Abstract
OBJECTIVES:
To compare QuantiFERON-TB gold in tube (QFT-GIT) test with tuberculin skin test (TST) in detecting latent tuberculosis infection (LTBI) among a general population in Saudi Arabia.
METHODS:
A population-based cross-sectional study was conducted between July 2010 and March 2013 among individuals randomly selected from the list of those receiving care at primary healthcare centers in three provinces of Saudi Arabia; Central, Western, and Eastern provinces. Those younger than 5 years, immunocompromised, had a current or previous history of active TB, LTBI, or who were receiving anti-TB medications were excluded. Informed consent was obtained before the study questionnaire was completed. Participants were then evaluated for LTBI using QFT-GIT test followed immediately by TST.
RESULTS:
Of the 1369 subjects included in the final analysis, QFT-GIT was positive in 124 (9.1%) and TST was positive in 127 (9.3%). Positive concordance was observed in 49 (3.6%) subjects while negative concordance was observed in 1167 (85.2%) subjects. The overall agreement between the two tests was 88.8% with a significant kappa (κ) test (κ = 0.332, P < 0.001). Concordance was significantly higher in younger age, female gender, single status, students, primary education, living in middle-sized families, and never smoked.
CONCLUSIONS:
The overall agreement of TST and QFT-GIT for the detection of LTBI among a Saudi general population was 88.8%. QFT-GIT is probably comparable to TST for detecting LTBI in an intermediate TB burden country with high at birth bacille calmette guerin vaccination coverage. Further prospective studies are needed to compare the ability of both tests to predict TB disease.
Keywords: Latent mycobacterium tuberculosis infection, QuantiFERON-TB gold in tube test, Saudi Arabia, tuberculin skin test
Approximately, one-third of the world's population is infected with Mycobacterium tuberculosis as estimated by the World Health Organization.[1] While early identification and treatment of latent tuberculosis infection (LTBI) may limit further dissemination of TB, this still remains a challenge. Diagnosis of LTBI has been dependent on the tuberculin skin test (TST) for many years, and a quick and reliable alternative is needed.[2] Placing and reading the TST requires experience and a second patient visit 48–72 h after placement to determine the reading. This has made the TST a difficult and challenging diagnostic tool for identifying LTBI cases.[3] QuantiFERON-TB gold in tube (QFT-GIT), on the other hand, has been developed using TB-specific antigens performed on a single blood sample.[4,5] As of 2005, QFT-GIT has been recommended for diagnosing LTBI by the United States Centers for Disease Control and Prevention.[6] While other countries, such as UK, Canada, Spain, and Italy, have recommended a two-step approach where the TST is followed by QFT-GIT.[7,8] In Saudi Arabia, TST continues to be preferred for diagnosing LTBI. Concerns for adopting QFT-GIT in Saudi Arabia do exist because of the relative lack of comparative population-based studies between QFT-GIT and TST in Saudi Arabia or from similar countries with moderate TB endemicity and a high bacille calmette guerin (BCG) coverage rate at birth.
The objective of the current study was to compare the performance of QFT-GIT to TST in identifying LTBI among a general Saudi population.
Methods
Study setting
The study was conducted at primary healthcare centers (PHC) of the Ministry of National Guard-Health Affairs in three provinces of Saudi Arabia. The Ministry of National Guard-Health Affairs serves a population over 1 million through, five main hospitals and over thirty PHCs are available in the three provinces. Primary health care centers in Riyadh, Jeddah, and Al-Ahsa were chosen as province representative primary study sites for patient enrollment.
Design
A population-based cross sectional study was conducted between July 2010 and March 2013. Required ethical approvals were obtained from King Abdullah International Medical Research Center.
Study population
The study was done among the population served by PHCs of the Ministry of National Guard-Health Affairs. Inclusion criteria included Saudi nationalities who had been residing in the country and were available to participate in the study. Exclusion criteria included age <5-year-old (to exclude the BCG vaccination effect on the results of the study as it is given routinely at birth for all newborn in Saudi Arabia), current or previous history of TB disease, current or previous history of exposure to anti-TB medications, immunocompromised persons including leukemia, lymphoma, other cancer under chemotherapy, hemodialysis, organ transplantation, chronic steroid or immunosuppressive therapy, or HIV.
Randomization and recruitment
Subjects were randomly chosen from the lists of medical record numbers of served population in the chosen PHCs. Chosen subjects were contacted by the study coordinator. Study aim, design, and tests were explained to potential adult participants or to one of the parents of potential child participants. Participants were booked for clinical visits for evaluation, responding to the questionnaire testing after signing the informed consent. Participants were then evaluated for LTBI using QFT-GIT test followed immediately by TST.
Tuberculin skin test and blood sample collection for QuantiFERON-TB gold in tube
During the initial clinic visit, 0.1 ml of purified protein derivative test using five unit ampoules (Sanofi Pasteur limited, Toronto, Ontario, Canada) was injected intradermally into the volar aspect of the subject's forearm. The transverse induration diameter was measured no sooner than 48 h and no later than 72 h by a trained nurse. In addition, during the initial visit, 3 ml blood sample was collected from the subject by venipuncture for the QFT-GIT assay. All information on the patients were coded and available only to the principal investigator, the research coordinator, and the statistician.
Statistical analysis
All categorical variables were presented as frequencies and percentages while continuous variables were presented as means and standard deviations or median and interquartile range, as appropriate. Agreement between the results of the TST and QFT-GIT tests was assessed by using kappa (k) coefficients. To detect the association of sociodemographic characteristics of the study participants with the concordance of the results of TST and QFT-GIT tests, Chi-square or Fisher's exact test, as appropriate, were used for categorical variables. All P values were two-tailed. A P < 0.05 was considered as significant. SPSS software (release 16.0, SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
A total 1443 out of 1745 (82.7%) subjects responded and accepted to participate in the study [Table 1]. A total of 74 out of 1443 subjects (5%) were excluded from the study due to different reasons: Unavailability of QFT-GIT test results due to poor handling and or transportation of the blood sample (n = 38), indeterminate results of QFT-GIT test (n = 27), unavailability of TST readings due to “clinic no show” within 72 h (n = 5), and age <5 years (n = 4). Data of the remaining 1369 subjects were included in the final analysis. A total 763 (55.7%), 313 (22.9%), and 293 (21.4%) subjects were from the Central, Eastern, and Western Provinces, respectively. Of those, 597 (43.6%) were male and the mean age of participants was 26.3 years. More than half of the studied population was single 782 (57.6%). Other demographic details are included in Table 1.
Table 1.
Sociodemographic characteristics of 1369 study participants enrolled to compare tuberculin skin test with QuantiFERON TB gold in tube in Saudi Arabia

LTBI test was positive by TST in 127 (9.3%) subjects and by QFT-GIT in 124 (9.1%) subjects. Only 49 (3.6%) subjects were positive for both tests whereas 202 (14.8%) were positive by either test [Figure 1]. Comparing the results of the QFT-GIT with those of the TST, both tests had a significant overall agreement of 88.8% ([1167 + 49]/{1369}; κ = 0.332; 95% confidence interval = 0.23–0.43;P < 0.001). The LTBI prevalence index was 0.82 and prevalence-adjusted kappa was 0.78 (P < 0.001). Negative concordance comprised 85.2% of the results, and positive concordance comprised 3.6%. However, positive TST but negative QFT-GIT comprised 5.7% of the results, and negative TST but positive QFT-GIT comprised 5.5% [Table 2].
Figure 1.

Results of tuberculin skin test and QuantiFERON-TB Gold in tube among 1369 study participants in Saudi Arabia
Table 2.
Agreement between the results of tuberculin skin test and QuantiFERON TB Gold in tube among 1369 study participants enrolled in Saudi Arabia

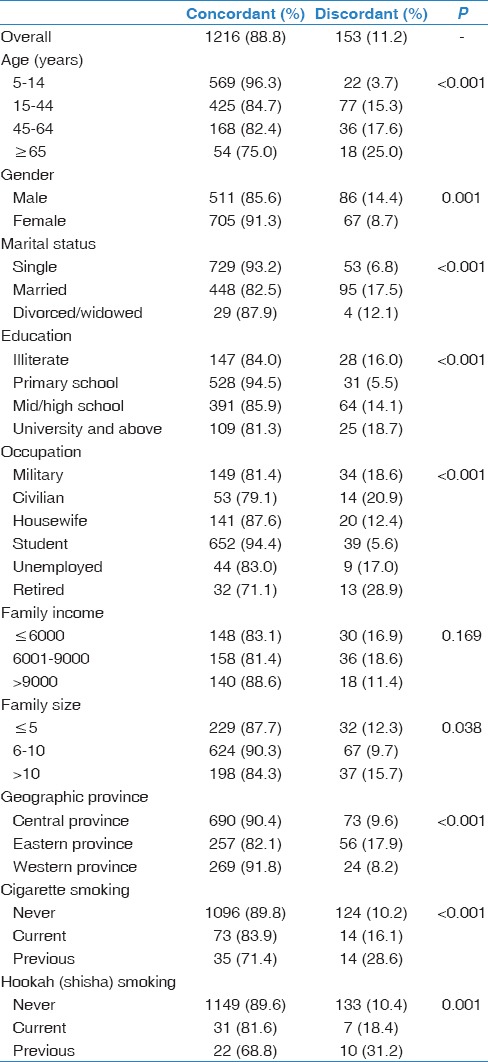
Concordance between TST and QFT-GIT tests among all study participants was 88.8%. This proportion was significantly higher among those of younger age 5–14 years (96. 3%, P < 0.001), female gender (91.3%, P < 0.05), unmarried subjects (93.2%, P < 0.001), primary school education (94.5%, P < 0.001), students (94.4%, P < 0.001), those living in Western province (91.8%, P < 0.001), and living in middle-sized (6–10) families (90.3%, P = 0.038), never smoked cigarette (89.8%, P < 0.001), and never smoked shisha (89.6%, P = 0.001). On the other hand, family income was not significantly associated with concordance (P = 0.169) [Table 3].
Table 3.
Concordance between tuberculin skin test and QuantiFERON TB Gold in tube by sociodemographic characteristics among 1369 study participants in Saudi Arabia
Discussion
In comparing QFT-GIT to TST in detecting LTBI, in a general population in Saudi Arabia, we found an agreement of 88.8% for both positive and negative concordance. Worldwide studies show a fair to good concordance from 65.4% to 92.5% among healthcare workers,[9,10,11,12] while others reported a much lower concordance rate among health care workers (HCWs).[13,14] Other studies showed total agreement of 82% among army personnel,[15]64.4% among lupus patients,[16]78.6% among hematopoietic stem cell patients,[17]85.1% among liver transplant candidates,[18]89.3% among HIV patients,[19] and 65% among hemodialysis patients.[20] In Saudi Arabia, almost all studies were among particular groups of patients or HCWs. While no studies were conducted in Saudi Arabia evaluating the agreement between the two tests among general population, a recent study among HCWs showed 73.7% overall agreement between the two tests (κ = 0.33, P < 0.01) with 60.1% negative concordance and 13.5% positive concordance.[21] Another recent study done on dialysis, patients showed 75.5% overall agreement between the two tests (κ = 0.34).[22] A third study reported overall agreement of TST and T spot QFT test OF 90.9% (κ = 0.46) among kidney donors.[23] However, the sample size of these studies was small, and the results may not be representative of a larger population.
In the current study, unadjusted kappa testing the agreement of the results of QTF-G and TST tests (considering positive TST at ≥10 ml induration size) was 0.33, which is considered fair agreement. However, we believe that the agreement in the studied population is considerably reduced by the relatively low prevalence of the disease (as indicated by a high prevalence index of 0.82). Therefore, when adjusting for the prevalence, adjusted kappa became 0.78, which is considered substantial agreement.
Despite the overall agreement of 88.8% for both positive and negative concordance, it is of concern that both tests being positive were in only 3.6% (49/1369) subjects whereas either test being positive was 14.8% (202/1369). The understanding that either test conducted alone for screening the population for LTBI will miss 5.5%, (75/1369) and 5.7%, (78/1368) for TST and QFT-GIT, respectively, is of concern. While screening a general population is rarely conducted outside national studies to identify prevalence rates, the shortcomings of using either test alone is a reasonable argument for conducting both tests simultaneously. In fact, guidelines from other countries such as the UK, Spain, Italy, and Canada have provided special scenarios in which a two-step testing is applied. For example, in the Canadian guidelines, both tests are preferred when the risk of infection or the likelihood of progression to TB disease is high.[8,24] The UK and European guidelines, on the other hand, highlight the need to use both tests, specifically in patients with HIV and a low CD4 count or any other condition leading to significant immunocompromise.[25,26,27] In all guidelines, however, it is clear that neither the TST nor the QFT-GIT should be used for the diagnosis of active TB disease for any age group.
In Saudi Arabia, as in many countries around the world, the BCG vaccine is administered at birth. However, it was found that the BCG vaccine may produce a temporary positive TST result that declines with age.[28,29,30] The timing between the vaccination and TST leading to such false positive TST result has not been clearly determined. For that reason, we excluded in our study children below the age of 5 years. On the hand, while the QFT-GIT test is not expected to be affected by the BCG vaccine, there is no clear data on the efficacy of the GFT-GIT tests for diagnosing LTBI in children. To date, using either test for diagnosing LTBI should be according to national guidelines, if available, and expert opinion.
The major strength of our study is the stratified random sampling technique used in recruitment, the large number of study subjects, and the inclusion of three geographic provinces in Saudi Arabia. Our limitations are that we did not plan to follow individuals with a positive TST or QFT-GIT for the development of TB disease and the fact that we excluded children below the age of 5 years. Both limitations will be addressed in a future study.
Conclusions
The overall agreement of TST and QFT-GIT for the detection of LTBI among a Saudi general population was 88.8%. QFT-GIT is probably comparable to TST for detecting LTBI in an intermediate TB burden country with high at birth bacille calmette guerin vaccination coverage. Further prospective studies are needed to compare the ability of both tests to predict TB disease.
Financial support and sponsorship
King Abdullah International Medical Research Center kindly funded this project through grant number RC-09-093, HB is the PI.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We greatly appreciate our colleagues who contributed in data collection, TST placement and reading, and blood collection: Samar Sobhy, Mashael Alonizy, Tahani Ahmad, Elizabeth Alba, Janet Evangelio, Mohamed Al Qassabi, Hesham Micky, and Jehad Al Hassan.
References
- 1.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: Global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–21. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 2.Arend SM, Engelhard AC, Groot G, de Boer K, Andersen P, Ottenhoff TH, et al. Tuberculin skin testing compared with T-cell responses to Mycobacterium tuberculosis-specific and nonspecific antigens for detection of latent infection in persons with recent tuberculosis contact. Clin Diagn Lab Immunol. 2001;8:1089–96. doi: 10.1128/CDLI.8.6.1089-1096.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taggart EW, Hill HR, Ruegner RG, Martins TB, Litwin CM. Evaluation of an in vitro assay for gamma interferon production in response to Mycobacterium tuberculosis infections. Clin Diagn Lab Immunol. 2004;11:1089–93. doi: 10.1128/CDLI.11.6.1089-1093.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356:1099–104. doi: 10.1016/s0140-6736(00)02742-2. [DOI] [PubMed] [Google Scholar]
- 5.Pai M, Riley LW, Colford JM., Jr Interferon-gamma assays in the immunodiagnosis of tuberculosis: A systematic review. Lancet Infect Dis. 2004;4:761–76. doi: 10.1016/S1473-3099(04)01206-X. [DOI] [PubMed] [Google Scholar]
- 6.Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49–55. [PubMed] [Google Scholar]
- 7.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection – United States, 2010. MMWR Recomm Rep. 2010;59:1–25. [PubMed] [Google Scholar]
- 8.Canadian Tuberculosis Committee (CTC). Updated recommendations on interferon gamma release assays for latent tuberculosis infection. An Advisory Committee Statement (ACS) Can Commun Dis Rep. 2008;34:1–13. [PubMed] [Google Scholar]
- 9.Caglayan V, Ak O, Dabak G, Damadoglu E, Ketenci B, Ozdemir M, et al. Comparison of tuberculin skin testing and QuantiFERON-TB Gold-In Tube test in health care workers. Tuberk Toraks. 2011;59:43–7. [PubMed] [Google Scholar]
- 10.Pai M, Gokhale K, Joshi R, Dogra S, Kalantri S, Mendiratta DK, et al. Mycobacterium tuberculosis infection in health care workers in rural India: Comparison of a whole-blood interferon gamma assay with tuberculin skin testing. JAMA. 2005;293:2746–55. doi: 10.1001/jama.293.22.2746. [DOI] [PubMed] [Google Scholar]
- 11.Khoury NZ, Binnicker MJ, Wengenack NL, Aksamit TR, Buchta WG, Molella RG. Preemployment screening for tuberculosis in a large health care setting: Comparison of the tuberculin skin test and a whole-blood interferon-gamma release assay. J Occup Environ Med. 2011;53:290–3. doi: 10.1097/JOM.0b013e31820c91c5. [DOI] [PubMed] [Google Scholar]
- 12.Zhao X, Mazlagic D, Flynn EA, Hernandez H, Abbott CL. Is the QuantiFERON-TB blood assay a good replacement for the tuberculin skin test in tuberculosis screening. A pilot study at Berkshire Medical Center? Am J Clin Pathol. 2009;132:678–86. doi: 10.1309/AJCPUHC34NBDGKKL. [DOI] [PubMed] [Google Scholar]
- 13.Jong Lee K, Ae Kang Y, Mi Kim Y, Cho SN, Wook Moon J, Suk Park M, et al. Screening for latent tuberculosis infection in South Korean healthcare workers using a tuberculin skin test and whole blood interferon-gamma assay. Scand J Infect Dis. 2010;42:672–8. doi: 10.3109/00365548.2010.485575. [DOI] [PubMed] [Google Scholar]
- 14.Talebi-Taher M, Javad-Moosavi SA, Entezari AH, Shekarabi M, Parhizkar B. Comparing the performance of QuantiFERON-TB Gold and Mantoux test in detecting latent tuberculosis infection among Iranian health care workers. Int J Occup Med Environ Health. 2011;24:359–66. doi: 10.2478/s13382-011-0046-7. [DOI] [PubMed] [Google Scholar]
- 15.Franken WP, Timmermans JF, Prins C, Slootman EJ, Dreverman J, Bruins H, et al. Comparison of Mantoux and QuantiFERON TB Gold tests for diagnosis of latent tuberculosis infection in Army personnel. Clin Vaccine Immunol. 2007;14:477–80. doi: 10.1128/CVI.00463-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yilmaz N, Zehra Aydin S, Inanc N, Karakurt S, Direskeneli H, Yavuz S. Comparison of QuantiFERON-TB Gold test and tuberculin skin test for the identification of latent Mycobacterium tuberculosis infection in lupus patients. Lupus. 2012;21:491–5. doi: 10.1177/0961203311430700. [DOI] [PubMed] [Google Scholar]
- 17.Moon SM, Lee SO, Choi SH, Kim YS, Woo JH, Yoon DH, et al. Comparison of the QuantiFERON-TB Gold In-Tube test with the tuberculin skin test for detecting latent tuberculosis infection prior to hematopoietic stem cell transplantation. Transpl Infect Dis. 2013;15:104–9. doi: 10.1111/j.1399-3062.2012.00765.x. [DOI] [PubMed] [Google Scholar]
- 18.Manuel O, Humar A, Preiksaitis J, Doucette K, Shokoples S, Peleg AY, et al. Comparison of quantiferon-TB gold with tuberculin skin test for detecting latent tuberculosis infection prior to liver transplantation. Am J Transplant. 2007;7:2797–801. doi: 10.1111/j.1600-6143.2007.02011.x. [DOI] [PubMed] [Google Scholar]
- 19.Luetkemeyer AF, Charlebois ED, Flores LL, Bangsberg DR, Deeks SG, Martin JN, et al. Comparison of an interferon-gamma release assay with tuberculin skin testing in HIV-infected individuals. Am J Respir Crit Care Med. 2007;175:737–42. doi: 10.1164/rccm.200608-1088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seyhan EC, Sökücü S, Altin S, Günlüoglu G, Trablus S, Yilmaz D, et al. Comparison of the QuantiFERON-TB Gold In-Tube test with the tuberculin skin test for detecting latent tuberculosis infection in hemodialysis patients. Transpl Infect Dis. 2010;12:98–105. doi: 10.1111/j.1399-3062.2009.00469.x. [DOI] [PubMed] [Google Scholar]
- 21.El-Helaly M, Khan W, El-Saed A, Balkhy HH. Pre-employment screening of latent tuberculosis infection among healthcare workers using tuberculin skin test and QuantiFERON-TB Gold test at a tertiary care hospital in Saudi Arabia. J Infect Public Health. 2014;7:481–8. doi: 10.1016/j.jiph.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Al Jahdali H, Ahmed AE, Balkhy HH, Baharoon S, Al Hejaili FF, Hajeer A, et al. Comparison of the tuberculin skin test and Quanti-FERON-TB Gold In-Tube (QFT-G) test for the diagnosis of latent tuberculosis infection in dialysis patients. J Infect Public Health. 2013;6:166–72. doi: 10.1016/j.jiph.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Hassan H, Shorman M, Housawi A, Elsammak M. Detecting latent tuberculosis infection prior to kidney transplantation in a tertiary hospital in Saudi Arabia: Comparison of the T-SPOT. TB test and tuberculin test. Br Microbiol Res J. 2013;3:116–27. [Google Scholar]
- 24.Pai M, Kunimoto D, Jamieson F, Menzies D. Diagnosis of latent tuberculosis infection. Canadian Tb standards. Can Respir J. 2013;20(Suppl A):23A. [Google Scholar]
- 25.National Institute for and H Clinical Excellence. Clinical Guideline 33. Tuberculosis: Clinical Diagnosis and Management of Tuberculosis and Measures for its Prevention and Control, 2006. London: NICE; 2006. [Last accessed on 2015 Jan 01]. Avaialble from: http://www.nice.org.uk/page.aspx?o=CG033NICEguideline . [PubMed] [Google Scholar]
- 26.National Institute for Health and Excellence C. Clinical Gridline 17. Tuberculosis: Clinical Diagnosis and Management of Tuberculosis and Measures for its Prevention and Control. London: NICE; 2011. [Last accessed on 2015 Jan 01]. Available from: http://www.nice.org.uk/nicemedia/live . [PubMed] [Google Scholar]
- 27.European Center for Disease Prevention and Control. Use of Interferon.Gamma Release Assays in Support of TB Diagnosis. Stockholm: European Center for Disease Prevention and Control. 2011. [Last accessed on 2015 Jan 01]. Available from: http://www.ecdc.europa.edu/en/publications .
- 28.Chadha VK, Jagannatha PS, Kumar P. Can BCG-vaccinated children be included in tuberculin surveys to estimate the annual risk of tuberculous infection in India? Int J Tuberc Lung Dis. 2004;8:1437–42. [PubMed] [Google Scholar]
- 29.Hill PC, Brookes RH, Fox A, Fielding K, Jeffries DJ, Jackson-Sillah D, et al. Large-scale evaluation of enzyme-linked immunospot assay and skin test for diagnosis of Mycobacterium tuberculosis infection against a gradient of exposure in the Gambia. Clin Infect Dis. 2004;38:966–73. doi: 10.1086/382362. [DOI] [PubMed] [Google Scholar]
- 30.Guwatudde D, Nakakeeto M, Jones-Lopez EC, Maganda A, Chiunda A, Mugerwa RD, et al. Tuberculosis in household contacts of infectious cases in Kampala, Uganda. Am J Epidemiol. 2003;158:887–98. doi: 10.1093/aje/kwg227. [DOI] [PMC free article] [PubMed] [Google Scholar]


