Abstract
PURPOSE
To characterize the clinical spectrum of class 1 and class 2 uveal melanomas and their relationship with intraocular proton radiation response.
DESIGN
Masked retrospective case series of uveal melanoma patients with fine needle biopsy– based molecular profiles.
METHODS
A total of 197 uveal melanoma patients from a single institution were analyzed for pathology, clinical characteristics, and response to radiation therapy.
RESULTS
A total of 126 patients (64%) had class 1 tumors and 71 (36%) had class 2 tumors. Patients with class 2 tumors had more advanced age (mean: 64 years vs 57 years; P = .001), had thicker initial mean ultrasound measurements (7.4 mm vs 5.9 mm; P = .0007), and were more likely to have epithelioid or mixed cells on cytopathology (66% vs 38%; P = .0004). Although mean pretreatment and posttreatment ultrasound thicknesses were significantly different between class 1 and class 2 tumors, there was no difference in the mean change in thickness 24 months after radiation therapy (mean difference: class 1=−1.64 mm, class 2=−1.47; P = .47) or in the overall rate of thickness change (slope: P = .64). Class 2 tumors were more likely to metastasize and cause death than class 1 tumors (DSS: P < .0001).
CONCLUSIONS
At the time of radiation therapy, thicker tumors, epithelioid pathology, and older patient age are significantly related to class 2 tumors, and class 2 tumors result in higher tumor-related mortality. We found no definitive clinical marker for differentiating class 1 and class 2 tumors.
Many uveal melanoma parameters are related to an increased risk of metastatic death.1 Genomic changes, most importantly loss of a single copy of chromosome 3, are associated with an increased risk of metastasis.2–5 Gene expression profiling of uveal melanomas has revealed 2 distinct classes of tumors that accurately predict prognosis. Class 1 tumors are unlikely to metastasize, while class 2 tumors are more likely to metastasize and cause death.6 The genetic signature of class 1 and class 2 tumors can be assessed from a traditional biopsy or from a fine needle aspiration biopsy (FNAB).7 This gene expression profiling has been shown to have superior predictive accuracy when compared to the analysis using fluorescence in situ hybridization or array comparative genomic hybridization looking only at monosomy 3.8,9
The goal of this study was to better characterize the clinical spectrum of class 1 and class 2 uveal melanomas and its possible relationship with intraocular proton radiation response.
METHODS
This study was a retrospective case series of patients with uveal melanoma treated with proton beam radiation therapy between November 13, 1997 and November 12, 2010, with the last follow-up in January 2011. All patients were recruited from a single institution. Patients who did not receive proton radiation, because of primary enucleation or iridocyclectomy without radiation, were excluded from the analysis (n = 25). The study examined the clinical characteristics and radiation response of 197 evaluable uveal melanoma patients. Each patient underwent FNAB (185) or tumor resection with biopsy (12), followed by proton radiation. The tissues from each case underwent molecular studies and were delineated into class 1 and class 2 tumors based on the genetic characteristics at 15 loci. The specifics of this gene expression profiling have been previously discussed.6 In addition, cytologic or histopathologic examination was performed to determine the predominant tumor cell type. Evaluation of the tissue was performed masked to all patient, disease, and outcome features.
Clinical data and fluorescein angiographic and ultrasound characteristics were obtained from patients’ initial visits. For each case, information obtained from clinical records and photographs included sex, age, ocular location, tumor area, and presence of orange pigmentation. Ultrasound data collected included tumor thickness, the presence of a collar button, and the presence of subretinal fluid.
To assess if an in-depth characterization of fluorescein angiographic or ultrasonographic characteristics might be related to molecular class, a subset of 36 irradiated patients with posterior class 1 or class 2 choroidal melanomas were selected based on tumor size and location (posterior to the equator). For these patients, additional ultrasound data (homogeneity, orbital shadowing, shape of posterior A-wave spike, and internal reflectivity) and fluorescein angiographic features (degree of blockage and leakage, presence of hot spots, tumor coloration, and the presence of intrinsic tumor vascularity) were assessed by 2 observers masked to all other clinical features and disease outcomes (M.C., D.H.C.).
Patients treated with proton beam radiation therapy had their follow-up clinical, photographic, and ultrasound thickness measurements gathered at each postoperative appointment. These data were then used to compare changes over time after radiation therapy.
Comparability between molecular classes in baseline disease and patient features was determined using Fisher exact test for categorical variables and a 2-group t test for continuous variables. Overall results were summarized using a logistic regression model to identify independent predictors of molecular class. Variables considered as potential predictors were those found to be related to molecular class univariately with a probability of less than 0.10 (Table). A likelihood ratio (LLR) test was used with a forward stepwise approach with a probability to be included in the model of .05 and a probability to be removed of .10. Linear regression analysis was performed to estimate the rate of change in tumor thickness over time using all follow-up measurements for each patient. The overall rate of change was summarized by the slope from this analysis. In addition, the estimate of change 24 months after proton therapy, the velocity, was calculated by the difference in tumor thickness at approximately 24 months from the pretreatment measurement divided by the follow-up time interval. A t statistic was used to compare the 2 molecular classes in terms of the velocity at 24 months, the mean change from baseline in tumor thickness to 24 months after therapy, and the mean rate of change using the slope for each patient.
TABLE.
Comparability of Baseline Features by Uveal Melanoma Molecular Class
| Class 1 (n = 126) | Class 2 (n = 71) | P Value | |
|---|---|---|---|
| Mean age at RT (y) | 57.2 | 64.1 | .001 |
| Range | 20.5–94.2 | 17.3–91.6 | |
| Number male (%) | 65 (52%) | 39 (55%) | .66 |
| Eyewall resection/irido, n (%) | 8 (6%) | 8 (11%) | .28 |
| Enucleation (post-RT), n (%) | 14 (11%) | 11 (15%) | .38 |
| FNAB, n (%) | 120 (95%) | 65 (92%) | .36 |
| Tumor location, n (%) | |||
| Crosses equator | 110 (89%) | 59 (83%) | .28 |
| Posterior to equator | 74 (60%) | 35 (49%) | .18 |
| Mean tumor area, mm | 61.2 | 68.8 | .25 |
| Range | 7.2–179.7 | 4.9–192.2 | |
| Ultrasound thickness, mm | 5.9 | 7.4 | .0007 |
| Range | 2.0–13.7 | 3.2–13.8 | |
| Number with OP (%) | 20 (16%) | 12 (17%) | 1.00 |
| Number with drusen (%) | 17 (14%) | 6 (8%) | .36 |
| Number with collar button (%) | 24 (19%) | 19 (27%) | .28 |
| Number with SRF (%) | 65 (52%) | 35 (50%) | .88 |
| Tumor cell type, n (%) | |||
| Spindle | 74 (62%) | 23 (34%) | |
| Epithelioid/mixed | 45 (38%) | 44 (66%) | .0004 |
FNAB = fine needle aspiration biopsy; Irido = iridocyclectomy; OP = orange pigment; RT = radiation therapy; SRF = subretinal fluid.
Bold font indicates statistically significant P values.
The durations of time to metastasis and disease-specific survival (DSS) were each measured from the start of proton beam radiation therapy. Failure for DSS was the date of death attributable to metastatic disease. Patients who were alive were censored at the date of last follow-up. Univariate analyses using the Cox proportional hazards model were carried out to determine which factors were predictors of time to metastasis and DSS. Statistical significance was analyzed using the LLR test. Variables found to be predictive of outcome with a probability value of less than .10 univariately were considered in multivariate analyses, again using the Cox proportional hazards model to identify independent predictors of time to metastases and DSS. A forward stepwise approach was used to build a model with a probability of .05 to include a variable and a probability of .10 to remove a variable in the model. Independent predictors were determined by a probability value of less than .05 using the LLR test, with the results summarized by the hazard ratio (HR). The Kaplan-Meier product limit method was used to graphically display the results and estimate 5-year time to metastases and DSS outcomes.
RESULTS
There were 197 evaluable patients with uveal melanoma defined by molecular classification treated with proton radiation between November 1997 and November 2010. One hundred and twenty-six (64%) had class 1 tumors and 71 (36%) had class 2 tumors. One hundred and four patients (53%) were male and 93 patients (47%) were female. The mean age of patients at the time of proton radiation therapy was significantly less in patients with class 1 tumors (57 years old) as compared to patients with class 2 tumors (64 years old) (P = .001). The mean tumor area for class 1 tumors (61.2 mm) was smaller than for class 2 tumors (68.8 mm). This difference was not statistically significant (P = .25). Mean pretreatment ultrasound thickness was greater for class 2 patients (7.4 mm) as compared to class 1 patients (5.9 mm) (P < .001) (Table).
There was no statistically significant difference between the 2 classes of tumors based on the presence of orange pigment (P = 1.00), drusen (P = .36), a collar button (P = .28), or subretinal fluid (P = .88). Analysis of gene profile and tumor cell type showed that a significantly greater number of tumors in class 1 were spindle cell (62%) as compared to class 2 (34%) (P = .0004) (Table).
A logistic regression model was developed to identify independent predictors of molecular class. The same 3 factors identified with the univariate analyses were found to be independent predictors of molecular classification. Using a LLR test the 3 variables were significant predictors in the following order. Having a class 2 tumor reflected an epithelioid cell type (P = .0003), being older at the time of radiotherapy (P = .002), and increased tumor thickness on ultrasound pretreatment (P = .03). Tumor location entirely posterior to the equator was not an independent predictor of molecular class.
Of the 197 patients treated with proton radiation, 187 had follow-up information available after radiation treatment (118 class 1, 69 class 2). There was no difference in the mean number of follow-up measurements between the 2 classes (P = .65). Overall, the median duration of disease follow-up for irradiated patients was 21.7 months (range 2.0–111.9 months) with no difference between the 2 classes (medians: class 1 = 25.7 months [2.6–111.9 months], class 2 = 18.0 months [2.0–94.3 months]) (P = .23). Univariate analyses were performed using the Cox proportional hazards model to evaluate factors predictive of time to metastases and DSS. The results indicated that molecular class (P < .0001), cell type (P = .05), and age of the patient at the time of radiation therapy (P = .04) were significantly predictive of time to metastases, whereas pretreatment ultrasound measurement (P = .11) was not.
Molecular class (P < .0001) was predictive of DSS with univariate analysis, while cell type (P = .24), age of the patient at time of radiation therapy (P = .056), and pretreatment ultrasound measurement (P = .21) were not. Multivariate analysis using the Cox proportional hazards model was performed to identify independent predictors of time to metastases and DSS. Variables found to be significant predictors univariately with a probability of less than .10 were considered as potential predictors. Only molecular class was an independent significant predictor of outcome for remaining metastasis-free (HR = 8.4; LLR: P < .0001) and DSS (HR = 12.3; LLR: P < .0001).
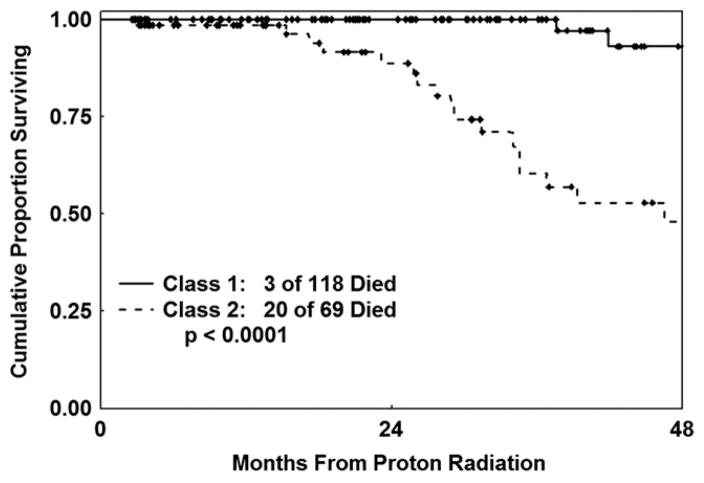
As of this analysis, 30 of the irradiated patients developed metastatic disease after proton radiation therapy, 6 of the class 1 patients and 24 of the class 2 patients (LLR test: P < .0001). Twenty-three patients died as a result of their metastatic melanoma, 3 patients with class 1 melanomas and 20 patients with class 2 melanomas (LLR test: P < .0001) (DSS: Figure 1). One of the class 1 patients whose disease metastasized (and who died of metastatic melanoma) was later reclassified as class 1B. It is unknown whether the other 5 class 1 patients were class 1A or 1B. At 5 years, the Kaplan-Meier estimate of the probability of metastatic death from uveal melanoma was 62% (95% CI: 44%–80%) for class 2 patients as compared to 7% (95% CI: 2%–25%) for class 1 patients.
FIGURE 1.
Disease-specific survival (DSS) by uveal melanoma molecular class.
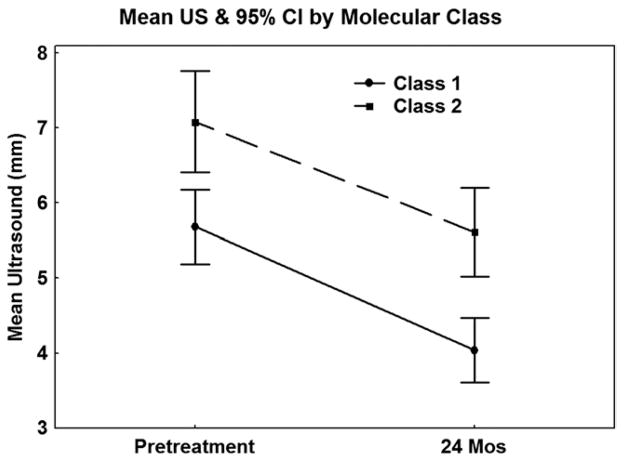
Among irradiated patients with multiple follow-up measurements, we analyzed alterations in ultrasound thickness measurements over time as related to melanoma molecular profile (n = 169). There was a significant difference in the mean pretreatment (class 1 = 5.7 mm, class 2 = 7.1 mm; P = .001) and approximate 24-month posttreatment (class 1 = 4.0 mm, class 2 = 5.6 mm; P < .0001) ultrasound thickness between class 1 and class 2 tumors (Figure 2). However, there was no significant difference between class 1 and class 2 tumors based on the extent of change in thickness at approximately 24 months (mean change: class 1=−1.64 mm, class 2 = −1.47 mm; P = .47), in the velocity (mean: class 1 = −0.09 mm/month, class 2 = −0.10 mm/month; P = −.71), or in the rate of thickness change when all follow-up measurements after proton therapy were summarized by the slope for each patient (P = −.64).
FIGURE 2.
Mean ultrasound thickness change as a function of uveal melanoma molecular class after treatment with proton radiation.
Twenty-five eyes were enucleated after proton therapy, 8 because of local radiation failure defined by enlargement with mitoses noted on histologic examination. Five of the 8 were class 1 melanomas and 3 were class 2 melanomas. None of these 8 cases had a preradiation molecular classification.
A subset of 36 irradiated posterior choroidal melanoma patients selected for in-depth assessment of molecular class, ultrasound, and fluorescein angiographic characteristics showed no statistically significant difference between class 1 and class 2 tumors for the following characteristics: ultrasound homogeneity, orbital shadowing, shape of posterior spike, internal reflectivity, fluorescein angiographic blockage, leakage, presence of hot spots, tumor coloration, and the presence of internal tumor vascularity. Because no difference attributable to molecular class was observed, no further analyses of these features were conducted.
DISCUSSION
This study reveals no definitive clinical, ultrasonographic, or fluorescein angiographic parameter that differentiates class 1 from class 2 uveal melanomas. It is uncertain whether class 1 and class 2 tumors arise from distinct cell lineages, or if class 1 tumors evolve into class 2 tumors.10
Patients with a class 2 tumor tend to be older than those with a class 1 tumor. The average age of the patients in our study was significantly different between class 1 (57 years) and class 2 tumors (64 years), as previously published.6 This molecular classification is in line with previous reports that show that mortality from metastatic melanoma is higher in people older than 60 years of age11,12 and risk continues to increase with further aging.13 Patients in this older age group also have a higher proportion of epithelioid cells than younger patients.14 A possible explanation for the link between class 2 uveal melanomas and age is that the tumors in older patients may have been present longer and have accumulated more mutations. Alternatively, it is possible that more aggressive mutations in genes such as BAP115 are more likely to occur in older patients. Yet another possible explanation is that the tumor-infiltrating macrophages in older individuals, which are more likely to be M2-polarized anti-inflammatory proangiogenic macro-phages, 16 induce more aggressive tumor behavior than those in younger patients.17
Class 2 melanomas were significantly thicker than class 1 melanomas before treatment, and this is consistent with previous studies that observed tumor thickness as an increased risk factor for growth and metastasis. 11,12,18,19 It is still uncertain whether the difference in tumor thickness between class 1 and class 2 tumors is a function of this genomic difference or a temporal difference in the natural history of the evolution and growth of these tumors.
We found no difference between class 1 and class 2 tumors in regard to tumor location, while previous studies have cited that tumor location has an effect on metastasis and disease-free survival.12,18,20 We also found no difference between class 1 and class 2 tumors based on fluorescein angiographic features or ultrasound characteristics other than thickness. As others have noted, there is minimal predictive capacity with fluorescein angiography.21,22
Class 2 melanomas are more likely to be of epithelioid cytology as compared to class 1 melanomas, and patients with class 2 melanomas are more likely to die from metastatic disease as of this report.
A surprising result from our study was that despite having a worse outcome and higher number of epithelioid cells, the class 2 tumors had no significant differences in rates of tumor shrinkage after proton beam radiation therapy when compared to class 1 tumors. Previous studies have stated that larger tumors have a faster shrinkage rate than smaller tumors when treated with brachytherapy,23 though this may be related to a larger radiation dose. After proton beam radiation therapy, rate of tumor shrinkage may not correlate with pretreatment tumor size.22,24 While some analyses have shown that after helium ion or proton beam irradiation there was a strong relationship between the rate of tumor regression and metastatic risk,25,26 our multivariate results indicated that only molecular class was a significant predictor of development of metastasis and DSS. Similarly, postradiation tumor recurrence was not predicted by molecular tumor class.
Patients with class 2 melanomas had a shorter interval between diagnosis of primary intraocular tumor and the detection of metastatic disease. Again, further investigation into genetic differences within the class 1 and class 2 subtypes of uveal melanomas will hopefully provide more insight into these discrepancies. The categorization of molecular profile by class is currently in flux. Recent data showed a subset of class 1 cases (class 1B) with an intermediate risk for metastasis.4 It is likely that some of the 5 class 1 cases that metastasized were class 1B tumors, but we have not yet reanalyzed our class 1 patients using this new categorization to confirm this.
After brachytherapy several groups have shown that tumor thickness shrinkage may be greater in melanomas with monosomy 3 as compared to disomy 3,27,28 although the methods of measuring tumor response were suboptimal. It is uncertain why there is a discrepancy between our analysis of response to proton therapy using molecular class and these other reports of a relationship between rates of tumor shrinkage and the presence of monosomy 3.
The findings in our study provide further evidence of the predictive accuracy of gene expression profiling for uveal melanomas. Not only can genetic classification of the tumors help determine the possibility for metastasis, but it allows us to consider adjunctive treatment in patients at high risk but without evidence of widespread disease. Tumor thickness and patient age at diagnosis are statistically different between class 1 and class 2 tumors, but there is a large variability in most clinical parameters in both classes that keeps these characteristics from definitively indicating a specific class.
Biographies

Michael C. Chappell, MD, is currently the ophthalmic plastic and reconstructive surgery fellow at the University of Washington in Seattle, Washington. He graduated from Hendrix College in Conway, Arkansas before earning his MD from the University of Arkansas for Medical Sciences. He then completed an ophthalmology residency at California Pacific Medical Center in San Francisco, California.

Devron H. Char is a pioneer in the diagnosis and treatment of patients with eyelid, conjunctival, iris, retinal, choroidal, and orbital tumors and patients with thyroid eye disease. He has written over 300 articles and six books, including the basic textbook on ocular oncology. Dr Char is the founding director of the Ocular Oncology unit at the UCSF Medical Center and is the current director of the Tumori Foundation, a non-profit research organization in San Francisco, California.
Footnotes
ALL AUTHORS HAVE COMPLETED AND SUBMITTED THE ICMJE FORM FOR DISCLOSURE OF POTENTIAL CONFLICTS OF Interest. Publication of this article was supported in part by a grant from The Tumori Foundation, San Francisco, California. Dr Char is the director of The Tumori Foundation (a nonprofit organization) and Tia Cole is an employee. Dr Harbour receives royalties from Castle Biosciences. Dr Phillips receives textbook royalties from Elsevier. Involved in design of study (M.C., D.C.); conduct of study (M.C., D.C., T.C.); collection (M.C., D.C.), management (T.C.), analysis (T.C., V.W.), and interpretation (M.C., D.C., W.H., K.M., T.P.) of data; and preparation, review, or approval of the manuscript (M.C., D.C., T.C., W.H., K.M., V.W., T.P.). The study and data accumulation were carried out with prior approval from the California Pacific Medical Center Research Institute (CPMCRI) Institutional Review Board, Informed Consent for the research was obtained from the patients, and the study is in accordance with HIPAA regulations.
References
- 1.Char DH. Tumors of the Eye and Ocular Adnexa. Hamilton: BC Decker Inc; 2001. pp. 121–140. [Google Scholar]
- 2.Scholes AG, Damato BE, Nunn J, Hiscott P, Grierson I, Field JK. Monosomy 3 in uveal melanoma: correlation with clinical and histologic predictors of survival. Invest Ophthalmol Vis Sci. 2003;44(3):1008–1011. doi: 10.1167/iovs.02-0159. [DOI] [PubMed] [Google Scholar]
- 3.Aalto Y, Eriksson L, Seregard S, Larsson O, Knuutila S. Concomitant loss of chromosome 3 and whole arm losses and gains of chromosome 1, 6, or 8 in metastasizing primary uveal melanoma. Invest Ophthalmol Vis Sci. 2001;42(2):313–317. [PubMed] [Google Scholar]
- 4.Ehlers JP, Worley L, Onken MD, Harbour JW. Integrative genomic analysis of aneuploidy in uveal melanoma. Clin Cancer Res. 2008;14(1):115–122. doi: 10.1158/1078-0432.CCR-07-1825. [DOI] [PubMed] [Google Scholar]
- 5.Prescher G, Bornfeld N, Hirche H, Horsthemke B, Jöckel KH, Becher R. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347(9010):1222–1225. doi: 10.1016/s0140-6736(96)90736-9. [DOI] [PubMed] [Google Scholar]
- 6.Onken MD, Worley LA, Ehlers JP, Harbour JW. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004;64(20):7205–7209. doi: 10.1158/0008-5472.CAN-04-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onken MD, Worley LA, Dávila RM, Char DH, Harbour JW. Prognostic testing in uveal melanoma by transcriptomic profiling of fine needle biopsy specimens. J Mol Diagn. 2006;8(5):567–573. doi: 10.2353/jmoldx.2006.060077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onken MD, Worley LA, Person E, Char DH, Bowcock AM, Harbour JW. Loss of heterozygosity of chromosome 3 detected with single nucleotide polymorphisms is superior to monosomy 3 for predicting metastasis in uveal melanoma. Clin Cancer Res. 2007;13(10):2923–2927. doi: 10.1158/1078-0432.CCR-06-2383. [DOI] [PubMed] [Google Scholar]
- 9.Worley LA, Onken MD, Person E, et al. Transcriptomic versus chromosomal prognostic markers and clinical outcome in uveal melanoma. Clin Cancer Res. 2007;13(5):1466–1471. doi: 10.1158/1078-0432.CCR-06-2401. [DOI] [PubMed] [Google Scholar]
- 10.Harbour JW. Eye cancer: unique insights into oncogenesis: the Cogan Lecture. Invest Ophthalmol Vis Sci. 2006;47(5):1736–1745. doi: 10.1167/iovs.05-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLean MJ, Foster WD, Zimmerman LE. Prognostic factors in small malignant melanomas of choroid and ciliary body. Arch Ophthalmol. 1977;95(1):48–58. doi: 10.1001/archopht.1977.04450010050004. [DOI] [PubMed] [Google Scholar]
- 12.Shammas HF, Blodi FC. Prognostic factors in choroidal and ciliary body melanomas. Arch Ophthalmol. 1977;95(1):63–69. doi: 10.1001/archopht.1977.04450010065005. [DOI] [PubMed] [Google Scholar]
- 13.Gragoudas E, Li W, Goitein M, Lane AM, Munzenrider JE, Egan KM. Evidence-based estimates of outcome in patients irradiated for intraocular melanoma. Arch Ophthalmol. 2002;120(12):1665–1671. doi: 10.1001/archopht.120.12.1665. [DOI] [PubMed] [Google Scholar]
- 14.Seddon JM, Polivogianis L, Hsieh CC, Albert DM, Gamel JW, Gragoudas ES. Death from uveal melanoma. Number of epithelioid cells and inverse SD of nucleolar area as prognostic factors. Arch Ophthalmol. 1987;105(6):801–806. doi: 10.1001/archopht.1987.01060060087039. [DOI] [PubMed] [Google Scholar]
- 15.Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330(6009):1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dace DS, Apte RS. Effect of senescence on macrophage polarization and angiogenesis. Rejuvenation Res. 2008;11(1):177–185. doi: 10.1089/rej.2007.0614. [DOI] [PubMed] [Google Scholar]
- 17.Bronkhorst IH, Ly LV, Jordanova ES, et al. Detection of M2-macrophages in uveal melanoma and relation with survival. Invest Ophthalmol Vis Sci. 2011;52(2):643–650. doi: 10.1167/iovs.10-5979. [DOI] [PubMed] [Google Scholar]
- 18.Augsburger JJ, Schroeder RP, Territo C, Gamel JW, Shields JA. Clinical parameters predictive of enlargement of melanocytic choroidal lesions. Br J Ophthalmol. 1989;73(11):911–917. doi: 10.1136/bjo.73.11.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shields CL, Shields JA, Kiratli H, De Potter P, Cater JR. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology. 1995;102(9):1351–1361. [PubMed] [Google Scholar]
- 20.Mooy CM, De Jong PT. Prognostic parameters in uveal melanoma: a review. Surv Ophthalmol. 1996;41(3):215–228. doi: 10.1016/s0039-6257(96)80024-5. [DOI] [PubMed] [Google Scholar]
- 21.Char DH, Stone RD, Irvine AR, et al. Diagnostic modalities in choroidal melanoma. Am J Ophthalmol. 1980;89(2):223–230. doi: 10.1016/0002-9394(80)90115-4. [DOI] [PubMed] [Google Scholar]
- 22.Wilkes SR, Gragoudas ES. Regression patterns of uveal melanomas after proton beam irradiation. Ophthalmology. 1982;89(7):840–844. doi: 10.1016/s0161-6420(82)34731-4. [DOI] [PubMed] [Google Scholar]
- 23.Kaiserman I, Anteby I, Chowers I, Blumenthal EZ, Kliers I, Pe’er J. Changes in ultrasound findings in posterior uveal melanoma after Ruthenium 106 brachytherapy. Ophthalmology. 2002;109(6):1137–1141. doi: 10.1016/s0161-6420(02)01054-0. [DOI] [PubMed] [Google Scholar]
- 24.Aziz S, Taylor A, McConnachie A, Kacperek A, Kemp E. Proton beam radiotherapy in the management of uveal melanoma: clinical experience in Scotland. Clin Ophthalmol. 2009;3:49–55. [PMC free article] [PubMed] [Google Scholar]
- 25.Char DH, Kroll SM, Castro J. Ten-year follow-up of helium ion therapy for uveal melanoma. Am J Ophthalmol. 1998;125(1):81–89. doi: 10.1016/s0002-9394(99)80238-4. [DOI] [PubMed] [Google Scholar]
- 26.Glynn RJ, Seddon JM, Gragoudas ES, Egan KM, Hart LJ. Evaluation of tumor regression and other prognostic factors for early and late metastasis after proton irradiation of uveal melanoma. Ophthalmology. 1989;96(10):1566–1573. doi: 10.1016/s0161-6420(89)32685-6. [DOI] [PubMed] [Google Scholar]
- 27.Shields CL, Bianciotto C, Rudich D, Materin MA, Ganguly A, Shields JA. Regression of uveal melanoma after plaque radiotherapy and thermotherapy based on chromosome 3 status. Retina. 2008;28(9):1289–1295. doi: 10.1097/IAE.0b013e31817f7b3e. [DOI] [PubMed] [Google Scholar]
- 28.Marathe OS, Wu J, Lee SP, et al. Ocular response of choroidal melanoma with monosomy 3 versus disomy 3 after iodine-125 brachytherapy. Int J Radiat Oncol Biol Phys. 2011;81(4):1046–1048. doi: 10.1016/j.ijrobp.2010.07.016. [DOI] [PubMed] [Google Scholar]