Abstract
Adolescence and excessive intake of saccharin have each been previously associated with enhanced vulnerability to drug abuse. In the present study, we focused on the relationship between these two factors using male adolescent and adult rats bred for high (HiS) and low (LoS) levels of saccharin intake. On postnatal day 25 (adolescents) or 150 (adults), rats were implanted with an intravenous catheter and trained to self-administer cocaine (0.4 mg/kg) using an autoshaping procedure that consisted of two 6-h sessions. In the first 6 h, rats were given noncontingent cocaine infusions at random intervals 10 times per hour, and during the second 6-h session, rats were allowed to self-administer cocaine under a fixed ratio 1 (FR 1) lever-response contingency. Acquisition was defined as a total of at least 250 infusions over 5 consecutive days, and rats were given 30 days to meet the acquisition criterion. Subsequently, saccharin intake was determined by comparing 24-h saccharin and water consumption in two-bottle tests. Adolescent LoS rats had a faster rate of acquisition of cocaine self-administration than adult LoS rats; however, adolescent and adult HiS rats acquired at the same rate. Both HiS and LoS adolescents had significantly higher saccharin preference scores than HiS and LoS adults, respectively. Additionally, saccharin score was negatively correlated with the number of days to meet the acquisition criterion for cocaine self-administration, but this was mostly accounted for by the HiS adolescents. These results suggest that during adolescence, rats have both an increased avidity for sweets and vulnerability to initiate drug abuse compared with adulthood.
Keywords: Acquisition, Adolescent, Adult, Cocaine, Genetic, Intravenous, Rats, Saccharin intake, Selective breeding, Self-administration
Introduction
Adolescence is a critical period when individuals may experience enhanced vulnerability to drug abuse [for reviews, see 1, 2, 3]. For example, adolescents exhibit higher rates of substance use and substance use disorders compared with adults [2]. Additionally, most individuals that were identified with an addictive disorder experienced the onset in adolescence [e.g., 1, 4], and others have shown that earlier onset of substance use is associated with greater addiction severity [e.g., 5, 6]. Individuals with adolescent onset of substance use also experience a more rapid progression from drug use to abuse and dependency [e.g., 6, 7, 8]. It has been suggested that adolescents are more vulnerable to substance abuse, and other impulsive and risky behaviors, due to greater motivation for novel experiences and to an underdeveloped inhibitory control system [2].
Animal models have provided useful tools for studying adolescent vulnerability to drug abuse [e.g., 1, 3, 4, 9]. For example, similar to humans, rats were more vulnerable to self-administering drugs of abuse in adulthood if they had previously been exposed to the drugs in adolescence [e.g., 10, 11, 12]. Adolescent rats self-administered more nicotine [13], alcohol [14, 15] and amphetamine [16] compared with adults. Adolescent rats also consumed significantly more of a solution that was previously paired with nicotine than adults [17]. Conversely, adolescent rats have reduced sensitivity to the negative aspects of nicotine [18] and alcohol [14, 19] withdrawal. Adolescents rats have also shown decreased sensitivity to other negative effects of drugs of abuse, for example, they were less sensitive to the motor-impairing [20] and sedative [21] effects of alcohol. Low doses of nicotine [22], alcohol [23], and morphine [24] produced conditioned place preference in adolescents, but not adults, suggesting that adolescents may be more sensitive to the rewarding effects of drugs of abuse; however, others have not found this effect [e.g., 25, 26, 27]. Adolescents appeared to be hyporesponsive to the locomotor activating effects of acute psychostimulant administration [28-33], but this may depend on dose, because when higher doses were administered, adolescents had greater behavioral responses than adults [28, 34, 35]. Furthermore, adolescents showed more behavioral sensitization than adults following repeated administration of psychomotor stimulants [11, 31, 36, 37, but see 27]. Taken together, these findings indicate that adolescents experience fewer of the negative effects of drugs of abuse, but more of the positive effects compared with adults. Parallels exist in human subjects; those reporting increases in the positive subjective effects of the drug chose drug over placebo; while those reporting decreases in positive subjective effects but increases in negative subjective effects chose placebo [38]. This suggests that adolescents (vs. adults) are more vulnerable to drug abuse, and it was a goal of this study to further examine this possibility in rats self-administering cocaine.
In addition to displaying similar responses to drugs of abuse, rodent models of adolescence mimic other behavioral aspects of adolescence in humans. For example, elevated levels of novelty seeking and impulsivity are hallmarks of adolescence in humans [for reviews, see 1, 2, 39], and adolescent rodents also display heightened novelty seeking [33, 36] and impulsivity [26] compared with adults. Impulsivity in both humans and rodent models may be related to excessive intake of palatable substances. In humans, adolescents scored higher on personality measures of impulsivity, and they also rated higher concentrations of sucrose as more pleasant than adults [40]. Young humans reported higher dietary preferences for sweets than older adults [41]. Similarly, adolescent rats were more impulsive than adults, and they showed a preference for sweeter sucrose solutions [40].
The present study was focused upon the acquisition of drug self-administration in adolescent and adult rats that were selectively bred for high (HiS) or low (LoS) levels of saccharin consumption. These selectively-bred lines show several differences in behavior motivated by drugs and dietary substances that are related to their saccharin phenotype. For example, HiS rats consumed more sucrose (and other sweet, salty, or starchy solutions) in a two-bottle choice test than LoS rats [42], they were more impulsive for food than LoS rats (Perry et al., unpublished data), and they showed greater drug-seeking behavior than LoS rats in several phases of drug abuse. Specifically, HiS rats consumed more ethanol [43], and acquired i.v. self-administration of cocaine faster and in greater numbers (percent of group) [44] than LoS rats. HiS rats showed greater escalation [45] and dysregulation [46] of cocaine intake compared with LoS rats. HiS females were also more sensitized to the locomotor-activating effects of cocaine than LoS females, LoS males, and HiS males (Carroll et al., unpublished data). Additionally, HiS female rats responded more than LoS female rats during reinstatement of cocaine-seeking behavior [45].
The purpose of the present study was to examine the interaction of age (adolescent v adult) and saccharin phenotype by comparing rates of acquisition of i.v. cocaine self-administration in adolescent and adult HiS and LoS male rats. Male rats were used to limit the number of groups and to minimize the influence of potentially different levels of circulating estrogen, a factor that influences acquisition of cocaine self-administration [47-49]. An autoshaping or autopriming procedure [50] that has been shown to be sensitive to individual differences [44, 51, 52] was used in the present study. Based on literature suggesting that adolescents exceed adults on a range of behaviors that have been shown to predict drug self-administration, such as novelty-seeking [53], impulsivity [52], and excessive intake of palatable substances [44, 54], we hypothesized that adolescents would acquire cocaine self-administration faster and in greater numbers (percent of group) than adults. Additionally, based on previous reports from our laboratory [44, 54], it was hypothesized that HiS adolescents and adults would acquire cocaine self-administration at a faster rate and in greater numbers than their LoS counterparts, although this effect is smaller in males than females [44].
Method
Animals
Experimentally naïve 150-day old (N = 24) and 25-day old (N = 24) Sprague Dawley (SD) male rats selectively bred for high (HiS) and low (LoS) saccharin intake were used in this experiment. Previous studies have defined adolescence in the rat as postnatal days (PND) 28-42 [4]. However, it has been suggested that adolescence may extend to PND 55-60 [55]. In the present study, rats were implanted with intravenous catheters at PND 25, began cocaine self-administration sessions on PND 29, and cocaine self-administration sessions were discontinued after 30 days if rats did not acquire cocaine self-administration. Therefore, the PND 25 group in the present study completed drug self-administration during a time period that has previously been defined as adolescence in the rat [4, 55]. Forty-eight rats were distributed into 4 groups of 12; HiS vs. LoS and adolescent vs. adult. Adult males weighed 440-680 g and adolescents weighed 70-120 g at the beginning of the experiment. The HiS and LoS lines have been maintained as previously described [44, 45] through pairings of rats with HiS or LoS phenotype scores, including no sibling, half-sibling, or first cousin matings [56]. Progenitor HiS and LoS rats were obtained from the Occidental stock (Occidental College, Los Angeles, CA, USA), but all rats used in the present study were bred from the Minnesota stock [44]. Occasional out-breeding (every 4-6 generations) with rats from the original breeding stock (Sprague-Dawley, Harlan Sprague-Dawley, Inc., Indianapolis, IN, USA) was used to maintain the vigor of both lines.
Rats were weaned at PND 20 and pair-housed until PND 25 (adolescent group) or PND 150 (adult group), and they were individually-housed in operant conditioning chambers during the drug self-administration procedure. Rats had ad libitum access to food and water prior to and during experimental testing. The experimental rooms were humidity and temperature (24° C) controlled and had a 12 h light/dark cycle, with lights on at 6:00 a.m. The University of Minnesota Institutional Animal Care and Use Committee approved the use of the animals in this experiment under Protocol No. 0140A64760. Laboratory facilities were approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), and recommended principles of laboratory animal care were followed [57].
Apparatus
Octagonal experimental chambers consisted of alternating Plexiglas and stainless steel panels, as previously described [44, 51, 52]. Two levers (1 retractable lever and 1 standard response lever, Coulbourn Instruments, Allentown, NJ) were positioned 3 cm from the wire mesh bottom of the cage. There were 3 colored stimulus lights (red, green, yellow, 4.6 W) mounted above each lever, and a white house light (4.6 W) was positioned at the top of each chamber. Experimental chambers were housed inside a sound-resistant wooden box that was equipped with a ventilation fan. Mounted to the outside of the wooden box was an infusion pump (model RHSYOCKC, Fluid Metering, Inc. Oyster Bay, NY) that was attached to a swivel (050-0022, Alice King Chatham, Hawthorne, CA) by Tygon tubing (1.52 mm o.d.; 0.51, mm i.d., Fisher Scientific, Springfield, NJ). The swivel was also attached to a spring-covered tube (C313CS, Plastics One, Roanoke, VA) that extended into the operant chamber. The spring-covered tubing was attached to a metal cannula (C3236, Plastics One) that was embedded in a plastic covance-infusion harness (CIH95 Instech Laboratories, Plymouth Meeting, PA), and the indwelling catheter was connected to the opposing end of the metal cannula. MED-PC software (Med Associates, St. Albans, VT) and IBM-compatible computers were programmed to control the experiments and collect the data.
Procedure
Ketamine (60 mg/kg, i.p.), xylazine (10 mg/kg, i.p.), atropine (0.02 mg/kg, s.c.) and dopram (5 mg/kg, s.c.) were given prior to implanting an intravenous silastic catheter (0.51 mm i.d., 0.94 mm o.d., Helix Medical, Inc., Carpinteria, CA) on PND 25 for adolescents or PND 150 for adults. These methods have been previously described in detail [e.g., 44, 52]. Briefly, the catheter was inserted in the right jugular vein, and the distal end of the catheter was led subcutaneously 1 cm caudal to the scapulae, where the catheter exited the body and was connected to the cannula on the harness. Gentamicin (2 mg/kg, i.v.) and heparinized saline (10 IU/kg, i.v.) were administered for 3 days post-surgery for adult rats and 4 days post-surgery for adolescent rats to prevent infection and catheter blockage. Buprenorphine (0.05 mg/kg, s.c.) was also administered every 12 h for 48 h following surgery. If the catheter became blocked, a second surgery was completed in which the catheter was inserted into the left jugular vein. After the experimental sessions began, catheter patency was checked once every 7 days with an injection of a solution containing 30 mg/ml ketamine and 1.5 mg/ml midazolam [58] dissolved in saline (10 ml total). Loss of the righting reflex confirmed catheter patency. Additionally, catheters were flushed with heparinized saline 4-5 days/week.
Cocaine self-administration sessions began 3 days following surgery for adult rats, and 4 days following surgery for adolescent rats. Adolescents were given a slightly longer recovery period following surgery because they appeared to be more sensitive to the surgical procedure. Experimental sessions began at 9:00 a.m., 7 days per week. Between 8:00 and 9:00 a.m., food and water were replenished, and intakes were measured and recorded. The drug autopriming procedure used in the present experiment was adapted from an autoshaping food program [59], and it had been previously used to quantify the rate of acquisition of i.v. drug self-administration [50-52, 60]. The autopriming procedure consisted of two 6-h components that occurred each day from 9:00 a.m. to 9:00 p.m. At the start of the first component (the autopriming component) the stimulus lights above both levers were illuminated. The retractable lever was extended into the chamber 10 times each hour under a random-interval 90 s (RI 90) schedule. Following a lever press or 15 s (whatever came first), the lever was retracted, and a cocaine infusion (0.4 mg/kg) was delivered after a 1 s delay. Cocaine infusions occurred during the first 13-15 minutes of each hour during the 6-h autopriming session, and during the remainder of each hour, the lever remained retracted and lever touches were counted, but had no consequences. Responses on a second, standard (nonretractable) response lever were also counted, and they resulted in illumination of the stimulus lights above the lever.
Following the autopriming component was a second 6-h component (the self-administration component), in which the retractable lever remained extended, and each cocaine infusion (0.4 mg/kg) was contingent upon a response under a fixed-ratio 1 (FR 1) schedule. Responding on the standard response lever was measured and resulted in illumination of the stimulus lights above the lever. At the end of the self-administration component, there was a 12-h time-out period when lights were extinguished and responding had no consequences until 9:00 a.m. the following day. The acquisition criterion for cocaine self-administration consisted of the self-administration of a total of at least 250 infusions during the self-administration components over 5 consecutive days. This criterion was derived from other studies in which the dose of cocaine used was half as much as used in the present study (0.2 mg/kg), and the acquisition criterion was twice as high (at least 500 infusions over 5 days) as in the present study [50-52, 61, 62]. A higher cocaine dose (0.4 mg/kg) was used in the present study to avoid obtaining possible floor effects because in the previous study [44], and in pilot work, very few adult LoS males acquired cocaine self-administration at a lower (0.2 mg/kg) dose.
Following the drug self-administration component of the experiment, the selective breeding was verified by calculating a saccharin consumption phenotype score for each rat [43, 44, 54]. The amount of water consumed in a 24-h period was measured over 2 days and averaged. This amount was compared with the amount of saccharin (0.1% w/v) consumed in a two-bottle choice test on the third day. The saccharin consumption phenotype score indicated a saccharin preference if it was positive, no preference if it was zero, and an aversion to saccharin if it was negative. The following equation was used to calculate each saccharin phenotype score:
Drugs
Cocaine HCl was provided by the National Institute of Drug Abuse (Research Triangle Institute, Research Triangle Park, NC). It was dissolved in sterile physiological saline and stored in glass reservoirs (500 ml) that were covered with aluminum foil. The rate of infusion for cocaine (0.4 mg/kg) was 0.03 ml/s. The infusion duration was 1 s per 100 g of body weight, and it was determined based on each individual animal's weight.
Data Analysis
The main dependent measures were the percent of each group meeting cocaine acquisition criterion, the mean number of days per group to meet the criterion, and mean number of infusions over the final 5 days before the acquisition criterion was met. Saccharin score, inactive lever responding, and mean food and water consumption during the acquisition period were also analyzed. The percentage of rats acquiring cocaine self-administration was compared between groups with the Kaplan-Meier survival analysis using the Log Rank statistic (GB Stat, Dynamic Microsystems, Inc., Silver Spring, MD). One-tailed tests were used because our a priori hypothesis was that adolescents would acquire faster than adults, and HiS rats would acquire faster than LoS rats. Mean number of days to meet the acquisition criterion, saccharin score, inactive lever responses, and mean food and water consumption during the acquisition period were compared using a 2-way analysis of variance (ANOVA, Statview, Abacus Concepts, Berkeley, CA). For rats that met the acquisition criterion, the mean number of infusions over the last 5 days before the acquisition criterion was met was compared using a 3-way repeated measures ANOVA. Fischer's LSD protected t-tests were used for post hoc comparisons. Additionally, saccharin score, number of days to meet the acquisition criterion, and mean number of infusions over the last 5 days before the acquisition criterion was met (for rats that met the acquisition criterion) were analyzed using a Pearson Product Moment Correlation (Statview, Abacus Concepts, Berkeley, CA). Results were considered statistically significant if p<0.05.
Results
Figure 1 shows the percent of rats in each group meeting the acquisition criterion for i.v. cocaine self-administration over the 30-day acquisition period. A survival analysis indicated that LoS adolescent rats had a significantly steeper acquisition curve than LoS adults (χ2=2.78, p<0.05), but there were no other significant differences due to phenotype or developmental period. A greater percentage of LoS adolescent rats (83%) than LoS adult rats (42%) met the acquisition criterion within 30 days. In both HiS adolescent rats and HiS adult rats, 58% met the acquisition criterion within 30 days.
Figure 1.
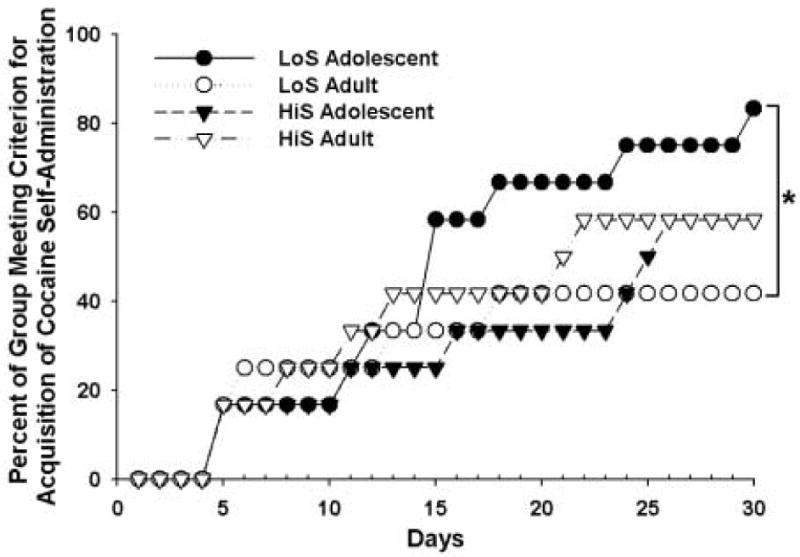
Cumulative percentage of rats in each group acquiring cocaine (0.4 mg/kg) self-administration over the 30-day acquisition period. A survival analysis indicated significant differences between LoS adolescents and LoS adults in their rates of acquisition (p < 0.05). Filled symbols indicate adolescents, and open symbols indicate adults. LoS rats are represented by circles, and HiS rats are represented by triangles.
The mean number of days for each group to acquire cocaine self-administration is shown in Figure 2. Rats that did not acquire cocaine self-administration during the 30-day acquisition period were given a score of 31 days, and the experiment was discontinued after that. LoS adolescents took an average (±SEM) of 17.7 (± 2.7) days to acquire cocaine self-administration, and HiS adolescents took 22.3 (± 4.5) days. LoS adults and HiS adults acquired cocaine self-administration in 22.0 (± 3.9) and 20.0 (± 3.8) days, respectively. There were no significant between-group differences in days to acquire cocaine self-administration. Figure 3 shows the average (±SEM) number of infusions per day over the last 5 days (for rats in each group that met the acquisition criterion). There was a main effect of day (F4,144 = 24.50, p<0.01), with all groups self-administering significantly more cocaine on Day 5 than on Day 1 (p<0.05). There were also significant interactions between developmental period and day (F4,144 = 3.34, p<0.01) and between phenotype and day (F4,144 = 3.91, p<0.01). Post-hoc analyses revealed that adult HiS males self-administered significantly less cocaine than adult LoS males on Day 3 (p<0.05), and on Day 5, adult HiS males self-administered significantly more cocaine than adult LoS males, adolescent HiS males, and adolescent LoS males (p<0.05).
Figure 2.
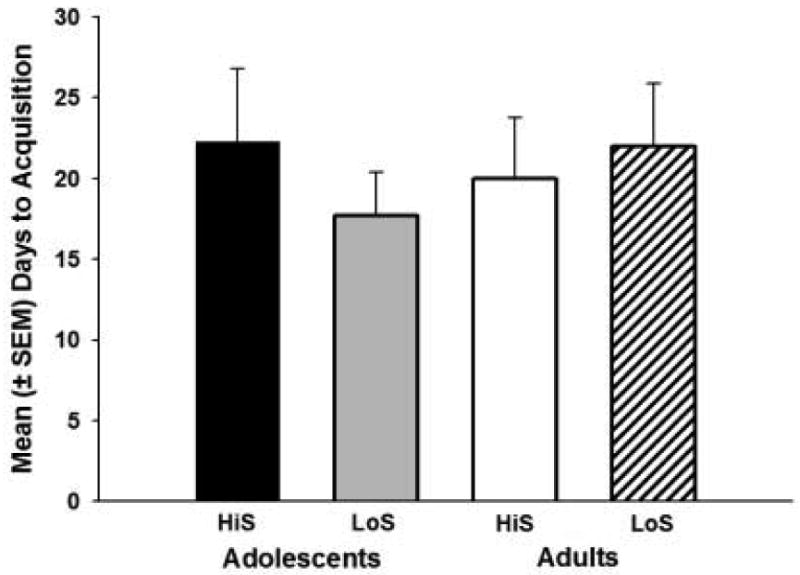
Mean (± SEM) number of days for rats in each group to meet the acquisition criterion for cocaine (0.4 mg/kg) self-administration. Rats that did not acquire cocaine self-administration during the 30-day acquisition period were given a score of 31 days. There were no significant differences between groups.
Figure 3.
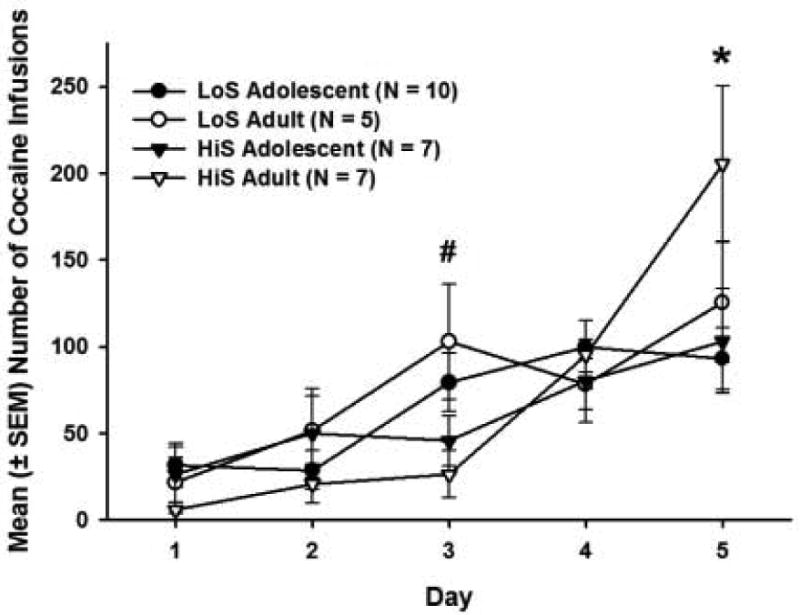
Mean (± SEM) number of cocaine (0.4 mg/kg) infusions over the last 5 days of acquisition for the rats that met the acquisition criterion in each group. Filled symbols indicate adolescents, and open symbols indicate adults. LoS rats are represented by circles, and HiS rats are represented by triangles. On Day 3, LoS adults self-administered significantly more cocaine infusions than HiS adults (# p < 0.05), and on Day 5, HiS adults self-administered significantly more cocaine infusions than all other groups (* p < 0.05).
When the data were collapsed across groups, there was a significant negative correlation between days to meet the acquisition criterion and the number of infusions in the last 5 days of acquisition (r=-0.80, p<0.01), such that fewer days to acquire was associated with a larger number of infusions over the last 5 days of the acquisition period. When the data for each group was analyzed separately, each group showed significant negative correlations between days to meet the acquisition criterion and number of infusions in the last 5 days of acquisition (HiS adolescents: r=-0.69, p<0.05; LoS adolescents: r=-.77, p<0.05; HiS adults: r=-0.91, p<0.05; LoS adults: r=-0.76, p<0.05). Over the last 5 days of acquisition, LoS adolescents self-administered an average (±SEM) of 66.5 (± 4.1) cocaine infusions, while HiS adolescents self-administered 60.9 (± 4.0) cocaine infusions. LoS adults self-administered 75.9 (± 10.7) and HiS adults self-administered 73.6 (± 7.9) cocaine infusions. The number of responses on the standard response lever during the acquisition period was minimal, and there were no group differences in this measure, thus, these data are not shown.
Table 1 shows food and water intake during the cocaine self-administration phase of the experiment and saccharin phenotype scores for each group. When saccharin scores were compared (Figure 4), there were significant effects of developmental period (F1,44 = 25.84, p<0.01) and phenotype (F1,44 = 13.69, p<0.01). Post hoc analyses revealed that HiS and LoS adolescents had significantly higher saccharin scores than their HiS and LoS adult counterparts (p<0.01). HiS adolescents and adults had higher saccharin scores than LoS adolescents and adults, respectively (p<0.05). Additionally, higher saccharin scores were negatively correlated with days to acquisition of cocaine self-administration (r=-0.30, p<0.05). When data from each group was analyzed separately, the HiS adolescent group was the only group to have a significant negative correlation between days to meet the acquisition criterion and the number of days to meet the acquisition criterion (r=-0.73, p<0.05). During the cocaine self-administration portion of the experiment, there were no significant differences in food intake; however, adolescents consumed significantly less water than adults (F1,47=5.55, p<0.05).
Table 1. Food and water intake during the cocaine self-administration period and saccharin phenotype score following cocaine self-administration.
| Group | n | Daily Food Intake (g) (± SEM) | Daily Water Intake (g) (± SEM) | Saccharin Phenotype Score (± SEM) |
|---|---|---|---|---|
| LoS Adolescents | 12 | 19.8 (± 1.1) | 38.6 (± 2.1) | 20.2 (± 2.4) |
| HiS Adolescents | 12 | 19.2 (± 0.9) | 34.6 (± 1.6) | 28.3 (± 3.9) |
| LoS Adults | 12 | 20.0 (± 2.7) | 44.5 (± 3.9) | 5.0 (± 1.5) |
| HiS Adults | 12 | 20.6 (± 2.0) | 41.9 (± 3.1) | 16.6 (± 2.0) |
Figure 4.
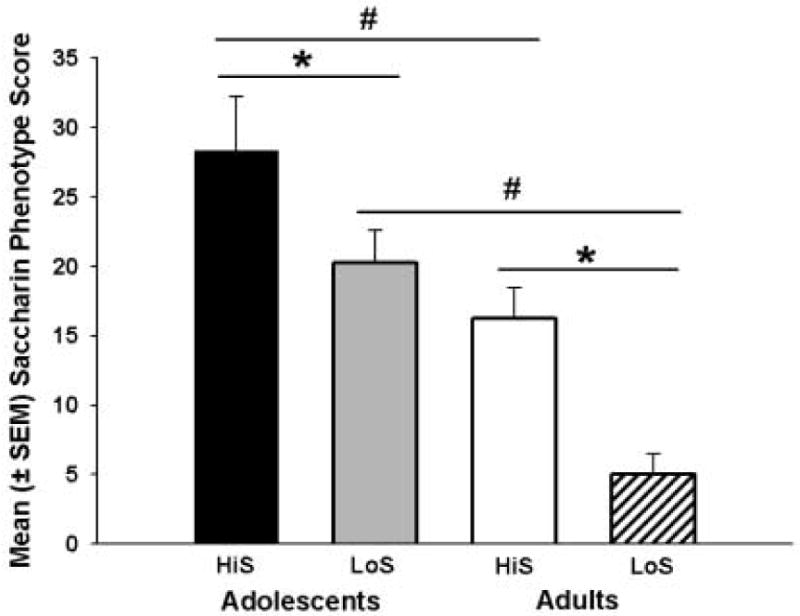
Mean (± SEM) saccharin phenotype score for rats in each group. HiS adolescents and adults had higher saccharin phenotype scores than LoS adolescents and adults, respectively (* p<0.05). HiS and LoS adolescents had higher saccharin phenotype scores than their HiS and LoS adult counterparts (# p<0.01).
Discussion
In the present experiment, acquisition of cocaine self-administration was compared in male adolescent and adult HiS and LoS rats. Adolescent LoS males acquired cocaine self-administration at a faster rate than adult LoS males; however, there were no differences in rates of acquisition between male HiS adolescents and adults. Adolescent HiS and LoS rats exhibited higher saccharin phenotype scores than adult HiS and LoS rats, respectively. Additionally, adolescent and adult HiS rats had higher saccharin phenotype scores than their LoS counterparts. Across all groups, saccharin phenotype score was significantly and negatively correlated with the number of days to acquire cocaine self-administration. Thus, higher scores (greater saccharin intake) predicted more rapid acquisition of cocaine self-administration.
The phenotypic differences in acquisition of cocaine self-administration in adult HiS and LoS males in the present experiment were similar to those of a previous experiment [44], although different doses of cocaine were used. In the present study, 58% of HiS and 42% of LoS male rats met the acquisition criteria for cocaine (0.4 mg/kg) self-administration. In a previous study [44], approximately 63% of HiS males and 18% of LoS males acquired cocaine (0.2 mg/kg) self-administration. Adult HiS and LoS males in the present study spent an average of approximately 20 and 22 days, respectively, in the acquisition period; whereas, in the previous study, adult HiS and LoS males were in the acquisition phase for an average of 18 and 27 days, respectively. As the cocaine dose doubled in the present compared to the previous study, the percentage of adult LoS male rats that met the acquisition criterion in 30 days also doubled, and the mean number of days spent in the acquisition period decreased in this group; however, the rate of acquisition in HiS males was similar in both experiments, possibly indicating a ceiling effect in this group.
Saccharin phenotype scores in the present experiment were similar to saccharin phenotype scores from previous experiments conducted in our laboratory. In the present study, adult LoS males had a saccharin phenotype score of 5.0, while adult HiS males had a score of 16.6. In previous experiments, adult LoS male rats had scores of -4.3 and 11.2, while HiS males had scores of 7.5 and 29.7, respectively [44, Carroll et al., unpublished data]. Comparable to HiS and LoS adults, HiS adolescent saccharin phenotype scores were higher than those of LoS adolescents. However, saccharin phenotype scores in adolescents were significantly higher than in adults in the present study. This avidity for sweets is consistent with previous reports that adolescent rats preferred sweeter sucrose solutions to a greater extent than did adult rats [40]. Also, studies in humans indicate greater sweet preference in younger vs. older subjects [41].
The current experiment extends previous research suggesting that adolescents are more sensitive to the reinforcing effects of drugs of abuse than adults [e.g., 9]. For example, several experiments have suggested that low doses of drugs produced conditioned place preference in adolescents, but not adults [22, 23, 24, but see 25, 26, 27]. Additionally, adolescents displayed heightened behavioral sensitization compared to adults following repeated administration of psychomotor stimulants [11, 31, 36, 37, but see 27]. In the present study, adolescent LoS rats acquired cocaine self-administration faster and in greater numbers than adult LoS rats; however, there were no significant differences in rate of acquisition between adolescent and adult HiS rats. This suggests that while adolescence may be a developmental period of enhanced vulnerability to drug abuse, genetic background also plays a role in this effect.
In previous studies of drug self-administration, adolescents had higher levels of nicotine [13], alcohol [14, 15], and amphetamine [16] intake than adults. This effect was not found in the present study when cocaine intake over the last 5 days of cocaine self-administration was compared between adolescent and adult HiS and LoS rats that had met the criterion for acquisition of cocaine self-administration. On the final day of cocaine self-administration, adult HiS rats self-administered significantly more cocaine infusions than adult LoS rats, adolescent HiS rats, and adolescent LoS rats, but in the 4 days prior to that, there were no significant differences between adolescent and adult groups. It is possible that we would have seen more differences between adolescent and adult groups if we had used a lower cocaine dose, as lower doses of psychostimulants tend to reveal subtle individual differences [63]. However, when the lower cocaine dose (0.2 mg/kg) was used in an earlier study with male Wistar rats, only 30% of the group acquired [51], and pilot work with LoS males yielded very few animals that acquired; thus, the dose was increased to 0.4 mg/kg. Therefore, one caveat of the present results is that differences due to phenotype and developmental phase may have been obtained at other doses. Future research should employ a range of doses to determine the role of dose in adolescent (vs. adult) cocaine self-administration.
In conclusion, male adolescent LoS rats acquired cocaine self-administration faster and in greater numbers (percent of group) than male adult LoS rats. However, there were no significant differences in rates of acquisition between male adolescent and adult HiS rats. Adolescent and adult HiS rats had higher saccharin phenotype scores than their LoS counterparts, and adolescent HiS and LoS rats had higher saccharin phenotype scores than adult HiS and LoS rats, respectively. Additionally, saccharin phenotype score was significantly and negatively correlated with days to acquire cocaine self-administration. Adolescents may experience enhanced vulnerability to drug abuse, and genetic factors (e.g., HiS vs. LoS) underlie this enhanced vulnerability. The data support previous findings that higher levels of intake of sweetened liquids are related to increased vulnerability to drug abuse. Future studies should address potential sex differences in adolescent vulnerability to drug abuse, and they should also determine the relationship between cocaine dose used and adolescent vulnerability. Further study of the relationship between adolescence, saccharin intake, and impulsivity will lead to a greater understanding of the ways in which these factors interact and additively influence vulnerability to drug abuse.
Acknowledgments
The authors would like to thank Justin J. Anker, Luke Gliddon, Erin B. Larson, Jennifer L. Newman, and Jason T. Ross for their helpful comments on earlier versions of this manuscript. This work was supported by NIH grants R01 DA03240 (MEC), K05 DA15267 (MEC), and F31 DA020237 (JLP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23:991–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- 2.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barron S, White A, Swartzwelder HS, Bell RL, Rodd ZA, Slawecki CJ, Ehlers CL, Levin ED, Rezvani AH, Spear LP. Adolescent vulnerabilities to chronic alcohol or nicotine exposure: findings from rodent models. Alcohol Clin Exp Res. 2005;29:1720–5. doi: 10.1097/01.alc.0000179220.79356.e5. [DOI] [PubMed] [Google Scholar]
- 4.Spear LP. The adolescent brain and age-related behavioral manifestation. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 5.Newcomb MD. Identifying high-risk youth: prevalence and patterns of adolescent drug abuse. NIDA Res Mono. 1985;156:7–37. [PubMed] [Google Scholar]
- 6.Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug Alcohol Depend. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- 7.Clark DB, Kirisci L, Tarter RE. Adolescent versus adult onset and the development of substance use disorders in males. Drug Alcohol Depend. 1998;49:115–121. doi: 10.1016/s0376-8716(97)00154-3. [DOI] [PubMed] [Google Scholar]
- 8.Estroff TW, Schwartz RH, Hoffmann NG. Adolescent cocaine abuse. Addictive potential, behavioral and psychiatric effects. Clin Pediatr (Phila) 1989;28:550–5. doi: 10.1177/000992288902801201. [DOI] [PubMed] [Google Scholar]
- 9.Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–59. [PubMed] [Google Scholar]
- 10.Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23:4712–6. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faraday MM, Elliott BM, Phillips JM, Grunberg NE. Adolescent and adult male rats differ in sensitivity to nicotine's activity effects. Pharmacol Biochem Behav. 2003;74:917–31. doi: 10.1016/s0091-3057(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 12.Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcohol Clin Exp Res. 2002;26:1632–41. doi: 10.1097/01.ALC.0000036301.36192.BC. [DOI] [PubMed] [Google Scholar]
- 13.Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacol. 2003;169:141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- 14.Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–53. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- 15.Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- 16.Frantz KJ, Shahbazi M. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharm. 2006 doi: 10.1038/sj.npp.1301130. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Wilmouth CE, Spear LP. Adolescent and adult rats' aversion to flavors previously paired with nicotine. Ann N Y Acad Sci. 2004;1021:462–4. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- 18.O'Dell LE, Bruijnzeel AW, Ghozland S, Markou A, Koob GF. Nicotine withdrawal in adolescent and adult rats. Ann N Y Acad Sci. 2004;1021:167–74. doi: 10.1196/annals.1308.022. [DOI] [PubMed] [Google Scholar]
- 19.Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–8. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 20.Silveri MM, Spear LP. Acute, rapid, and chronic tolerance during ontogeny: observations when equating ethanol perturbation across age. Alcohol Clin Exp Res. 2001;25:1301–8. [PubMed] [Google Scholar]
- 21.Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–6. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- 22.Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–14. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- 23.Philpot RM, Badanich KA, Kirstein CL. Place conditioning: age-related changes in the rewarding and aversive effects of alcohol. Alcohol Clin Exp Res. 2003;27:593–9. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- 24.Bolanos CA, Garmsen GM, Clair MA, McDougall SA. Effects of the kappa-opioid receptor agonist U-50,488 on morphine-induced place preference conditioning in the developing rat. Eur J Pharmacol. 1996;317:1–8. doi: 10.1016/s0014-2999(96)00698-x. [DOI] [PubMed] [Google Scholar]
- 25.Campbell JO, Wood RD, Spear LP. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiol Behav. 2000;68:487–93. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- 26.Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: two behavioral features of adolescence in mice. Behav Neurosci. 2003;117:695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- 27.Schochet TL, Kelley AE, Landry CF. Differential behavioral effects of nicotine exposure in adolescent and adult rats. Psychopharmacol. 2004;175:265–73. doi: 10.1007/s00213-004-1831-9. [DOI] [PubMed] [Google Scholar]
- 28.Adriani W, Laviola G. A unique hormonal and behavioral hyporesponsivity to both forced novelty and d-amphetamine in periadolescent mice. Neuropharmacol. 2000;39:334–46. doi: 10.1016/s0028-3908(99)00115-x. [DOI] [PubMed] [Google Scholar]
- 29.Spear LP, Brake SC. Periadolescence: Age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- 30.Bolanos CA, Glatt SJ, Jackson D. Subsensitivity to dopaminergic drugs in periadolescent rats: a behavioral and neurochemical analysis. Dev Brain Res. 1998;111:25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- 31.Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exp Ther. 1995;275:345–57. [PubMed] [Google Scholar]
- 32.Torres-Reveron A, Dow-Edwards D. Repeated administration of methylphenidate in young, adolescent, and mature rats affects the response to cocaine later in adulthood. Psychopharmacol. 2005;181:38–47. doi: 10.1007/s00213-005-2221-7. [DOI] [PubMed] [Google Scholar]
- 33.Wooters TE, Dwoskin LP, Bardo MT. Age and sex differences in the locomotor effect of repeated methylphenidate in rats classified as high or low novelty responders. Psychopharmacol. 2006;188:18–27. doi: 10.1007/s00213-006-0445-9. [DOI] [PubMed] [Google Scholar]
- 34.Caster JM, Walker QD, Kuhn CM. Enhanced behavioral response to repeated-dose cocaine in adolescent rats. Psychopharmacol. 2005;183:218–225. doi: 10.1007/s00213-005-0159-4. [DOI] [PubMed] [Google Scholar]
- 35.Catlow B, Kirstein CL. Heightened cocaine-induced locomotor activity in adolescent compared to adult female rats. J Psychopharmacol. 2005;19:443–447. doi: 10.1177/0269881105056518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112:1152–66. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- 37.Faraday MM, Elliott BM, Grunberg NE. Adult vs. adolescent rats differ in biobehavioral responses to chronic nicotine administration. Pharmacol Biochem Behav. 2001;70:475–89. doi: 10.1016/s0091-3057(01)00642-6. [DOI] [PubMed] [Google Scholar]
- 38.Chutuape M, de Wit H. Relationship between subjective effects and drug preferences: Ethanol and diazepam. Drug Alcohol Depend. 1994;34:243–251. doi: 10.1016/0376-8716(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 39.Kelley AE, Schochet T, Landry CF. Risk taking and novelty seeking in adolescence. Ann N Y Acad Sci. 2004;1021:27–32. doi: 10.1196/annals.1308.003. [DOI] [PubMed] [Google Scholar]
- 40.Vaidya JG, Grippo AJ, Johnson AK, Watson D. A comparative developmental study of impulsivity in rats and humans: the role of reward sensitivity. Ann N Y Acad Sci. 2004;1021:395–8. doi: 10.1196/annals.1308.051. [DOI] [PubMed] [Google Scholar]
- 41.Pelchat ML. Food cravings in young and elderly adults. Appetite. 1997;28:103–113. doi: 10.1006/appe.1996.0063. [DOI] [PubMed] [Google Scholar]
- 42.Dess NK. Responses to basic taste qualities in rats selectively bred for high versus low saccharin intake. Physiol Behav. 2000;69:247–257. doi: 10.1016/s0031-9384(99)00246-2. [DOI] [PubMed] [Google Scholar]
- 43.Dess NK, Badia-Elder NE, Thiele TE, Kiefer SW, Blizard DA. Ethanol consumption in rats selectively bred for differential saccharin intake. Alcohol. 1998;16:275–8. doi: 10.1016/s0741-8329(98)00010-x. [DOI] [PubMed] [Google Scholar]
- 44.Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacol. 2002;161:304–13. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- 45.Perry JL, Morgan AD, Anker JJ, Dess NK, Carroll ME. Escalation of i.v. cocaine self-administration and reinstatement of cocaine-seeking behavior in rats bred for high and low saccharin intake. Psychopharmacol. 2006;186:235–45. doi: 10.1007/s00213-006-0371-x. [DOI] [PubMed] [Google Scholar]
- 46.Carroll ME, Anderson MM, Morgan AD. Regulation of intravenous cocaine self-administration in rats selectively bred for high (HiS) and low (LoS) saccharin intake. Psychopharmacol. 2006 doi: 10.1007/s00213-006-0600-3. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 47.Roth ME, Casimir AG, Carroll ME. Influence of estrogen in the acquisition of intravenously self-administered heroin in female rats. Pharmacol Biochem Behav. 2002;72:313–8. doi: 10.1016/s0091-3057(01)00777-8. [DOI] [PubMed] [Google Scholar]
- 48.Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;68:641–6. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 49.Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacol. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- 50.Carroll ME, Lac ST. Autoshaping i.v. cocaine self-administration in rats: effects of nondrug alternative reinforcers on acquisition. Psychopharmacol. 1993;110:5–12. doi: 10.1007/BF02246944. [DOI] [PubMed] [Google Scholar]
- 51.Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacol. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- 52.Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacol. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- 53.Bardo MT, Dwoskin LP. Biological connection between novelty- and drug-seeking motivational systems. Nebr Symp Motiv. 2004;50:127–58. [PubMed] [Google Scholar]
- 54.Perry JL, Morgan AD, Anker JJ, Dess NK, Carroll ME. Escalation of i.v. cocaine self-administration and reinstatement of cocaine-seeking behavior in rats bred for high and low saccharin intake. Psychopharmacol. 2006;186:235–45. doi: 10.1007/s00213-006-0371-x. [DOI] [PubMed] [Google Scholar]
- 55.Odell WD. Sexual maturation in the rat. In: Grumbach MM, Sizonenko PC, Aubert ML, editors. Control of the Onset of Puberty. Baltimore, MD: Williams and Wilkins; 1990. pp. 182–210. [Google Scholar]
- 56.Badia-Elder NE, Kiefer SW, Dess NK. Taste reactivity in rats selecively bred for high versus low saccharin consumption. Physiol Behav. 1996;59:749–755. doi: 10.1016/0031-9384(95)02131-0. [DOI] [PubMed] [Google Scholar]
- 57.National Research Council Guidelines for the care and use of mammals in Neuroscience and Behavioral Research. Washington D.C.: The National Academies Press; 2003. p. 209. [PubMed] [Google Scholar]
- 58.Caine SB, Negus SS, Mellow NK, Bergman J. Effects of dopamine D(1-like) and D(2-like) agonists in rats that self-administer cocaine. J Pharmacol Exp Ther. 1999;291:353–360. [PubMed] [Google Scholar]
- 59.Messing RB, Sparber SB. Greater task difficulty amplifies the facilitatory effect of des-glycinamide arginine vasopressin on appetitively motivated learning. Behav Neurosci. 1985;99:1114–9. doi: 10.1037//0735-7044.99.6.1114. [DOI] [PubMed] [Google Scholar]
- 60.Campbell UC, Carroll ME. Acquisition of drug self-administration: environmental and pharmacological interventions. Exp Clin Psychopharmacol. 2000;8:312–25. doi: 10.1037//1064-1297.8.3.312. [DOI] [PubMed] [Google Scholar]
- 61.Carroll ME, Lac ST. Dietary additives and the acquisition of cocaine self-administration in rats. Psychopharmacol. 1998;137:81–9. doi: 10.1007/s002130050596. [DOI] [PubMed] [Google Scholar]
- 62.Carroll ME, Lac ST. Acquisition of i.v. amphetamine and cocaine self-administration in rats as a function of dose. Psychopharmacol. 1997;129:206–14. doi: 10.1007/s002130050182. [DOI] [PubMed] [Google Scholar]
- 63.Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–9. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]


