Abstract
BACKGROUND:
Middle East Respiratory Syndrome (MERS) is a disease of the lower respiratory tract and is characterized by high mortality. It is caused by a beta coronavirus (CoV) referred to as MERS-CoV. Majority of MERS-CoV cases have been reported from Saudi Arabia.
AIM:
We investigated the human leukocyte antigen (HLA) Class II alleles in patients with severe MERS who were admitted in our Intensive Care Unit.
METHODS:
A total of 23 Saudi patients with severe MERS-CoV infection were typed for HLA class II, results were compared with those of 161 healthy controls.
RESULTS:
Two HLA class II alleles were associated with the disease; HLA-DRB1*11:01 and DQB1*02:02, but not with the disease outcome.
CONCLUSIONS:
Our results suggest that the HLA-DRB1*11:01 and DQB1*02:02 may be associated with susceptibility to MERS.
Keywords: Human leukocyte Antigen Class II, Middle East respiratory syndrome-coronavirus, Saudi Arabia
Middle East respiratory syndrome (MERS) is caused by a novel coronavirus (MERS-CoV).[1] In Saudi Arabia, Zaki et al. reported the first case of MERS presenting with acute pneumonia that subsequently lead to renal failure and fatal outcome.[1] This was followed by multiple outbreaks although so far the majority of the reported cases are from Saudi Arabia.[2] MERS presents as acute respiratory syndrome, but majority of cases suffer from shock, acute kidney injury, and thrombocytopenia which lead to high morbidity.[3] It is unclear whether the clustering of cases in Saudi Arabia is related to host genetic predisposition such as the human leukocyte antigen (HLA).
HLA Class I and II genes encode protein receptors that orchestrate the immune response by presenting foreign or modified self-antigens to T-cells.[4] MERS-CoV is closely related to severe acute respiratory syndrome coronavirus (SARS-CoV). Studies on the HLA associations in SARS showed conflicting results. Xiong et al. showed no HLA allele association in 95 Chinese SARS patients,[5] whereas Lin et al. showed that severe SARS was associated with HLA-B*46:01 in Taiwanese patients.[6]
To date, it remains unclear why most cases have been reported in Saudi Arabia although evidence for infection among camels have been demonstrated in a much geographically dispersed area including East Africa and Spain.[7] In addition, it remains unclear why some patients develop severe MERS-CoV illness with high mortality.
In this study, we recruited MERS patients admitted to Intensive Care Unit (ICU) at King Abdulaziz Medical City to study the association between HLA Class II alleles and severe MERS disease.
Methods
This study comprised 23 consecutive Saudi MERS patients admitted to ICU at King Abdulaziz Medical City, Riyadh. All patients confirmed to have laboratory-confirmed MERS-CoV infection by real-time reverse transcription-polymerase chain reaction of nasopharyngeal swabs or tracheal aspirates (TIB Molbiol GMbH, Berlin, Germany). The study was approved by the local Institutional Review Board Committee and written informed consent was obtained from the patient or next of kin.
Control group
A group of 161 healthy individuals were available for comparison. All were Saudi with a mean age of 36 years and almost equal distribution of male and female gender.
Human leukocyte antigen-typing
HLA typing was carried out using high definition kits LABType® SSO HD (One Lambda Inc., Canoga Park, CA, USA). The HLA typing was carried out according to the manufacturers’ instructions. Briefly, the HLA typing procedure comprised DNA extraction, amplification, hybridization, reading on a Luminex machine (LABScan™ 100, One Lambda, Canoga Park, CA, USA), and interpretation was carried out using HLA Fusion™ software (One Lambda, Canoga Park, CA, USA).
Statistical analysis
Statistical analysis was carried out using STATA 12.0 software (Stata Corporation, College Station, TX, USA). Differences in the HLA-DRB1 and HLA-DQB1 allele frequencies between two groups were compared using Fisher's exact test separately for all alleles and P < 0.05 was considered to be statistically significant, after Bonferroni correction for multiple testing. Odds ratio (OR) and 95% confidence interval (CI) were calculated using 2 × 2 tables.
Results
As can be seen from Table 1, most patients were male and old with very high mortality. The comparison of HLA-DRB1 results between cases and controls revealed one association. HLA-DRB1*11:01 carried a significant association with severe MERS in this Saudi cohort (OR = 6.11, 95% CI 1.36-24.76, P = 0.0016, P c = 0.022) [Table 2]. HLA-DRB1*04:03, 04:05, and 13:02 alleles were overrepresented in the MERS cases but did not reach statistical significance. Table 3 demonstrates the comparison between the cases and controls for the HLA-DQB1 alleles. HLA-DQB1*02:02 showed positive association with severe MERS infection (OR = 2.64, 95% CI 0.99-7.10, P = 0.027, P c = 0.27) but this association did not reach significance after correcting for multiple allele testing [Table 3]. HLA-DQB1*02:01, 05:01, and 05:02 allele frequencies were reduced in the cases compared to controls but did not reach statistical significance, whereas HLA-DQB1*03:01, 03:02, 06:02, and 06:04 were raised in the cases compared to controls but again did not reach statistical significance [Table 3].
Table 1.
Demographic characteristics of Middle East respiratory syndrome patients

Table 2.
Distribution of HLA-DRB1 alleles in Middle East respiratory syndrome-coronavirus patients and controls*
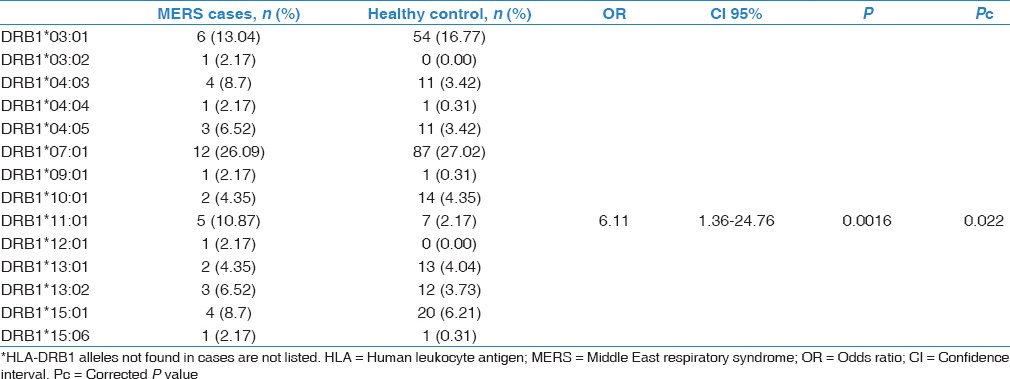
Table 3.
Distribution of HLA-DQB1 alleles in Middle East respiratory syndrome-coronavirus patients and controls*

Discussion
Until today, the vast majority of MERS-CoV cases came from Saudi Arabia.[2] The disease is so severe affecting mainly elderly people with comorbidity. Arabs have HLA allele and haplotype distribution that is different from other ethnicities, for example, the HLA-A2, B50, DR7 alleles and haplotype are found mainly in Arabs.[8] Up to our knowledge, this is the first report on HLA associations with MERS infection. Our results demonstrate the association of severe MERS with HLA Class II alleles, namely DRB1*11:01 and DQB1*02:02. These alleles are also common in the Korean population[9] where the second biggest outbreak of MERS infection occurred.[10]
Multiple logistic regression analysis revealed no association between any of the HLA alleles and 28-day mortality (data not shown).
Conflicting results on HLA association were reported in SARS infection; a closely related emerging corona virus. While Xiong et al. and Ng et al.[5,11] reported no HLA associations, others showed association with HLA-A*46:01.[6]
Conclusion
Here, we show association between severe MERS infection and HLA Class II alleles. Our sample size is small and this is one of the major limitations of this study. Studies with larger sample size are needed to evaluate these associations.
Financial support and sponsorship
This work was sponsored by a grant from KAIMRC.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. Musharaf Sadat for her help in collecting the demographic data of the patients.
References
- 1.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–20. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.Al-Tawfiq JA, Memish ZA. Middle East respiratory syndrome coronavirus: Epidemiology and disease control measures. Infect Drug Resist. 2014;7:281–7. doi: 10.2147/IDR.S51283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arabi YM, Arifi AA, Balkhy HH, Najm H, Aldawood AS, Ghabashi A, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389–97. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 4.Klein J, Sato A. The HLA system. First of two parts. N Engl J Med. 2000;343:702–9. doi: 10.1056/NEJM200009073431006. [DOI] [PubMed] [Google Scholar]
- 5.Xiong P, Zeng X, Song MS, Jia SW, Zhong MH, Xiao LL, et al. Lack of association between HLA-A,-B and-DRB1 alleles and the development of SARS: A cohort of 95 SARS-recovered individuals in a population of Guangdong, Southern China. Int J Immunogenet. 2008;35:69–74. doi: 10.1111/j.1744-313X.2007.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin M, Tseng HK, Trejaut JA, Lee HL, Loo JH, Chu CC, et al. Association of HLA class I with severe acute respiratory syndrome coronavirus infection. BMC Med Genet. 2003;4:9. doi: 10.1186/1471-2350-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chastel C. Middle East respiratory syndrome (MERS): Bats or dromedary, which of them is responsible? Bull Soc Pathol Exot. 2014;107:69–73. doi: 10.1007/s13149-014-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajeer AH, Al Balwi MA, Aytül Uyar F, Alhaidan Y, Alabdulrahman A, Al Abdulkareem I, et al. HLA-A,-B,-C,-DRB1 and-DQB1 allele and haplotype frequencies in Saudis using next generation sequencing technique. Tissue Antigens. 2013;82:252–8. doi: 10.1111/tan.12200. [DOI] [PubMed] [Google Scholar]
- 9.In JW, Roh EY, Oh S, Shin S, Park KU, Song EY. Allele and haplotype frequencies of human leukocyte antigen-A,-B,-C,-DRB1, and-DQB1 from sequence-based DNA typing data in koreans. Ann Lab Med. 2015;35:429–35. doi: 10.3343/alm.2015.35.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowling BJ, Park M, Fang VJ, Wu P, Leung GM, Wu JT. Preliminary epidemiological assessment of MERS-CoV outbreak in South Korea, May to June 2015. Euro Surveill. 2015;20:7–13. doi: 10.2807/1560-7917.es2015.20.25.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng MH, Cheng SH, Lau KM, Leung GM, Khoo US, Zee BC, et al. Immunogenetics in SARS: A case-control study. Hong Kong Med J. 2010;16(5 Suppl 4):29–33. [PubMed] [Google Scholar]


