Abstract
Aims
To characterise a histologically unusual paediatric uveal melanoma by gene expression and karyotypic profiling and assess prognosis.
Methods
The tumour was studied by histopathology, karyotype analysis, single nucleotide polymorphism and gene expression profile analysis for correlation with clinical outcome.
Results
The tumour had predominantly epithelioid histology. Karyotype analysis showed none of the poor prognosis features normally associated with uveal melanoma. single nucleotide polymorphism analysis revealed no imbalance at chromosome 3. Gene expression profiling indicated low risk disease.
Conclusions
We report a child remaining relapse-free 6 years after diagnosis of a very rare uveal melanoma, with poor prognosis epithelioid histology, but gene expression profiling that accurately predicted low risk disease.
INTRODUCTION
Uveal melanoma is extremely rare in children, the incidence ranging from 0.8% for those <20 years and 0.12% for those <10 years of age.1 Clinical presentation and treatment do not differ from adults,2 however there is a suggestion that paediatric tumours may be smaller and seed less than those in adults.3 Poor prognosis is associated with large tumours (>10-mm diameter, >2-mm height),4 epithelioid histology, vascular, highly proliferative and necrotic tumours, monosomy 3,5 and trisomy 8 or 8q.5–7 Other karyotypic abnormalities including loss of a sex chromosome, and partial loss of 6q, confer a better prognosis.5,7 Metastases commonly affect liver, lung, bone and skin. Median relapse-free survival is 3 years.8
We report a child remaining relapse-free 6 years after diagnosis of a rare uveal melanoma with a novel karyotype, poor prognosis epithelioid histology, but gene expression profiling that accurately predicted low risk disease.
MATERIALS AND METHODS
Clinical care
Standard clinical care protocols were used to diagnose and treat the patient, including clinical examination, examination under anaesthesia for imaging using RetCam, ultrasonography, axial CT and MRI. When the eye was enucleated, tumour was sent for molecular analyses, and the remaining globe was fixed in 4% formalin and embedded in paraffin.
Histopathology
The formalin-fixed, paraffin-embedded eye was cut into 5-µm sections and stained with H&E and Mart-1 (Cell Marque, clone M2-7C10; dilution 1:100; detection with red chromogen). Images were captured using a Nikon Super Coolscan 5000 ED slide scanner.
Karyotyping
Mechanically disaggregated direct preparations and short-term collagenase-treated cultures were prepared and G-banded according to standard cytogenetic techniques.
Single nucleotide polymorphism analysis and gene expression profiling
Single nucleotide polymorphism analysis was performed for chromosome 3, a hotspot for uveal melanoma abnormalities. Gene expression profiling was performed on a microfluidics multiplex-PCR platform as previously described,9 and analysed mathematically using principal component analysis against a set of previously validated tumours.
RESULTS
Clinical findings
A 4-year-old girl presented with loss of vision for 1-day in the left eye that had ‘turned outwards’ for 1 month. She had congenital left sensorineural deafness. Her left eye was exotropic with 20/400 vision, with a large pigmented subretinal mass with yellow flecks abutting the left optic nerve, causing a serous macular detachment. Right eye was normal.
Cutaneous melanoma was diagnosed in a maternal first cousin at age 34 years and a paternal grandfather at age 40 years. The patient had no atypical cutaneous naevi.
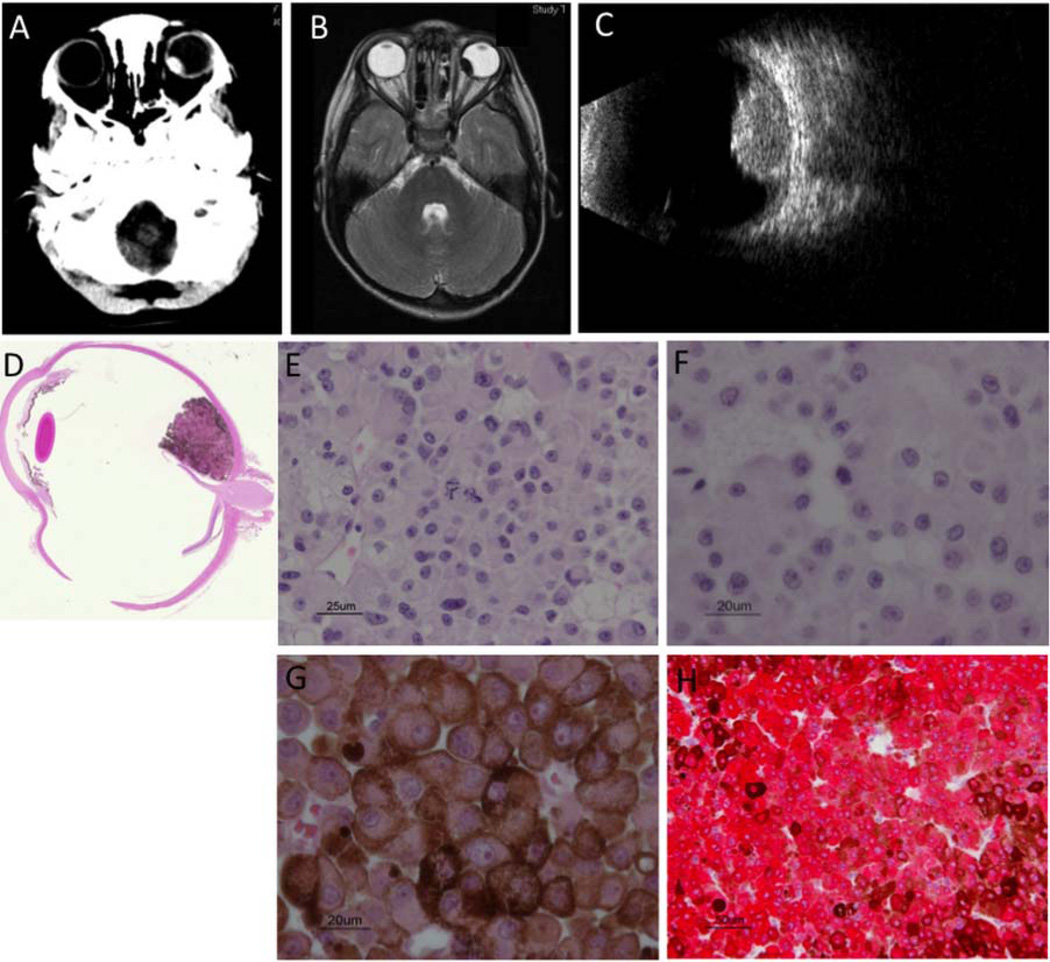
CT orbits showed a left ocular hyperdense calcified dome-shaped lesion (figure 1A). MRI showed that the 11.6-mm width×6.3-mm height mass was T1-hyperintense (not shown) and T2-hypointense (figure 1B), and ultrasonography showed a large calcified10 lesion (figure 1C). Examination under anaesthesia showed a grayish-black, homogeneous, non-haemorrhagic mass with early hyperfluorescence and late staining on fluorescein angiography. Differential diagnoses included uveal melanoma, melanocytoma, amelanotic schwannoma, neurogenic tumour, pigment cell hamartoma, retinoblastoma and metastatic tumour. Biopsy was avoided because of concern of extraocular seeding. The left eye was enucleated 2 months later because of a slight enlargement and poor vision.
Figure 1.
Radiology and histopathology (A) Axial CT orbits showed a dome-shaped hyperdense lesion in the left eye with calcification along the anterolateral margin. (B) Axial MRI of the head with gadolinium showed a T2 hypointense lesion measuring 11.6-mm width, 6.3-mm height. (C) Ultrasound showed calcified mass 6.49 mm in height. H&E stain showed tumour confined to globe (D) and predominantly epithelioid cell morphology (E) 40× (F) 60× bleached (G) 60× unbleached. (H) Tumour stained with antibody to melanocytic marker, Mart-1.
The melanocytic tumour, confined to the left globe (figure 1D), occupied the choroid and showed predominantly epithelioid histology, consisting of large, irregular, polygonal cells with prominent nuclei, abundant cytoplasm and varying levels of pigmentation (figure 1E–G), fitting the Callender classification.11 It was positive for the melanocytic marker Mart-1 (figure 1H), showed few mitotic figures and low MIB-1 staining, with no necrosis, vasculogenic mimicry nor extension into sclera or optic nerve.
The original and subsequent metastatic surveillance of MRI head and orbits, liver and total-body MRI, liver ultrasonography and liver function tests, showed that she is relapse-free 6 years later.
Cytogenetic and molecular findings
Tumour karyotype by G-band analysis showed rearrangements of chromosomes 9, 19 and 22, and deletion of chromosome 13: (45,XX,der(9)t(9;22)(p13;?q13),13,der(19)t(13;19)(q12;p13), der(22)t(9;22)(p13;q11.2)[3]/46,XX[7]). However, most of 13q was translocated to the short arm chromosome 19, with overall karyotypic imbalance from loss of 13pter to proximal 13q, and loss of most of the long arm of chromosome 22. Chromosomes 3 and 8, often lost and gained, respectively, in adult uveal melanoma, were normal.
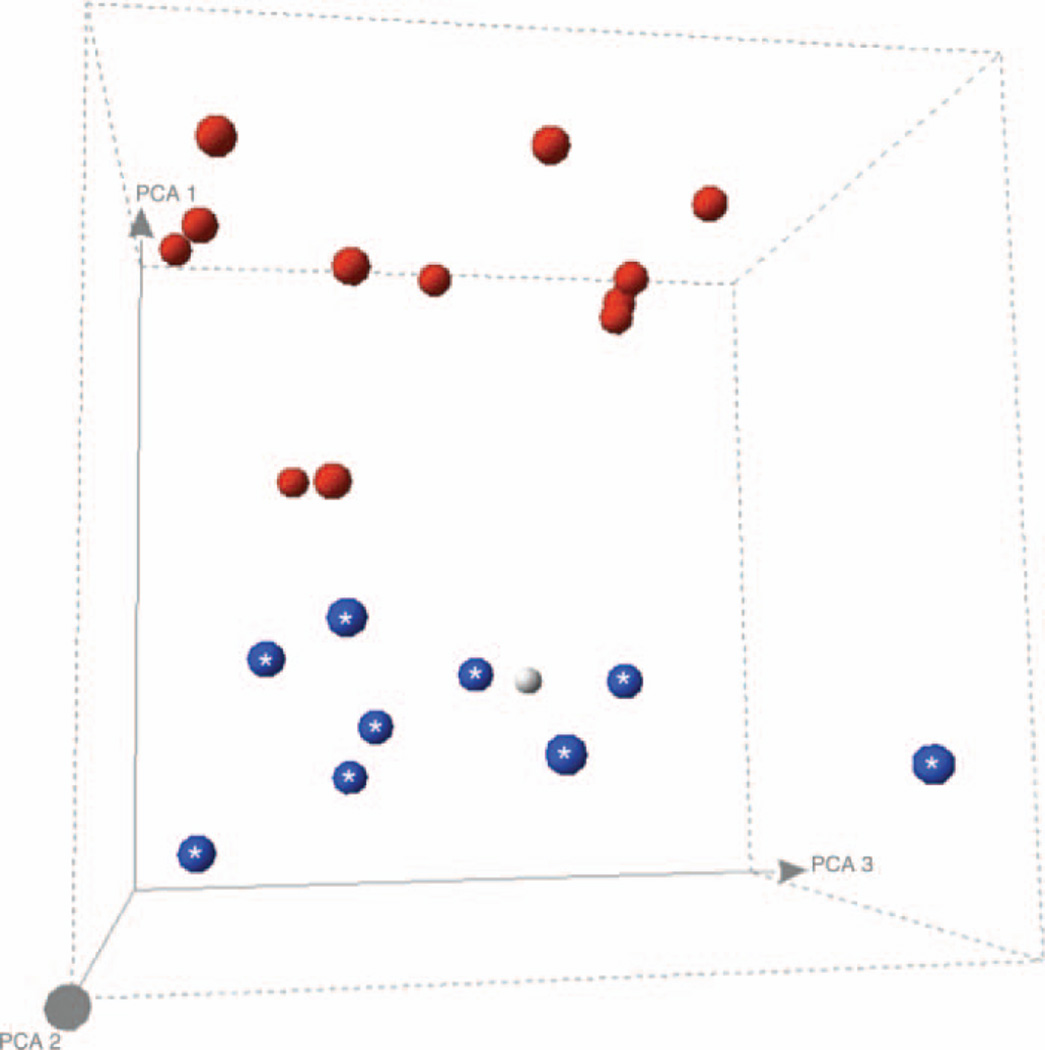
The NF1 gene was not studied. There was no chromosome 3 loss of heterozygosity by single nucleotide polymorphism analysis. Gene expression profiling revealed a low-risk Class 1 gene expression signature (figure 2). This was confirmed using weighted voting (not shown), a rigorous predictive algorithm, which had a zero error rate in a known set of tumours.
Figure 2.
Gene Expression Profiling Principal Component Analysis (PCA) of the uveal melanoma against previously analysed and validated tumours. Spheres with asterisks=Class 1 gene expression signature with favourable prognosis; Black spheres=Class 2 gene expression signature with less favourable prognosis; Grey sphere=our patient’s uveal melanoma, which co-localises with the favourable-prognosis tumours with Class 1 gene expression signature. This figure is only reproduced in colour in the online version.
DISCUSSION
There are several case series of uveal melanoma in children and adolescents (<20 years of age),1,3,4,12 and individual reports documenting cases presenting under the age of 15 years.2,13–18 However only three of these publications reported cases presenting in children under the age of 5 years,1,2,18 similar to our case.
Distinct histological, karyotypic and clinical features of uveal melanoma are used to predict prognosis. Poor prognosis was suggested for our patient with a very rare paediatric uveal melanoma, since it was large in size and displayed a predominantly epithelioid histology. Adults with uveal melanoma with a diameter between 11–15 mm have a 40% 10-year mortality.19 Poore survival is suggested by pure epithelioid histology compared with tumours with partially epithelioid or completely spindlecell histology.19 Prognosis is better in the absence of tumour vascularity, invasion, necrosis and mitoses.19 Our patient’s predominantly epithelioid tumour showed no pleomorphism, low proliferation, and no evidence of necrosis, vasculogenic mimicry, nor infiltration of the sclera or optic nerve.
Recurrent chromosomal abnormalities identified in uveal melanoma include monosomy 3, trisomy 8 or 8q, loss of a sex chromosome and loss of 6q.20 Our patient with paediatric uveal melanoma displayed a novel tumour karyotype, compared with previous reports. The only other reported case of a large, low-mitotic and non-necrotic paediatric uveal melanoma differed genomically and histologically from our patient, showing chromosome 8 gain by fluorescent in situ hybridization and predominantly spindle cell morphology.2 The history of melanoma in the cousin and grandparent of our patient may suggest the presence a familial mutation in the NF1 gene, which has been correlated to a higher risk of skin and ocular melanomas;16 however this gene was not studied in our patient.
Although the clinical and histological features predicted a poor prognosis for our patient, the karyotypic features did not support this (no chromosome 3 or 8 abnormalities). Monosomy 3 is predictive of poor prognosis, and is strongly correlated with epithelioid histology,21 yet the presence of epithelioid histology is not predictive of monosomy 3,22 nor is the absence of monosomy 3 indicative of low risk, particularly in the presence of poor prognosis histology. To further define the prognosis, we looked at the tumour’s molecular features by gene expression analysis. A Class 1 gene expression signature was observed in our patient, which suggested a low metastatic potential, confirmed to date by her 6-year relapse-free survival. Together with karyotypic analysis, gene expression profiling may prove to be a very useful prognostic factor of the very rare paediatric uveal melanoma.
Acknowledgments
This study was supported in part by grants to Dr Chan from The Ontario Institute for Cancer Research and The Terry Fox Research Institute, and to Dr Gallie from the Canadian Retinoblastoma Society and the Royal Arch Masons of Canada.
Footnotes
Contributors The authors contributed to the manuscript as follows: Conception and design: HD, HSLC, JWH, EH and BLG; Analysis and interpretation of data: all authors; Drafting the article: HD; Revising it critically for important intellectual content: MVP, GK, ERS, AA, WH, MS, JWH, EH, BLG and HSLC; Final approval of the version to be published: all authors. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests None.
Ethics approval The Hospital for Sick Children REB.
REFERENCES
- 1.Singh AD, Shields CL, Shields JA, et al. Uveal melanoma in young patients. Arch Ophthalmol. 2000;118:918–923. [PubMed] [Google Scholar]
- 2.Kanthan GL, Grigg J, Billson F, et al. Paediatric uveal melanoma. Clin Experiment Ophthalmol. 2008;36:374–376. doi: 10.1111/j.1442-9071.2008.001739.x. [DOI] [PubMed] [Google Scholar]
- 3.Shields CL, Kaliki S, Shah SU, et al. Iris melanoma: features and prognosis in 317 children and adults. J Aapos. 2012;16:10–16. doi: 10.1016/j.jaapos.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Barr CC, McLean IW, Zimmerman LE. Uveal melanoma in children and adolescents. Arch Ophthalmol. 1981;99:2133–2136. doi: 10.1001/archopht.1981.03930021009003. [DOI] [PubMed] [Google Scholar]
- 5.Kilic E, van Gils W, Lodder E, et al. Clinical and cytogenetic analyses in uveal melanoma. Invest Ophthalmol Vis Sci. 2006;47:3703–3707. doi: 10.1167/iovs.06-0101. [DOI] [PubMed] [Google Scholar]
- 6.Prescher G, Bornfeld N, Hirche H, et al. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347:1222–1225. doi: 10.1016/s0140-6736(96)90736-9. [DOI] [PubMed] [Google Scholar]
- 7.Horsman DE, White VA. Cytogenetic analysis of uveal melanoma. Consistent occurrence of monosomy 3 and trisomy 8q. Cancer. 1993;71:811–819. doi: 10.1002/1097-0142(19930201)71:3<811::aid-cncr2820710325>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 8.Kath R, Hayungs J, Bornfeld N, et al. Prognosis and treatment of disseminated uveal melanoma. Cancer. 1993;72:2219–2213. doi: 10.1002/1097-0142(19931001)72:7<2219::aid-cncr2820720725>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Chang SH, Worley LA, Onken MD, et al. Prognostic biomarkers in uveal melanoma: evidence for a stem cell-like phenotype associated with metastasis. Melanoma Res. 2008;18:191–200. doi: 10.1097/CMR.0b013e3283005270. [DOI] [PubMed] [Google Scholar]
- 10.Chan TK, Atta HR, Scott GB. Ossification in choroidal melanoma. Br J Ophthalmol. 1995;79:705–706. doi: 10.1136/bjo.79.7.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gamel JW, McLean IW, Foster WD, et al. Uveal melanomas: correlation of cytologic features with prognosis. Cancer. 1978;41:1897–1901. doi: 10.1002/1097-0142(197805)41:5<1897::aid-cncr2820410534>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Shields CL, Kaliki S, Furuta M, et al. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8,033 cases. Retina. 2012;32:1363–1372. doi: 10.1097/IAE.0b013e31824d09a8. [DOI] [PubMed] [Google Scholar]
- 13.Fong A, Lee L, Glasson W. Pediatric choroidal melanoma in a 13-year-old girl–a clinical masquerade. J Aapos. 2011;15:305–307. doi: 10.1016/j.jaapos.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Gunduz K, Shields CL, Shields JA, et al. Iris mammillations as the only sign of ocular melanocytosis in a child with choroidal melanoma. Arch Ophthalmol. 2000;118:716–717. doi: 10.1001/archopht.118.5.716. [DOI] [PubMed] [Google Scholar]
- 15.Gunduz K, Shields JA, Shields CL, et al. Choroidal melanoma in a 14-year-old patient with ocular melanocytosis. Arch Ophthalmol. 1998;116:1112–1114. doi: 10.1001/archopht.116.8.1112. [DOI] [PubMed] [Google Scholar]
- 16.Russo A, Coupland SE, O’Keefe M, et al. Choroidal melanoma in a 7-year-old child treated by trans-scleral local resection. Graefes Arch Clin Exp Ophthalmol. 2010;248:747–749. doi: 10.1007/s00417-009-1295-z. [DOI] [PubMed] [Google Scholar]
- 17.Soni S, Lee DS, DiVito J, Jr, et al. Treatment of pediatric ocular melanoma with high-dose interleukin-2 and thalidomide. J Pediatr Hematol Oncol. 2002;24:488–491. doi: 10.1097/00043426-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Grabowska A, Abelarias J, Peralta J, et al. Uveal melanoma in a 19-month-old child. J Aapos. 2011;15:606–608. doi: 10.1016/j.jaapos.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Woll E, Bedikian A, Legha SS. Uveal melanoma: natural history and treatment options for metastatic disease. Melanoma Res. 1999;9:575–581. [PubMed] [Google Scholar]
- 20.White VA, Chambers JD, Courtright PD, et al. Correlation of cytogenetic abnormalities with the outcome of patients with uveal melanoma. Cancer. 1998;83:354–359. [PubMed] [Google Scholar]
- 21.Damato B, Duke C, Coupland SE, et al. Cytogenetics of uveal melanoma: a 7-year clinical experience. Ophthalmology. 2007;114:1925–1931. doi: 10.1016/j.ophtha.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Scholes AG, Damato BE, Nunn J, et al. Monosomy 3 in uveal melanoma: correlation with clinical and histologic predictors of survival. Invest Ophthalmol Vis Sci. 2003;44:1008–1011. doi: 10.1167/iovs.02-0159. [DOI] [PubMed] [Google Scholar]