casali P. G.; Picci P. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J. Clin. Oncol. 2012, 30, 850–856. 10.1200/JCO.2011.37.7218. [DOI] [PubMed] [Google Scholar]
- Frustaci S.; Gherlinzoni F.; De Paoli A.; Bonetti M.; Azzarelli A.; Comandone A.; Olmi P.; Buonadonna A.; Pignatti G.; Barbieri E.; Apice G.; Zmerly H.; Serraino D.; Picci P. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J. Clin. Oncol. 2001, 19, 1238–1247. [DOI] [PubMed] [Google Scholar]
- Yang J. C.; Chang A. E.; Baker A. R.; Sindelar W. F.; Danforth D. N.; Topalian S. L.; DeLaney T.; Glatstein E.; Steinberg S. M.; Merino M. J.; Rosenberg S. A. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J. Clin. Oncol. 1998, 16, 197–203. [DOI] [PubMed] [Google Scholar]
- Holloway C. L.; Delaney T. F.; Alektiar K. M.; Devlin P. M.; O’Farrell D. A.; Demanes D. J. American Brachytherapy Society(ABS) consensus statement for sarcoma brachytherapy. Brachytherapy 2013, 12, 179–190. 10.1016/j.brachy.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Miller E. D.; Xu-Welliver M.; Haglund K. E. The role of modern radiation therapy in the management of extremity sarcomas. J. Surg. Oncol. 2015, 111, 599–603. 10.1002/jso.23823. [DOI] [PubMed] [Google Scholar]
- Harrison D. J.; Schwartz C. Survivorship. J. Surg. Oncol. 2015, 111, 648–655. 10.1002/jso.23844. [DOI] [PubMed] [Google Scholar]
- Ganjoo K. N.; Cranmer L. D.; Butrynski J. E.; Rushing D.; Adkins D.; Okuno S. H.; Lorente G.; Kroll S.; Langmuir V. K.; Chawla S. P. A phase I study of the safety and pharmacokinetics of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma. Oncology 2011, 80, 50–56. 10.1159/000327739. [DOI] [PubMed] [Google Scholar]
- Chawla S. P.; Cranmer L. D.; Van Tine B. A.; Reed D. R.; Okuno S. H.; Butrynski J. E.; Adkins D. R.; Hendifar A. E.; Kroll S.; Ganjoo K. N. Phase II study of the safety and antitumor activity of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma. J. Clin. Oncol. 2014, 32, 3299. 10.1200/JCO.2013.54.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sborov D.; Chen J. L. Targeted therapy in sarcomas other than GIST tumors. J. Surg. Oncol. 2015, 111, 632–640. 10.1002/jso.23802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom H.; Blay J. Y.; Seddon B. M.; Leahy M.; Ray-Coquard I.; Sleijfer S.; Kerst J. M.; Rutkowski P.; Bauer S.; Ouali M.; Marreaud S.; van der Straaten R. J.; Guchelaar H. J.; Weitman S. D.; Hogendoorn P. C.; Hohenberger P. Brostallicin versus doxorubicin as first-line chemotherapy in patients with advanced or metastatic soft tissue sarcoma: an European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group randomised phase II and pharmacogenetic study. Eur. J. Cancer 2014, 50, 388–396. 10.1016/j.ejca.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Albright C. F.; Graciani N.; Han W.; Yue E.; Stein R.; Lai Z.; Diamond M.; Dowling R.; Grimminger L.; Zhang S. Y.; Behrens D.; Musselman A.; Bruckner R.; Zhang M.; Jiang X.; Hu D.; Higley A.; Dimeo S.; Rafalski M.; Mandlekar S.; Car B.; Yeleswaram S.; Stern A.; Copeland R. A.; Combs A.; Seitz S. P.; Trainor G. L.; Taub R.; Huang P.; Oliff A. Matrix metalloproteinase-activated doxorubicin prodrugs inhibit HT1080 xenograft growth better than doxorubicin with less toxicity. Mol. Cancer Ther. 2005, 4, 751–760. [DOI] [PubMed] [Google Scholar]
- Le Cesne A.; Ouali M.; Leahy M. G.; Santoro A.; Hoekstra H. J.; Hohenberger P.; Van Coevorden F.; Rutkowski P.; Van Hoesel R.; Verweij J.; Bonvalot S.; Steward W. P.; Gronchi A.; Hogendoorn P. C.; Litiere S.; Marreaud S.; Blay J. Y.; Van Der Graaf W. T. Doxorubicin-based adjuvant chemotherapy in soft tissue sarcoma: pooled analysis of two STBSG-EORTC phase III clinical trials. Ann. Oncol. 2014, 25, 2425–2432. 10.1093/annonc/mdu460. [DOI] [PubMed] [Google Scholar]
- Eggermont A. M.; ten Hagen T. L. Isolated limb perfusion for extremity soft-tissue sarcomas, in-transit metastases, and other unresectable tumors: credits, debits, and future perspectives. Curr. Oncol. Rep. 2001, 3, 359–367. 10.1007/s11912-001-0090-8. [DOI] [PubMed] [Google Scholar]
- Colombo C.; Baratti D.; Kusamura S.; Deraco M.; Gronchi A. The role of hyperthermic intraperitoneal chemotherapy(HIPEC) and isolated perfusion(ILP) interventions in sarcoma. J. Surg. Oncol. 2015, 111, 570–579. 10.1002/jso.23808. [DOI] [PubMed] [Google Scholar]
- Baratti D.; Pennacchioli E.; Kusamura S.; Fiore M.; Balestra M. R.; Colombo C.; Mingrone E.; Gronchi A.; Deraco M. Peritoneal sarcomatosis: is there a subset of patients who may benefit from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy?. Ann. Surg. Oncol. 2010, 17, 3220–3228. 10.1245/s10434-010-1178-x. [DOI] [PubMed] [Google Scholar]
- Koscielniak E.; Klingebiel T. H.; Peters C.; Hermann J.; Burdach S. T.; Bender-Gotze C.; Muller-Weihrich S. T.; Treuner J. Do patients with metastatic and recurrent rhabdomyosarcoma benefit from high-dose therapy with hematopoietic rescue? Report of the German/Austrian Pediatric Bone Marrow Transplantation Group. Bone Marrow Transplant. 1997, 19, 227–231. 10.1038/sj.bmt.1700628. [DOI] [PubMed] [Google Scholar]
- Judson I.; Radford J. A.; Harris M.; Blay J. Y.; van Hoesel Q.; le Cesne A.; van Oosterom A. T.; Clemons M. J.; Kamby C.; Hermans C.; Whittaker J.; Donato di Paola E.; Verweij J.; Nielsen S. Randomised phase II trial of pegylated liposomal doxorubicin(DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur. J. Cancer 2001, 37, 870–877. 10.1016/S0959-8049(01)00050-8. [DOI] [PubMed] [Google Scholar]
- Park K. Lessons learned from thermosensitive liposomes for improved chemotherapy. J. Controlled Release 2014, 174, 219. 10.1016/j.jconrel.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Hanaoka H.; Katagiri T.; Fukukawa C.; Yoshioka H.; Yamamoto S.; Iida Y.; Higuchi T.; Oriuchi N.; Paudyal B.; Paudyal P.; Nakamura Y.; Endo K. Radioimmunotherapy of solid tumors targeting a cell-surface protein, FZD10: therapeutic efficacy largely depends on radiosensitivity. Ann. Nucl. Med. 2009, 23, 479–485. 10.1007/s12149-009-0265-1. [DOI] [PubMed] [Google Scholar]
- van der Graaf W. T.; Gelderblom H. New systemic therapy options for advanced sarcomas. Curr. Treat. Options Oncol. 2012, 13, 306–317. 10.1007/s11864-012-0196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetri G. D.; von Mehren M.; Jones R. L.; Hensley M. L.; Schuetze S. M.; Staddon A.; Milhem M.; Elias A.; Ganjoo K. N.; Tawbi H.; Van Tine B. A.; Spira A.; Dean A.; Khokhar N. Z.; Park Y. C.; Knoblauch R. E.; Parekh T. V.; Maki R. G.; Patel S. R. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2016, 34, 786–793. 10.1200/JCO.2015.62.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. K. Trabectedin and the L-Sarcomas: A Decade-Long Odyssey. J. Clin. Oncol. 2016, 34, 769–771. 10.1200/JCO.2015.63.5938. [DOI] [PubMed] [Google Scholar]
- Hendifar A. E.; Ahlmann E.; Allison D. C.; Hu J.; Menendez L.; Chawla S. P. Moving beyond response criteria: new measures of success in the treatment of sarcomas. Curr. Treat. Options Oncol. 2012, 13, 299–305. 10.1007/s11864-012-0197-1. [DOI] [PubMed] [Google Scholar]
- Minchinton A. I.; Tannock I. F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592. 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- Rossin R.; Renart Verkerk P.; van den Bosch S. M.; Vulders R. C.; Verel I.; Lub J.; Robillard M. S. In vivo chemistry for pretargeted tumor imaging in live mice. Angew. Chem., Int. Ed. 2010, 49, 3375–3378. 10.1002/anie.200906294. [DOI] [PubMed] [Google Scholar]
- Devaraj N. K.; Thurber G. M.; Keliher E. J.; Marinelli B.; Weissleder R. Reactive polymer enables efficient in vivo bioorthogonal chemistry. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 4762–4767. 10.1073/pnas.1113466109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteegen R. M.; Rossin R.; ten Hoeve W.; Janssen H. M.; Robillard M. S. Click to release: instantaneous doxorubicin elimination upon tetrazine ligation. Angew. Chem., Int. Ed. 2013, 52, 14112–14116. 10.1002/anie.201305969. [DOI] [PubMed] [Google Scholar]
- Brudno Y.; Desai R. M.; Kwee B. J.; Joshi N. S.; Aizenberg M.; Mooney D. J. In vivo targeting through click chemistry. ChemMedChem 2015, 10, 617–620. 10.1002/cmdc.201402527. [DOI] [PubMed] [Google Scholar]
- Hu Z.; Jiang X.; Albright C. F.; Graciani N.; Yue E.; Zhang M.; Zhang S. Y.; Bruckner R.; Diamond M.; Dowling R.; Rafalski M.; Yeleswaram S.; Trainor G. L.; Seitz S. P.; Han W. Discovery of matrix metalloproteases selective and activated peptide-doxorubicin prodrugs as anti-tumor agents. Bioorg. Med. Chem. Lett. 2010, 20, 853–856. 10.1016/j.bmcl.2009.12.084. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Sun J. D.; Wang J.; Ahluwalia D.; Baker A. F.; Cranmer L. D.; Ferraro D.; Wang Y.; Duan J. X.; Ammons W. S.; Curd J. G.; Matteucci M. D.; Hart C. P. TH-302, a hypoxia-activated prodrug with broad in vivo preclinical combination therapy efficacy: optimization of dosing regimens and schedules. Cancer Chemother. Pharmacol. 2012, 69, 1487–1498. 10.1007/s00280-012-1852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongu M.; Harashima N.; Monma H.; Inao T.; Yamada T.; Kawauchi H.; Harada M. Metronomic chemotherapy with low-dose cyclophosphamide plus gemcitabine can induce anti-tumor T cell immunity in vivo. Cancer Immunol. Immunother. 2013, 62, 383–391. 10.1007/s00262-012-1343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkens L. H.; van Tinteren H.; May A.; ten Tije A. J.; Creemers G. J.; Loosveld O. J.; de Jongh F. E.; Erdkamp F. L.; Erjavec Z.; van der Torren A. M.; Tol J.; Braun H. J.; Nieboer P.; van der Hoeven J. J.; Haasjes J. G.; Jansen R. L.; Wals J.; Cats A.; Derleyn V. A.; Honkoop A. H.; Mol L.; Punt C. J.; Koopman M. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer(CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet 2015, 385, 1843–1852. 10.1016/S0140-6736(14)62004-3. [DOI] [PubMed] [Google Scholar]
- Li J.; Jia S.; Chen P. R. Diels-Alder reaction-triggered bioorthogonal protein decaging in living cells. Nat. Chem. Biol. 2014, 10, 1003–1005. 10.1038/nchembio.1656. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Li J.; Xie R.; Fan X.; Liu Y.; Zheng S.; Ge Y.; Chen P. R. Bioorthogonal Chemical Activation of Kinases in Living Systems. ACS Cent. Sci. 2016, 2, 325–331. 10.1021/acscentsci.6b00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agustin E.; Asare Okai P. N.; Khan I.; Miller M. R.; Wang R.; Sheng J.; Royzen M. A fast click-slow release strategy towards the HPLC-free synthesis of RNA. Chem. Commun. (Cambridge, U. K.) 2016, 52, 1405–1408. 10.1039/C5CC05392G. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.