Abstract
Background:
Peripartum cardiomyopathy (PPCM) is common in North-Western Nigeria. This study aimed to describe the 1-year survival and left ventricular reverse remodeling (LVRR) in a group of patients with PPCM from three referral hospitals in Kano, Nigeria.
Methods:
PPCM was defined according to recommendations of the Heart Failure (HF) Association of the European Society of Cardiology Working Group on PPCM. LVRR was defined as absolute increase in left ventricular ejection fraction (LVEF) by ≥10.0% and decrease in left ventricular (LV) end-diastolic dimension indexed to body surface area ≤33.0 mm/m2, while recovered LV systolic function as LVEF ≥55%, at 12 months follow-up.
Results:
A total of 54 newly diagnosed PPCM patients with mean age of 26.6 ± 6.7 years, presented with classical features of predominantly left-sided HF and 33 of them qualified for follow-up. Of the 17 survivors at 12 months, 8 patients (47.1%) satisfied the criteria for LVRR, of whom 5 (29.4%) had recovered LV systolic function (LVEF ≥55%), but LVRR was not predicted by any variable in the regression models. The prevalence of normal LV diastolic function increased from 11.1% at baseline to 35.3% at 12 months (P = 0.02). At 1-year follow-up, 41.4% of patients had died (two-thirds of them within the first 6 months), but mortality was not predicted by any variable including LVRR.
Conclusions:
In Kano, PPCM patients had modest LVRR but high mortality at 1-year. Further studies should be carried out to identify reasons for the high mortality and how to curb it.
Key words: Left ventricular reverse modeling, mortality, Nigeria, peripartum cardiomyopathy, survival
INTRODUCTION
Although peripartum cardiomyopathy (PPCM) was first described in 1880, much is still unknown about its etiology, epidemiology, characteristics, and clinical outcome.[1,2] The disease has a wide geographic spread, and is an important cause of heart failure (HF) in Northern Nigeria; it is responsible for as high as 18.7% of all HF cases, and is as important as hypertension as a cause of HF among women.[3,4] It is believed that left ventricular (LV) function recovers in 23–41% of PPCM patients over time, and mortality rates also vary widely and could be as high as 16% at 6 months.[5] However, robust outcome data from many parts of the world with high disease prevalence is still lacking.[5] It is, therefore, imperative to study the disease in Northern Nigeria which has a modest health care capacity, in an attempt to better understand the disease.
Therefore, the present study aimed to describe the 1-year survival and left ventricular reverse remodeling (LVRR) in a cohort of PPCM patients from three referral hospitals in Kano, Nigeria.
METHODS
This was a longitudinal study carried out in three referral hospitals in Kano, Nigeria.
Clinical evaluation
Before the commencement of the study, the research protocol was approved by the Research Ethics Committees of the study centers and Kano State Hospitals Management Board. The study conformed to the ethical guidelines of the declaration of Helsinki, on the principles for medical research involving human subjects.[6]
Patients' inclusion criteria were: (i) New diagnosis of PPCM before commencement of medical treatment; (ii) onset of HF symptoms between last few months of pregnancy and first 5 months postpartum; (iii) presenting between last few months of pregnancy and first 9 months postpartum; (iv) at least 18 years of age; (v) contact telephone number, except patients who gave reassurance that they were willing to attend the follow-up visits; and (vi) giving written informed consent.
PPCM was defined according to the recommendations of the HF Association of the European Society of Cardiology Working Group on PPCM, and LV systolic dysfunction was defined as left ventricular ejection fraction (LVEF) <50%.[5]
At the study sites, physicians and obstetricians were approached and requested to refer all patients with suspected PPCM to the principal investigator (PI) for further evaluation at no cost. Patients were then interviewed, clinically evaluated, and recruited consecutively. Hospital in-patients with PPCM were clinically evaluated and underwent investigations within the first 48 h of admission. Demographic data, relevant aspects of history and physical signs, results of investigations, medications, co-morbid conditions, and complications were included in a detailed questionnaire. For patients who did not have contact telephone number but who agreed to participate, attending the 1st month follow-up with the supervising physician was used as evidence of commitment and for inclusion.
All patients were given appointment cards as a reminder, and were also telephoned to be informed of the 6 and 12 months follow-up visits. At the recruitment visit, each subject underwent a 12-lead electrocardiogram (ECG) at rest and transthoracic echocardiogram at the study centers according to standard recommendations.[7] The echocardiographic examination was carried out using Sonoscape S8 Doppler ultrasound system (Shenzhen, China, 2010). Other baseline investigations recommended for the management of patients with PPCM were carried out in the laboratories of the study center, including plasma hemoglobin; serum urea, electrolytes and creatinine; blood counts; liver enzymes; and serum albumin.[5]
The PI re-evaluated the patients at 6 and 12 months follow-up, using the same protocol as at recruitment including ECG and echocardiogram examinations, but blood tests were not repeated.
Systemic hypertension was defined using standard cut-off values for systolic blood pressure (SBP) ≥140 mmHg and or diastolic blood pressure ≥90 mmHg. Anemia was defined as hemoglobin <12 g/dl, according to the recommendation of the World Health Organization in nonpregnant women, renal impairment as serum creatinine >176 µmol/L (>2 mg/dl), hyponatremia as serum sodium <135 mmol/L, and hypoalbuminemia as serum albumin <35 g/L.[8]
Cardiac function assessment
Of the standard echocardiographic recordings obtained, LVRR was defined as the presence of both absolute increase in LVEF ≥10.0% and decrease in left ventricular end-diastolic dimension indexed to body surface area (LVEDDi) ≤33.0 mm/m2, while recovered LV systolic function as LVEF ≥55%, at 12 months follow-up.[9]
LV diastolic function was defined and graded using transmitral flow and LV myocardial tissue Doppler imaging velocities at the mitral annular level as follows:[7,10]
Normal LV diastolic function: E:A ratio 1–2, deceleration time (DT) 160–230 ms and E/e' <8.
Grade I left ventricular diastolic dysfunction (LVDD) (impaired myocardial relaxation): E:A <1.0 and DT >240 ms.
Grade II LVDD (pseudonormalized pattern): E:A 1–1.5, DT 160–230 ms, e' <7 cm/s, and E/e' >15.0.
Grade III LVDD (restrictive filling): E:A >2.0, DT <160 ms, e' <7 cm/s, and E/e' >15.0.
Statistical analysis
Frequencies, mean, median, and inter-quartile ranges (IQR) were used to describe patients' characteristics. Chi-squared, Fisher's exact, Student t and Mann–Whitney U-tests were used to compare categorical and continuous variables as appropriate. Spearman correlation coefficient and logistic regression models were used to assess potential associations between LVRR or mortality and variables of interest. The statistical analysis was carried out using (SPSS for Windows, Version 16.0. Chicago, SPSS Inc. Released 2007). Two-sided P < 0.05 was considered as the minimum level of statistical significance.
RESULTS
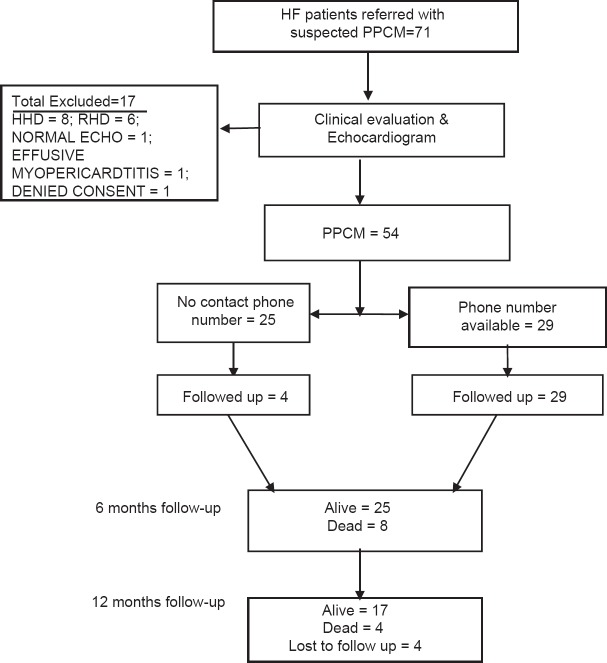
A total of 72 patients were referred to the investigators with a diagnosis of PPCM based on clinical features and findings on chest radiograph. After further evaluation including echocardiography, 18 subjects (25.0%) were excluded and the remaining 54 (75.0%) were confirmed to have PPCM, and all were of Hausa–Fulani ethnic group [Figure 1]. Of these 54 patients, 29 had contact phone numbers and were contacted and the remaining 25 (46.3%) did not have contact phone numbers, 4 (16.0%) of whom qualified for follow-up. Overall, therefore, 33 patients were followed up as shown in Figure 1. When the 21 patients who did not have contact phone numbers and did not attend follow-up were compared with the 33 followed up patients, their baseline characteristics were similar (P > 0.05) except for the lower mean hemoglobin in the former group (11.5 ± 2.0 g/dL) compared with the latter group (12.8 ± 1.6 g/dL) (P = 0.026).
Figure 1.
Flowchart of recruitment and follow-up of patients
Patients' baseline demographics and clinical characteristics
The age of the patients ranged between 18 and 45 years with a mean of 26.6 ± 6.7 years, and 19 of them (35.2%) were between 18 and 20 years, 24 (44.5%) between 20 and 30 years, and the remaining 11 (20.4%) were older than 30 years. No patient had a history of smoking, diabetes mellitus, alcohol drinking, stroke, or morbid arrhythmias. One patient had LV thrombus and developed lower limb gangrene needing bilateral below knee amputations. Screening for human immunodeficiency virus was not carried out and none of the recruited patients was known to have the disease.
Patients' body mass index (BMI) <18.5kg/m2 (under-weight) was found in 14 (25.9%), 18.5–24.9 kg/m2 (normal body weight) in 29 (53.7%), 25.0–29.9 kg/m2 in 8 (14.8%), and ≥30.05 kg/m2 in only 2 (3.7%) patients. Nine (16.7%) patients had hypotension (SBP <100 mmHg) and 25 (46.3%) had pregnancy-induced hypertension at presentation. Two patients became pregnant again before the 6 months follow-up, and both survived follow-up; their LVEF increased from 43.5 ± 5.0% at baseline to 52.5 ± 5.0% and 54.5 ± 3.5% at 6 and 12 months follow-up, respectively.
Left ventricular reverse remodeling
Of the 17 survivors at 12 months follow-up, 5 (29.4%) had recovered LV systolic function (LVEF ≥55%), 10 (58.8%) had increased LVEF of at least 10%, and 8 (47.1%) had reduced LVEDDi ≤33.0 mm/m2 [Table 1]. Overall, 8 patients (47.1%) satisfied the criteria for LVRR. There was no relationship between the use of angiotensin converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARB), beta-blockers (P = 0.203), other medications or echocardiographic variables (P > 0.05) and LVRR (P = 0.325), in binary logistic regression models.
Table 1.
Characteristics of patients with and without LV reverse remodeling
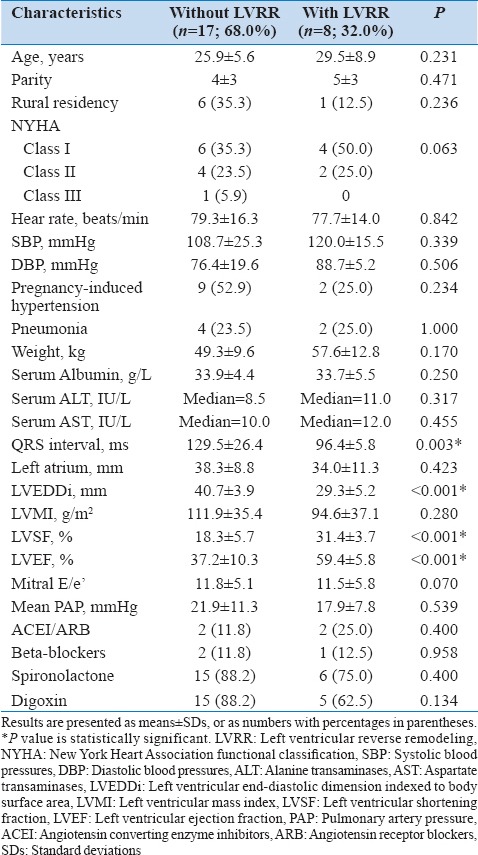
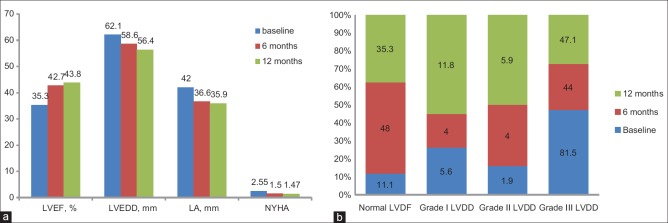
Of note, mean LVEF, LVEDD, as well as left atrial (LA) size and New York Heart Association (NYHA) functional class all significantly improved at 6 months (P < 0.05), but the differences between the 6 and 12 months follow-upvalues were not statistically significant (P > 0.05) [Figure 2a]. Furthermore, patients with LVRR had significantly shorter QRS duration than those without (P = 0.003). At 12 months, QRS duration correlated negatively with LVEF (r = −0.602; P = 0.018) and positively with left ventricular mass index (LVMI) (r = 0.612; P = 0, 012). However, these associations were not significant (P > 0.05) at baseline or 6 months follow-up.
Figure 2.
(a)Pattern of changes in left ventricular ejection fraction and end-diastolic dimension, left atrial dimension, and symptoms in peripartum cardiomyopathy patients over 1-year, (b) Pattern of changes in left ventricular diastolic dysfunction in peripartum cardiomyopathy patients over 1-year
Left ventricular diastolic function
The pattern and changes over 1-year of the LVDD are illustrated in Figure 2b. At baseline, LVDD was found in a total of 48 patients (88.9%) and 6 (11.1%) had normal function. Of the patients with LVDD, 44 (91.7%) had Grade III, 1 (2.1%) had Grade II, while the remaining 3 (6.3%) had Grade I LVDD. At 6 months follow-up, 12 patients (48.0%) had normal LV diastolic function and 13 (52.0%) had LVDD (P = 0.003 compared with baseline results), while at 12 months 6 patients (35.3%) had normal LV diastolic function and 11 (64.7%) had LVDD (P = 0.02 compared with baseline results). However, the prevalence of LVDD at 6 and 12 months follow-ups did not differ (P = 0.414).
Mortality
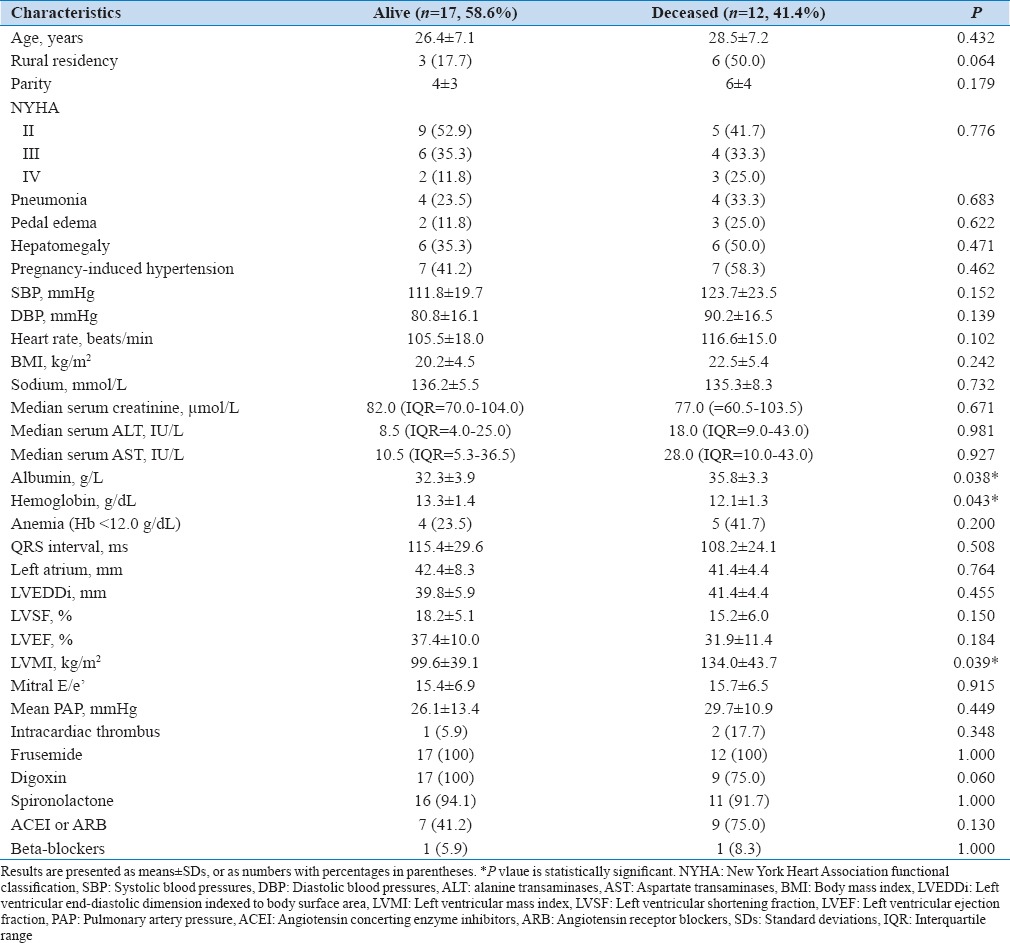
Of the 33 patients followed up, 12 died (36.4%), 8 (66.7%) within the first 6 months and the remaining 4 (33.3%) before the 12 months follow-up, four patients were lost to follow-up (12.1%), and 17 (51.5%) completed the follow-up [Figure 1]. The baseline characteristics of the 17 survivors (58.6%) were similar to the deceased (41.4%) except for lower serum albumin and LVMI, and higher plasma hemoglobin (P < 0.05) [Table 2]. The mean NYHA class for survivors reduced from 2.53 ± 0.80 at baseline to 1.47 ± 0.62 at 1-year follow-up (P < 0.001). None of these or other variables including LVRR predicted mortality in the regression models (>0.05). The median survival time from diagnosis for the deceased patients was 20.0 (IQR = 9–26) weeks.
Table 2.
Baseline characteristics of survivors and deceased patients
DISCUSSION
The present study describes the 1-year mortality and LVRR of a cohort of PPCM patients recruited from three referral hospitals in Kano, Nigeria. Of the 33 patients (80% <30 years old) followed up, 47.1% had LVRR and 29.4% recovered LV systolic function. Of the 29 remaining patients after excluding the four lost to follow-up, 17 survived (58.6%) and 12 died (41.4%) during the follow-up period, with a median survival time of 20 weeks.
Left ventricular reverse remodeling
Of the 17 survivors at 12 months follow-up, 47.1% satisfied the criteria for LVRR while 29.4% recovered LV systolic function. There was no relationship between the use of HF medications or other variables and LVRR in the regression models. In addition, significant improvement in mean LVEF, LVEDD, LA size, LVDD, and NYHA functional class had already manifested at 6 months, with no further significant improvement at 12 months follow-up. LVDD was similar; showing a maximum fall in its prevalence from 89% at baseline to 52% at 6 months follow-up. Therefore, LV recovery in our PPCM patients involved both systolic and diastolic functions, with maximum improvement achieved at 6 months follow-up in most patients, independent of ACEIs, ARBs, and beta-blockers. However, almost all the patients were treated with frusemide, spironolactone, and digoxin, as a treatment for HF.
Our findings on LVRR seem to be similar to those previously reported from South Africa (21%) and Haiti (28%) but lower than what was reported from USA (54%) likely because of the differences in the level of health care between the three countries.[11,12,13] Furthermore, the achievement of maximum recovery within 6 months in our cohort of patients is also supported by Fett et al. and Elkayam.[12,13] Finally, our inability to identify predictors of recovery of LV function or reverse remodeling, including HF medications, was similarly reported by Fett et al.[12] However, Blauwet et al. found older age and smaller LV end-systolic dimension to be significant predictors of LV recovery among South Africans.[11]
In addition, our results show that patients with LVRR had significantly shorter QRS duration than those without, and QRS duration correlated with LVEF and with LVMI, but only at 1-year follow-up. Thus, aside from “mechanical remodeling,” our results also show that “electrical remodeling” does occur in PPCM, and is likely related to the progression or otherwise of the LV disease. Similar observations were previously made by Shamim et al. among patients with dilated and ischemic cardiomyopathies.[14]
Progressive LV disease eventually results in increased myocardial stiffness and a rise in diastolic pressures. The resulting increase in LV filling pressures and consequent ischemia of the subendocardium predispose the latter to conduction “depolarization” delay, which if prolonged could result in subendocardial fibrosis and permanent conduction disturbance.[14] Thus, in dilated and dysfunctioning ventricles, progressive broadening of the QRS complex can be taken as a marker of worsening of the ventricular disease.[14]
Mortality in peripartum cardiomyopathy patients
Of the 29 patients with complete 12 months follow-up, 58.6% had significantly lower serum albumin and LVMI, and higher plasma hemoglobin than the deceased. However, none of these or other variables could predict mortality in regression models. Two-thirds of the deaths occurred within 6 months of diagnosis while the remaining one-third died before the 12 months follow-up. Much lower mortality rates of 13.0% over 6 months in South Africa, 15.3% over 2 years in Haiti, and 28.0% over 5 years in USA, respectively, have been reported.[11,12,15] Although some researchers did not identify any predictors of mortality in agreement with our finding, others reported inconsistent predictors of mortality including younger age at diagnosis, lower BMI, and echocardiographic variables.[11,12,13] It is clearly difficult to compare mortality results between studies given the varying follow-up times and several other challenges including the possible strong influence of sociopolitical crises on the mortality.
Similar to our findings, 53.3% of those who died in the Haiti study did so within 60 days after diagnosis.[12] Whitehead et al. also reported that in a cohort of PPCM patients in the USA, 18% of deaths occurred within 1-week and 87% within 6 months of diagnosis.[16] Therefore, most of the mortality in PPCM, as well as recovery, seem to occur within 6 months of diagnosis. Although the reasons for these observations are not clear, they seem to give hope to patients of better outcomes once they survive the first 6 months of the disease. It is, therefore, imperative that patients are supported with optimal medical treatment to be able to survive the first 6 months of the disease.
Study limitations
The present study has some limitations. First, natriuretic peptides and other biomarkers of HF were not assessed because the tests were not readily available at the study centers. Second, 21 out of 54 patients could not be followed up because they lacked phone numbers and so could not be contacted, and did not turn up for follow-up in spite of adequate counseling. However, these patients had similar baseline characteristics as those followed up except for their lower mean hemoglobin levels.
CONCLUSIONS
This study has described the LVRR and 1-year survival of PPCM in three referral hospitals in Kano, Nigeria. LVRR occurred in almost half the patients at 6 months but was not predicted by any clinical or echocardiographic variable. Overtime, the prevalence of normal LV diastolic function tripled independent of HF medications. Our results also showed that LVRR was associated with shorter QRS duration which correlated with LVEF and LVMI at 1-year follow-up, thus signifying “electrical remodeling” in PPCM. Finally, none of the variables predicted the 41.4% mortality, of which two-thirds occurred before the first 6 months follow-up. The authors feel strongly that further studies should be carried out to better understand the disease.
ACKNOWLEDGMENTS
We wish to acknowledge the efforts of Dr. Hadiza Sa'idu and Mr. Ibrahim Armaya'u, both staff of MMSH Kano, in the recruitment of patients at the Hospital.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Virchow R. De L'influence Réciproque de la Grossesse et des Maladies du Coeur, Thesis, Paris. 1880. Sitzing der Berliner Geburtshilflisher Gersellskhalt. Cited by Porak C. [Google Scholar]
- 2.Porak C. De L'influence Réciproque de la Grossesse et des Maladies du Coeur, Thesis, Paris. 1880. [Google Scholar]
- 3.Karaye KM, Saidu H, Bala MS, Yahaya IA. Prevalence, clinical characteristics and outcome of pulmonary hypertension among admitted heart failure patients. Ann Afr Med. 2013;12:197–204. doi: 10.4103/1596-3519.122685. [DOI] [PubMed] [Google Scholar]
- 4.Karaye KM, Sani MU. Factors associated with poor prognosis among patients admitted with heart failure in a Nigerian tertiary medical centre: A cross-sectional study. BMC Cardiovasc Disord. 2008;8:16. doi: 10.1186/1471-2261-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12:767–78. doi: 10.1093/eurjhf/hfq120. [DOI] [PubMed] [Google Scholar]
- 6.World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J Postgrad Med. 2002;48:206–8. [PubMed] [Google Scholar]
- 7.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. WHO Technical Report Series, No 405. Geneva, Switzerland: World Health Organization; 1968. Nutritional Anemia. Report of a WHO Scientific Group. [PubMed] [Google Scholar]
- 9.Merlo M, Pyxaras SA, Pinamonti B, Barbati G, Di Lenarda A, Sinagra G. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J Am Coll Cardiol. 2011;57:1468–76. doi: 10.1016/j.jacc.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Oh JK, Park SJ, Nagueh SF. Established and novel clinical applications of diastolic function assessment by echocardiography. Circ Cardiovasc Imaging. 2011;4:444–55. doi: 10.1161/CIRCIMAGING.110.961623. [DOI] [PubMed] [Google Scholar]
- 11.Blauwet LA, Libhaber E, Forster O, Tibazarwa K, Mebazaa A, Hilfiker-Kleiner D, et al. Predictors of outcome in 176 South African patients with peripartum cardiomyopathy. Heart. 2013;99:308–13. doi: 10.1136/heartjnl-2012-302760. [DOI] [PubMed] [Google Scholar]
- 12.Fett JD, Christie LG, Carraway RD, Murphy JG. Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clin Proc. 2005;80:1602–6. doi: 10.4065/80.12.1602. [DOI] [PubMed] [Google Scholar]
- 13.Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol. 2011;58:659–70. doi: 10.1016/j.jacc.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 14.Shamim W, Yousufuddin M, Cicoria M, Gibson DG, Coats AJ, Henein MY. Incremental changes in QRS duration in serial ECGs over time identify high risk elderly patients with heart failure. Heart. 2002;88:47–51. doi: 10.1136/heart.88.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witlin AG, Mabie WC, Sibai BM. Peripartum cardiomyopathy: an ominous diagnosis. Am J Obstet Gynecol. 1997;176:182–8. doi: 10.1016/s0002-9378(97)80033-6. [DOI] [PubMed] [Google Scholar]
- 16.Whitehead SJ, Berg CJ, Chang J. Pregnancy-related mortality due to cardiomyopathy: United States, 1991-1997. Obstet Gynecol. 2003;102:1326–31. doi: 10.1016/j.obstetgynecol.2003.08.009. [DOI] [PubMed] [Google Scholar]