Abstract
Aim
To determine whether BAP1 gene and protein expression associates with different prognostic parameters in uveal melanoma and whether BAP1 expression correctly identifies patients as being at risk for metastases, following enucleation of the primary tumour.
Methods
Thirty cases of uveal melanoma obtained by enucleation between 1999 and 2004 were analysed for a variety of prognostic markers, including histological characteristics, chromosome aberrations obtained by fluorescence in situ hybridisation (FISH) and single nucleotide polymorphism (SNP) analysis and gene expression profiling. These parameters were compared with BAP1 gene expression and BAP1 immunostaining.
Results
The presence of monosomy of chromosome 3 as identified by the different chromosome 3 tests showed significantly increased HRs (FISH on isolated nuclei cut-off 30%: HR 11.6, p=0.002; SNP analysis: HR 20.3, p=0.004) for death due to metastasis. The gene expression profile class 2, based on the 15-gene expression profile, similarly provided a significantly increased HR for a poor outcome (HR 8.5, p=0.005). Lower BAP1 gene expression and negative BAP1 immunostaining (50% of 28 tumours were immunonegative) were both associated with these markers for prognostication: FISH cut-off 30% monosomy 3 (BAP1 gene expression: p=0.037; BAP1 immunostaining: p=0.001), SNP-monosomy 3 (BAP1 gene expression: p=0.008; BAP1 immunostaining: p=0.002) and class 2 profile (BAP1 gene expression: p<0.001; BAP1 immunostaining: p=0.001) and were themselves associated with an increased risk of death due to metastasis (BAP1 gene expression dichotomised: HR 8.7, p=0.006; BAP1 immunostaining: HR 4.0, p=0.010).
Conclusions
Loss of BAP1 expression associated well with all of the methods currently used for prognostication and was itself predictive of death due to metastasis in uveal melanoma after enucleation, thereby emphasising the importance of further research on the role of BAP1 in uveal melanoma.
INTRODUCTION
Uveal melanoma is a rare primary malignancy of the eye. Up to 50% of the patients may develop metastases, which are fatal in almost all cases.1 A correct risk assessment is necessary in order to effectively select patients for inclusion in clinical trials, now that more effective drugs are being developed. An analysis of 8033 uveal melanomas showed a 10-year metastasis rate of 12% for small tumours (up to 3 mm thick) and 49% for large tumours (>8 mm thick).2 It is therefore especially important to properly assess this risk in individuals with large tumours, such as those that need enucleation. Prognostic factors include histological factors such as cell type, involvement of the ciliary body, extrascleral extension and several chromosomal aberrations.1 The parameter size, ciliary body involvement and extrascleral extension are often combined into one parameter used in the TNM classification, which provides a better prognostic value than any of these parameters individually.3 Different techniques such as fluorescence in situ hybridisation (FISH) on tumour sections or isolated nuclei, or chromosome analysis techniques can be used to assess the tumour’s chromosome status.4–6 originally, loss of one chromosome 3 was identified as an important marker of poor prognosis, and this has been substantiated in many studies. However, later studies have also identified the importance of other chromosomes: gain of chromosome 8q is also correlated with death due to metastases,5–14 while an extra chromosome 6p is associated with a better survival.8,10,15–18 In separate studies, gene expression profiling has also been identified as a reliable method for prognostication.19,20
The pathophysiology of the importance of chromosome 3 loss was demonstrated by Harbour et al, who observed that loss of one copy of chromosome 3 together with inactivating mutations in the metastasis-suppressor gene encoding for BRCA1-asssociated protein 1 (BAP1) on the remaining copy of chromosome 3 was associated with the development of metastases. BAP1 is a deubiquitinating enzyme that is a member of the polycomb group proteins of transcriptional repressors and exhibits tumour suppressive activity.21–23 Inactivation of BAP1 at the chromosome level may be the driving force for the development of metastases, and BAP1 levels may therefore influence survival.7,24–26
For early detection of metastases and for studies on adjuvant treatment, it is important to know which techniques accurately predict the patient’s prognosis. As loss of chromosome 3 is an essential step in the inactivation of BAP1,27 we decided to compare BAP1 gene and protein expression in 30 cases of enucleated uveal melanoma of at least 8-year follow-up, with the results of a variety of techniques assessing chromosome aberrations, and with gene expression profiling based on the 15-gene classification assay described by Onken et al.20,24,28
MATERIALS AND METHODS
Study population
Fresh-frozen material and formalin-fixed, paraffin-embedded specimens were obtained from the 30 uveal melanoma of which enough frozen material was left and good quality DNA was available. All 30 uveal melanomas had been enucleated at the Leiden University Medical Center (LUMC), Leiden, The Netherlands, between 1999 and 2004. Following enucleation, fresh tumour material was obtained immediately after the bulbus had been opened. Survival data and information on cause of death were obtained from the patient’s charts and from the Dutch National Registry, and updated in November 2013. Each tumour sample was processed for conventional histopathological evaluation, including cell-type assessment according to the modified Callender classification at that time.29 The collection of material for research had been agreed upon by the Medical Ethics Committee of the LUMC and the research protocol adhered to Dutch law and the current version of the tenets of the Declaration of Helsinki (World Medical Association of Declaration 1964; ethical principles for medical research involving human subjects).
Chromosome aberrations and gene expression
Three different techniques were applied to determine the presence of aberrations of chromosomes 3 and 8: FISH on isolated nuclei (for chromosome 3) and single nucleotide polymorphism (SNP) analysis. FISH analysis on isolated nuclei was performed as described before.30,31
DNA and RNA were isolated from fresh-frozen tissue. DNA for SNP analysis was extracted with the QIAmp DNA Mini kit and RNA for gene expression profiling with the RNeasy mini Kit (both from Qiagen, Venlo, the Netherlands). SNP analysis was performed with the Affymetrix 250K_NSP microarray chip (Affymetrix, Santa Clara, California, USA) on all 30 uveal melanomas. Gene expression analysis on BAP1 was carried out on RNA of 28 tumours using the Illumina HT-12v4 chip (Illumina, San Diego, California, USA). RNA obtained from frozen material from all 30 uveal melanomas was tested in the 15-gene classification assay as described by Onken et al28 and results sent to the Department of Ophthalmology and Visual Sciences of Washington University School of Medicine (St. Louis, Missouri, USA) for class assignment.
BAP1 immunohistochemistry
Immunohistochemical staining was performed for 28 patients from whom enough tumour material was available using the Ventana Benchmark ULTRA fully automated staining system (Ventana Medical Systems Inc, Tucson, Arizona, USA) with an alkaline phosphatase red detection kit.32 In short, sections were deparaffinised and then heated using Heat-induced Epitope Retrieval for 64 min at 97C°. The sections were then incubated for 32 min at 37C° with the primary BAP1 antibody (sc-28383, concentration 1:50, Santa Cruz Biotechnology, Dallas, Texas, USA). Target amplification was performed and then followed by incubation with haematoxylin II counterstain for 8 min. An additional counterstain was performed with blueing reagent (Ventana Medical Systems Inc.).
Liver, tonsil, breast and surrounding non-malignant tissue, as well as intratumoral vessels and inflammatory cells, were used as positive controls. As negative control sections without antibody were used.32 Tumours were scored either negative or positive for the BAP1 nuclear staining.
Statistical analysis
For data analysis, we used the statistical programming language R V.3.0.1 (R: A Language and Environment for Statistical Computing, R Core Team, R foundation for Statistical Computing, Vienna, Austria, 2014, http://www.R-project.org) supplemented with specialised packages for SNP and RNA analysis. The main package used for SNP analysis was aroma.affymetrix,33–35 supported by ‘DNAcopy’ (Venkatraman E. Seshan and Adam Olshen, DNAcopy: DNA copy number data analysis. R package V.1.34.0), ‘sfit’ (Henrik Bengtsson and Pratyaksha Wirapati (2013), sfit: Multidimensional simplex fitting. R package V.0.3.0/r185, http://R-Forge.R-project.org/projects/matrixstats/) and ‘R.utils’ (Henrik Bengtsson (2014), R.utils: Various programming utilities, R package V.1.29.8, http://CRAN.R-project.org/package=R.utils). As reference set, we used the data of 84 healthy controls obtained with the same Affymetrix 250K Nsp chip (Affymetrix, Santa Clara, California, USA) by the Department of Human Genetics at our centre. The ‘Aroma.Affymetrix’ package made it possible to use these SNP microarrays to determine copy number values.33–35 The packages used for RNA microarray analysis were ‘limma’ V.3.16.836 and the specific packages for Illumina microarrays: ‘lumi’ V.2.12.0,37–40 ‘annotate’ (R. Gentleman, annotate: Annotation for microarrays, R package V.1.38.0), and the database package ‘illuminaHumanv4.db’ (Mark Dunning, Andy Lynch and Matthew Eldridge, illuminaHumanv4.db: Illumina HumanHT12v4 annotation data (chip illuminaHumanv4), R package V.1.18.0).
Mann–Whitney U test for non-parametric analysis was used to assess differences in the amount of BAP1 gene expression, and χ2 tests for comparing the prognostic parameters with the BAP1 staining on immunohistochemistry (IHC). Univariate Cox regression was applied with events determined as ‘death due to UM’ and right censoring. Cases of which the cause of death was unknown were censored as well. To calculate the respective HRs of the different parameters, the BAP1 gene expression was dichotomised at the median to create two equal groups. All analyses were performed with SPSS V.20.0.1 (IBM SPSS Statistics, IBM Corporation, Armonk, New York, USA).
RESULTS
Patients
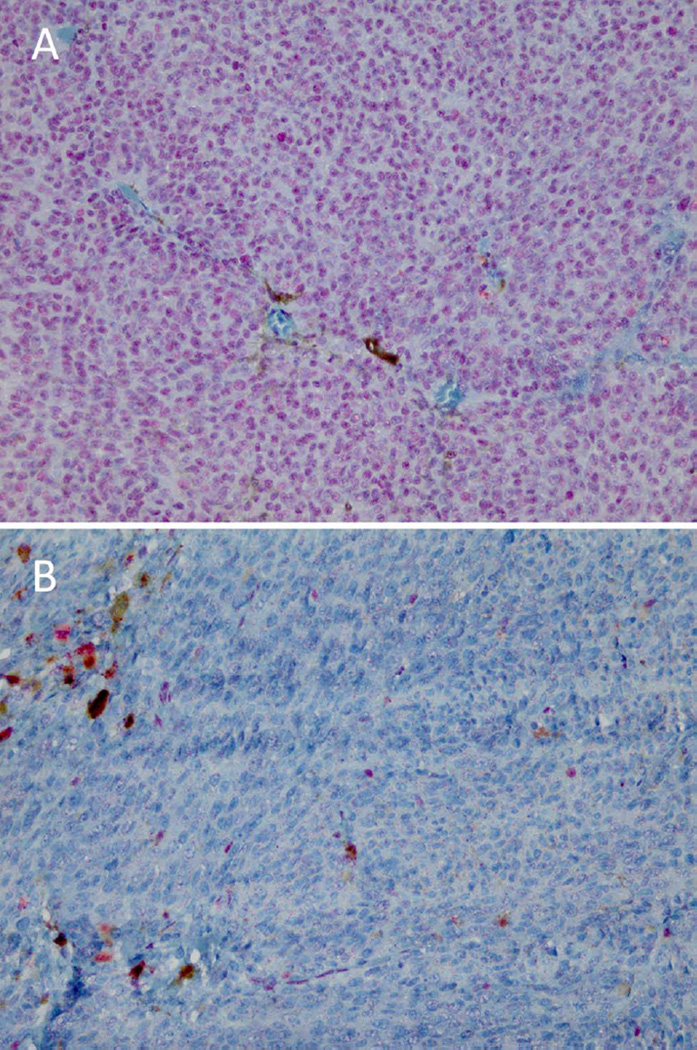
We studied a group of 30 uveal melanoma patients, consisting of 13 men and 17 women with an average age at the time of enucleation of 61.7 years (range 28–84 years; median 66.5 years) and a mean follow-up of 77.5 months (range 14–155 months). Of these patients, 14 had died of uveal melanoma metastases (mean survival 36.7 months; range 14– 96 months) and 3 of unknown causes (mean survival 85.0 months; range 63–126 months), while no patient has been lost to follow-up. The mean largest basal tumour diameter was 13.7 mm (range 8–18 mm; median 13.5 mm), with an average prominence of 7.3 mm (range 2–12 mm; median 7.0 mm). The pathological TNM stages showed stage I in 4, stage IIA in 5, stage IIB in 10, stage IIIA in 9 and stage IIIB in 2 cases.41 The ciliary body was involved in 13 cases. The histological cell type was spindle in 11, mixed in 14 and epithelioid in 5 cases. Immunohistochemical staining for BAP1 (figure 1) was positive in 14 of the 28 patients who could be tested. BAP1 staining was either present on more than 95% of the tumour cells or almost completely absent. An overview of all variables per patient is available (see online supplementary table S1).
Figure 1.
Examples of a BAP1-immunopositive (A) and a BAP1-immunonegative (B) tumour.
Chromosome aberrations
FISH analysis of chromosome 3 on isolated nuclei with a cut-off value of 5% indicated 19 cases of monosomy of chromosome 3, while a cut-off value of 30% identified 15 cases.
SNP analysis revealed 16 cases with monosomy of chromosome 3, and one case with a partial deletion from 3q28 till the end of the chromosome. With this technique, 15 out of the 16 monosomy 3 tumours had a gain of the long arm of chromosome 8 (8q) compared with four of the disomy 3 tumours.
Gene expression profiling
The 15-gene expression assay of Onken et al, performed on all 30 tumours, classified 14 of the tumours as class 1 (good prognosis) and 16 as class 2 (poor prognosis) uveal melanoma.
Associations
A univariate Cox regression analysis was performed to compare the impact of all clinical, histological, chromosomal and gene expression data on death due to metastases. All categorical variables fulfilled the proportional hazards assumption. All parameters, except gender and age at enucleation, were associated with increased risk of death due to metastasis, with the chromosomal aberrations analysed with SNP, and the stage groups having relatively wide CIs (table 1). When the cases for which the cause of death was unknown were attributed to death due to metastasis, the results were similar (see online supplementary table S2). Multivariate analysis of all parameters led to results containing one in the 95% CI, or having infinite as upper limit, or both (data not shown).
Table 1.
Comparison of different prognostic parameters with survival in 30 uveal melanoma patients
| Univariate analysis—unknown cases attributed to death due to other causes |
|||||
|---|---|---|---|---|---|
| 95% confidence interval | |||||
| Parameters | n | HR | Lower | Upper | p Value |
| Standard clinical/histological parameters | |||||
| Gender | |||||
| Male | 17 | – | – | – | – |
| Female | 13 | 1.3 | 0.5 | 3.7 | 0.63 |
| Ciliary body involvement | |||||
| No | 17 | – | – | – | – |
| Yes | 13 | 6.3 | 1.9 | 21.0 | 0.002 |
| Stage group | |||||
| I and IIA | 9 | – | – | – | – |
| IIB | 10 | 5.0 | 0.6 | 45.0 | 0.14 |
| IIIA | 9 | 16.9 | 2.1 | 138.8 | 0.009 |
| IIIB | 2 | 5.8 | 0.4 | 92.6 | 0.22 |
| Age at enucleation (years; low to high; continuous) | 30 | 1.0 | 1.0 | 1.1 | 0.26 |
| Largest basal diameter (mm; low to high; continuous) | 30 | 1.4 | 1.1 | 1.7 | 0.008 |
| Chromosomal aberrations | |||||
| FISH on isolated nuclei, cut-off at 5% | |||||
| Disomy chromosome 3 | 11 | – | – | – | – |
| Monosomy chromosome 3 | 19 | 12.1 | 1.6 | 93.2 | 0.017 |
| FISH on isolated nuclei, cut-off at 30% | |||||
| Disomy chromosome 3 | 15 | – | – | – | – |
| Monosomy chromosome 3 | 15 | 11.6 | 2.5 | 52.5 | 0.002 |
| SNP on tumour DNA | |||||
| Disomy chromosome 3 | 14 | – | – | – | – |
| Monosomy chromosome 3 | 16 | 20.3 | 2.6 | 156.7 | 0.004 |
| Disomy chromosome 8q | 11 | – | – | – | – |
| Gain chromosome 8q | 19 | 11.7 | 1.5 | 89.7 | 0.018 |
| Disomy chrom 3+disomy chrom 8q | 10 | – | – | – | – |
| Monosomy chrom 3+gain chrom 8q | 15 | 8.9 | 2.0 | 40.4 | 0.004 |
| Gene expression | |||||
| 15-gene expression assay class | |||||
| Class 1 | 14 | – | – | – | – |
| Class 2 | 14 | 8.5 | 1.9 | 38.3 | 0.005 |
| BAP1 gene expression (dichotomised at median) | |||||
| High | 14 | – | – | – | – |
| Low | 14 | 8.7 | 1.9 | 39.7 | 0.006 |
| BAP1 gene expression (high to low; continuous) | 28 | 4.0 | 1.5 | 10.6 | 0.006 |
| Immunohistochemistry | |||||
| Positive BAP1 immunostaining | 14 | – | – | – | – |
| Negative BAP1 immunostaining | 14 | 5.5 | 1.5 | 20.1 | 0.010 |
An univariate Cox regression analysis was performed.
FISH, fluorescence in situ hybridization; SNP, single nucleotide polymorphism.
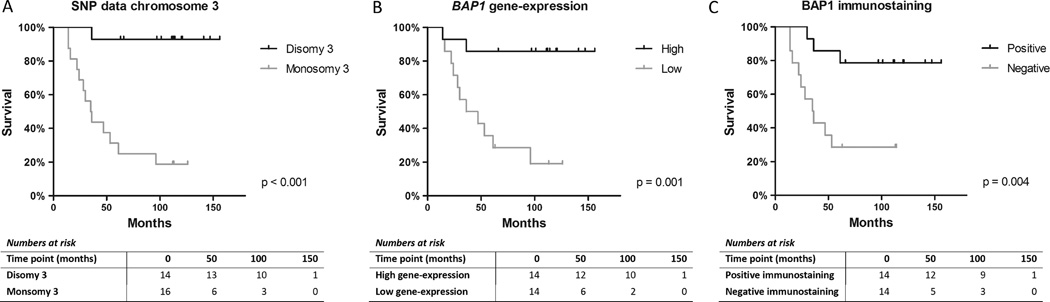
Loss of chromosome 3 was associated with death due to metastasis (figure 2A). Dividing the tumors in two groups based on chromsome 3 and 8q (either both disomic or both altered: loss of chromosome 3 together 8q gain), as determined by SNP analysis, associated with the 15-gene expression classes of Harbour (χ2 test p<0.001; one cell, had an expected count less than 5).
Figure 2.
Kaplan–Meier (log-rank test) survival graphs for single nucleotide polymorphism data on chromosome 3 status (A), BAP1 gene expression dichotomised at the median (B), and for BAP1 negative and positive staining as seen on immunohistochemistry (C).
The Kaplan–Meier survival graphs showed a discriminative function for BAP1 gene expression and the BAP1 immunostainings with regards to death due to metastasis (figure 2B, C).
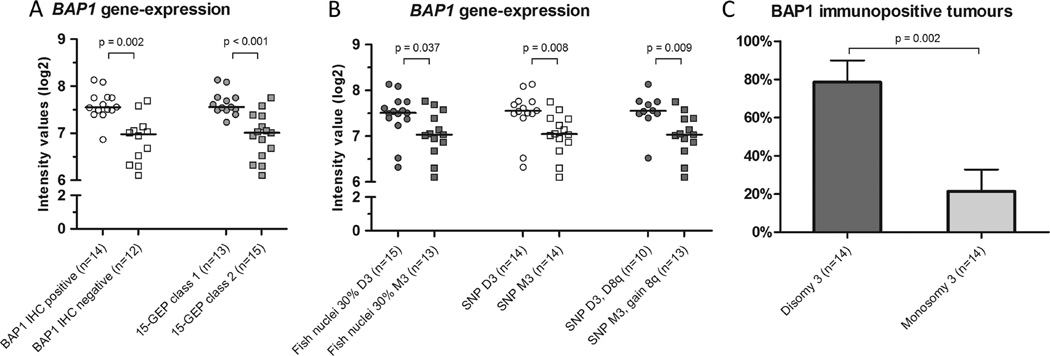
Low BAP1 gene expression (RNA) was associated with lack of immunohistochemical staining of BAP1 on uveal melanoma cells (p<0.001 for dichotomised data, χ2 test; p<0.001 for continuous data, independent t test). When the RNA expression values of BAP1 were compared with the 15-gene expression assay classes, and the different methods assessing chromosome 3 aberrations, significant associations were seen for all comparisons, with lower RNA values for the gene expression class and the aberrations that were associated with poor prognosis (figure 3A, B). Especially the strong association between the RNA values of BAP1 and the 15 gene expression profile is striking.
Figure 3.
Median BAP1 gene expression compared with the immunohistochemistry of BAP1 and different parameters used for prognostication (A, B), and a bar graph showing the distribution of BAP1 immunopositive tumours with regards to disomy and monosomy of chromosome 3 (C). p Values were calculated with Mann–Whitney U test and corrected for multiple comparisons (Benjamini–Hochberg; n=7) for A and B, and with the χ2 test for C. GEP, gene expression profiling; D3, disomy of chromosome 3; M3, monosomy of chromosome 3; D8q, disomy of chromosome 8q.
The BAP1 immunostaining corresponded to the expected Harbour gene expression class in 23 of the 28 cases. Similar to the findings at the RNA level, loss of chromosome 3 as seen by the two different methods was associated with a negative BAP1 immunostaining (figure 3C).
DISCUSSION
The different methods that identify monosomy of chromosome 3 as well as the gene expression-based classifications had increased HRs for death due to metastasis. These parameters were all associated with lower RNA levels of BAP1 and negative immunohistochemical staining for BAP1, and moreover, these BAP1 expression levels themselves were predictive for death due to metastasis of uveal melanoma (p=0.01).
Harbour et al27 identified an important role in the development of metastases in uveal melanomas for a specific gene on chromosome 3, that is, BAP1, and suggested that loss of one copy of chromosome 3 may unmask inactivating mutations in the metastasis-suppressor gene BAP1 on the remaining copy of chromosome 3. We were able to classify uveal melanoma easily into two groups using gene expression profiling (15-gene expression profile classification), as has already previously been shown by Tschentscher, Onken and van Gils.19,20,42 In our study, the loss of one copy of chromosome 3 together with gain of chromosome 8q was highly correlated to the 15 gene expression profile class 2, while we had only enough tumour material to perform a gene expression analysis in 28 of the 30 tumours and our number of samples was relatively small.
Interestingly, one case was staged as stage IIA and still alive after 9 years’ follow-up, despite having a monosomy of chromosome 3 together with gain of chromosome 8q and being a class 2 tumour (15-gene expression assay). In this case, the BAP1 immunostaining scored negative as well.
In large tumours, strong positive associations have been described between the prognostically poor 15-gene expression profile known as class 2 and the presence of epithelioid cells, the extracellular matrix pattern known as networks and largest basal tumour diameter, all of which associations have also been described to be related to monosomy of chromosome 3.19,20,43,44 As we observed an almost perfect association between the presence of monosomy of chromosome 3 plus 8q gain and the 15 gene expression profile class 2, we hypothesised that both should associate with loss of BAP1. We indeed noted a significant association for lower BAP1 gene expression (dichotomised and continuous data) and negative BAP1 immunostaining, with the combined presence of monosomy of chromosome 3 and chromosome 8q gain, as well as with the 15 gene expression prolife class 2. This is identical to the results published by Harbour et al.27
Our data show that BAP1 gene expression correlated with the findings of the BAP1 immunohistochemistry. Previously Harbour et al27 showed this for six tumour samples. The association between RNA and immunohistochemistry suggests that a cut-off value of the BAP1 gene expression, for example, measured with a quantitative PCR, could be made to predict loss of BAP1 immunoreaction. Shah et al45 and members of our group32 have shown that immunohistochemistry for BAP1 protein expression might be an easy way to discriminate between long and short survival, and may even replace mutation analysis of BAP1 in uveal melanoma patients. In large uveal melanoma, loss of one copy of chromosome 3, together with gain of chromosome 8q, clearly leads to this specific gene expression profile known as class 2, which is associated with loss of BAP1 gene expression, and with metastases formation.20,27 This loss of BAP1 plays an important role in developing malignant tumour behaviour,27 and it may also be involved in the development of an inflammatory phenotype, as this was previously found to be associated with monosomy of chromosome 3.46 Although Harbour’s group originally reported a strong association between the presence of the 15-gene expression profile class 2 and monosomy of chromosome 3, they stated in their most recent papers that monosomy 3 is not a good prognostic marker.47 This was based on the use of different tests on material obtained from enucleated uveal melanoma as well as on biopsies.47 However, the small amount of material obtained from biopsies may not have been sufficient for the specific SNP assay that they used, or small tumours may not represent the same profile in all parts of the tumours.
Now that several prognostication techniques have been developed that work accurately in the highest risk patients, that is, those that undergo enucleation, the next challenge is to determine the exact way how these inactivation mutations in BAP1 lead to metastasis formation.
In summary, our results show that monosomy 3/8q gain and the class 2 gene expression profile are both highly associated with lower BAP1 gene expression and negative BAP1 immunostaining, and that both methods for assessing BAP1 levels are predictive for death due to metastasis in uveal melanoma after enucleation. This emphasises the importance of further research on the role of BAP1 in the development of the inflammatory phenotype and the pathophysiology of the role of BAP1 in metastasis formation in uveal melanoma.
Supplementary Material
Acknowledgments
This study was supported by the SNOO (Stichting Nederlands Oogheelkundig Onderzoek).
Funding Dutch Cancer Society (KWF) UL2011-4991.
Footnotes
Contributors THvE: conception and design of the study, performed statistical analysis with SPSS, interpreted the data and drafted and revised whole article. SIvP: conception, design, statistical analysis with R and interpretation of data. MV: conception, evaluated patient samples for genetic testing and reviewed the article. IHGB: conception, collected clinical patient data. SGvD: conception, processed tissue samples and reviewed the article. MM: conception, acquired clinical data. WGMK and CALR: conception, genetic testing, analysis and reviewed the article. SS: conception, genetic testing, analysis and reviewed the article. AdK: evaluated patients’ samples for immunohistochemical staining and reviewed the article. EK: analysed and evaluated patients’ samples for immunohistochemical staining and reviewed the article. WH: genetic testing, analysis and reviewed the article. GPML: conception, acquired clinical patient tissue/data and reviewed the article. PAvdV: conception, design, interpreted the data and reviewed the article. RV: performed immunohistochemical staining and analysed and evaluated the samples, and reviewed the article. MJJ: conception, design and organisation of the study, interpreted the data and critically reviewed and revised the article.
Competing interests None.
Ethics approval Medical Ethics Committee of the LUMC and the research protocol adhered to Dutch law.
Data sharing statement There are additional unpublished data from the study. This data are available for our own department and collaborators in this research project. Please feel free to inquire for collaborations.
REFERENCES
- 1.Mooy CM, de Jong PT. Prognostic parameters in uveal melanoma: a review. Surv Ophthalmol. 1996;41:215–228. doi: 10.1016/s0039-6257(96)80024-5. [DOI] [PubMed] [Google Scholar]
- 2.Kujala E, Damato B, Coupland SE, et al. Staging of ciliary body and choroidal melanomas based on anatomic extent. J Clin Oncol. 2013;31:2825–2831. doi: 10.1200/JCO.2012.45.2771. [DOI] [PubMed] [Google Scholar]
- 3.Kivela T, Kujala E. Prognostication in eye cancer: the latest tumor, node, metastasis classification and beyond. Eye (Lond) 2013;27:243–252. doi: 10.1038/eye.2012.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronow M, Sun Y, Saunthararajah Y, et al. Monosomy 3 by FISH in uveal melanoma: variability in techniques and results. Surv Ophthalmol. 2012;57:463–473. doi: 10.1016/j.survophthal.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 5.van den Bosch T, van Beek JG, Vaarwater J, et al. Higher percentage of FISH-determined monosomy 3 and 8q amplification in uveal melanoma cells relate to poor patient prognosis. Invest Ophthalmol Vis Sci. 2012;53:2668–2674. doi: 10.1167/iovs.11-8697. [DOI] [PubMed] [Google Scholar]
- 6.Damato B, Dopierala JA, Coupland SE. Genotypic profiling of 452 choroidal melanomas with multiplex ligation-dependent probe amplification. Clin Cancer Res. 2010;16:6083–6092. doi: 10.1158/1078-0432.CCR-10-2076. [DOI] [PubMed] [Google Scholar]
- 7.Coupland SE, Lake SL, Zeschnigk M, et al. Molecular pathology of uveal melanoma. Eye (Lond) 2013;27:230–242. doi: 10.1038/eye.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White VA, Chambers JD, Courtright PD, et al. Correlation of cytogenetic abnormalities with the outcome of patients with uveal melanoma. Cancer. 1998;83:354–359. [PubMed] [Google Scholar]
- 9.Prescher G, Bornfeld N, Becher R. Nonrandom chromosomal abnormalities in primary uveal melanoma. J Natl Cancer Inst. 1990;82:1765–1769. doi: 10.1093/jnci/82.22.1765. [DOI] [PubMed] [Google Scholar]
- 10.Prescher G, Bornfeld N, Hirche H, et al. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347:1222–1225. doi: 10.1016/s0140-6736(96)90736-9. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Rahman MH, Cebulla CM, Verma V, et al. Monosomy 3 status of uveal melanoma metastases is associated with rapidly progressive tumors and short survival. Exp Eye Res. 2012;100:26–31. doi: 10.1016/j.exer.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilic E, van GW, Lodder E, et al. Clinical and cytogenetic analyses in uveal melanoma. Invest Ophthalmol Vis Sci. 2006;47:3703–3707. doi: 10.1167/iovs.06-0101. [DOI] [PubMed] [Google Scholar]
- 13.van Beek JG, Koopmans AE, Vaarwater J, et al. The prognostic value of extraocular extension in relation to monosomy 3 and gain of chromosome 8q in uveal melanoma. Invest Ophthalmol Vis Sci. 2014;55:1284–1291. doi: 10.1167/iovs.13-13670. [DOI] [PubMed] [Google Scholar]
- 14.Ewens KG, Kanetsky PA, Richards-Yutz J, et al. Genomic profile of 320 uveal melanoma cases: chromosome 8p-loss and metastatic outcome. Invest Ophthalmol Vis Sci. 2013;54:5721–5729. doi: 10.1167/iovs.13-12195. [DOI] [PubMed] [Google Scholar]
- 15.Damato B, Coupland SE. Translating uveal melanoma cytogenetics into clinical care. Arch Ophthalmol. 2009;127:423–429. doi: 10.1001/archophthalmol.2009.40. [DOI] [PubMed] [Google Scholar]
- 16.Harbour JW. Molecular prognostic testing and individualized patient care in uveal melanoma. Am J Ophthalmol. 2009;148:823–829. doi: 10.1016/j.ajo.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholes AG, Damato BE, Nunn J, et al. Monosomy 3 in uveal melanoma: correlation with clinical and histologic predictors of survival. Invest Ophthalmol Vis Sci. 2003;44:1008–1011. doi: 10.1167/iovs.02-0159. [DOI] [PubMed] [Google Scholar]
- 18.Mudhar HS, Parsons MA, Sisley K, et al. A critical appraisal of the prognostic and predictive factors for uveal malignant melanoma. Histopathology. 2004;45:1–12. doi: 10.1111/j.1365-2559.2004.01874.x. [DOI] [PubMed] [Google Scholar]
- 19.Tschentscher F, Husing J, Holter T, et al. Tumor classification based on gene expression profiling shows that uveal melanomas with and without monosomy 3 represent two distinct entities. Cancer Res. 2003;63:2578–2584. [PubMed] [Google Scholar]
- 20.Onken MD, Worley LA, Ehlers JP, et al. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004;64:7205–7209. doi: 10.1158/0008-5472.CAN-04-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen DE, Proctor M, Marquis ST, et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998;16:1097–1112. doi: 10.1038/sj.onc.1201861. [DOI] [PubMed] [Google Scholar]
- 22.Scheuermann JC, de Ayala Alonso AG, Oktaba K, et al. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature. 2010;465:243–247. doi: 10.1038/nature08966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ventii KH, Devi NS, Friedrich KL, et al. BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res. 2008;68:6953–6962. doi: 10.1158/0008-5472.CAN-08-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carbone M, Yang H, Pass HI, et al. BAP1 and cancer. Nat Rev Cancer. 2013;13:153–159. doi: 10.1038/nrc3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aoude LG, Vajdic CM, Kricker A, et al. Prevalence of germline BAP1 mutation in a population-based sample of uveal melanoma cases. Pigment Cell Melanoma Res. 2013;26:278–279. doi: 10.1111/pcmr.12046. [DOI] [PubMed] [Google Scholar]
- 26.Laurent C, Gentien D, Piperno-Neumann S, et al. Patient-derived xenografts recapitulate molecular features of human uveal melanomas. Mol Oncol. 2013;7:625–636. doi: 10.1016/j.molonc.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onken MD, Worley LA, Tuscan MD, et al. An accurate, clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma. J Mol Diagn. 2010;12:461–468. doi: 10.2353/jmoldx.2010.090220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLean IW, Foster WD, Zimmerman LE, et al. Modifications of Callender’s classification of uveal melanoma at the Armed Forces Institute of Pathology. Am J Ophthalmol. 1983;96:502–509. doi: 10.1016/s0002-9394(14)77914-0. [DOI] [PubMed] [Google Scholar]
- 30.Maat W, Jordanova ES, van Zelderen-Bhola SL, et al. The heterogeneous distribution of monosomy 3 in uveal melanomas: implications for prognostication based on fine-needle aspiration biopsies. Arch Pathol Lab Med. 2007;131:91–96. doi: 10.5858/2007-131-91-THDOMI. [DOI] [PubMed] [Google Scholar]
- 31.Bronkhorst IH, Maat W, Jordanova ES, et al. Effect of heterogeneous distribution of monosomy 3 on prognosis in uveal melanoma. Arch Pathol Lab Med. 2011;135:1042–1047. doi: 10.5858/2010-0477-OAR1. [DOI] [PubMed] [Google Scholar]
- 32.Koopmans AE, Verdijk RM, Brouwer RW, et al. Clinical significance of immunohistochemistry for detection of BAP1 mutations in uveal melanoma. Mod Pathol. doi: 10.1038/modpathol.2014.43. Published Online First: 14 Mar 2014. [DOI] [PubMed] [Google Scholar]
- 33.Bengtsson H, Simpson K, Bullard J, et al. Tech Report #745. Berkeley: Department of Statistics, University of California; 2012. aroma.affymetrix: A generic framework in R for analyzing small to very large Affymetrix data sets in bounded memory. [Google Scholar]
- 34.Bengtsson H, Irizarry R, Carvalho B, et al. Estimation and assessment of raw copy numbers at the single locus level. Bioinformatics. 2008;24:759–767. doi: 10.1093/bioinformatics/btn016. [DOI] [PubMed] [Google Scholar]
- 35.Bengtsson H, Wirapati P, Speed TP. A single-array preprocessing method for estimating full-resolution raw copy numbers from all Affymetrix genotyping arrays including GenomeWideSNP 5 & 6. Bioinformatics. 2009;25:2149–2156. doi: 10.1093/bioinformatics/btp371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smyth GK. In: Bioinformatics and computational biology solutions using {R} and bioconductor. Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W, editors. New York: Springer; 2005. pp. 397–420. [Google Scholar]
- 37.Du P, Kibbe WA, Lin SM. lumi: a pipeline for processing Illumina microarray. Bioinformatics. 2008;24:1547–1548. doi: 10.1093/bioinformatics/btn224. [DOI] [PubMed] [Google Scholar]
- 38.Du P, Zhang X, Huang CC, et al. Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics. 2010;11:587. doi: 10.1186/1471-2105-11-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du P, Kibbe WA, Lin SM. nuID: a universal naming scheme of oligonucleotides for illumina, affymetrix, and other microarrays. Biol Direct. 2007;2:16. doi: 10.1186/1745-6150-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin SM, Du P, Huber W, et al. Model-based variance-stabilizing transformation for Illumina microarray data. Nucleic Acids Res. 2008;36:e11. doi: 10.1093/nar/gkm1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th. New York: Springer; 2010. pp. 547–560. [Google Scholar]
- 42.van Gils W, Lodder EM, Mensink HW, et al. Gene expression profiling in uveal melanoma: two regions on 3p related to prognosis. Invest Ophthalmol Vis Sci. 2008;49:4254–4262. doi: 10.1167/iovs.08-2033. [DOI] [PubMed] [Google Scholar]
- 43.Meir T, Zeschnigk M, Masshofer L, et al. The spatial distribution of monosomy 3 and network vasculogenic mimicry patterns in uveal melanoma. Invest Ophthalmol Vis Sci. 2007;48:1918–1922. doi: 10.1167/iovs.06-1308. [DOI] [PubMed] [Google Scholar]
- 44.Onken MD, Lin AY, Worley LA, et al. Association between microarray gene expression signature and extravascular matrix patterns in primary uveal melanomas. Am J Ophthalmol. 2005;140:748–749. doi: 10.1016/j.ajo.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 45.Shah AA, Bourne TD, Murali R. BAP1 protein loss by immunohistochemistry: a potentially useful tool for prognostic prediction in patients with uveal melanoma. Pathology. 2013;45:651–656. doi: 10.1097/PAT.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 46.Maat W, Ly LV, Jordanova ES, et al. Monosomy of chromosome 3 and an inflammatory phenotype occur together in uveal melanoma. Invest Ophthalmol Vis Sci. 2008;49:505–510. doi: 10.1167/iovs.07-0786. [DOI] [PubMed] [Google Scholar]
- 47.Worley LA, Onken MD, Person E, et al. Transcriptomic versus chromosomal prognostic markers and clinical outcome in uveal melanoma. Clin Cancer Res. 2007;13:1466–1471. doi: 10.1158/1078-0432.CCR-06-2401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.