Abstract
The discovery of microtube structures that link tumour cells in some invasive brain tumours reveals how these cancers spread, and how they resist treatment.
Hardly any diagnosis is as distressing as that of a primary brain tumour. These tumours, often known as gliomas, are a varied group that originate from immature stem cells or from glial cells, which support and protect neuronal networks throughout the brain1. Gliomas proliferate uncontrollably, destroying surrounding brain tissue and causing profound neurological damage. They are responsible for around 14,000 deaths each year in the United States alone2, and are almost always deadly, owing to their resistance to radiation therapy and ability to infiltrate healthy brain tissue. In a paper online in Nature, Osswald et al.3 shed light on what confers these destructive abilities.
Gliomas have long frustrated neurosurgeons4, because cancerous cells invade the surrounding brain before diagnosis is possible, making surgical removal of these tumours inefficient. The tumours move into the brain through extracellular spaces5, often following the outside of blood vessels or nerve tracts — in contrast to most other cancers, which disseminate through the blood or lymphatic systems. The invading cells must be killed if treatment is to be successful, and so patients typically undergo aggressive radiation therapy in combination with chemotherapy.
Many gliomas, including the most malignant varieties, resist both radiation and chemotherapy. But a small subgroup called oligodendrogliomas, which harbour deletions in two chromosomal regions dubbed 1p and 19q, respond well to radiation treatment and carry a more favourable prognosis6. Osswald and colleagues set out to determine what accounts for this difference in radiation sensitivity.
The authors labelled patient-derived glioma cells taken from a variety of tumours before transplanting them into the brains of mice. Using in vivo microscopy, they visualized tumour growth and invasion for up to one year through a window implanted in the animals’ heads. Invading glioma cells without the 1p and 19q co-deletion extended long, thin, contractile processes into the surrounding brain tissue. The researchers called these structures tumour microtubes.
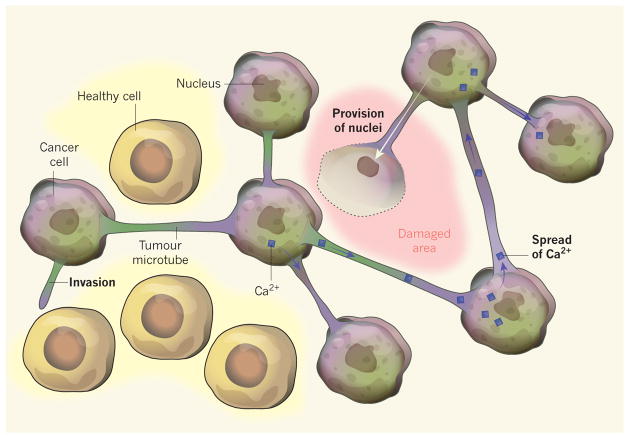
The microtubes were rich in the proteins actin and myosin, which are known7 to propel neuronal growth cones (structures at the tips of developing neurons that project out to seek other cells with which to connect). Some microtubes explored, and eventually invaded, the healthy brain. By contrast, others contacted neighbouring tumour cells, forming cytoplasmic bridges between adjacent cells (Fig. 1). This effectively turned the cells into a single organismal unit (a syncytium). The connections between microtubes from each tumour cell were formed by cytoplasm-filled pores called gap junctions, composed of hexameric Cx43 proteins.
Figure 1. A network of neighbours.
Osswald et al.3 report that, in some types of brain tumour, structures called microtubes connect tumour cells, allowing them to act as a single, organism-like unit. Tumour microtubes facilitate invasion into healthy brain tissue. They permit the spread of toxic molecules such as calcium ions (Ca2+) that build up during radiation therapy, allowing the whole unit to share the burden of toxicity. Furthermore, if tumour tissue is surgically removed, newly synthesized nuclei donated by tumour cells can travel down the microtube to the cell-free site to form new tumour cells.
What advantage might the tumour gain from growing as a single organismal unit? Osswald et al. found that cells of the syncytium were highly resistant to radiation therapy. In unconnected cells, radiation caused an increase in intracellular calcium-ion levels that triggered cell death. But in cells of the syncytium, Ca2+ levels remained more stable, presumably because the extra Ca2+ was distributed among all cells. Furthermore, when the nucleus of a connected cell was ablated by laser, the neighbouring cell extended a microtube into the affected tissue to deliver a newly synthesized cell nucleus, thus replacing the dead neighbour with a newly nucleated cell — a remarkable feat. The connected cells can thus be thought of as forming a neighbourhood watch group, protecting one another by sharing toxic exposure and even going as far as replacing dead neighbours with part of themselves.
These results indicate that those gliomas that are sensitive to radiation should not be part of a protective neighbourhood. Indeed, when Osswald et al. analysed human biopsies from oligodendrogliomas, fewer than 1% contained microtubes and Cx43 expression was almost absent. The authors also found that microtubes and Cx43 were lacking when they transplanted oligodendroglioma-derived cells harbouring the 1p and 19q co-deletion into mice.
Because microtubes are at the heart of the glioma network, the authors next searched for genes that regulate microtube formation. Through Ingenuity Pathway Analysis (a computer-aided method for detecting genes linked to biological traits), they identified Gap-43 as a candidate. The GAP-43 protein aids neuronal migration and the formation of neural growth cones during development8. It was prominently expressed on the invading tips of tumour microtubes, and was conspicuously absent in 1p and 19q co-deleted tumours. Moreover, forced expression of GAP-43 in oligodendroglioma-derived transplants produced highly invasive tumours that acted in protective, microtube-connected networks and resisted radiation. Thus, GAP-43 seems to mediate tumour-microtube formation.
This carefully executed study advances our understanding of brain-tumour growth. For instance, it is now clear that the gap junctions formed by Cx43 are central to the success of glioma neighbourhoods. This protein has been thought to act as a tumour suppressor, preventing cell division in cancers, including gliomas9. By contrast, Osswald and colleagues’ findings imply that tumour growth is enhanced by the presence of Cx43. This difference can be reconciled when one considers the organism-like growth of the syncytium — in this scenario, the connected cells protect and support one other, allowing the tumour mass to grow even when exposed to radiation.
Although the authors hypothesize that the sharing of Ca2+ confers resistance to radiation, many other mechanisms could be at work. Gap junctions allow the passage of many macromolecules between cells, including ATP, amino acids and even microRNAs. One molecule deserving of consideration is the antioxidant glutathione, which readily permeates gap junctions to directly protect cells from radiation damage7.
From a therapeutic perspective, Cx43 is a challenging pharmacological target. The protein is expressed throughout the body, and is required both for glial transport of cellular metabolites, signals and waste products, and to ensure that heart cells contract in synchrony. Drugs that block Cx43 have been used to heal chronic skin ulcers in humans10 and to enhance the effectiveness of the chemotherapeutic drug temozolamide in treating gliomas in mice11. However, Osswald and colleagues’ discovery that GAP-43 is responsible for establishing glioma networks might point to a more effective target for combating these destructive cancers.
References
- 1.Stiles CD, Rowitch DH. Neuron. 2008;58:832–846. doi: 10.1016/j.neuron.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 2.Central Brain Tumour Registry of the United States. Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2008. CBTRUS; 2012. [Google Scholar]
- 3.Osswald M, et al. Nature. 2015 http://dx.doi.org/10.1038/nature16071.
- 4.Sontheimer H. In: Diseases of the Nervous System. Sontheimer H, editor. Academic; 2015. pp. 259–288. [Google Scholar]
- 5.Seifert S, Sontheimer H. J Physiol (Lond) 2014;592:5109–5127. doi: 10.1113/jphysiol.2014.274498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamberlain MC, Born D. J Neurooncol. 2015;125:249–251. doi: 10.1007/s11060-015-1906-y. [DOI] [PubMed] [Google Scholar]
- 7.Smith SJ. Science. 1988;242:708–715. doi: 10.1126/science.3055292. [DOI] [PubMed] [Google Scholar]
- 8.Denny JB. Curr Neuropharmacol. 2006;4:293–304. doi: 10.2174/157015906778520782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naus CC, Laird DW. Nature Rev Cancer. 2010;10:435–441. doi: 10.1038/nrc2841. [DOI] [PubMed] [Google Scholar]
- 10.Grek CL, Rhett JM, Ghatnekar GS. FEBS Lett. 2014;588:1349–1364. doi: 10.1016/j.febslet.2014.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy SF, et al. Cancer Res. http://dx.doi.org/10.1158/0008-5472.CAN-15-1286 in the press.