Abstract
INTRODUCTION:
Pediatric lung abscesses can be primary or secondary, and there is limited data regarding response to treatments and patient outcomes.
OBJECTIVES:
To assess the clinical and microbiologic profile of pediatric patients with lung abscess and assess the differences in outcomes for patients treated with medical therapy or medical plus surgical therapy.
METHODS:
A retrospective review of all pediatric patients ≤ 18 years of age that were treated as an inpatient for lung abscess between the dates of August 2004 and August 2014 was conducted. Patients were divided into two subgroups based on the need for surgical intervention.
RESULTS:
A total of 39 patients with lung abscess (30 treated with medical therapy alone, 9 also required surgical interventions) were included. Fever, cough, and emesis were the most common presenting symptoms, and most of the patients had underlying respiratory (31%) or neurologic disorders (15%). Staphylococcus aureus was the most common organism in those that had culture results available, and ceftriaxone with clindamycin was the most common combination of antibiotics used for treatment. Comparison of medical and surgical subgroups identified the duration of fever and abscess size as risk factors for surgical intervention.
CONCLUSIONS:
Pediatric lung abscesses can be managed with medical therapy alone in most cases. Presence of prolonged duration of fever and larger abscess size may be predictive of the need for surgical intervention. Good clinical response to prolonged therapy with ceftriaxone and clindamycin was noted.
Keywords: Abscess, anaerobes, antibiotics, bacterial, empyema, infections, lung, microbiology pediatric, pneumonia, surgery
Lung abscesses are thick-walled cavities that contain purulent material and result from an acute pulmonary infection that has led to suppurative necrosis and destruction of the involved lung parenchyma.[1,2,3] Treatment with prolonged duration of antibiotics with or without drainage of the abscess cavity has been the standard of care. It is also important to recognize the complications associated with lung abscesses in children, and there could be several factors that predispose some patients or increase their risk for having those complications.[1]
Lung abscesses have been classified as primary (without any lung or systemic disorders) or secondary (with preexisting lung or systemic disorders). Primary pediatric lung abscesses are most commonly caused by Streptococcus pneumoniae,[4] Staphylococcus aureus, and oral bacteria.[5] For secondary lung abscesses, the most common pathogen is Pseudomonas aeruginosa.[4] Multiple studies have also advocated for assessment and coverage for anaerobic bacteria[1,6] but there are no conclusive data regarding the prevalence of these organisms in pediatric lung abscess patients. Fungal infections can be found in patients with secondary lung abscesses. Treatment with empiric broad-spectrum antibiotics is initiated until the causative organism is identified[2,4,6] through respiratory cultures, but in many cases, cultures are not clinically feasible. Invasive procedures such as surgical open drainage, lobectomy and/or percutaneous drainage are reserved for cases with persistent infection despite antibiotic therapy.[1,2,4] Prognosis is good for primary lung abscesses,[1,4] while the nature of the underlying disease is important for determining the outcome of secondary lung abscesses.[2]
For many of the aspects related to the management of pediatric lung abscess (such as optimal duration of antibiotics, need for open surgical drainage), there is still inadequate evidence in the literature. Hence, this study was initiated to understand the clinical characteristics, causative organisms, treatments utilized, associated complications, and the final outcomes of pediatric lung abscesses treated at a single center over 10 years.
Methods
We conducted a retrospective chart review of all cases of pediatric lung abscesses that were treated as an inpatient at the Children's Hospital of Michigan (CHM) over 10 years (August 2004 to August 2014). The patients were screened from their hospital discharge diagnoses using the ICD-9 codes for pediatric lung abscesses (513.0, 519.9, and 510.9). We included all patients ranging from newborns to 18 years of age, with a diagnosis of lung abscess who were admitted for inpatient therapy at our institution. We excluded all instances of repeat admissions for a known case of lung abscess already undergoing treatment. The study was approved by the Wayne State University's Institutional Review Board (Protocol #045614MP4E).
Information about the clinical presentation such as presenting symptoms and their duration; laboratory studies and culture results (when available); the type of organisms identified and the treatment choices made for empiric and subsequent antibiotic therapy and its duration were gathered from patient records. We also screened the charts for any pulmonary and systemic complications related to lung abscesses. Imaging studies were used to determine the size of abscess (using the largest dimension at admission), the affected sites (single lobe versus multilobar) and the types of imaging obtained were also recorded. The final antibiotic choice was recorded from discharge summary and/or outpatient clinic follow-up, and duration of therapy was determined by last outpatient clinic note. For patients who required surgical intervention, the indications, type of procedures performed, and any complications stemming from the intervention were also recorded. Resolution status was determined by last pediatric infectious disease outpatient clinic note that documented the discontinuation of antibiotics.
Statistical analysis
For this descriptive study, the variables assessed (as noted above) in the study population are presented as frequencies or percentages of the total. For statistical analysis, we used SPSS software (version 19.0, International Business Machines, Armonk, NY, USA) and utilized the Chi-square test to compare clinical variables. P ≤0.05 was determined to be statically significant.
Results
Over the 10-year period covered in this study, there were 874 patients that met the initial inclusion criteria. From chart review of each of these individual inpatient encounters, 40 patients were finally identified to have a true lung abscess. The remaining 834 charts were not included as there was evidence of pneumonia without abscess formation. One patient had to be excluded because the patient was subsequently found to have an infected teratoma rather than lung abscess. Therefore, 39 total patients were included in this study, of which 22 were male (56%) [Table 1]. Further subgroups were created based on subsequent course – if patients required treatment with intravenous (IV) antibiotics alone, they were allocated in the “medical” sub-group. However, if they required surgical interventions (chest tube placement, open drainage of abscess, or lobectomy), then they were placed in the “surgical” sub-group. The demographic and clinical characteristics for our study population are presented in Table 1. The most common race encountered in our cohort was African American (n = 23, 59%), and also included Caucasian (n = 9, 23%), Hispanic (n = 4, 10%), Middle Eastern (n = 2, 5%), and one patient (n = 1, 11%) for whom race was not specified. The average age of patients in this study was 7.6 years, although ranged from 2 weeks of life to 18 years.
Table 1.
Demographic and clinical profile of pediatric patients with lung abscess
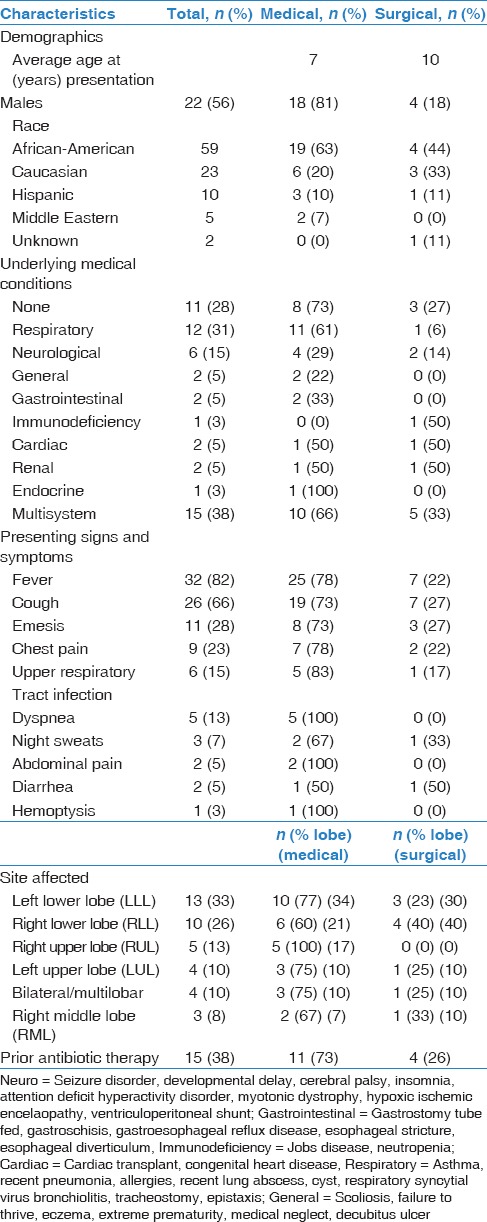
The most common presenting symptoms were fever (n = 32, 82%), cough (n = 26, 66%), and emesis (n = 11, 28%). Even after categorizing patients into medical and surgical groups, we found the most common presenting symptoms continued to be fever (medical n = 25, 83%, surgical n = 7, 78%), cough (medical n = 19, 63%, surgical n = 7, 78%), and emesis (medical n = 11, 27%, surgical n = 3, 33%) in both groups. Eleven of the 39 (28%) patients had no underlying medical condition while the most common medical history was asthma with 21%. Of note, one patient was found to have hyperimmunoglobulin E syndrome after the second admission for a recurrent lung abscess. Other notable patients included one patient with each of the following: Post-heart transplant on immunosuppressive therapy, extreme prematurity, seizure disorder, diabetes mellitus type 1, esophageal diverticulum, myotonic dystrophy, tracheostomy and gastric tube dependent, and cerebral palsy. Of note, 4 patients had a history of recent pneumonia, as well as one with a history of recurrent pneumonias. When grouped into categories, the most common underlying medical condition was a respiratory disease (n = 12, 31%), followed by neurological disease (n = 6, 15%). Prior to admission, 24 of 39 (62%) patients were not treated with any antibiotic therapy. Of the remaining 38%, the most common antibiotic therapy patients received prior to admission was amoxicillin (53% of patients who received prior therapy).
The most common location was noted to be the left lower lobe (n = 13, 33%) while the least common site was right middle lobe (n = 3, 8%). Of note, there were 4 patients with bilateral or multilobar lung abscesses. Of the left lower lobe abscess site, 77% were treated medically while 23% were treated surgically. The most common medically treated site was the left lower lobe (n = 10, 34%) while the most common surgically treated site was right lower lobe (n = 3, 30%). The most common imaging obtained for confirmation of infection was chest radiograph followed by computed tomography (CT) of chest (n = 34, 87%). Four patients had chest radiographs alone and 1 patient had undergone chest ultrasound.
Table 2 outlines the results of the microbiological cultures obtained as well as imaging studies. After admission, 12 patients did not have any culture results for the identification of organisms causing the lung abscess. Sixteen patients had sputum cultures; 6 underwent a bronchoalveolar lavage, 5 underwent abscess incision and drainage, and 1 had an associated empyema that was drained. Of note, one patient was admitted with the diagnosis of pyelonephritis and urosepsis and did have a positive blood culture, which showed Fusobacterium necrophorum. Of the respiratory cultures obtained, the organisms most commonly identified were methicillin-sensitive S. aureus and methicillin-resistant S. aureus (MRSA), S. pneumoniae, Streptococcus mitis and Peptostreptococcus. Other organisms obtained included Achromobacter sp., Aspergillus fumigatus, Moraxella caterhallis, Klebsiella sp. (extended spectrum beta-lactamase producing), Enterobacter sp., viridans group streptococci, and Eikenella sp.
Table 2.
Diagnostic testing, imaging, and microbiology results for pediatric patients with lung abscess
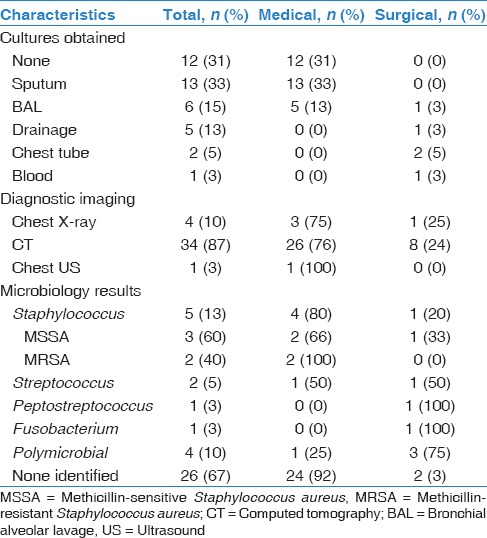
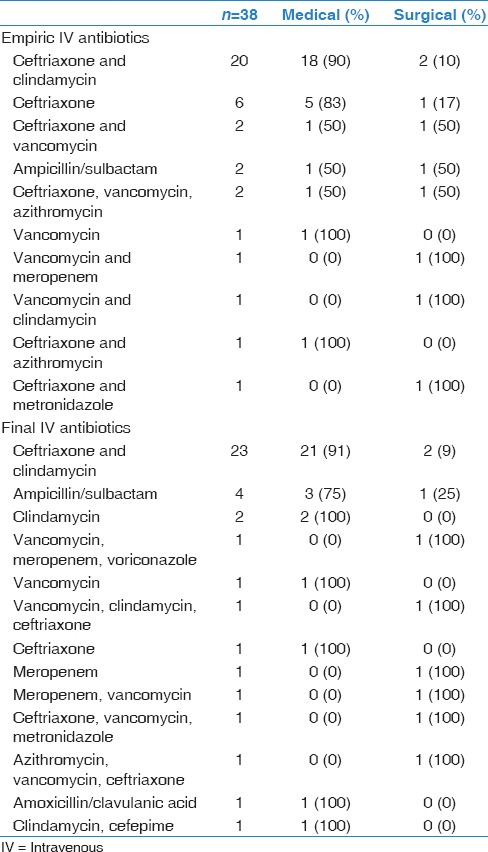
The most common empiric antibiotic combination [Table 3] used for treatment in our cohort of 39 patients was ceftriaxone with clindamycin (n = 20, 53%). It should be noted that one patient was directly started on oral medication, and one history and physical could not be reviewed. Some patients underwent changes in their antibiotic treatment plans based on culture results or clinician preferences. The most common final antibiotic choice was also ceftriaxone with clindamycin (n = 23, 59%). Of the 20 patients that were started on ceftriaxone and clindamycin, 18 (90%) were treated medically. IV antibiotic duration had been recorded in 34/39 patients. The average length of IV treatment was 24.6 days, ranging from 3 days to 51 days.
Table 3.
Empiric and final antibiotic therapies used for pediatric patients with lung abscess
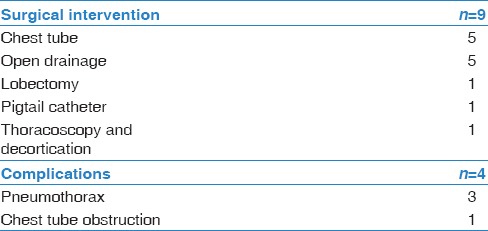
There were 9 (23%) patients that underwent operative or procedural intervention [Table 4]. Interventions included chest tube placement (5/9), thoracoscopy with decortication (1/9), drainage by interventional radiology (5/9), lobectomy (1/9), and pigtail catheter placement (1/9). The most common complications from these interventions were pneumothorax (3/9), followed by chest tube obstruction (1/5). Of the 39 patients, 31 had a complete resolution, while 8 were lost to follow-up.
Table 4.
Surgical interventions and their outcomes
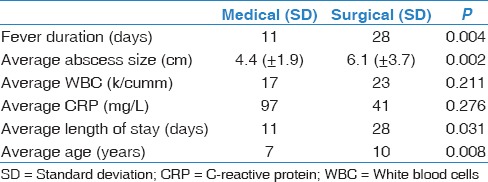
We compared the differences between medical versus surgical patients in relation to fever duration, average abscess size, average length of stay, average age, average white blood cell, and average C-reactive protein (CRP) [Table 5]. Medically treated patients were found to have an average fever duration of 11 days compared to surgically treated patients at 28 days (P = 0.004). Medically treated patients had an average abscess size of 4.4 cm compared to surgically treated patients at 6.1 cm (P = 0.002). Medically treated patients had an average length of stay 11 days while surgically treated patients were found to have an average stay of 28 days (P = 0.031). Medically treated patients were slightly younger, with an average age of 7 years; while surgically treated patients had an average age of 10 years (P = 0.008). Initial white blood count and CRP did not vary significantly between the two groups.
Table 5.
Statistical analysis of risk factors for need for surgical intervention in pediatric patients with lung abscess
Discussion
This study was conducted to highlight the epidemiologic profile, clinical presentation, microbiologic spectrum, and pharmacologic management of pediatric patients with lung abscess at a single institution. While there were not enough patients to enable comparisons of outcomes based on antibiotic regimens that were utilized, it does give some meaningful information about the clinical management of these patients. Assessment of the risk factors for the need for surgical intervention further helps in treatment decisions given that children with longer duration of fever and larger size of the abscess are more likely to require surgical intervention.
There have been few case series of pediatric lung abscesses previously published,[4,5,7,8] but there have been no reports since 2005.[2,5] The most common presenting signs and symptoms reported in these studies included the fever, cough, and dyspnea.[1,2,4,5] Most previous case series have reported the right lung to be the most commonly affected side.[1,2,3,4] There are additional reports about surgical treatment of pediatric lung abscesses[9,10] and some recent publications have highlighted the fact that the treatment approach is now shifting toward the use of less invasive procedures, such as CT-guided needle aspiration[11] or thoracoscopic resection[12] in cases that are refractory to medical therapy. The overall treatment outcomes and incidence of complications in pediatric patients with lung abscess still remains poorly defined.
In our study, we found that fever and cough were the most common presenting symptoms. This was also demonstrated in previous studies.[1,5,8] Puligandla and Laberge reported that tachypnea, cough, and fever were the most common symptoms, and may develop over weeks.[3] Emesis and chest pain were the third and fourth most common symptoms in our cohort, and Chan et al. also reported similar rates of chest pain among their cohort.[5] The most common site of involvement of lung varies between studies. We found the most common location to be left lower lobe while other studies have reported right upper lobe[5] and right lower lobe[1] to be the most common sites. Another study found lung abscess secondary to aspiration to be more prevalent.[6] One study found that lung abscess developed on the right side greater than left,[1] which was consistent with our overall results (18 right sided vs. 17 left sided). We also had four patients with bilateral lung abscess, which has rarely been reported in literature.[13] Imaging plays an important role in diagnosis and management. Stark reported that the ideal method to image a suspected lung abscess is via CT scan[14] and majority of our patients did undergo a chest CT for establishing the diagnosis and for treatment planning.
We found that Staphylococcus spp. and Streptococcus spp. were the most common single pathogens, which correlates with previously reported studies.[1,5] Macdonald goes as far as stating that S. pneumoniae was the preantibiotic era pathogen while now the most common pathogen is Staphylococcus or polymicrobial anaerobic bacteria,[15] which is also consistent in our study data. Brook and Finegold also gave emphasis to how the microbiological cultures are obtained, stating that unless the cultures were obtained by bypassing the oropharynx, the test result could not be verified.[6] In their study, they were able to find both aerobic and anaerobic bacteria group A beta-hemolytic Streptococcus and Peptostreptococci, respectively. Emanuel and Shulman had reported 18 cases with primary lung abscess, of which one was found to have S. pneumoniae while the rest had bacteria from the upper respiratory tract or of uncertain significance.[1] Tan found that 27% of isolates were anaerobes.[2] Puligandla and Laberge found Streptococcus, anaerobic species, S. aureus, and Klebsiella to be the most common pathogens in their study.[3] It would seem through the results of these studies that antibiotic coverage for both Streptococcus and Staphylococcus is imperative, along with anaerobic coverage. The specimen collection method is also very important, as sputum culture seems to provide little or no help in clinical decision-making and is difficult to obtain in the majority of the pediatric patients.
Our study found that mean duration of therapy with IV antibiotics was 24 days. In another study, the mean duration of antibiotic therapy separated into primary versus secondary lung abscess, was found to be 28 and 45 days, respectively.[5] Brook and Finegold found that patients were treated for an average of 30 days.[6] When choosing antimicrobials, Tan et al. recommended selection based on the clinical situation, stating that it should include a penicillinase-resistant agent and clindamycin,[2] which was consistent with our study findings. Puligandla and Laberge recommended using penicillins as the first line but warned that beta-lactam resistance can be problematic. This author also states that adding clindamycin for S. aureus can help to overcome this, along with a third generation cephalosporin.[3] Similarly, Holston et al. recommended adding a third generation cephalosporin if clindamycin is used.[16] Given that S. pneumoniae and S. aureus are the likely pathogens, a reasonable option of initial antibiotic choice can be ceftriaxone combined with clindamycin. In our study, this was the most common antibiotic of choice to result in resolution of the lung abscess. Of note, while we did find cases of MRSA, most patients in our study did not have an identifiable organism as no culture was obtained, or cultures only showed normal oral flora. This choice of antibiotics in our cohort may reflect institutional preference and antibiotic regimens can vary between centers. At CHM, approximately 90% of all our S. aureus isolates retained susceptibility to clindamycin during the study period.
Surgical intervention is not commonly required for the management of pediatric lung abscess. We found only 9 cases in a 10 year period that required surgical intervention while another study reported it for 21 cases over a period of 25 years.[10] There are reports giving some guidance as to when surgical intervention is necessary. One instance states that if a patient cannot provide an adequate cough and is failing medical management, surgical intervention may be necessary.[8] There are many different types of surgical intervention. Wong recommends that early ultrasound-guided percutaneous aspiration be attempted in peripherally located abscesses as this can be diagnostic and therapeutic.[17] Cuestas argues that to optimize percutaneous drainage, the abscess should be verified to be an abscess (preferably by obtaining cross-sectional imaging), and be peripherally located.[18] Previously reported risk factors that can lead to need for surgical intervention are inadequate antimicrobial coverage and anatomical malformations.[10]
As in our study, while initial concerns for lung abscess are valid, they are not always easy to detect. One patient in our study, who was excluded, was initially treated as lung abscess until he was found to have an infected cyst, and then subsequently diagnosed as teratoma in the anterior mediastinum. There are case reports of congenital cystic adenomatoid malformation presenting as a lung abscess.[19,20] In another case report, an adolescent male with a history of acute lymphoblastic leukemia was admitted for sepsis and initial radiographic studies showed features concerning for pulmonary aspergilloma. After enucleation of the mass and cavity was performed, the patient was found to have Pseudomonas sp. lung abscess.[21]
There are several limitations of this study, which include its retrospective nature, it being limited to a single center and the lack of follow-up data for some of the patients. Due to the transition from paper charts to electronic medical records at our institution in the mid-2000s and the variability in documentation, there were some difficulties in obtaining complete records for all the inpatients. Another limitation of this study is the paucity of culture data and the organisms identified. There was a wide array of antibiotic regimens used with limited number of patients per group, which limited our statistical analysis.
Conclusion
To conclude, pediatric lung abscesses are likely polymicrobial and frequently include Staphylococcus and Streptococcus spp. along with oral anaerobes. Surgical intervention is not required for majority of the cases, but the presence of prolonged fever and large abscess size may help predict the need for surgical intervention. However, optimal selection and duration of antimicrobial therapy are still not clearly defined. Larger studies are needed to address the questions of appropriate empiric antibiotic selection and duration of therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Emanuel B, Shulman ST. Lung abscess in infants and children. Clin Pediatr (Phila) 1995;34:2–6. doi: 10.1177/000992289503400101. [DOI] [PubMed] [Google Scholar]
- 2.Tan TQ, Seilheimer DK, Kaplan SL. Pediatric lung abscess: Clinical management and outcome. Pediatr Infect Dis J. 1995;14:51–5. doi: 10.1097/00006454-199501000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Puligandla PS, Laberge JM. Respiratory infections: Pneumonia, lung abscess, and empyema. Semin Pediatr Surg. 2008;17:42–52. doi: 10.1053/j.sempedsurg.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Yen CC, Tang RB, Chen SJ, Chin TW. Pediatric lung abscess: A retrospective review of 23 cases. J Microbiol Immunol Infect. 2004;37:45–9. [PubMed] [Google Scholar]
- 5.Chan PC, Huang LM, Wu PS, Chang PY, Yang TT, Lu CY, et al. Clinical management and outcome of childhood lung abscess: A 16-year experience. J Microbiol Immunol Infect. 2005;38:183–8. [PubMed] [Google Scholar]
- 6.Brook I, Finegold SM. Bacteriology and therapy of lung abscess in children. J Pediatr. 1979;94:10–2. doi: 10.1016/s0022-3476(79)80341-8. [DOI] [PubMed] [Google Scholar]
- 7.Tumwine JK. Lung abscess in children in Harare, Zimbabwe. East Afr Med J. 1992;69:547–9. [PubMed] [Google Scholar]
- 8.Kosloske AM, Ball WS, Jr, Butler C, Musemeche CA. Drainage of pediatric lung abscess by cough, catheter, or complete resection. J Pediatr Surg. 1986;21:596–600. doi: 10.1016/s0022-3468(86)80413-4. [DOI] [PubMed] [Google Scholar]
- 9.Wu MH, Tseng YL, Lin MY, Lai WW. Surgical treatment of pediatric lung abscess. Pediatr Surg Int. 1997;12:293–5. doi: 10.1007/BF01372153. [DOI] [PubMed] [Google Scholar]
- 10.Cowles RA, Lelli JL, Jr, Takayasu J, Coran AG. Lung resection in infants and children with pulmonary infections refractory to medical therapy. J Pediatr Surg. 2002;37:643–7. doi: 10.1053/jpsu.2002.31629. [DOI] [PubMed] [Google Scholar]
- 11.Lee SK, Morris RF, Cramer B. Percutaneous needle aspiration of neonatal lung abscesses. Pediatr Radiol. 1991;21:254–7. doi: 10.1007/BF02018616. [DOI] [PubMed] [Google Scholar]
- 12.Garrido-Pérez JI, Lasso-Betancor CE, Escassi-Gil Á. Thoracoscopic treatment of pediatric lung abscess. Arch Bronconeumol. 2012;48:382–3. doi: 10.1016/j.arbres.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni K, Bakshi AS, Jerath N. Bilateral lung abscesses in a 9-month-old healthy infant. BMJ Case Rep 2010. 2010 doi: 10.1136/bcr.06.2009.2046. pii: Bcr0620092046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stark DD, Federle MP, Goodman PC, Podrasky AE, Webb WR. Differentiating lung abscess and empyema: Radiography and computed tomography. AJR Am J Roentgenol. 1983;141:163–7. doi: 10.2214/ajr.141.1.163. [DOI] [PubMed] [Google Scholar]
- 15.Macdonald KS, de Carvalho VM, Liebert L, Embree JE. Streptococcus pneumoniae: A cause of primary lung abscess in a child. Can J Infect Dis. 1993;4:232–4. doi: 10.1155/1993/860767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holston AM, Miller JR. Primary lung abscess caused by multidrug-nonsusceptible Streptococcus pneumoniae in a child. Pediatr Infect Dis J. 2006;25:182–3. doi: 10.1097/01.inf.0000199320.87812.d9. [DOI] [PubMed] [Google Scholar]
- 17.Wong KS, Yeow KM, Huang YC, Lin TY. Early echo-guided percutaneous aspiration of peripheral lung abscesses in children: Report of two cases. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1997;38:145–8. [PubMed] [Google Scholar]
- 18.Cuestas RA, Kienzle GD, Armstrong JD., 2nd Percutaneous drainage of lung abscesses in infants. Pediatr Infect Dis J. 1989;8:390–2. doi: 10.1097/00006454-198906000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Kumar KJ, Anilkumar MG, Shivamurthy YL, M Kumar P. Congenital cystic adenomatoid malformation presenting as lung abscess in a child. Tuberk Toraks. 2012;60:389–92. [PubMed] [Google Scholar]
- 20.Sheu JN, Lee MT, Hsieh JC, Chang H. Lung abscess in congenital cystic adenomatoid malformation: Report of one case. Acta Paediatr Taiwan. 2001;42:162–5. [PubMed] [Google Scholar]
- 21.Dieks JK, von Bueren AO, Schaefer IM, Menke J, Lex C, Krause U, et al. Always expect the unexpected: Lung abscess due to pseudomonas aeruginosa mimicking pulmonary aspergilloma in acute B-cell leukemia. Klin Padiatr. 2013;225:347–9. doi: 10.1055/s-0033-1354390. [DOI] [PubMed] [Google Scholar]


