Abstract
Statins have the potential to reduce breast cancer incidence and recurrence as shown in both epidemiologic and laboratory studies. The purpose of this study was to evaluate the effect of a lipophilic statin, atorvastatin, on breast cancer biomarkers of risk [mammographic density (MD) and insulin growth factor 1 (IGF-1)] in high-risk premenopausal women.
Premenopausal women at increased risk for breast cancer received either 40 mg of atorvastatin or placebo for 1 year. Biomarker assessment was performed prior to initiation and at completion of study medication. MD was determined using both Breast Imaging Reporting and Data System and the visual analogue scale. Serum IGF-1 was determined by ELISA assay at the end of the study.
Sixty-three women were enrolled between December 2005 and May 2010. Sixteen (25%) women withdrew. The mean age of participants was 43 (range, 35–50), 100% were white, and the average body mass index (BMI) was 26.4. The statin group demonstrated a significant decrease in cholesterol and low-density lipoprotein (LDL), suggesting compliance with study medication. After accounting for BMI, there was no difference in change in MD between groups. There was a significant increase in serum IGF-1 in the statin group.
In this multi-institutional randomized prospective clinical trial of premenopausal women at increased risk for breast cancer, we did not see an effect of atorvastatin on MD. Further investigation of statins may be warranted; however, design of prior trials and potential mechanism of action of the agent need to be considered in the design of future trials.
Introduction
There are several chemoprevention options for breast cancer prevention; however, these agents [selective estrogen response modifiers (SERMs) and aromatase inhibitors] only prevent estrogen receptor–positive (ER+) breast cancer (1, 2). In addition, toxicity and tolerability are significant barriers to utilization of these agents (3, 4). Agents which prevent ER− breast cancer with improved tolerability are needed.
Statins are well tolerated and may prevent both ER+ and ER− breast cancer (5–7). There are strong biologic data supporting a preventive effect (8–10). Epidemiologic studies have generally demonstrated a favorable effect of statins on breast cancer (7, 11–13); however, other studies have not shown this same effect (10, 14, 15). Meta-analyses of both observational studies and randomized controlled trials have not shown benefit of statins in breast cancer (16–21), although many of the authors and others suggest that short follow-up and inclusion of both lipophilic and hydrophilic statins may account for the lack of breast cancer prevention effect (22, 23).
Several short-term single-arm intervention studies evaluating statin effects on breast cancer biomarkers have been performed also with mixed results (24–26). The authors suggest that sample size, drug dose, and/or duration may have contributed to negative results (24, 25). We sought to evaluate the effect of statins for longer duration in a randomized placebo-controlled trial of high-risk, premenopausal women.
Among the available biomarkers for breast cancer risk, mammographic density (MD) is the most widely accepted (27, 28). MD is associated with a 4- to 6-fold increased risk of breast cancer for women with highest MD (defined as extremely dense or >75% dense; ref. 29). In addition, MD is modifiable (30–33), and modification is associated with change in risk (34, 35). Serum insulin growth factor-1 (IGF-1) is another important breast cancer biomarker (36–39). IGF-1 levels have been associated with risk, especially for premenopausal women (40–45). There is evidence to suggest that inhibition of mevalonate (by statins and other compounds) may affect both expression of IGF and levels of free IGF-1 (46). Given the acceptance of MD as an intermediate biomarker, and the association of MD and risk, this was chosen as our primary endpoint. IGF-1 levels were chosen as a secondary endpoint given the association of IGF-1 and premenopausal breast cancer and effect of statins on IGF levels (40–46). Importantly, both of these biomarkers have been used in prior biomarker studies involving statins and other potential breast cancer prevention agents (24, 25, 47). Given the cost and resources involved in large-scale randomized trials of chemoprevention agents, we sought to gather more definitive data supporting a chemopreventive effect of statins through a longer exposure (1 year) in a placebo-controlled trial of high-risk premenopausal women examining biomarker endpoints.
Materials and Methods
This was a multi-institutional, randomized, placebo-controlled trial examining the effect of 1 year of atorvastatin (40 mg), or placebo on both MD and serum IGF-1 in high-risk premenopausal women. This study was conducted at several institutions, including the University of Vermont, Dana Farber Cancer Institute, Southern Nevada Cancer Research Foundation, Southeastern Medical Oncology Center, and Delaware Christiana Care. The study was approved by the Institutional Review Board at each of the sites.
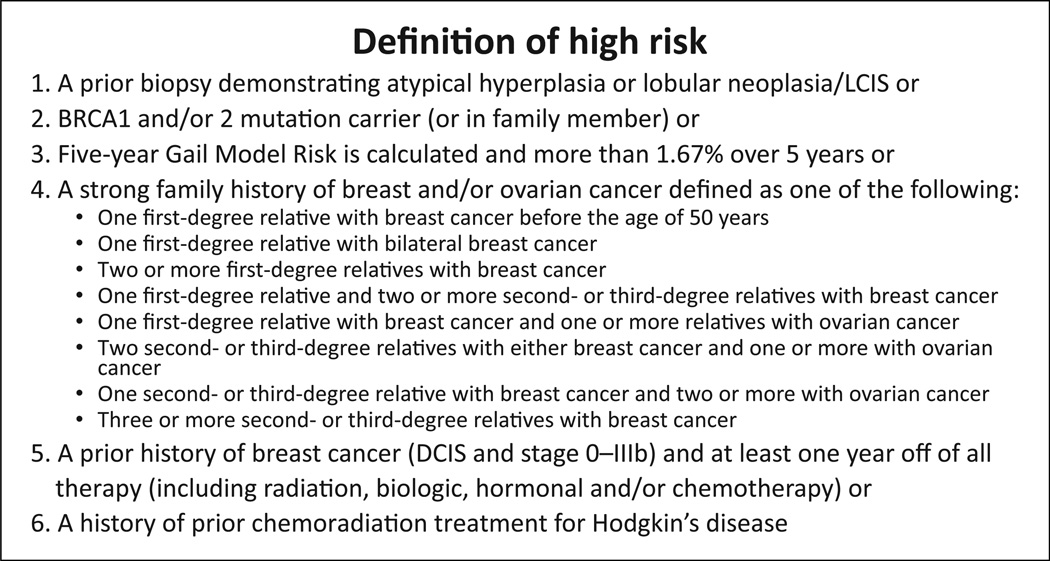
Women were eligible if age was 35 to 55 years, they were premenopausal (defined as having at least 4 menstrual cycles in 6 months, or a serum estradiol level in the premenopausal range for women with a hysterectomy) and at high risk for breast cancer (defined as meeting one of the criteria outlined in Fig. 1). Women were excluded if they had taken statins in the prior 6 months, were taking hormonal replacement therapy or a SERM or taking drugs that increase the risk of statin-induced myopathy or rhabdomyolysis (i.e., niacin, protease inhibitors, verapamil, gemfibrozil, cyclosporine, clofibrate/fenofibrate, or any CYP3A4 inhibitor). Women who were pregnant, planning to become pregnant, were breastfeeding, had underlying liver disease, or had known hypersensitivity to atorvastatin were excluded.
Figure 1.
Definition of high risk (61).
After signing written informed consent and completing an on-study evaluation [including physical examination, body mass index (BMI), system review, and questionnaires], women were randomized to receive either daily placebo or 40 mg of atorvastatin. Women were monitored for sensitivity to statins [liver enzymes, creatine phosphokinase (CPK), and muscle injury] at 6 weeks, 3, 6, and 9 months. Study participants completed questionnaires regarding medical history, diet, and physical activity at baseline (on-study) and 12 months. Lipid profiles were determined at baseline and 12 months.
Serum IGF-1
Blood was collected at baseline and 1 year for measurement of serum IGF-1. Serum IGF-1 concentrations were analyzed by a sandwich ELISA (human IGF-1 Quantikine Immunoassay; R&D Systems) after acid extraction of samples to remove endogenous binding proteins. Standards were recombinant human IGF-1. All samples were analyzed in a single run with an intra-assay coefficients of variation (CV) for rhIGF-1 of 9.2% and a sensitivity of 29 pg/mL. The investigator measuring IGF-1 was blinded to patient characteristics and treatment.
Mammographic density
Mammograms were obtained at baseline and 12 months. MD was determined using the Breast Imaging Reporting and Data System (BIRADs) category from the clinical report (48) in which BIRADs 1 correlates with an almost entirely fatty breast (<25% glandular); BIRADs 2 shows scattered fibroglandular densities (25%–50% glandular); BIRADs 3 shows the breast tissue is heterogeneously dense (51%–75% glandular); and BIRADS 4 shows the breast tissue is extremely dense (>75% glandular). MD was also determined using the visual analogue scale (VAS; ref. 49) by M. Wood and B. Sussman. VAS density scoring was performed twice for a single view of each mammogram, and a median score was calculated. Investigators determining MD were blinded to patient characteristics and treatment.
Statistical considerations
Based on the review of literature related to change in MD for women taking either hormone replacement therapy (HRT) or Tamoxifen, a decrease of 7.9% (33) would be considered clinically significant, and the control group would be expected to change 3.5%. This study was therefore designed to enroll 100 women, which would give an 80% power to detect a 3.5% difference in density (at the alpha = 0.05 level).
Descriptive statistical methods were used to show the distribution of study participant characteristics at baseline. Means and SDs were calculated to summarize continuous variables, whereas frequencies and percentages were determined to summarize categorical variables. Univariate inferential techniques, t tests, and χ2 tests were used to compare baseline characteristics for the placebo and atorvastatin groups.
To examine the change in outcomes over time, 95% confidence intervals were constructed to estimate the change in total cholesterol, high-density lipoprotein (HDL), LDL, IGF-1, and VAS for both the atorvastatin and placebo groups. Dependent t tests were used to identify statistically significant differences over time. Independent t tests were used to compare changes in these measures between groups. χ2 methods were used to examine changes in BIRADs categories between the two study groups over time. Regression methods were used to examine the effect of BMI on outcome measures.
Results
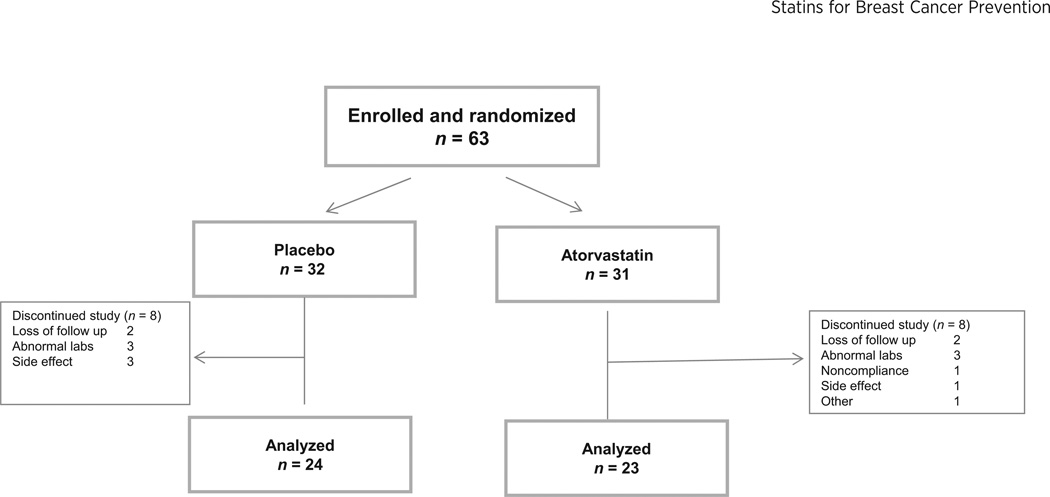
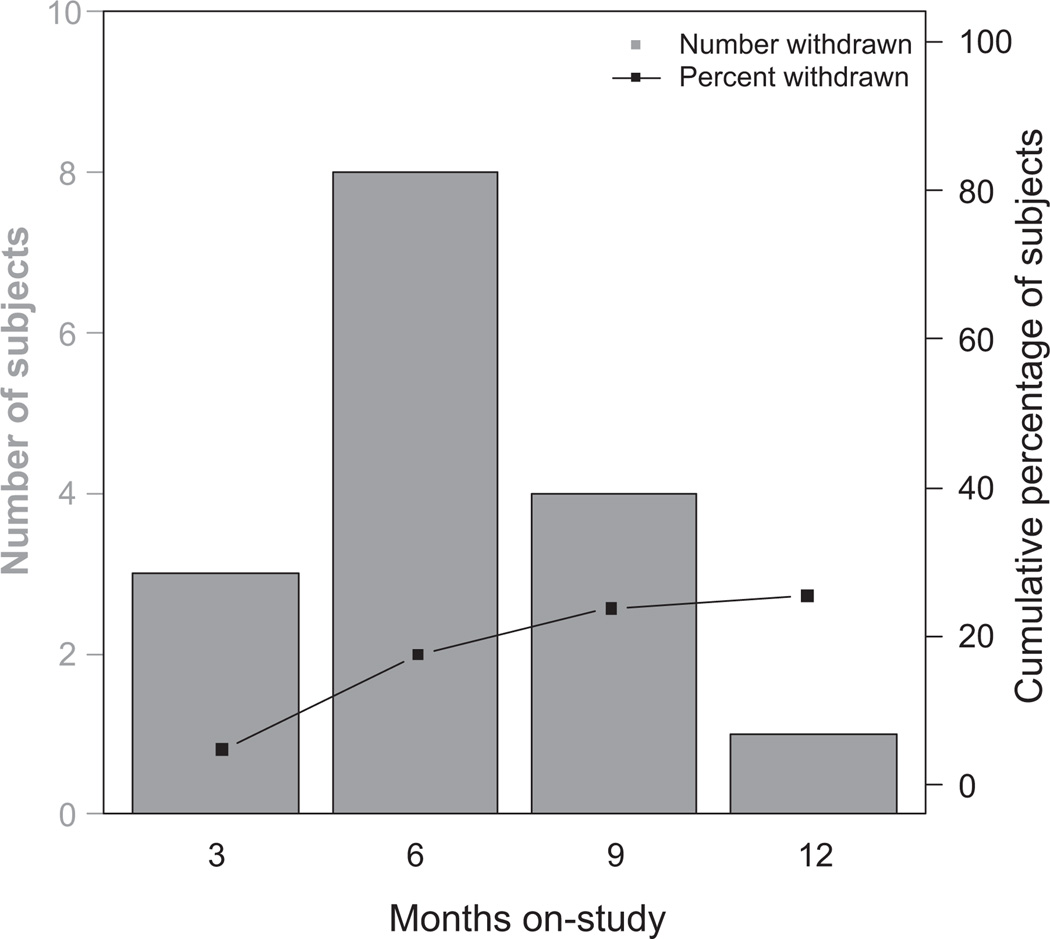
Sixty-three women were enrolled between December 2005 and May 2010. Sixteen women (25%) withdrew from the study; six due to laboratory abnormalities, four due to side effects, and four were lost to follow-up (see Fig. 2 for further detail). The dropout rate over time (Fig. 3) reveals a dropout rate at 6 months of 17%. Forty-seven women (74%) completed 1 year of study drug and were included in the outcome analysis. Patient characteristics are shown in Table 1. Women ranged in age from 35 to 50 years, 100% were Caucasian, average BMI was 26, and 66% of women entered had a strong family history of breast cancer. The two groups (placebo and atorvastatin) were well balanced, with no statistically significant differences in baseline characteristics (Table 1).
Figure 2.
Trial design.
Figure 3.
Patient withdrawals over time.
Table 1.
Baseline characteristics
| Characteristic | Placebo (n = 24) Mean (SD) or n (%) |
Atorvastatin (n = 23) Mean (SD) or n (%) |
P value |
|---|---|---|---|
| Age | 43 (4.5) | 43.7 (4.1) | 0.6772 |
| BMI | 25.8 (4.5) | 27.0 (6.8) | 0.5108 |
| Race, white | 22 (100%) | 23 (100%) | >0.999 |
| Eligibility risk criteria | |||
| Strong family history | 14 (63%) | 16 (70%) | |
| History of ADH/LCIS | 6 | 6 | |
| BRCA mutation carrier | 1 | 0 | |
| History of BC/DCIS | 1 | 0 | |
| Gail risk alone | 0 | 1 | |
| Calculated Gail risk (61) | |||
| 5-year risk | 2.3 (1.8) | 2.9 (2.0) | 0.3430 |
| Lifetime risk | 23.9 (8.6) | 28.2 (11.4) | 0.1834 |
| Total cholesterol mg/dl | 184.5 (28.6) | 196.0 (34.5) | 0.2224 |
| HDL mg/dl | 63.2 (17.1) | 62.2 (12.0) | 0.8269 |
| LDL mg/dl | 102.3 (26.8) | 114.1 (35.6) | 0.2036 |
| IGF ng/mL | 87.8 (27.7) | 85.0 (22.4) | 0.7171 |
| Density measurements | |||
| VAS percent density | 33.7 (18.5) | 31.4 (24.8) | 0.7311 |
| BIRAD density category | |||
| 1 | 2 (8%) | 1 (4%) | |
| 2 | 1 (4%) | 8 (35%) | 0.060 |
| 3 | 16 (67%) | 12 (52%) | |
| 4 | 4 (17%) | 2 (9%) |
Abbreviations: ADH, atypical ductal hyperplasia; BC, breast cancer; DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ.
Toxicity
Little toxicity was reported by women in this study. Elevated CPK was seen in four participants (two on placebo). Elevations in liver enzymes were seen in three individuals (one on placebo).
Lipid profiles
Paired baseline and posttreatment fasting lipid profiles were available for 89% (n = 42) of the study participants. The average total cholesterol in the placebo group was 184.5 mg/dl at baseline and was unchanged (186.3 mg/dl, P > 0.05) after 1 year. By contrast, cholesterol levels in the atorvastatin group were 196.0 mg/dl at baseline and were significantly reduced after 1 year (152.4 mg/dl; P < 0.05).
Results for HDL and LDL levels over time were available for 57% (n = 13) of the atorvastatin group and 67% (n = 16) of the placebo group. In the atorvastatin group, there was a significant decrease in LDL over time (P < 0.001) but no change in HDL. Results are summarized in Table 2.
Table 2.
Change in cholesterol, baseline to 12-month follow-up
| Placebo | Atorvastatin | ||||
|---|---|---|---|---|---|
| Measure | N | Mean change (95% CI) | N | Mean change (95% CI) | P value |
| Total cholesterol (mg/dl) | 22 | 1.1 (−11.4–13.5) | 20 | −45.1 (−61–29.1) | <0.001 |
| HDL (mg/dl) | 12 | −1.6 (−7.0–3.8) | 8 | −1.9 (−7.8–4.0) | 0.9370 |
| LDL (mg/dl) | 13 | −3.5 (−15.0–8.1) | 8 | −49.1 (−74.8–23.5) | <0.001 |
Abbreviation: CI, confidence interval.
Mammographic density
MD was measured at baseline and after 1 year using both the BIRADs and VAS methods. BIRADs measures were available for 94% of study participants (n = 44), and VAS calculations were determined for 85% (n = 40). Although not statistically significant, a greater proportion of women in the placebo group had increased MD (BIRADs 3 or 4) compared with the atorvastatin group at baseline (84% and 61%, respectively). The BIRADs measure of MD in the majority of women in both groups did not change after 1 year (Table 3). Moreover, the changes in BIRADs category over time were not significant (P = 0.244; Table 3).
Table 3.
Change in MD, baseline to 12-month follow-up
| Placebo (n = 21) | Atorvastatin (n = 23) | ||
|---|---|---|---|
| BIRAD category | N (%) | N (%) | P value |
| No change | 14 (58%) | 16 (70%) | |
| Increase | 1 (4%) | 3 (13%) | 0.244 |
| Decrease | 6 (25%) | 4 (17%) | |
| Placebo (n = 20) | Atorvastatin (n = 20) | ||
| VAS | Mean change (95% CI) | Mean change (95% CI) | |
| −0.3 (−3.8–3.2) | 0 (−3.9–3.9) | 0.9056 | |
The mean MD at baseline using the VAS method was similar for both groups (33.7% for placebo vs. 31.4% for the atorvastatin group). There was no significant change in VAS density in either group over time (Table 3). This was true whether change was examined for the group as a whole or when determining change for each participant.
Serum IGF-1 analysis
Paired samples for baseline and 1-year IGF-1 levels were available for 87% (n = 41) of study participants. Mean values for both groups at baseline were similar (87.8 ng/mL for the placebo and 85.0 ng/mL for the atorvastatin group, P = 0.71). At 12months, the placebo group had an average decrease of 3.3 ng/mL (not significant), whereas the atorvastatin group had an average increase of 14.7 ng/mL (P = 0.021). The difference in change was significantly different between the two groups (P = 0.031; Table 4).
Table 4.
Change in IGF-1, baseline to 12-month follow-up
| Placebo (n = 21) | Atorvastatin (n = 20) | ||
|---|---|---|---|
| Mean change (95% CI) | Mean change (95% CI) | P value | |
| IGF-1 (ng/mL) | −3.3 (−14.7–8.2) | 14.7 (2.4–27.0) | 0.0308 |
Discussion
In this multicenter, randomized, placebo-controlled trial involving high-risk premenopausal women, we were unable to demonstrate an effect of the lipophilic statin, atorvastatin, on MD (the primary endpoint of the study). We did demonstrate a decrease in cholesterol (suggesting a high degree of compliance) and observed an increase in IGF-1 with atorvastatin.
Our findings are somewhat similar to other short-term biomarker studies of statins for breast cancer prevention. Higgins and colleagues evaluated the effects of 24 to 28 weeks of simvastatin on the contralateral breast in 45 women (ranging in age from 38–74) with stage 0 to III breast cancer (25). They observed a decrease in cholesterol but no change in MD. They did find a decrease in CRP and estrone sulfate. IGF-1 was not evaluated. Vinayak and colleagues evaluated 6 months of lovastatin in 26 high-risk women ranging in age from 25 to 62 (24). They saw no change in cholesterol profiles or biomarkers (cytologic atypia, MD, IGF levels, or CRP). Compared with these studies, our study was larger, treated women for a longer time, and was placebo controlled.
Our study was originally designed to enroll 100 women, giving an 80% power to detect a 3.5% difference in density (at alpha = 0.05). We were forced to stop the study early due to lack of funding. This study should therefore be considered underpowered, with an increased likelihood of a Type I error. In addition, interpretation of MD is known to be subjective with high intra-and inter-reader variability (50, 51). The methods we used may have been too imprecise to see a significant change in MD. The BIRADs categories each span 25 percentage points, so a change of less than 25% might not have been detected if the category did not change. In addition, MD done by the VAS method led to clustering of measurements around whole numbers, potentially missing smaller differences.
Given that our and other studies have failed to demonstrate a change in MD while identifying significant change in other biomarkers, it is important to consider that statins may not act via change in MD. Other studies of agents known to prevent breast cancer, such as aromatase inhibitors, have not identified change in MD (47). Statins have however, been shown to modulate other biomarkers. Garwood and colleagues demonstrated reduced proliferation and increased apoptosis as indicated by changes in Ki-67 and cleaved caspase-3, respectively, in women with stage 0 to 1 breast cancer treated with 3 to 6 weeks of fluvastatin (26). Higgins and colleagues identified a significant change in hsCRP and estrone sulfate levels after 6 months of simvastatin (25).
Our observation of increased IGF-1 after 1 year of atorvastatin was unexpected. Given the randomized design of this study and that the two groups were well balanced, this is likely a real effect. We are, however, at a loss to explain the mechanism of this effect. Circulating IGF-1 is affected by numerous variables, including age, sex, exercise, stress level, and circadian rhythm. Recent studies show that IGF-1 levels are associated with HDL levels, and are inversely associated with total cholesterol and LDL (52, 53). We did not find this same association, but our sample size may have been too small to see this effect.
Despite our findings, there is a strong biologic rationale for statins as a preventive therapy. Statins decrease both mevalonate and its downstream lipid products which interfere with the function of RAS and other G proteins leading to decreased proliferation and survival (54). Statins are also known to cause cell-cycle arrest by affecting the G1 to S transition, partially due to increased CDK (p21/p27) activity (55–57). Campbell reported that statin use suppressed the MAPK pathway and increased the expression of p21. Such effects were more pronounced in ER− cell lines (9). Induction of apoptosis by statins may be another mechanism of cancer prevention (58). One recent study indicated such an inhibitory effect may work through upregulated PTEN expression (59). The effects of statins on proliferation and apoptosis provide a strong biologic rationale for a preventive effect (60).
This study has several strengths: it is multi-institutional and placebo controlled, women were treated for 1 year (longer than other studies), and our cohort was not only high risk but premenopausal. In addition, there was a high degree of compliance, demonstrated by lowered lipid levels over time. There are, however, limitations that must be considered including the small sample size and high dropout rate. Our dropout rate is higher than other studies of statins and biomarkers (24, 25). However, the dropout rate at 6 months of 17% is similar to the prior two studies of 6-month duration (10% and 13%; refs. 24, 25).
In conclusion, the strong biologic rationale and observational studies supporting the effect of statins as prevention therapy for ER− and ER+ breast cancer suggest that additional investigation of statins may be warranted. Design of these trials might focus on biomarkers that have shown effects of statins, such as proliferation, apoptosis, and inflammation. However, these biomarkers are not as strongly linked to breast cancer development as MD. In addition, more precise measurement of MD (such as fully automated methods) may reveal a significant change.
Acknowledgments
Grant Support
This study is sponsored by grants from Breast Cancer Research Breast Cancer Research Foundation, New York, NY, and Cancer and Leukemia Group B (prevention pilot grant) to M.E. Wood.
Footnotes
This study was registered with the NCI as NCT NCT00914017 (http://clinicaltrials.gov/show/NCT00914017).
Disclosure of Potential Conflicts of Interest
J.E. Garber is a consultant/advisory board member for Pfizer. No potential conflicts of interest were disclosed by the other authors.
Authors' Contributions
Conception and design: Y. Ji, H.B. Muss, M.E. Wood
Development of methodology: Y. Ji, A. Crocker, B. Sussman, R.C. Hovey, M.E. Wood
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): Y. Ji, A. Crocker, R.C. Hovey, F. Kingsley, H.B. Muss, M.E. Wood
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): Y. Ji, T. Rounds, A. Crocker, B. Sussman, H.B. Muss
Writing, review, and/or revision of the manuscript: Y. Ji, A. Crocker, B. Sussman, R.C. Hovey, H.B. Muss, J.E. Garber, M.E. Wood
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): Y. Ji, T. Rounds, A. Crocker, F. Kingsley, H.B. Muss
Study supervision: M.E. Wood
References
- 1.Visvanathan K, Hurley P, Bantug E, Brown P, Col NF, Cuzick J, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31:2942–2962. doi: 10.1200/JCO.2013.49.3122. [DOI] [PubMed] [Google Scholar]
- 2.Cuzick J, DeCensi A, Arun B, Brown PH, Castiglione M, Dunn B, et al. Preventive therapy for breast cancer: A consensus statement. Lancet Oncol. 2011;12:496–503. doi: 10.1016/S1470-2045(11)70030-4. [DOI] [PubMed] [Google Scholar]
- 3.Dent SF, Gaspo R, Kissner M, Pritchard KI. Aromatase inhibitor therapy: Toxicities and management strategies in the treatment of postmenopausal women with hormone-sensitive early breast cancer. Breast Cancer Res Treat. 2011;126:295–310. doi: 10.1007/s10549-011-1351-3. [DOI] [PubMed] [Google Scholar]
- 4.Makubate B, Donnan PT, Dewar JA, Thompson AM, McCowan C. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer. 2013;108:1515–1524. doi: 10.1038/bjc.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minder CM, Blumenthal RS, Blaha MJ. Statins for primary prevention of cardiovascular disease: The benefits outweigh the risks. Curr Opin Cardiol. 2013;28:554–560. doi: 10.1097/HCO.0b013e32836429e6. [DOI] [PubMed] [Google Scholar]
- 6.Mueck AO, Seeger H, Wallwiener D. Effect of statins combined with estradiol on the proliferation of human receptor-positive and receptor-negative breast cancer cells. Menopause. 2003;10:332–336. doi: 10.1097/01.GME.0000055485.06076.00. [DOI] [PubMed] [Google Scholar]
- 7.Kumar AS, Benz CC, Shim V, Minami CA, Moore DH, Esserman LJ. Estrogen receptor-negative breast cancer is less likely to arise among lipophilic statin users. Cancer Epidemiol Biomarkers Prev. 2008;17:1028–1033. doi: 10.1158/1055-9965.EPI-07-0726. [DOI] [PubMed] [Google Scholar]
- 8.Seeger H, Wallwiener D, Mueck AO. Statins can inhibit proliferation of human breast cancer cells in vitro. Exp Clin Endocrinol Diabetes. 2003;111:47–48. doi: 10.1055/s-2003-37501. [DOI] [PubMed] [Google Scholar]
- 9.Campbell MJ, Esserman LJ, Zhou Y, Shoemaker M, Lobo M, Borman E, et al. Breast cancer growth prevention by statins. Cancer Res. 2006;66:8707–8714. doi: 10.1158/0008-5472.CAN-05-4061. [DOI] [PubMed] [Google Scholar]
- 10.Pocobelli G, Newcomb PA, Trentham-Dietz A, Titus-Ernstoff L, Hampton JM, Egan KM. Statin use and risk of breast cancer. Cancer. 2008;112:27–33. doi: 10.1002/cncr.23129. [DOI] [PubMed] [Google Scholar]
- 11.Cauley JA, Zmuda JM, Lui LY, Hillier TA, Ness RB, Stone KL, et al. Lipid-lowering drug use and breast cancer in older women: A prospective study. J Womens Health (Larchmt) 2003;12:749–756. doi: 10.1089/154099903322447710. [DOI] [PubMed] [Google Scholar]
- 12.Farwell WR, Scranton RE, Lawler EV, Lew RA, Brophy MT, Fiore LD, et al. The association between statins and cancer incidence in a veterans population. J Natl Cancer Inst. 2008;100:134–139. doi: 10.1093/jnci/djm286. [DOI] [PubMed] [Google Scholar]
- 13.Karp I, Behlouli H, Lelorier J, Pilote L. Statins and cancer risk. Am J Med. 2008;121:302–309. doi: 10.1016/j.amjmed.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Blais L, Desgagne A, LeLorier J. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case-control study. Arch Intern Med. 2000;160:2363–2368. doi: 10.1001/archinte.160.15.2363. [DOI] [PubMed] [Google Scholar]
- 15.Boudreau DM, Gardner JS, Malone KE, Heckbert SR, Blough DK, Daling JR. The association between 3-hydroxy-3-methylglutaryl conenzyme A inhibitor use and breast carcinoma risk among postmenopausal women: a case-control study. Cancer. 2004;100:2308–2316. doi: 10.1002/cncr.20271. [DOI] [PubMed] [Google Scholar]
- 16.Taylor ML, Wells BJ, Smolak MJ. Statins and cancer: A meta-analysis of case-control studies. Eur J Cancer Prev. 2008;17:259–268. doi: 10.1097/CEJ.0b013e3282b721fe. [DOI] [PubMed] [Google Scholar]
- 17.Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Use of statins and breast cancer: A meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol. 2005;23:8606–8612. doi: 10.1200/JCO.2005.02.7045. [DOI] [PubMed] [Google Scholar]
- 18.Undela K, Srikanth V, Bansal D. Statin use and risk of breast cancer: A meta-analysis of observational studies. Breast Cancer Res Treat. 2012;135:261–269. doi: 10.1007/s10549-012-2154-x. [DOI] [PubMed] [Google Scholar]
- 19.Peto R, Emberson J, Landray M, Baigent C, Collins R, Clare R, et al. Analyses of cancer data from three ezetimibe trials. N Engl J Med. 2008;359:1357–1366. doi: 10.1056/NEJMsa0806603. [DOI] [PubMed] [Google Scholar]
- 20.Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: A meta-analysis. JAMA. 2006;295:74–80. doi: 10.1001/jama.295.1.74. [DOI] [PubMed] [Google Scholar]
- 21.Browning DR, Martin RM. Statins and risk of cancer: A systematic review and metaanalysis. Int J Cancer. 2007;120:833–843. doi: 10.1002/ijc.22366. [DOI] [PubMed] [Google Scholar]
- 22.Prowell TM, Stearns V, Trock B. Lipophilic statins merit additional study for breast cancer chemoprevention. J Clin Oncol. 2006;24:2128–2129. doi: 10.1200/JCO.2005.05.1649. author reply 2129. [DOI] [PubMed] [Google Scholar]
- 23.Sprague JR, Wood ME. Statins and breast cancer prevention: Time for randomized controlled trials. J Clin Oncol. 2006;24:2129–2130. doi: 10.1200/JCO.2005.05.5392. author reply 2130–2121. [DOI] [PubMed] [Google Scholar]
- 24.Vinayak S, Schwartz EJ, Jensen K, Lipson J, Alli E, McPherson L, et al. A clinical trial of lovastatin for modification of biomarkers associated with breast cancer risk. Breast Cancer Res Treat. 2013;142:389–398. doi: 10.1007/s10549-013-2739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins MJ, Prowell TM, Blackford AL, Byrne C, Khouri NF, Slater SA, et al. A short-term biomarker modulation study of simvastatin in women at increased risk of a new breast cancer. Breast Cancer Res Treat. 2012;131:915–924. doi: 10.1007/s10549-011-1858-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garwood ER, Kumar AS, Baehner FL, Moore DH, Au A, Hylton N, et al. Fluvastatin reduces proliferation and increases apoptosis in women with high grade breast cancer. Breast Cancer Res Treat. 2010;119:137–144. doi: 10.1007/s10549-009-0507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyd NF, Martin LJ, Stone J, Greenberg C, Minkin S, Yaffe MJ. Mammographic densities as a marker of human breast cancer risk and their use in chemoprevention. Curr Oncol Rep. 2001;3:314–321. doi: 10.1007/s11912-001-0083-7. [DOI] [PubMed] [Google Scholar]
- 28.Heine JJ, Malhotra P. Mammographic tissue, breast cancer risk, serial image analysis, and digital mammography. Part 1. Tissue and related risk factors. Acad Radiol. 2002;9:298–316. doi: 10.1016/s1076-6332(03)80373-2. [DOI] [PubMed] [Google Scholar]
- 29.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 30.Greendale GA, Reboussin BA, Slone S, Wasilauskas C, Pike MC, Ursin G. Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst. 2003;95:30–37. doi: 10.1093/jnci/95.1.30. [DOI] [PubMed] [Google Scholar]
- 31.Stuedal A, Ma H, Bjorndal H, Ursin G. Postmenopausal hormone therapy with estradiol and norethisterone acetate and mammographic density: Findings from a cross-sectional study among Norwegian women. Climacteric. 2009;12:248–258. doi: 10.1080/13697130802638458. [DOI] [PubMed] [Google Scholar]
- 32.Atkinson C, Warren R, Bingham SA, Day NE. Mammographic patterns as a predictive biomarker of breast cancer risk: Effect of tamoxifen. Cancer Epidemiol Biomarkers Prev. 1999;8:863–866. [PubMed] [Google Scholar]
- 33.Cuzick J, Warwick J, Pinney E, Warren RM, Duffy SW. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96:621–628. doi: 10.1093/jnci/djh106. [DOI] [PubMed] [Google Scholar]
- 34.Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: A quantitative review to 2011. Ann Oncol. 2012;23:1403–1415. doi: 10.1093/annonc/mds113. [DOI] [PubMed] [Google Scholar]
- 35.Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: A nested case-control study. J Natl Cancer Inst. 2011;103:744–752. doi: 10.1093/jnci/djr079. [DOI] [PubMed] [Google Scholar]
- 36.Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 37.Toniolo P, Bruning PF, Akhmedkhanov A, Bonfrer JM, Koenig KL, Luka-nova A, et al. Serum insulin-like growth factor-I and breast cancer. Int J Cancer. 2000;88:828–832. doi: 10.1002/1097-0215(20001201)88:5<828::aid-ijc22>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Fletcher O, Gibson L, Johnson N, Altmann DR, Holly JM, Ashworth A, et al. Polymorphisms and circulating levels in the insulin-like growth factor system and risk of breast cancer: A systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:2–19. [PubMed] [Google Scholar]
- 39.Allen NE, Roddam AW, Allen DS, Fentiman IS, Dos Santos Silva I, Peto J, et al. A prospective study of serum insulin-like growth factor-I (IGF-I), IGF-II, IGF-binding protein-3 and breast cancer risk. Br J Cancer. 2005;92:1283–1287. doi: 10.1038/sj.bjc.6602471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 41.Sugumar A, Liu YC, Xia Q, Koh YS, Matsuo K. Insulin-like growth factor (IGF)-I and IGF-binding protein 3 and the risk of premenopausal breast cancer: A meta-analysis of literature. Int J Cancer. 2004;111:293–297. doi: 10.1002/ijc.20253. [DOI] [PubMed] [Google Scholar]
- 42.Shi R, Yu H, McLarty J, Glass J. IGF-I and breast cancer: a meta-analysis. Int J Cancer. 2004;111:418–423. doi: 10.1002/ijc.20233. [DOI] [PubMed] [Google Scholar]
- 43.Schernhammer ES, Holly JM, Pollak MN, Hankinson SE. Circulating levels of insulin-like growth factors, their binding proteins, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:699–704. doi: 10.1158/1055-9965.EPI-04-0561. [DOI] [PubMed] [Google Scholar]
- 44.Vatten LJ, Holly JM, Gunnell D, Tretli S. Nested case-control study of the association of circulating levels of serum insulin-like growth factor I and insulin-like growth factor binding protein 3 with breast cancer in young women in Norway. Cancer Epidemiol Biomarkers Prev. 2008;17:2097–2100. doi: 10.1158/1055-9965.EPI-08-0212. [DOI] [PubMed] [Google Scholar]
- 45.Endogenous H, Breast Cancer Collaborative G, Key TJ, Appleby PN, Reeves GK, Roddam AW. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: Pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11:530–542. doi: 10.1016/S1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCarty MF. Suppression of dolichol synthesis with isoprenoids and statins may potentiate the cancer-retardant efficacy of IGF-I down-regulation. Med Hypotheses. 2001;56:12–16. doi: 10.1054/mehy.2000.1073. [DOI] [PubMed] [Google Scholar]
- 47.Cigler T, Richardson H, Yaffe MJ, Fabian CJ, Johnston D, Ingle JN, et al. A randomized, placebo-controlled trial (NCIC CTG MAP.2) examining the effects of exemestane on mammographic breast density, bone density, markers of bone metabolism and serum lipid levels in postmenopausal women. Breast Cancer Res Treat. 2011;126:453–461. doi: 10.1007/s10549-010-1322-0. [DOI] [PubMed] [Google Scholar]
- 48.Balleyguier C, Ayadi S, Van Nguyen K, Vanel D, Dromain C, Sigal R. BIRADS classification in mammography. Eur J Radiol. 2007;61:192–194. doi: 10.1016/j.ejrad.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 49.Duffy SW, Nagtegaal ID, Astley SM, Gillan MG, McGee MA, Boggis CR, et al. Visually assessed breast density, breast cancer risk and the importance of the craniocaudal view. Breast Cancer Res. 2008;10:R64. doi: 10.1186/bcr2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicholson BT, LoRusso AP, Smolkin M, Bovbjerg VE, Petroni GR, Harvey JA. Accuracy of assigned BI-RADS breast density category definitions. Acad Radiol. 2006;13:1143–1149. doi: 10.1016/j.acra.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Ooms EA, Zonderland HM, Eijkemans MJ, Kriege M, Mahdavian Delavary B, Burger CW, et al. Mammography: Interobserver variability in breast density assessment. Breast. 2007;16:568–576. doi: 10.1016/j.breast.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 52.Succurro E, Arturi F, Grembiale A, Iorio F, Laino I, Andreozzi F, et al. Positive association between plasma IGF1 and high-density lipoprotein cholesterol levels in adult nondiabetic subjects. Eur J Endocrinol. 2010;163:75–80. doi: 10.1530/EJE-10-0113. [DOI] [PubMed] [Google Scholar]
- 53.Colangelo LA, Liu K, Gapstur SM, Study CMH. Insulin-like growth factor-1, insulin-like growth factor binding protein-3, and cardiovascular disease risk factors in young black men and white men: The CARDIA Male Hormone Study. Am J Epidemiol. 2004;160:750–757. doi: 10.1093/aje/kwh289. [DOI] [PubMed] [Google Scholar]
- 54.Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003;9:10–19. [PubMed] [Google Scholar]
- 55.Denoyelle C, Vasse M, Korner M, Mishal Z, Ganne F, Vannier JP, et al. Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits the signaling pathways involved in the invasiveness and metastatic properties of highly invasive breast cancer cell lines: an in vitro study. Carcinogenesis. 2001;22:1139–1148. doi: 10.1093/carcin/22.8.1139. [DOI] [PubMed] [Google Scholar]
- 56.Wachtershauser A, Akoglu B, Stein J. HMG-CoA reductase inhibitor mevastatin enhances the growth inhibitory effect of butyrate in the colorectal carcinoma cell line Caco-2. Carcinogenesis. 2001;22:1061–1067. doi: 10.1093/carcin/22.7.1061. [DOI] [PubMed] [Google Scholar]
- 57.Keyomarsi K, Sandoval L, Band V, Pardee AB. Synchronization of tumor and normal cells from G1 to multiple cell cycles by lovastatin. Cancer Res. 1991;51:3602–3609. [PubMed] [Google Scholar]
- 58.Kotamraju S, Williams CL, Kalyanaraman B. Statin-induced breast cancer cell death: Role of inducible nitric oxide and arginase-dependent pathways. Cancer Res. 2007;67:7386–7394. doi: 10.1158/0008-5472.CAN-07-0993. [DOI] [PubMed] [Google Scholar]
- 59.Ghosh-Choudhury N, Mandal CC, Ghosh-Choudhury N, Ghosh Choudhury G. Simvastatin induces derepression of PTEN expression via NFkappaB to inhibit breast cancer cell growth. Cell Signal. 2010;22:749–758. doi: 10.1016/j.cellsig.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vinayak S, Kurian AW. Statins may reduce breast cancer risk, particularly hormone receptor-negative disease. Curr Breast Cancer Rep. 2009;1:148–156. doi: 10.1007/s12609-009-0021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]