Abstract
Previous research in rats indicates that delay discounting for food, a model of impulsivity, predicted the rate of acquisition of cocaine self-administration. In other studies, rats bred for high saccharin intake (HiS) acquired cocaine self-administration at higher rates than those with low saccharin intake (LoS), and female (F) rats acquired cocaine self-administration more rapidly than males (M). The purpose of this study was to examine a possible connection between impulsivity, saccharin intake, and sex by comparing M and F rats from the HiS and LoS selectively bred lines on measures of impulsivity; i.e., their rate of delay discounting for food or i.v. cocaine infusions. The adjusting delay procedure allowed rats access to 2 response levers, and a pellet dispenser or an i.v. drug infusion pump. In 4 groups (HiS M, HiS F, LoS M, LoS F) responses under a fixed-ratio (FR) 1 schedule on one lever resulted in one 45 mg pellet immediately, and responses on the other lever resulted in 3 or 6 pellets after a delay. Four additional groups received either a small cocaine (0.2, 0.4, or 0.8 mg/kg) infusion immediately or a delayed larger infusion (3 × the amount of the small infusions). The delay to the larger reinforcer began at 6 sec and increased or decreased by 1 s following responses on the delay or immediate levers, respectively. A mean adjusted delay (MAD) was calculated over 30 choice trials during each daily 3-hr session, and it was used as a quantitative measure of impulsivity. In groups maintained by food, HiS rats were more impulsive (lower MADs) than LoS rats, and LoS females were more impulsive than LoS males. There were no phenotype or sex differences in delay discounting for cocaine. Understanding the relationship between impulsivity and other predictors of drug abuse (e.g., sex, saccharin intake) is important in developing prevention and treatment strategies.
Keywords: Adjusting Delay, Cocaine, Delay Discounting, Food, Impulsivity, Saccharin Preference, Selective Breeding, Sex
1. Introduction
It has been argued that drug abuse and impulsivity are closely related because those who abuse drugs value the immediate reward of the drug's effects over other delayed rewards such as good health, good relationships, or job-related productivity (e.g., Madden et al., 1997). Human drug-using populations valued future monetary rewards less than their non-drug abusing counterparts (Allen et al., 1998; Petry, 2003). This is true for cigarette smokers (Baker et al., 2003; Bickel et al., 1999; Mitchell, 1999; Reynolds et al., 2004 but see Ohmura et al. 2005), problem drinkers (Petry, 2001; Vuchinich and Simpson, 1998 but see Kirby and Petry 2004), opioid-dependent individuals (Kirby and Petry, 2004; Kirby et al., 1999; Madden et al., 1997), and crack/cocaine abusers (Coffey et al., 2003; Heil et al., 2005; Kirby and Petry, 2004). It is unclear, however, whether impulsivity precedes and predicts drug abuse, whether drug abuse influences impulsivity, whether both of these conditions exist, or whether there are additional factors, such as other forms or expressions of excessive behavior or stress that underlie and interact with both drug abuse and impulsivity.
Considering the first possibility, there are several lines of evidence suggesting that impulsivity precedes and predicts drug abuse. In one study, rats that frequently chose a small-immediate food reward over a large-delayed reward consumed significantly more of a 12% w/v ethanol solution than rats that chose the small-immediate reward less frequently (Poulos et al., 1995). In a second study, rats were divided into high (HiI) and low (LoI) impulsive groups based on performance on an adjusting delay task for food reward, and subsequently, HiI rats acquired cocaine self-administration faster and in greater numbers than LoI rats (Perry et al., 2005; Perry et al., 2007b). Finally, mice that were more impulsive on a delay discounting task exhibited less locomotor stimulation after initial exposure to ethanol and greater sensitization following repeated exposure to ethanol (Mitchell et al., 2006).
The hypothesis that drugs of abuse affect impulsivity has also been supported by both animal and human laboratory studies. In rodents, administration of psychomotor stimulants had mixed effects on delay discounting. Amphetamine (van Gaalen et al., 2005; Wade et al., 2000), d-amphetamine (Isles et al., 2003), methylphenidate (van Gaalen et al., 2005), and methamphetamine (Richards et al., 1999) decreased impulsivity, while d-amphetamine (Charrier and Thiebot, 1996; Evenden and Ryan, 1996) and cocaine (Logue et al., 1992) increased impulsivity in rodents. The discrepancies in these results may be due to type of reinforcer, cues present during the delay to the presentation of the larger reinforcer, drug dose, or dosing regimen (for a review, see Perry and Carroll, 2007); however, they clearly show that drugs influence impulsive behavior.
That additional factors may interact with and influence both impulsivity and drug abuse has not been frequently examined in the preclinical literature; however, selectively bred rodent lines (e.g., Lewis/Fischer 344) may be useful when studying the role of genetic differences in impulsive behavior. Lewis rats more readily self-administered drugs of abuse than Fischer 344 rats (Kosten et al., 1997; Martin et al., 1999; Suzuki et al., 1988), and they showed increased cocaine (Kosten et al., 1994) and nicotine (Horan et al., 1997) conditioned place preference compared with Fischer 344 rats. Additionally, Lewis rats were more impulsive than Fischer 344 rats in a delay discounting task (Anderson and Woolverton, 2005). It is possible that the differences in impulsivity and drug-related behaviors in these rat strains were due to underlying genetic factors.
Dietary preference has also emerged as a major factor underlying drug abuse vulnerability, and proclivity for preferred dietary substances predicts many aspects of drug abuse (e.g., Carroll et al., 2002; Perry et al., 2006a). For example, rats that have been selectively bred for high (HiS) and low (LoS) levels of saccharin intake also show corresponding differences in drug-related behaviors. HiS rats consumed more ethanol than LoS rats (Dess et al., 1998); they acquired i.v. cocaine self-administration faster than LoS rats (Carroll et al., 2002) and reinstated cocaine-seeking behavior after a period of abstinence more than LoS rats (Perry et al., 2006a). HiS rats also showed greater escalation (Perry et al., 2006a) and dysregulation (Carroll et al., 2007b) of cocaine intake compared with LoS rats. HiS female rats also showed greater sensitization to the locomotor-activating effects of cocaine than LoS females, LoS males, and HiS males (Carroll et al., 2007a). The HiS and LoS rats were used in the present study to examine the interaction of impulsivity with another major vulnerability factor that strongly predicts drug abuse (dietary preference). Given the comparable differences in drug-related behaviors in HiS vs. LoS rats and HiI vs. LoI rats, we hypothesized that HiS rats would be more impulsive than LoS rats. One study has demonstrated that sucrose preference and impulsivity were higher in adolescent (vs. adult) rats and humans (Vaidya et al., 2004); however, we are not aware of other studies that have assessed the relationship between avidity for sweetened dietary substances and impulsivity.
In addition to avidity for sweetened dietary substances, sex appears to be a major factor in human drug abuse and in animal models of drug abuse, with females exhibiting greater drug-seeking behavior than males under a wide range of conditions (for reviews, see Carroll et al., 2004; Lynch et al., 2002; Roth et al., 2004). For example, female rats exceeded males in acquisition of drug self-administration (e.g., Carroll et al., 2000; Lynch and Carroll, 1999), escalation of drug intake under extended access conditions (Carroll et al., 2005; Roth and Carroll, 2004), extinction, and cocaine-primed reinstatement (Lynch and Carroll, 2000). Females also regulated their drug intake less precisely (Lynch and Carroll, 2001) and showed more binge-like patterns (Morgan et al., 2002) than males. That females exceed males in a number of drug-related behaviors is influenced by hormonal status (e.g., Carroll et al., 2004; Roth et al., 2004); however, it may also be enhanced by elevated impulsivity, another major vulnerability factor, in females. Clinical studies of sex differences in impulsivity have reported mixed findings, that women have lower (Kirby and Marakovic, 1996), higher (Wallace, 1979), or the same (Fillmore and Weafer, 2004; Skinner et al., 2004) levels of impulsivity compared to males. In a preclinical experiment addressing the relationship between sex and impulsivity, Jentsch and Taylor (2003) used a Go/No-go task in which rats were trained to respond for a food pellet upon illumination of a stimulus light (the Go period). When the light was not illuminated (the No-go period), males had higher levels of responding than females, indicating higher levels of impulsivity. However, males and females were fed the same amount of food despite differing body weights, which may have increased the motivation to respond more in males (vs. females). In a second preclinical study of sex differences in impulsivity, male and female rats performed similarly on a delay discounting task for food; however, the results may have been due to a ceiling effect since more than 70% of the rats (both male and female) were highly impulsive on the task (Perry et al., 2007b).
The goal of the present experiment was to determine whether there were differences in impulsive choices for food and cocaine in male and female HiS and LoS rats. Based on other forms of drug-seeking behavior (e.g., HiS>LoS, F>M) during several phases of addiction, we hypothesized that HiS rats would be more impulsive than LoS rats and that females would be more impulsive than males using delay discounting procedures maintained by food pellets or i.v. cocaine infusions. Locomotor testing was conducted prior to the delay discounting training to determine whether there were underlying sex or phenotypic differences.
2. Methods
2.1. Animals
Eighty-six 90-day old experimentally naïve Sprague-Dawley rats that were selectively bred for HiS or LoS as previously described (Carroll et al., 2002; Perry et al., 2006a) were used as subjects. Briefly, the HiS/LoS rat lines were started by pairing an avid saccharin-drinking male with several females that had average levels of saccharin consumption. In subsequent generations, HiS males with were paired with HiS females, and LoS males were paired with LoS females (Dess and Minor, 1996). By the third generation, the saccharin intake of the HiS and LoS lines diverged. A group of rats in the 17-18th generations were obtained from a breeding program at Occidental College (Los Angeles, CA) for continued breeding in our laboratory at the University of Minnesota (Minneapolis, MN). The outbred status of the lines has been maintained through periodic mating with the original parental stock (Holtzman, HSD from Harlan Sprague-Dawley, Inc., Indianapolis, IN), and siblings, half-siblings, or first cousins were never paired for mating.
During the food and cocaine self-administration portions of the experiments, all rats were supplemented after their daily sessions to maintain them at 85% of their free-feeding weight (16 g for females and 20 g for males). At the onset of the study, females weighed approximately 260-300 g and males weighed 430-480 g. Prior to the experiments, rats were housed in same-sex pairs in plastic holding cages with ad libitum food and water. In the locomotor testing phase and in the food delay discounting phase, rats continued to be housed individually in plastic holding changes, and they were moved to experimental chambers for daily sessions. Rats working for i.v. cocaine under a delay discounting schedule were implanted with a chronic indwelling catheter in the right jugular vein, and they subsequently lived in individual experimental chambers. Upon completion of the cocaine self-administration portion of the study, rats were housed individually in plastic holding cages for determination of their saccharin phenotype score. Throughout the experiments, animals were housed in rooms that were maintained at a constant temperature (24°C) and humidity level and had a 12 h light/dark cycle (lights on at 6:00 a.m.). Use of animals for this protocol was approved by the University of Minnesota Institutional Animal Care and Use Committee (protocol #0410A64760). Laboratory facilities were accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), and recommended principles of animal care were followed (National Research Council, 2003).
2.2. Apparatus
2.2.1. Locomotor activity
A circular stainless steel locomotor track (inner diameter 46 cm; outer diameter 71 cm) similar to that reported by Piazza et al. (1989; 1990) was used to measure locomotor activity over 2 days. The walls of the track were 25 cm high and 5 cm above the floor of the track, 4 infrared sensors (SE612CV, Banner Engineering Corp., Minneapolis, MN) were mounted on the outer wall at 0°, 90°, 180°, and 270°. Sensors were connected to a VersaMax programmable logic controller (IC200UDR001, GE Fanuc Automation, Charlottesville, VA), and data were recorded using PCs and VersaPro software (GE Fanuc Automation, Charlottesville, VA).
2.2.2. Delay discounting for food
As previously described (Perry et al., 2005), eight-sided operant chambers consisting of alternating Plexiglas and stainless-steel walls were used for the food delay discounting experiment. A 45 mg pellet feeder attached to a pellet delivery trough (Coulbourn Instruments, Allentown, NJ) was mounted on one of the stainless steel walls, and standard response levers were mounted on the stainless steel walls on either side of it. Three colored (red, yellow, green) stimulus lights (4.6 W) were mounted above each lever, and a white 4.6 W house light was located at the top of each chamber. Each chamber was enclosed in a wooden sound-attenuating enclosure equipped with a small ventilation fan. PCs and MED-PC software (Med Associates, St. Albans, VT) were used for data collection and experimental programming.
2.2.3. Delay discounting for cocaine
Operant chambers identical to those described above were used for the cocaine delay discounting, with the exception that the pellet feeder was replaced with an insertion for a food jar, and the stainless steel walls of the cage were rearranged so that the levers were placed on two consecutive stainless-steel walls. Each rat was implanted with an indwelling catheter that was connected to a metal cannula (C3236, Plastics One, Roanoke, VA) embedded in the center of a soft plastic covance-infusion harness (CIH95 Instech Laboratories, Plymouth Meeting, PA). Spring-covered infusion tubing (C313CS, Plastics One, Roanoke, VA) connected the metal cannula to a swivel (050-0022, Alice King Chatham, Hawthorne, CA) that was connected to a syringe pump (PHM-100 Med Associates, St. Albans, VT) with Tygon tubing (1.52 mm o.d.; 0.51 mm i.d., Fisher Scientific, Springfield, NJ). Syringes (30 ml) and syringe pumps were mounted on the inside of each rat's wooden enclosure. Experiments were programmed and data were recorded using PCs and MED-PC software (Med Associates, St. Albans, VT).
2.3. Drugs
Cocaine HCl was obtained from the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC), dissolved in sterile physiological saline, and stored in 30 ml syringes that were placed in syringe pumps. Drug solutions were made biweekly and refrigerated, and they were added to syringes at room temperature as needed. Each cocaine infusion was delivered at a rate of 0.03 ml/s, and the infusion duration was 1 s/100 g of body weight.
2.4. Procedure
2.4.1. Locomotor activity
Because locomotor activity in a novel environment has been shown to predict subsequent drug self-administration (Mantsch et al., 2001; Piazza et al., 1989; Piazza et al., 1990), the purpose of this part of the experiment was to determine whether differences in delay discounting were related to locomotor activity. To obtain measures of novelty reactivity (Day 1) and basal locomotor activity (Day 2), each rat was placed in a circular locomotor track and allowed to run for 45 min on 2 consecutive days as previously described (Perry et al., 2005). The track was covered with a sheet of clear Plexiglas during the trials. Locomotor testing was conducted between 8:00 a.m. and 4:00 p.m., and rats were tested at the same time each day. Locomotor counts were only included if the rat interrupted 2 infrared sensors in succession, and these counts were totaled and recorded in 5 min increments. Behavior on the first day (considered a measure of reactivity to a novel environment) and the second day (a measure of basal locomotor activity) were analyzed.
2.4.2. Delay discounting for food
As previously described (Perry et al., 2005), daily sessions began at either 8 a.m. or 11:30 a.m. (with each rat being tested at the same time each day), and they ended after the completion of 60 trials or 3 h, whichever occurred first. Each session consisted of 15 4-trial blocks, and in each block, the first trial was a forced-choice trial on the left lever, and the second trial was a forced choice on the right lever. These forced choice trials were signaled by illumination of the stimulus lights above the lever requiring a response on that trial. The third and fourth trials in each block were free-choice trials, and the stimulus lights above both levers were illuminated. A response on one lever yielded immediate delivery of one grain-based 45 mg pellet (PJA1-0045, Research Diets Inc., New Brunswick, NJ); whereas, a response on the other lever resulted in 3 grain-based 45 mg pellets delivered after a delay. The lever side associated with the immediate or delayed reward alternated daily. An intertrial interval (ITI) was imposed immediately following each lever press so that regardless of the delay associated with the lever press, each trial would last exactly 60 s. During the ITI, the stimulus and house lights were turned off, and responses on the levers had no programmed consequences.
At the start of each experiment, the initial delay to the delivery of the larger reward was set at 6 s, and it increased or decreased depending on responding during the free-choice trials (i.e., the third and forth trials in each block). A response on the larger-delayed reward lever yielded a 1 s increase in the delay, and a response on the small-immediate reward lever resulted in a 1 s decrease in the delay. The adjusting delay determined on the fourth trial of each block was used as the delay in the forced-choice trials in the next block of trials. The final adjusting delay at the end of 60 trials was used for the initial delay in the next daily session. A mean adjusted delay (MAD) was calculated for each session by taking the average of all adjusting delays on the free-choice trials (30 trials per session). This procedure was repeated until the MAD stabilized, which was defined by variation of less than 5 s across 5 days and no steadily increasing or decreasing trends over the 5 days. Following stability, the quantity of the large-delayed food reinforcer was changed from 3 to 6, and adjusting delay procedures continued until stability was reached again. Stable MAD values were used as a quantitative measure of impulsivity, with lower MADs indicating higher levels of impulsivity. The magnitude of the small-immediate vs. large-delayed was changed from 3 to 6 in the food-reinforced task because preliminary data indicated that offering more food pellets during any given session would result in the animals completing fewer than 60 trials within the 3 hr time limit.
2.4.3. Delay discounting for cocaine
In the delay discounting for cocaine portion of the experiment, indwelling i.v. catheters were implanted. Rats were anesthetized with ketamine (60 mg/kg) and xylazine (10 mg/kg), and they were also given dopram (5 mg/kg) and atropine (0.15 cc) to facilitate respiration. They were subsequently implanted with a chronic indwelling silastic catheter (0.51 mm i.d., 0.94 mm o.d.; Helix Medical Inc., Carpinteria, CA) in the right jugular vein to the level of the right atrium as previously described (Carroll et al., 2002; Perry et al., 2005). Each catheter was approximately 15 cm long, and it had two beads of prosthetic silicone elastomer (MDX4-4210; Factor II, Inc., Lakeside, AZ) 3.0 and 3.5 cm from one end. The distal end of the catheter was led subcutaneously to a medial incision made 1 cm caudal to the scapulae and connected to the harness via the cannula. Rats were administered heparin (10 IU/kg, i.v.) and gentomycin (2.0 mg/kg, i.v.) each day during a 3-day recovery period after surgery. Additionally, buprenorphine hydrochloride (0.2 mg/kg) was given subcutaneously once every 12 hours for 48 hours following surgery. Following the recovery period, the cocaine-reinforced delay discounting sessions were conducted 7 days per week. Catheter patency was checked approximately every 7 days by administering a solution containing 30 mg/ml ketamine and 1.5 mg/ml midazolam (0.1-0.2 ml, i.v., Caine et al., 1999). Patency was assumed if a loss of the righting reflex was observed immediately following administration.
The cocaine-reinforced delay discounting sessions were identical to the food-reinforced sessions, with the exception that i.v. cocaine infusions, instead of food pellets, were delivered contingent upon a lever press response. The small-immediate cocaine reward was initially a 0.4 mg/kg infusion, and after MAD values stabilized, it was changed to 0.2 and 0.8 mg/kg in nonsystematic order. Stability was defined as MADs varying less than 4 sec over 3 days, with no steadily increasing or decreasing trends. The MAD was assessed over a more narrow range of values (4 sec vs. 5 sec) and over a shorter number of days (3 days vs. 5 days) in cocaine sessions (vs. food sessions) because requiring a shorter stability period decreased the likelihood of maintaining functioning catheters during the course of the experiment. The large-delayed cocaine reward was 3 times (1.8, 3.6, and 7.2 mg/kg) the amount of the small-immediate reward (0.6, 1.2, and 2.4 mg/kg, respectively). The small-immediate cocaine doses were chosen because preliminary data from our laboratory suggested that these doses were all on the ascending limb of the dose-response curve in HiS and LoS rats (unpublished data). Additionally, we wanted to keep the 1:3 ratio from the initial food condition, and 3 times each of these small-immediate doses would result in a cocaine dose that would be self-administered readily (i.e., the large-delayed cocaine doses were not too large for the rats to self-administer).
2.4.4. Saccharin phenotype score testing
Following the completion of testing impulsivity measures, rats were subjected to a 24-h two-bottle choice test with 0.1% (w/v) sodium saccharin and water to measure saccharin intake (Dess et al., 1998). The difference in consumption of saccharin in the two-bottle choice test and a previous measure of 24-hour water intake (when only water was present) were calculated as a percentage of the rat's body weight using the following equation:
Using this equation, a positive score indicated saccharin preference, a negative score indicated a saccharin aversion, and a score of zero reflected no aversion or preference for saccharin (Dess et al., 1998). This test was conducted at the end of the experiment so that saccharin intake would not influence cocaine- or food-maintained responding.
2.5. Data Analysis
Data for the food and cocaine groups were analyzed independently. In each of these, the main dependent measures were the Day 1 mean and the Day 2 mean number of locomotor counts, MAD values, latency to respond on the right and left levers during both free and forced choice trials, and number of reinforcers earned per session. The Day 1 and Day 2 mean numbers of locomotor counts were analyzed in 5-min increments between HiS and LoS males and females using a 3-way repeated measures analysis of variance (ANOVA; sex × phenotype × time; GB Stat, Dynamic Microsystems, Inc., Silver Spring, MD). MAD values, response latencies, and number of reinforcers earned per session were also analyzed using a 3-way repeated measures ANOVA (sex × phenotype × food reinforcer magnitude for food groups and sex × phenotype × drug dose for drug groups). For illustrative purposes, the distribution of MAD values for each group under each condition was also graphed, and the skewness and kurtosis of each distribution was calculated. Within-food or drug groups, saccharin phenotype scores and number of days to reach initial stability criterion were analyzed using a 2-way ANOVA (sex × phenotype). Between-group food vs. drug group comparisons were made using a 2-way ANOVA (saccharin group × food/drug). Post hoc comparisons were made with Fisher's LSD protected t-tests, and results were considered statistically significant if p ≤ 0.05.
3. Results
3.1. Delay discounting for food
3.1.1. Locomotor activity
Figure 1 shows the Day 1 (left panels) and the Day 2 (right panels) locomotor counts for female (upper panels) and male (lower panels) groups. In all figures, activity counts per 5-min interval decreased in an exponential pattern over the 45 min sessions. On Day 1, there was a main effect of phenotype (F1,332 = 4.48, p < 0.05) and interval (F8,332 = 198.46, p < 0.01), and there was a sex × phenotype interaction (F1,332 = 12.54, p < 0.01), a phenotype × interval interaction (F8,332 = 3.17, p < 0.01), and a sex × phenotype × interval interaction (F8,332 = 2.09, p < 0.05). Post hoc analyses revealed that LoS females had higher locomotor activity than HiS females during all but the third interval (p < 0.05), and they had higher locomotor activity than LoS males during all but the fifth interval (p < 0.05). HiS females had less locomotor activity than HiS males during the second 5-min interval (p < 0.05). Males showed fewer phenotype differences over the 9 time intervals. LoS males had higher locomotor activity than HiS males during the fifth interval, and HiS males had higher locomotor activity than LoS males during the last two 5-min intervals (p < 0.05).
Figure 1.
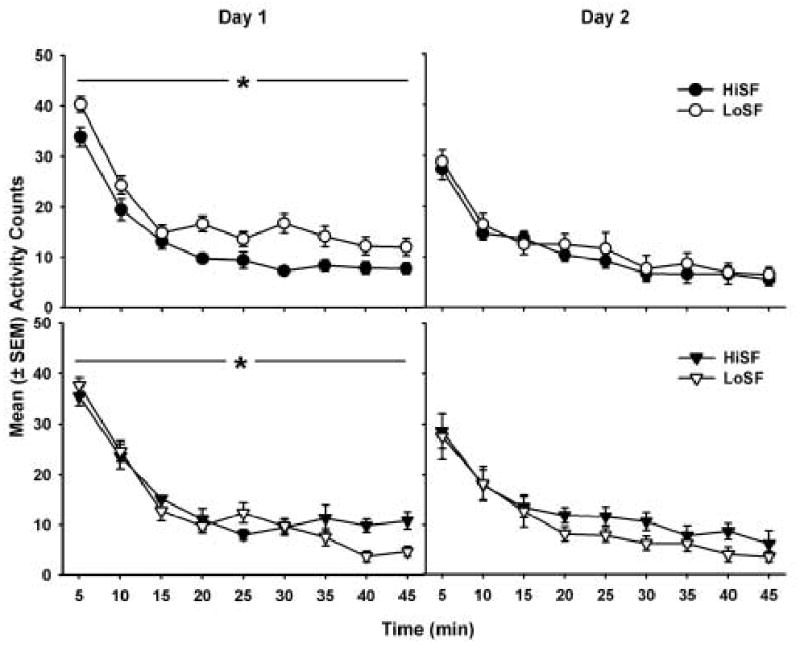
Day 1 (left panels) and Day 2 (right panels) locomotor counts (± SEM) in the HiS and LoS female (top panels) and male (bottom panels) groups over a 45-min period. Filled symbols refer to HiS and open symbols refer to LoS groups. Activity significantly decreased in an exponential pattern over the 45 min. Overall, LoS rats had significantly greater locomotor activity than HiS rats on Day 1 (* p < 0.05).
The locomotor activity on Day 2 of testing showed significant effects of 5-min interval (F8,341 = 91.10, p < 0.01). All groups had significantly higher locomotor activity in the first time interval compared with the subsequent 8 intervals (p < 0.05). Overall, data from Day 1 indicated that phenotype differences were more pronounced and more consistent in females vs. males, and sex differences were more apparent in LoS vs. HiS groups. On Day 2, however, there were no phenotypic or sex differences.
3.1.2. MADs
Figure 2 shows the average MAD values for HiS and LoS males and females during the last 5 days of the food delay discounting procedure grouped by sex (upper frames) and phenotype (lower frames). Overall, HiS male and female rats had significantly lower MADs (were more impulsive) compared with LoS male and females (F1,77 = 5.60, p < 0.05). There was also a sex × reinforcer magnitude (1 vs. 3 or 1 vs. 6) interaction (F1,77 = 6.43, p < 0.05). HiS females were more impulsive (had lower MADs) than LoS males when 1 vs. 3 or 1 vs. 6 pellets were available (p < 0.05). LoS females were more impulsive than LoS males when choosing between 1 vs. 3 pellets (p < 0.05), and this was also the condition (1 vs. 3, LoS) under which the highest MADs (lowest impulsivity) occurred. There were no sex differences in HiS rats, and these were the conditions (1 vs. 3 and 1 vs. 6, HiS) under which the lowest MADs (highest impulsivity) were found. There were no significant changes in impulsivity as a function of reinforcer magnitude. Overall, these results indicated more significant differences in the phenotype than the sex variable.
Figure 2.

Mean (± SEM) adjusted delay (sec) in the HiS and LoS female and male groups delay discounting for food. Filled symbols refer to HiS and open symbols refer to LoS groups. Circles indicate females and triangles indicate males. Overall, HiS rats had lower MADs (higher impulsivity) than LoS rats (* p < 0.05). LoS females had lower MADs than LoS males in the 1 vs. 3 pellet condition (# p < 0.05).
3.1.3. MAD distributions
A distribution of the average MAD values for all groups in the 1 vs. 3 (a) and 1 vs. 6 (b) pellet conditions over the 5 stable days of each condition is presented in Figure 3. When the reinforcer magnitude was 1 vs. 3, HiS female, LoS female, and HiS male groups had distributions that were centered around 2-10 sec, while the distribution for LoS males showed a greater spread. In the 1 vs. 3 pellet condition, the distributions for HiS females, LoS females, and LoS males were slightly flatter than a normal distribution (kurtosis: 0.24, -1.64, and 0.22, respectively), while the distribution for HiS males was peaked compared to a normal distribution (kurtosis: 5.80). All groups had distributions that were skewed to the right (skewness: HiS females = 1.04, LoS females = 0.34, HiS males = 2.29, and LoS males = 1.26).
Figure 3.
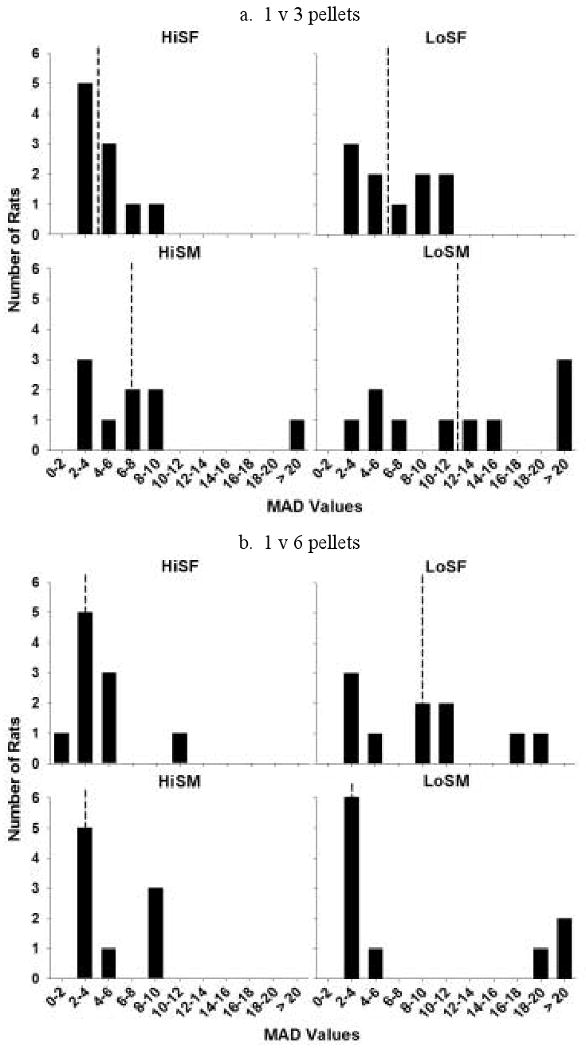
Distribution of MAD scores in the HiS and LoS female and male groups in the 1 vs. 3 (a) and 1 vs. 6 (b) pellet conditions. Dotted lines denote the median score for each group.
In the 1 vs. 6 pellet condition, HiS females and HiS males continued to have distributions that were centered around 2-10 sec. The distribution for LoS females, HiS males, and LoS males was flattened compared to a normal distribution (kurtosis: -0.67, -1.56, and -0.01), and the distribution for HiS females was peaked compared to both the normal distribution and the HiS females distribution for the 1 vs. 3 pellet condition. Again, all groups had distributions that were skewed to the right (skewness: HiS females = 2.18, LoS females = 0.56, HiS males = 0.82, and LoS males = 1.32), with the HiS females' distribution becoming more skewed, and the HiS males' distribution becoming less skewed. Overall, the center of the HiS rats' distributions were shifted left compared to LoS rats. There were no consistent trends in MAD distributions as a function of sex or number of larger-delayed pellets. Thus, phenotype and sex differences in MADs (Figure 3) were due to leftward shifts (HiS) in the MAD distribution rather than distinctly different distribution patterns.
3.1.4. Number of reinforcers earned
Figure 4 shows the mean (± SEM) number of food pellets earned in each group. Overall, male HiS and LoS rats earned more food pellets than female HiS and LoS rats (F1,77 = 16.26, p < 0.01), HiS rats earned more pellets than LoS rats (F1,77 = 6.23, p < 0.05), and all groups earned significantly more food pellets when the reinforcer magnitude was 1 vs. 6 compared to 1 vs. 3 (F1,77 = 104.28, p < 0.01). Additionally, there was a sex × reinforcer magnitude interaction (F1,77 = 20.65, p < 0.01). In the 1 vs. 6 pellet condition, HiS and LoS males earned more food pellets than HiS and LoS females, respectively (p < 0.01).
Figure 4.
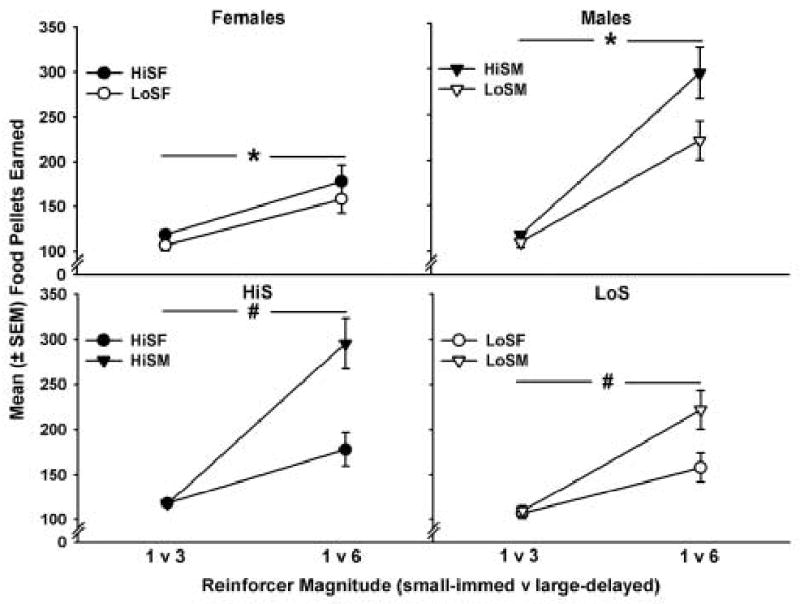
Mean (± SEM) number of food pellets earned in the HiS and LoS female and male groups delay discounting for food. Filled symbols refer to HiS and open symbols refer to LoS groups. Circles indicate females and triangles indicate males. Overall, HiS rats earned more than LoS rats (* p < 0.05), males earned more food pellets than females (# p < 0.05), and all groups earned more pellets when the reinforcer magnitude was 1 vs. 6 compared to 1 vs. 3.
3.1.5. Days to stable MADs, response latencies and saccharin phenotype scores
Table 1 lists the number of days to reach stable MAD values, latencies to respond on the left and right levers during the free and forced choice trials, and the saccharin phenotype scores for all food groups. Females took significantly longer than males to reach initial MAD stability (F1,38 = 7.90, p < 0.01), but there were no differences due to phenotype.
Table 1. Mean (SEM) number of days to reach initial MAD stability, response latencies and saccharin phenotype scores for groups responding under delay discounting schedules for food.
| N | Days to MAD Stability | 1 vs. 3 Pellet Condition | 1 vs. 6 Pellet Condition | Saccharin Phenotype Score | |||
|---|---|---|---|---|---|---|---|
| Left Response Latency (sec) | Right Response Latency (sec) | Left Response Latency (sec) | Right Response Latency (sec) | ||||
| HiSF | 10 | 50.5(7.0) | 37.6(10.6)c | 42.7(14.6)c | 298.3(74.1)a,c | 172.8(30.2)a,c | 50.5(7.0)c |
| LoSF | 10 | 21.2(2.4) | 45.1(10.8) | 92.1(45.4) | 340.8(51.8)a | 249.1(63.8)a | 21.2(2.4) |
| HiSM | 9 | 20.4(5.0)b | 20.2(2.0)b,c | 26.5(5.8)b,c | 84.7(42.4)a,b,c | 53.6(25.9)a,b,c | 20.4(5.0)b,c |
| LoSM | 10 | 10.3(1.9)b | 92.7(33.5)b | 46.5(10.6)b | 191.9(43.3)a,b | 165.5(69.3)a,b | 10.9(2.0)b |
significantly greater than the 1 vs. 3 pellet condition
significantly less than HiS and LoS females
significantly different than LoS males and females
Analysis of the latencies to respond on the left lever showed that HiS and LoS females had significantly longer response latencies than HiS and LoS males (F1,77 = 7.04, p < 0.05), and response latencies were significantly longer in the 1 vs. 6 pellet condition compared with the 1 vs. 3 pellet condition (F1,77 = 48.13, p < 0.01). There was also a sex × reinforcer magnitude interaction (F1,77 = 14.29, p < 0.01), such that when the reinforcer magnitude was 1 vs. 6 (but not when it was 1 vs. 3) HiS and LoS females had longer response latencies than HiS and LoS males (p < 0.05). Analysis of the right response latencies revealed a main effect of reinforcer magnitude (F1,77 = 20.86, p < 0.01), with all groups taking longer to respond during the 1 vs. 6 pellet condition compared with the 1 vs. 3 pellet condition. Overall, response latencies were longer in the 1 vs. 6 pellet condition compared with the 1 vs. 3 pellet condition, and there was a tendency for males to have shorter response latencies than females in the 1 vs. 6 pellet condition.
Analysis of saccharin phenotype scores (presented in Table 1) revealed that females had significantly higher saccharin phenotype scores than males (F1,36 = 18.68, p < 0.01), and HiS rats had significantly higher saccharin phenotype scores than LoS rats (F1,36 = 17.24, p < 0.01). Additionally, there was a sex × phenotype interaction (F1,36 = 4.52, p < 0.05), such that HiS females had significantly higher saccharin phenotype scores than LoS females, HiS males, and LoS males (p < 0.01). Thus, the saccharin intake status of the selectively bred groups was confirmed.
3.2. Delay discounting for cocaine
3.2.1. Locomotor activity
Figure 5 shows the mean Day 1 (left panels) and Day 2 (right panels) locomotor counts for female (upper panels) and male (lower panels) cocaine groups. In all figures, activity counts per 5-min interval decreased in an exponential pattern over the 45 min. On Day 1, LoS rats had significantly greater locomotor activity than HiS rats (F1,386 = 9.42, p < 0.01). On both Day 1 and Day 2 of locomotor testing, there was a significant main effect of interval (Day 1: F8,386 = 163.20, p < 0.01, Day 2: F1,368 = 89.91, p < 0.01), with all groups having greater locomotor activity in the first 5-min interval than in the subsequent intervals.
Figure 5.
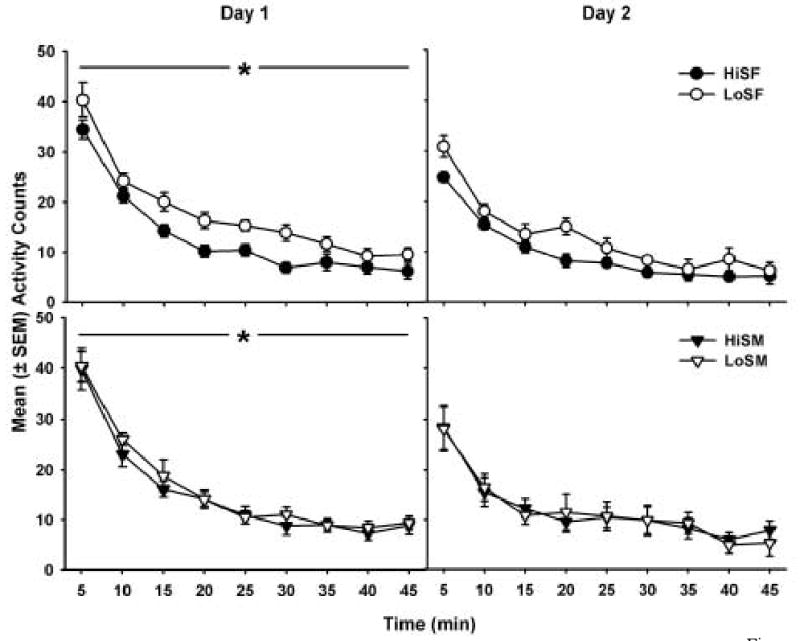
Day 1 (left panels) and Day 2 (right panels) locomotor counts (± SEM) in the HiS and LoS female (top panels) and male (bottom panels) groups over a 45-min period. In all figures, activity counts significantly decreased in an exponential pattern over the 45 min. On Day 1 of testing, LoS rats had significantly greater locomotor activity than HiS rats (* p < 0.05).
3.2.2. MADs
Figure 6 shows MADs (s) over 3 cocaine doses in each group. There was a significant effect of dose (F2,140 = 3.10, p = 0.05), indicating that generally impulsivity increased (lower MADs) with higher doses (possibly due to the direct drug effects); however, no significant phenotype or sex differences were found. MADs for cocaine were in the same range as they were for food (Figure 2). There was not a distinct decrease in MAD for females (v males) or HiS (v LoS) as there was with food (Figure 2).
Figure 6.

Mean (± SEM) adjusted delay (sec) in the HiS and LoS female and male groups delay discounting for cocaine. Filled symbols refer to HiS and open symbols refer to LoS groups. Circles indicate females and triangles indicate males. Generally, impulsivity increased (lower MADs) significantly at higher doses.
3.2.3. MAD distributions
In Figure 7, the distribution of MAD values is presented when the small-immediate reinforcer was 0.2 (a), 0.4 (b), and 0.8 (c) mg/kg cocaine. The MAD distributions for cocaine show similar kurtosis to the distributions for food, and also similar to the food distributions, they are skewed to the right. When 0.2 mg/kg cocaine was the small-immediate reinforcer, the distribution for HiS females was slightly peaked compared to the normal distribution (kurtosis: 3.80); while the distributions for LoS females, HiS males, and LoS males were flatter near the mean for each group (kurtosis: 1.44, -1.66, and 0.55, respectively). All distributions were skewed to the right, and HiS females', LoS females', and LoS males' distributions were more skewed (skewness: 1.69, 1.55, 1.18, respectively) than HiS males' distribution (skewness: 0.34).
Figure 7.
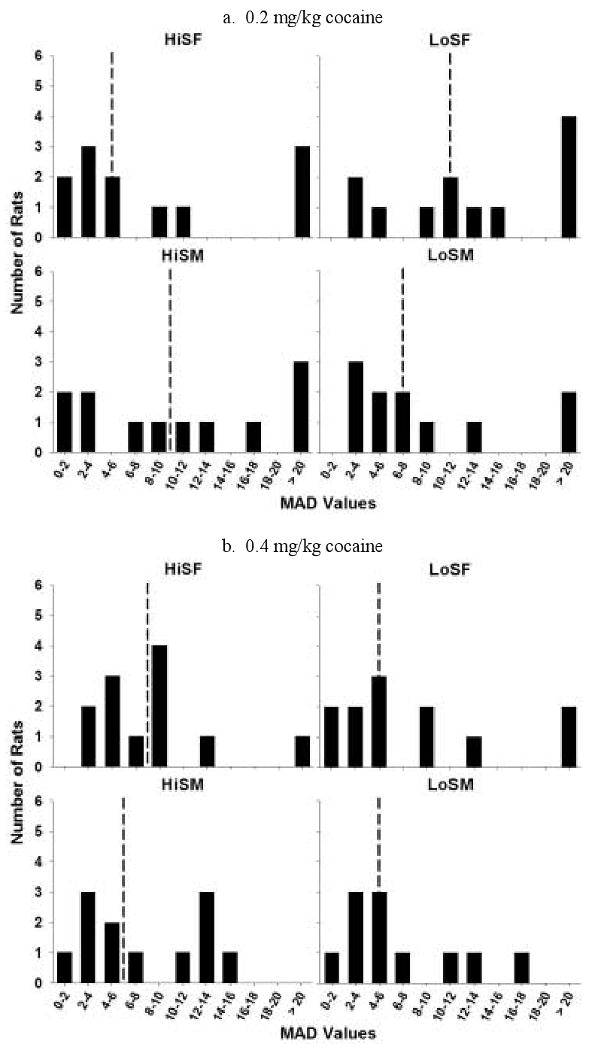

Distribution of MAD scores in the HiS and LoS female and male groups when the small, immediate reward was 0.2 (a), 0.4 (b), and 0.8 (c) mg/kg cocaine. Dotted lines denote the median score for each group.
When the small-immediate reinforcer was 0.4 mg/kg cocaine, HiS females, LoS females, and HiS males had flatter distributions than the normal distribution (kurtosis: HiS females = 0.78, LoS females = 1.83, and HiS males = 0.37); however, the LoS males' distribution was more peaked (kurtosis: 4.64). All distributions were skewed to the right, and again, the HiS males' distribution had the most symmetry (skewness: HiS females = 1.45, LoS females = 1.30, HiS males = 0.76, and LoS males = 2.16). As the dose of the small-immediate reinforcer was increased from 0.4 to 0.8 mg/kg, the distribution of the HiS males' MADs became more peaked around the mean (kurtosis: 8.12), while the HiS females', LoS females', and LoS males' distributions were flatter (kurtosis: 1.62, -0.09, and -1.11, respectively). The distributions for LoS females and LoS males displayed the most symmetry (skewness: 0.93 and 0.20, respectively), while the HiS females' and HiS males' distributions were more skewed to the right (skewness: 1.26 and 2.77, respectively). Similar to the distributions for delay discounting for food, the means of HiS rats were shifted to the left compared to LoS rats especially when 0.8 mg/kg cocaine was the low immediate dose.
3.2.4. Number of reinforcers earned
The mean (± SEM) number of cocaine infusions earned in the HiS and LoS female and male groups is presented in Figure 8. HiS and LoS females earned significantly more cocaine infusions than HiS and LoS males (F1,140 = 6.03, p < 0.05). Additionally, HiS rats earned more infusions than LoS rats (F1,140 = 5.20, p < 0.05), and in all groups, there were dose-dependent decreases in the number of cocaine infusions self-administered (F2,140 = 54.69, p < 0.01). Drug intake at the highest dose was approximately twice the intake at the lowest dose in all groups.
Figure 8.
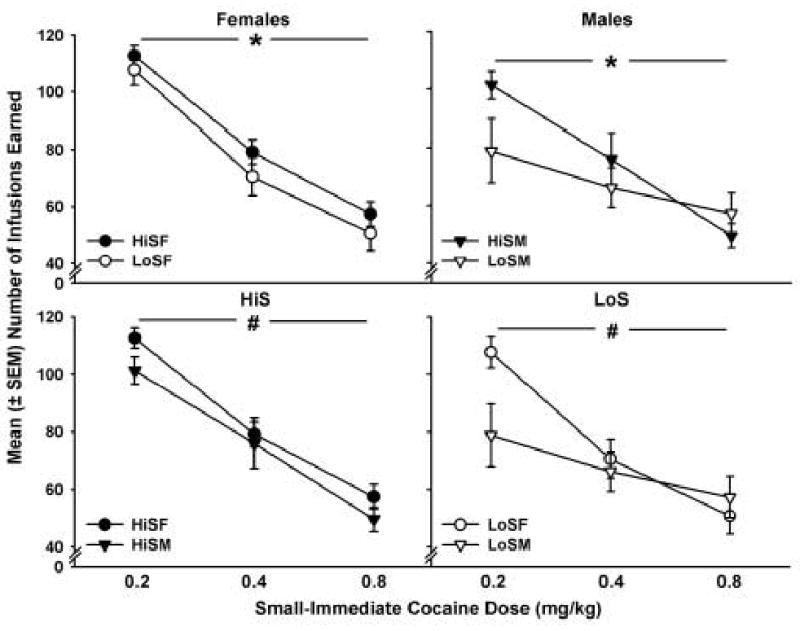
Mean (± SEM) number of infusions earned in the HiS and LoS female and male groups. Filled symbols refer to HiS and open symbols refer to LoS groups. Circles indicate females and triangles indicate males. Overall, HiS rats earned more infusions than LoS rats (* p < 0.05) and females earned more cocaine infusions than males and HiS rats earned more infusions than LoS rats (# p < 0.05). There were significant dose-dependent decreases in the number of infusions earned.
3.2.5. Days to stable MADs, response latencies, and saccharin phenotype scores
The number of days to reach initial MAD stability, response latencies on the left and right levers during both the free and forced choice trials, and saccharin phenotype scores are presented in Table 2. Females took significantly longer to reach stability than males (F1,45 = 6.68, p < 0.05), and HiS rats took significantly longer than LoS rats to reach stability (F1,45 = 6.51, p < 0.05).
Table 2. Mean (SEM) number of days to reach initial MAD stability, response latencies, and saccharin phenotype scores for groups responding under delay discounting schedules for cocaine.
| N | Days to MAD Stability | 0.2 vs. 0.6 mg/kg Cocaine | 0.4 vs. 1.2 mg/kg Cocaine | 0.8 vs. 2.4 mg/kg Cocaine | Saccharin Phenotype Score | ||||
|---|---|---|---|---|---|---|---|---|---|
| Left Response Latency (sec) | Right Response Latency (sec) | Left Response Latency (sec) | Right Response Latency (sec) | Left Response Latency (sec) | Right Response Latency (sec) | ||||
| HiSF | 12 | 23.7(4.3) | 288.5(51.8) | 217.7(20.2) | 125.4(22.4) | 108.2(13.7) | 393.0(38.8) | 352.2(23.4)a | 23.5(3.6) |
| LoSF | 12 | 13.8(1.5)c | 315.9(36.9) | 228.1(35.4) | 236.1(81.5) | 142.2(19.6) | 801.4(386.2) | 471.6(85.9) | 9.6(2.7)c |
| HiSM | 11 | 13.7(2.1)b | 331.4(65.0) | 255.1(61.3) | 119.3(21.1) | 134.7(31.4) | 415.3(90.4) | 406.6(94.0)a | 12.8(2.9)b |
| LoSM | 12 | 10.5(1.8)b,c | 339.8(39.7) | 298.1(80.5) | 300.9(146.2) | 454.8(199.2) | 527.6(117.0) | 304.3(81.7)a | 4.0(1.2)b,c |
significantly greater than the 0.2 vs. 0.6 mg/kg cocaine condition
significantly less than HiS and LoS females
significantly less than HiS males and females
There was a significant effect of dose on latency to respond on the left lever (F2,80 = 6.30, p < 0.01), with the longest response latencies occurring at the highest cocaine doses (i.e., 0.8 vs. 2.4 mg/kg). Right response latencies were higher in LoS rats compared to HiS rats (F1,80 = 4.60, p < 0.05). Similar to left latencies, right latencies were dose-dependent (F2,80 = 12.09, p < 0.01), and the longest latencies typically occurred at the highest cocaine dose. There were also sex × dose (F2,80 = 4.66, p < 0.05) and sex × phenotype × dose (F2,80 = 5.88, p < 0.01) interactions. In HiS males, HiS females, and LoS males, response latencies were significantly higher at the highest dose (0.8 vs. 2.4 mg/kg) compared with the lowest dose (0.2 vs. 0.6 mg/kg; p < 0.05). LoS males had significantly longer right response latencies than HiS males, HiS females, and LoS females when the small, immediate reinforcer was 0.2 mg/kg cocaine (p < 0.05). As with food reward (Table 1), overall, in each group response latencies on the right or left lever were longer at the highest dose of cocaine compared with the lowest dose of cocaine.
Analysis of saccharin phenotype scores (presented in Table 2) revealed that females had significantly higher scores than males (F1,39 = 9.11, p < 0.01), and HiS rats had significantly higher scores than LoS rats (F1,39 = 17.61, p < 0.01), confirming the selective breeding. HiS and LoS females in the delay discounting for drug groups had significantly lower saccharin phenotype scores compared with HiS and LoS females in the delay discounting for food groups, respectively (p < 0.05). There were no differences in saccharin phenotype scores between food or drug groups in HiS and LoS males.
4. Discussion
In the present experiment, HiS females and males were more impulsive than LoS females and males, respectively, for food reinforcers. When the reinforcer magnitude was 1 vs. 3 pellets, LoS females were more impulsive than LoS males. Thus, saccharin phenotype and sex were significantly related to impulsivity for food. The magnitude of delayed pellet reinforcement (3 vs. 6) did not have a significant effect, suggesting that this variable was not strongly related to impulsivity. This study is the first to examine impulsive choices for cocaine reinforcers; however, there were no significant phenotype or sex differences. Generally, impulsivity increased with higher doses on the delay discounting for cocaine task, and this may have been due to the direct, dose-dependent stimulating effects of cocaine on operant behavior.
4.1. Delay discounting for food
The purpose of the locomotor testing was to determine whether differences in impulsivity as measured by the delay discounting task could be due to underlying differences in locomotor activity or reactivity to a novel task. In rats that performed the delay discounting task for food, LoS females had a higher exploratory response to the novel locomotor track (based on Day 1 performance) than HiS females and LoS males; however, there were no phenotypic or sex differenced in basal locomotor activity (based on Day 2 performance). That LoS females were more active on Day 1 of testing is consistent with previous reports of running-wheel activity; female LoS rats ran more on a running wheel than HiS rats under both food-restricted and free-feeding conditions (Dess et al., 2000). Locomotor activity in a novel environment was predictive of acquisition of drug self-administration in other studies (Mantsch et al., 2001; Piazza et al., 1989; Piazza et al., 1990), and impulsivity also predicted acquisition of drug self-administration (Perry et al. 2005). Therefore, locomotor activity was assessed to determine the relationship between these three factors (locomotor activity, impulsivity, saccharin intake) that have been shown to predict vulnerability to drug abuse. In the present experiment, there was no relationship between impulsivity and locomotor activity, and this is consistent with previous results (Perry et al. 2005). LoS females had more locomotor activity in a novel environment (Day 1) and were less impulsive than HiS females; however, LoS females had more locomotor activity than LoS males, but were more impulsive. This suggests that differences in impulsivity are due to phenotypic differences in incentive motivation and not to locomotor activity in a novel environment. Additionally, while locomotor activity in a novel environment, impulsivity, and saccharin preference are all vulnerability factors in drug abuse, they are not necessarily related to each other.
In the delay discounting for food paradigm, there were higher levels of impulsivity (lower MADs) in HiS rats compared to LoS rats, respectively, and higher impulsivity in LoS females compared to LoS males (when choosing between 1 vs. 3 pellets). In other experiments, we have not found sex differences in impulsivity, possibly due to a ceiling effect because most rats tend to be highly impulsive (Perry et al. 2007b). The difference may have emerged in the present study due to the selective breeding which yielded a wider range of MAD values. Similar to the Wistar rats in our other experiment (Perry et al. 2007b), the majority of HiS rats had MADs between 2 and 6 sec. LoS rats, however, had more spread out distributions, which may have allowed for the expression of sex differences. For example, in the present experiment there were sex differences in LoS rats, while the lack of sex differences in HiS rats may have been due to a floor effect on MAD values (or a ceiling effect on impulsiveness).
In the food portion of this study, there was no main effect of large-delayed reinforcer magnitude. In humans, increasing reinforcer magnitude has consistently decreased impulsive choice (e.g., Baker et al., 2003; Johnson and Bickel, 2002). However, in rodents the results are mixed; increasing the amount of the delayed reinforcer decreased impulsive choice (Wade et al., 2000) or had no effect (Green et al., 2004; Richards et al., 1997). In another study, increasing the magnitude (concentration) of a sucrose solution also increased impulsive choice (Farrar et al., 2003). As the larger, delayed reinforcer was increased from 3 to 6 pellets, all groups earned more pellets (because more pellets were available to self-administer) and had longer response latencies. The longer response latencies in the 1 vs. 6 pellet condition may have been a result of satiation, since more pellets were typically earned in this condition compared to the 1 vs. 3 pellet condition. Response latencies for the 1 vs. 3 pellet condition in the present study were similar to response latencies in other studies that used this procedure (Perry et al., 2005; 2007b). However, it is possible that response latencies would be shortened under all conditions if retractable levers were used, making stimulus changes more salient. Additionally, in the 1 vs. 6 pellet condition, HiS and LoS males earned significantly more pellets and had significantly shorter response latencies than their HiS and LoS female counterparts. This may have been due to a larger body weight in males compared to females.
4.2. Delay discounting for cocaine
Patterns of locomotor activity in rats that performed the delay discounting task for cocaine were similar to those in rats responding for food. Specifically, in all groups, locomotor activity decreased in an exponential pattern over the 45-min test period. On Day 1, locomotor activity was higher in LoS rats compared with HiS rats. This is similar to the locomotor activity obtained in the food groups, with the exception that there were no sex differences on Day 1 of testing in the LoS rats. The lack of sex differences on Day 1 of testing in the cocaine groups compared with the food groups may have been due to low levels of locomotor activity in two of the LoS males in the delay discounting for food group.
That there were no significant phenotypic or sex differences in impulsive choices for cocaine at any dose tested may be difficult to interpret because as cocaine is self-administered, its direct effects may influence impulsivity. A previous study in rats showed that cocaine increased impulsivity on a delay discounting for food task (Logue et al., 1992). Therefore, in the present study, cocaine may be increasing impulsivity as the session progresses, and as the cocaine dose increases, and this may indicate that higher cocaine doses increase impulsivity to a greater extent than lower doses. It is unclear whether cocaine's ability to increase impulsivity would be uniform across HiS/LoS male/female groups, as these groups are differentially sensitive to cocaine self-administration in several phases of addiction (Carroll et al., 2007a, b; Carroll et al., 2002, Perry et al., 2006a). Thus, it would be interesting to measure delay discounting for food in HiS and LoS male and female rats both before and after cocaine self-administration to determine whether cocaine self-administration alters delay discounting for food in the same way it seems to alter delay discounting for cocaine.
It is also possible that the phenotypic differences in delay discounting for food, but not cocaine, can be attributed to the palatability of food. HiS rats showed higher impulsivity than LoS rats only when the reinforcer was a palatable substance. Previous research has indicated that HiS rats showed greater preferences for a variety of sweet, salty, and starchy solutions compared with LoS rats (Dess, 2000). This may reflect differing levels of incentive motivation for a variety of foods in HiS and LoS groups, and it may have influenced their behavior in the delay discounting task when food was a reinforcer. In the present study, grain-based pellets were used, however, it would be interesting to assess performance on this task using several different types of food pellets (e.g., sucrose pellets) to determine whether the type of food reinforcer used plays a role in impulsive choices in HiS and LoS rats.
The magnitude of the difference between the small, immediate and large, delayed reinforcer was held constant in the delay discounting for cocaine task (that is, the large, delayed reinforcer was always 3× the small, immediate reinforcer). However, in the delay discounting for food task, the magnitude of the large reinforcer varied from 3× to 6× the small reinforcer. It is possible that there would have been more sex or phenotypic differences due to cocaine dose if we had altered the magnitude of the immediate vs. delayed reinforcers, as we did with the food reinforcers.
The descending limb of the dose-response curve was produced in this experiment, and response latencies for all groups increased at higher doses (compared to lower doses). Female rats earned more infusions than male rats, and HiS rats earned more infusions than LoS rats. These results are consistent with data showing that females (Carroll et al., 2004) and HiS rats (Carroll et al., 2002) have a greater propensity to self-administer drugs of abuse than males and LoS rats, respectively.
The saccharin phenotype scores obtained during the 2-bottle choice test were comparable to scores obtained in previous experiments. For example, HiS females in the present experiment had saccharin phenotype scores of 50.48 (± 6.97; food group) and 23.52 (± 3.59; cocaine group); whereas, in previous studies, they had scores of 24.4 and 31.1 (Carroll et al., 2002) or 33.7, 39, and 32.9 (Perry et al., 2006a). LoS females in the present study had scores of 21.18 (± 2.39; food group) and 9.62 (± 2.68; cocaine group), and in previous studies they had scores of 13.4 and 8.7 (Carroll et al., 2002) or 21.5, 9.0, and 17.6 (Perry et al., 2006a). There was a similar pattern for the HiS and LoS males; such that in both the present and in recent studies (Carroll et al., 2002; Dess et al., 2005), HiS rats had higher saccharin phenotype scores than LoS rats, and females had higher saccharin phenotype scores than males. In the present study, HiS and LoS female food groups had significantly higher saccharin phenotype scores than their respective drug groups. It is possible that exposure to drugs of abuse influenced saccharin phenotype scores in females. Alternatively, the delay discounting for food procedure have increased subsequent consumption of a saccharin solution more in females than males.
Overall, females exceed males in drug-seeking and drug-related behavior across several phases (Carroll et al., 2004) of the addiction process (e.g., acquisition, escalation, reinstatement). Similarly, HiS rats outperform LoS rats under many of the same drug-related conditions, such as acquisition (Carroll et al., 2002), escalation (Perry et al., 2006a), and reinstatement (Perry et al., 2006a) of drug-seeking behavior. Interestingly, one area where males show a greater drug effect than females is in disruptions in operant behavior during drug withdrawal (Perry et al., 2006b), and recent evidence suggests that LoS rats also exceed HiS rats in their behavioral suppression during drug withdrawal (Dess et al., 2005). The literature and the present results emphasize the salience of selective breeding for saccharin intake and sex as factors that influence vulnerability to drug abuse.
Recent evidence suggests that impulsivity is an additional vulnerability factor for drug abuse (e.g., Mitchell et al., 2006; Perry et al., 2005, 2007b; Poulos et al., 1995). In the present study, HiS rats were more impulsive than LoS rats when food was the reinforcer, suggesting that there may be a relationship between impulsivity and excessive saccharin intake. Additionally, female LoS rats were more impulsive than male LoS rats on the delay discounting for food task. Thus, under some conditions, sex and impulsivity may also be related. Since any one of these vulnerability factors predicts vulnerability to drug abuse, it is possible that having two or more of these factors may have additive effects, yielding even greater vulnerability to drug abuse. For example, in male and female Wistar rats selected for high (HiI) and low (LoI) impulsivity based on an adjusting delay task, female HiI rats reinstated cocaine-seeking behavior following a cocaine prime to a greater extent than female LoI rats and male HiI and LoI rats (Perry et al. 2007b).
In summary, the results of this experiment suggest that HiS rats are more impulsive for food rewards than LoS rats, and that LoS females were more impulsive than LoS males. There were no group differences in impulsive choices for cocaine; however, these results may have been influenced by the effect of cocaine on impulsivity during the session, as there was a decrease in MAD (increased impulsivity) as cocaine dose (and consumption) increased. This experiment provides evidence supporting additive vulnerability of factors, such as sex or excessive intake of a dietary substance that may influence both impulsivity and drug abuse. Future research focusing on the neurobiological mechanisms underlying the relationship between excessive saccharin consumption, impulsivity, and drug–seeking behavior may clarify the relationship among these three factors.
Acknowledgments
The authors would like to thank Justin Anker and Dr. Erin Larson for their technical assistance, and Jason Ross for his helpful comments on earlier versions of the manuscript. We are grateful for support provided by NIDA: R01 DA03240-22 and K05 DA15267-05 (MEC) and F31 DA020237-01 (JLP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen TJ, Moeller FG, Rhoades HM, Cherek DR. Impulsivity and history of drug dependence. Drug Alcohol Depend. 1998;50:137–45. doi: 10.1016/s0376-8716(98)00023-4. [DOI] [PubMed] [Google Scholar]
- Anderson KG, Woolverton WL. Effects of comipramine on self-control choice in Lewis and Fischer 344 rats. Pharmacol Biochem Behav. 2005;80:387–93. doi: 10.1016/j.pbb.2004.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: similarities and differences across commodity, sign, and magnitude. J Abnorm Psychol. 2003;112:382–92. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: Delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacol. 1999;146:447–54. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mellow NK, Bergman J. Effects of dopamine D(1-like) and D(2-like) agonists in rats that self-administer cocaine. J Pharmacol Exp Ther. 1999;291:353–60. [PubMed] [Google Scholar]
- Carroll ME, Anderson MM, Morgan AD. Cocaine-induced locomotor sensitization in rats selectively bred for high and low saccharin intake. 2007a in preparation. [Google Scholar]
- Carroll ME, Anderson MM, Morgan AD. Regulation of intravenous cocaine self-administration in rats selectively bred for high (HiS) and low (LoS) saccharin intake. Psychopharmacol. 2007b;190:331–41. doi: 10.1007/s00213-006-0600-3. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Batulis D, Landry K, Morgan AD. Sex differences in the escalation of oral phencyclidine (PCP) self-administration under FR and PR schedules in rhesus monkeys. Psychopharmacol. 2005;180:414–26. doi: 10.1007/s00213-005-2182-x. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–9. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacol. 2002;161:304–13. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Roth ME, Voeller RK, Nguyen PD. Acquisition of oral phencyclidine self-administration in rhesus monkeys: effect of sex. Psychopharmacol. 2000;149:401–8. doi: 10.1007/s002130000389. [DOI] [PubMed] [Google Scholar]
- Charrier D, Thiebot MH. Effects of psychotropic drugs on rat responding in an operant paradigm involving choice between delayed reinforcers. Pharmacol Biochem Behav. 1996;54:149–57. doi: 10.1016/0091-3057(95)02114-0. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- de Wit H, Richards JB. Dual determinants of drug use in humans: reward and impulsivity. Nebr Symp Motiv. 2004;50:19–55. [PubMed] [Google Scholar]
- Dess NK. Responses to basic taste qualities in rats selectively bred for high versus low saccharin intake. Phys Behav. 2000;69:247–57. doi: 10.1016/s0031-9384(99)00246-2. [DOI] [PubMed] [Google Scholar]
- Dess NK, Arnal J, Chapman CD, Siebel S, VanderWeele DA, Green KF. Exploring adaptations to famine: Rats selectively bred for differential intake of saccharin differ on deprivation-induced hyperactivity and emotionality. Int J Comp Psychol. 2000;13:34–52. [Google Scholar]
- Dess NK, Badia-Elder NE, Thiele TE, Kiefer SW, Blizard DA. Ethanol consumption in rats selectively bred for differential saccharin intake. Alcohol. 1998;16:275–8. doi: 10.1016/s0741-8329(98)00010-x. [DOI] [PubMed] [Google Scholar]
- Dess NK, Minor TR. Taste and emotionality in rats selectively bred for high versus low saccharin intake. Anim Learn Behav. 1996;24:105–15. [Google Scholar]
- Dess NK, O'Neill P, Chapman CD. Ethanol withdrawal and proclivity are inversely related in rats selectively bred for differential saccharin intake. Alcohol. 2005;37:9–22. doi: 10.1016/j.alcohol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacol. 1996;128:161–70. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Farrar AM, Kieres AK, Hausknecht KA, de Wit H, Richards JB. Effects of reinforcer magnitude on an animal model of impulsive behavior. Behav Processes. 2003;64:261–71. doi: 10.1016/s0376-6357(03)00139-6. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Weafer J. Alcohol impairment of behavior in men and women. Addiction. 2004;99:1237–46. doi: 10.1111/j.1360-0443.2004.00805.x. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, Holt DD, Slevin JR, Estle SJ. Discounting of delayed food rewards in pigeons and rats: is there a magnitude effect? J Exp Anal Behav. 2004;81:39–50. doi: 10.1901/jeab.2004.81-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict Behav. 2006;31:1290–4. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Horan B, Smith M, Gardner EL, Lepore M, Ashby CR. (-)-Nicotine produces conditioned place preference in Lewis, but not Fischer 344 rats. Synapse. 1997;26:93–4. doi: 10.1002/(SICI)1098-2396(199705)26:1<93::AID-SYN10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Isles AR, Humby T, Wilkinson LS. Measuring impulsivity in mice using a novel operant delayed reinforcement task: effects of behavioural manipulations and d-amphetamine. Psychopharmacol. 2003;170:376–82. doi: 10.1007/s00213-003-1551-6. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Sex-related differences in spatial divided attention and motor impulsivity in rats. Behav Neurosci. 2003;117:76–83. doi: 10.1037//0735-7044.117.1.76. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav. 2002;77:129–46. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Marakovic NN. Delay-discounting probabilistic rewards: Rates decrease as amounts increase. Psychonom Bull Rev. 1996;3:100–4. doi: 10.3758/BF03210748. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–71. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kosten T, Miserendino M, Chi S, Nestler EJ. Fischer and Lewis rat strains show differential cocaine effects in conditioned place preference and behavioral sensitization but not in locomotor activity or conditioned taste aversion. J Pharmacol Exp Ther. 1994;269:137–44. [PubMed] [Google Scholar]
- Kosten T, Miserendino M, Haile C, DeCaprio J, Jatlow P, Nestler EJ. Acquisition and maintenance of intravenous cocaine self-administration in Lewis and Fischer inbred rat strains. Brain Res. 1997;778:418–29. doi: 10.1016/s0006-8993(97)01205-5. [DOI] [PubMed] [Google Scholar]
- Logue AW, Tobin H, Chelonis JJ, Wang RY, Geary N, Schachter S. Cocaine decreases self-control in rats: a preliminary report. Psychopharmacol. 1992;109:245–7. doi: 10.1007/BF02245509. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Exp Clin Psychopharmacol. 2001;9:131–43. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacol. 2000;148:196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacol. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacol. 2002;164:121–37. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256–62. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Ho A, Schlussman SD, Kreek MJ. Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacol. 2001;157:31–9. doi: 10.1007/s002130100744. [DOI] [PubMed] [Google Scholar]
- Martin S, Manzanares J, Corchero J, Garcia-Lecumberri C, Crespo JA, Fuentes JA, Ambrosio E. Differential basal proenkephalin gene expression in dorsal striatum and nucleus accumbens, and vulnerability to morphine self-administration in Fischer 344 and Lewis rats. Brain Res. 1999;821:350–5. doi: 10.1016/s0006-8993(99)01122-1. [DOI] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Qualitative Analyses of Behavior: The Effect of Delay and of Intervening Events on Reinforcement Value. Hillsdale, NJ: Erlbaum; 1987. pp. 55–73. [Google Scholar]
- Mitchell S. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacol. 1999;146:455–64. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Mitchell SH, Reeves JM, Li N, Phillips TJ. Delay discounting predicts behavioral sensitization to ethanol in outbred WSC mice. Alcohol Clin Exp Res. 2006;30:429–37. doi: 10.1111/j.1530-0277.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- Morgan D, Brebner K, Lynch WJ, Roberts DC. Increases in the reinforcing efficacy of cocaine after particular histories of reinforcement. Behav Pharmacol. 2002;13:389–96. doi: 10.1097/00008877-200209000-00012. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the care and use of mammals in Neuroscience and Behavioral Research. Washington D.C.: The National Academies Press; 2003. p. 209. [PubMed] [Google Scholar]
- Ohmura Y, Takahashi T, Kitamura N. Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacol. 2005;182:508–15. doi: 10.1007/s00213-005-0110-8. [DOI] [PubMed] [Google Scholar]
- Olmstead MC. Animal models of drug addiction: Where do we go from here? Q J Exp Psychol. 2006;59:625–53. doi: 10.1080/17470210500356308. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. Models of impulsivity and drug abuse. 2007a In preparation. [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacol. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry JL, Morgan AD, Anker JJ, Dess NK, Carroll ME. Escalation of i.v. cocaine self-administration and reinstatement of cocaine-seeking behavior in rats bred for high and low saccharin intake. Psychopharmacol. 2006a;186:235–45. doi: 10.1007/s00213-006-0371-x. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition and reinstatement of i.v. cocaine self-administration in male (v female) rats. 2007b doi: 10.1007/s00213-004-1994-4. Under review. [DOI] [PubMed] [Google Scholar]
- Perry JL, Normile LM, Morgan AD, Carroll ME. Sex differences in physical dependence on orally self-administered phencyclidine (PCP) in rhesus monkeys. Exp Clin Psychopharmacol. 2006b;14:68–78. doi: 10.1037/1064-1297.14.1.68. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacol. 2001;154:243–50. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Petry NM. Discounting of money, health, and freedom in substance abusers and controls. Drug Alcohol Depend. 2003;71:133–41. doi: 10.1016/s0376-8716(03)00090-5. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Maccari S, Mormede P, Le Moal M, Simon H. Individual reactivity to novelty predicts probability of amphetamine self-administration. Behav Pharmacol. 1990;1:339–45. doi: 10.1097/00008877-199000140-00007. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995;6:810–4. [PubMed] [Google Scholar]
- Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behav Processes. 2004;65:35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Richards JB, Mitchell SH, de Wit H, Seiden LS. Determination of discount functions in rats with an adjusting-amount procedure. J Exp Anal Behav. 1997;67:353–66. doi: 10.1901/jeab.1997.67-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, de Wit H. Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacol. 1999;146:432–9. doi: 10.1007/pl00005488. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev. 2004;28:533–46. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Skinner MD, Aubin HJ, Berlin I. Impulsivity in smoking, nonsmoking, and ex-smoking alcoholics. Addict Behav. 2004;29:973–8. doi: 10.1016/j.addbeh.2004.02.045. [DOI] [PubMed] [Google Scholar]
- Suzuki T, George FR, Meisch RA. Differential establishment and maintenance of oral ethanol reinforced behavior in Lewis and Fischer 344 inbred rat strains. J Pharmacol Exp Ther. 1988;245:164–70. [PubMed] [Google Scholar]
- Vaidya JG, Grippo AJ, Johnson AK, Watson D. A comparative developmental study of impulsivity in rats and humans: the role of reward sensitivity. Ann N Y Acad Sci. 2004;1021:395–8. doi: 10.1196/annals.1308.051. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer A, Vanderschuren L. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Exp Clin Psychopharmacol. 1998;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacol. 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Wallace CJ. The effects of delayed rewards, social pressure, and frustration on the responses of opiate addicts. NIDA Res Mono. 1979;25:6–25. [PubMed] [Google Scholar]


