Abstract
Objective:
Malaria rapid diagnostic test (MRDT) provides a good alternative to malaria microscopy diagnosis, particularly in resource-constrained settings. This study therefore evaluated MRDT in private retail pharmacies (PRPs) as a critical step in community case malaria management.
Methods:
In a prospective, cross-over, validation survey at six PRPs in the Ashanti Region of Ghana, 1200 patients presenting with fever in the preceding 48 h were sampled. Fingerstick blood samples were collected for preparation of thick and thin blood films for malaria microscopy. Categorized patients (600 each) went through the processes of MRDT or presumptive diagnosis (PD) of malaria. The malaria disease prevalence of the study area was established. Selectivity (Se), specificity (Sp), positive predictive value (PPV) along with false discovery rate (FDR), and negative predictive value (NPV) along with the false omission rate (FOR), and diagnostic odds ratio (DOR) of MRDT were then calculated.
Findings:
While 43.0% tested positive using the MRDT, 57.0% tested negative. However, 62.0% MRDT-negative patients in addition to all the MRDT positives were given artemether-lumefantrine. Of those diagnosed by PD, 98.2% were prescribed with an antimalarial (microscopy however confirmed only 70.3% as positive). Se and Sp of the MRDT were 90.68 ± 11.18% and 98.68 ± 1.19%, respectively. Malaria prevalence was estimated to be 43.3%. PPV was 98.0%, FDR was 2.0%, NPV was 98.0%, FOR was 2.0%, and DOR was 2366.43.
Conclusion:
Results highlighted good performance of MRDTs at PRPs which could inform decision toward its implementation.
Keywords: Diagnostic odds ratio, fingerstick blood samples, Malaria, positive predictive value
INTRODUCTION
Malaria remains an important public health challenge worldwide, particularly in Sub-Saharan Africa.[1] It presents a huge economic burden on society, especially in developing countries where accessibility to effective malaria diagnosis is limited. Among the numerous presentations of malaria, fever is the most common. Although innovative methods have been used to increase access to the most effective antimalarial drugs in the recent past, these efforts will be incomplete and unsustainable without similar efforts to incorporate accurate and prompt diagnosis of malaria into private retail pharmacies (PRPs) which remain the first point of call for patients seeking malaria treatment.[2] Often times, diagnosis of malaria has been centered on presenting symptoms only (presumptive diagnosis [PD]), without parasitological confirmation. In recent times, however, the WHO policy guideline on malaria has been mandatory parasitological testing,[3] with the introduction of accessible, easy to use, and affordable malaria rapid diagnostic test (MRDT) kits.
MRDT provides a good alternative to microscopy, particularly in resource-constrained settings and has the added advantage of providing prompt and accurate results.[4] The test which has a sensitivity of 82–97%[5] and an accuracy of 98–100% is used to diagnose Plasmodium falciparum and other forms of plasmodial infections. MRDT strips detect histidine-rich protein 2 (HRP-2), Plasmodium aldolase, or parasitic specific lactate dehydrogenase by immunochromatographic assay, with monoclonal antibodies directed against the target parasite antigen impregnated on a test strip.[6] MRDT is cost-effective, simple to perform, easy to interpret, and easy to transport and is recommended for situations exceeding microscopic capability such as an outbreak.[7]
The health care practitioner's perspective on the introduction of MRDT at registered retail pharmacies in Ghana indicated willingness to implement the policy as PD was the practice.[8] Another study purported to examine the diagnostic procedure of uncomplicated malaria and patients’ understanding and satisfaction of treatment at community health care facilities in Ghana, indicated that not a single PRP used MRDT for diagnosis 3 years after the deployment of MRDT, with the unscientifically proven excuse that results were mainly false negatives (FNs).[8] Nontesting before treatment often results in extensive overuse of antimalarial drugs,[9] which has economic implications and may even border on safety as far as the individual is concerned. The questions therefore were as follows: Is the MRDT effective in diagnosing malaria? What are the strengths and weaknesses of the MRDT? This study therefore was conducted to evaluate MRDTs at PRPs in Kumasi and some districts in the Ashanti Region of Ghana.
METHODS
The study was a prospective, longitudinal, and cross-over validation survey conducted in Kumasi, Ejisu, and Asokore Mampong Districts of the Ashanti Region of Ghana from April to September 2014. These areas were selected to have fair representations of the pharmaceutical practice in the district, municipal, and metropolitan administrative segments. By this segmentation, it would be easier to advise policy makers as regards to the management of malaria in the retail pharmacies within those jurisdictions. A purposive, convenient, and random sampling method was applied with the help of the Ashanti Regional Pharmacy Council Secretariat (ARPCS), to select six PRPs within the study area.
The factors considered in the selection processes were geographical range and logistic feasibility in reaching a standard laboratory facility, adequate facility utilization rates (to achieve the desired sample size), and representation of areas of ARPCS supervision. In addition to the aforementioned factors, the PRP should have: been operating for at least 5 years, a superintendent pharmacist, and dispensing technologist with a minimum of 5 years working experience, already been using MRDT or health care providers were willing to incorporate it into their activities, a minimum of 8 working hours, especially between 7.00 a.m. to 10.00 p.m. every day of the week, and an adequate stock level of diagnostic kits.
A cross-over validation period of 2 weeks was designed to evaluate PD and MRDT. Practitioners in the selected PRPs were trained on the technique and usage of the MRDT kit (which uses the HRP-2 detection system for P. falciparum), approved by the National Malaria Control Programme, in accordance with the WHO quality control standards. The trained staff was assigned the role of daily consultations with the eligible patients. A total of 1200 patients, who presented with fever (axillary temperature ≥37.5°C) or a history of fever in the preceding 48 h, were selected. This sample size was calculated based on expectation that approximately 34% of patients with clinical diagnosis of malaria would be parasitemic and MRDT-positive.[10] This sample size was also sufficient to evaluate other health facility survey variables. The patients were categorized into those on which the MRDT was to be performed and those for PD. Effectively 200 patients per PRP were sampled; 100 each for MRDT and PD. Fingerstick blood samples were collected to prepare thick and thin blood films for malaria microscopy (Gold standard) for both MRDT and PD categorized patients. Patients for MRDT then went through the test processes while those for PD also went ahead for their medical attention. Data obtained was recorded on a standardized case reporting form (CRF). The CRFs were studied daily by the principal researcher. Pregnant women, persons presenting with extremely elevated body temperature, chills, and rigor, and those refusing or unable to provide informed consent were excluded from the study.
For uniformity, the malaria P. falciparum/Plasmodium vivax rapid test device, “Blue Aid Malaria Test Kit” (Core Technology Co., Ltd., Beijing, China) was used. The kit which was within the recommended shelve life was stored at 19–30°C (within the recommended temperature). MRDT was performed and the results interpreted as per the manufacturer instruction. Results were recorded and classified as true positive (TP), true negative (TN), false positive (FP), or FN. A TP or TN is the event where the MRDT makes a positive or negative prediction, and the subject also obtains a positive or negative result under the gold standard, while a FP or FN is the event where the MRDT makes a positive or negative prediction, and the subject has the opposite result under the gold standard. The test's selectivity (Se), specificity (Sp), positive predictive value (PPV) along with false discovery rate (FDR), and negative predictive value (NPV) along with the false omission rate (FOR) were then calculated. The diagnostic odds ratio (DOR) was computed and employed to measure the effectiveness of the diagnostic test. Standard malaria disease prevalence (MDP) of the study area was determined based on TP and FN in relation to the total study population. For each value, a 95% confidence interval (95% CI) was used.
Formulae for the calculation of the parameters mentioned above are as follows:
MDP = Tdx/Tsp × 100: where Tdx = number of individuals with disease; Tsp = total study population
Se = TP/(TP + FN) × 100
Sp = TN/(TN + FP) × 100
PPV = TP/(TP + FP) = TP/number of positive calls
FDR = (1 − PPV) or FP/(TP + FP)
NPV = TN/(TN + FN) = TP/number of negative calls
FOR = (1 − NPV) or FN/(TN + FN)
DOR = Se × Sp/([1 − Se] × [1 − Sp]).
Microscopy examination was used as the “gold standard” for confirmation of diagnoses obtained from MRDT and PD. This was done, according to the WHO standard protocol, in an agreed accredited medical laboratory organized by research assistants. The blood films were stained (within 3 days of collection) for 30 min with 3% Giemsa. The films were examined independently by two experienced laboratory technicians. For the thick film examination, parasites were counted against 200–500 white blood cells by microscopists who were blinded to the MRDT results and the readings of the other microscopists. Reading of the smear was done using a Leica DM750 light microscope (Leica Microsystems CM5 GmbH, Wetzlar, Germany) under oil immersion (×1000 magnification). Smears were considered negative if the examination did not reveal any parasites. The discordant results were checked and confirmed by a senior technician.
RESULTS
Of the 600 patients subjected to MRDT, 258 (43%) tested positive while 342 tested negative. All the 258 (43%) participants who tested positive to the MRDT were given artemisinin-based combination therapy consisting of artemether-lumefantrine. A total of 212 (62.0%) of the 342 (57.0%) MRDT negative patients were also given this antimalarial [Table 1]. However, the remaining 130 (38.0%) of MRDT negative patients were not prescribed with antimalarial therapy. Of the 600 patients who were diagnosed using PD, 98.2% were also given artemether-lumefantrine. However, microscopy examination confirmed that only 70.3% were positive while 29.7% were negative [Table 2]. The test's Se and Sp for the MRDT were 90.68 ± 11.18% and 98.68 ± 1.19%, respectively. For instance, all the MRDT positives recorded in patients ≤5-year-old maintained their status as positive on comparison of their malaria status using the microscopy method; TP is therefore 100%. A total of 16 (94.1%) MRDT negatives within the same age limit remained negative after microscopy (TN). Thus, Se and Sp were 75% and 100%, respectively. Similarly, in patients between 11 and 15 years, 94 of the 95 MRDT positive results were TP (i.e., 98.9%) after microscopy while 84 of the 85 MRDT negatives were TN (i.e., 98.8%) [Table 3]. Malaria prevalence in the study area was estimated to be 43.3%, i.e., Tdx = TP + FN = 260, and Tsp = 600. Therefore, prevalence of disease = 260/600 × 100 = 43.3%. Based on the Se and Sp of the MRDT kit and the disease prevalence, the performance characteristics of the kit were ascertained. Predictive values and DOR which are the basis for assessing the performance of MRDT were estimated (as shown below) with results confirmed by microscopy.
Table 1.
Diagnosis using malaria rapid diagnostic test and artemisinin-based combination therapy treatment given for 600 patients of all ages
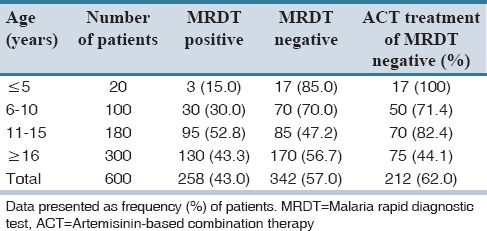
Table 2.
Presumptive diagnosis, microscopic diagnosis, and antimalarial treatment
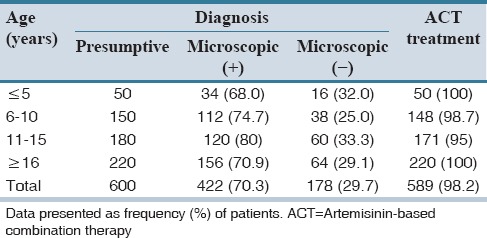
Table 3.
Comparing the results for 258 malaria rapid diagnostic test positive patients with their microscopy results to determine “selectivity” and “specificity”
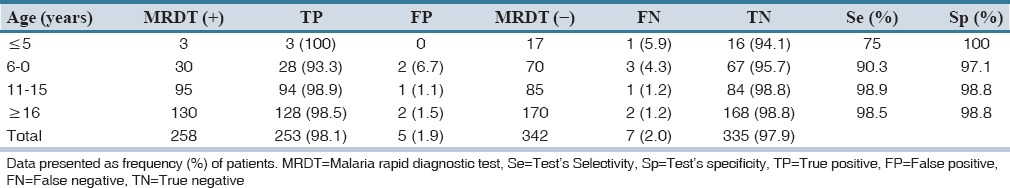
Se = TP/(TP + FN) × 100 = 253/(253 + 7) × 100 = 97.3%
Sp = TN/(TN + FP) × 100 = 335/(335 + 5) × 100 = 98.5%
PPV = TP/(TP + FP) =253/(253 + 5) = 0.98. Therefore, at 95% CI, PPV = 0.98 × 100 = 98.0%
FDR = 1 − PPV = 1 − 0.98 = 0.02; or FDR = FP/(TP + FP) = FP/number of positive calls = 5/258 = 0.019 = 0.02. Therefore at 95% CI, FDR = 2.0%
NPV = TN/(TN + FN) = TN/number of negative calls = 335/(335 + 7) = 0.98. Therefore, at 95% CI, NPV = 0.98 × 100 = 98.0%
FOR = 1 − NPV = 1 − 0.98 = 0.02; or FOR = FN/(TN + FN) = 7/(340 + 7) = 0.02. Therefore at 95% CI: FOR = 0.02 × 100 = 2.0%
DOR = Se × Sp/([1 − Se] × [1 − Sp]) = 0.973 × 0.985/([1 − 0.973] × [1 − 985]) = 0.958405/0.000405 = 2366.43.
DISCUSSION
This study is the first one to be conducted in PRPs in Ghana. It was designed to evaluate the diagnostic performance of MRDT, a few years after its introduction, as a rapid diagnostic tool for malaria. It was undertaken as a follow-up to a study by the same authors, which established a link between the poor usage of MRDT and practitioners’ poor perception of the Sp and sensitivity of the kit. In this study, therefore, Se, Sp, PPV along with FDR, and NPV along with the FOR, and DOR of MRDT were evaluated.
The performance of any diagnostic procedure in health care delivery is reflected in its sensitivity and Sp. This is very important for MRDT because patients who test negative, including FNs, PRPs may not always go to a health facility for additional care.[11] In this study, sensitivity of the MRDT was 90.68 ± 11.18% which is within that of the manufacturer (91.3%) range and the 95% recommendation by WHO. Sp (the fraction of those without disease who will have negative test results) was 98.5% which was almost concordant with that by the manufacturer (98.6%) and that quoted in other studies.[12] The inability of the MRDT to sometimes detect high parasitemia had been observed previously.[13] This, however, is a very rare event and most likely to be due to the presence of a mutation or deletion within the HRP-2 and Plasmodium lactate dehydrogenase genes.[14] This may explain a lower Sp when individuals received previous antimalarial treatment, or when individuals have a higher immunity against malaria.[15] Other studies have also been cited for high Sp.[16] Varying levels of Sp which could be a potential threat to the success of treatment have however been reported as it could lead to misdiagnoses of other febrile illnesses.[17] It is a fact that comparative assessment is difficult because trials do not share common guidelines. Clinical and epidemiologic characteristics of the study populations (especially the parasitemia level) vary; reference standards are different and products of different batches may differ in quality, or may have been compromised probably due to inappropriate storage condition, for example, extreme temperatures. The errors in measuring the Se and Sp of a test will arise if the “gold standard” test itself does not have 100% Se and Sp, which is frequently the case.
In this study, PPV and NPV were 98.0% each while FDR and FOR were 2.0% each. These are indication of good performance of the MRDT. The PPV and NPV are the proportions of positive and negative results in statistics and diagnostic tests that are TP and TN results.[18] Moreover, they describe the performance of the diagnostic test; therefore, high results indicate the accuracy of the research findings. The PPV and NPV are not intrinsic to the test – it depends also on the disease prevalence which in this study was determined as 43.3%. FDR and FOR procedures were designed to complement PPV and NPV, respectively; hence, the lower the FDR and FOR values, the better the performance of the diagnostic test. DOR, a measure of the effectiveness of a diagnostic test,[19] was established as 2366.43. The rationale for establishing the DOR is that it is a single indicator of test performance, but is independent of prevalence and is presented as odds ratio. It ranges from zero to infinity; higher DORs are indicative of better test performance.
All patients who were MRDT-positive together with a vast majority of MRDT negative patients were given artemether-lumefantrine, contrary to the guidelines. Studies have demonstrated more variable adherence level because health care providers often rely on clinical judgment instead of MRDT results,[20] and the fear of FN results may tempt them to treat negative patients, especially in the case of subjects with no or little malaria immunity. Often, the irrational use of tests and drugs is based on perceived shortcomings of the tests. A common concern among health staff (which is likely to apply to workers in PRPs) is that negative tests do not definitively rule out malaria, but trials that withheld antimalarials in febrile children with negative test results have shown no additional malaria risk to patients in moderate-to-high transmission settings.[20] Support for adhering to guidelines for negative results will therefore be required. All the PD patients were also given antimalarial treatment, but microscopic examination confirmed that 29.7% were not Plasmodium-infected patients. This is an indication that treatment decisions based on clinical signs and symptoms are neither sensitive nor specific. Both situations result in extensive overuse of antimalarial drugs[9] which could lead to developing resistant plasmodia strains. There is wastage of antimalarials and unnecessary expenditure to budgetary allocation of the NHIS.
Despite limitations of the currently available evidence, the results of the survey highlighted good performance of the MRDTs at the PRP and could inform decision toward its implementation. Recommendations can be made to healthcare practitioners toward adherence to test results; in line with WHO directives aimed at proper case management of malaria.
AUTHORS’ CONTRIBUTION
RA, BPA, and GAK, brought up the concept and designed the study. BPA, GAK, and KOB defined the intellectual content. RA and AAA did the literature search, data acquisition, and data analysis. RA, GAK, AAA prepared the manuscript. BPA, KOB, and AAA edited the final manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Researchers wish to acknowledge all the pharmacy and laboratory staff, as well as the staff of Kama Health Services, KNUST, Kumasi, Ghana, and participants who by their efforts saw to the success of the research.
REFERENCES
- 1.Ansah EK, Narh-Bana S, Affran-Bonful H, Bart-Plange C, Cundill B, Gyapong M, et al. The impact of providing rapid diagnostic malaria tests on fever management in the private retail sector in Ghana: A cluster randomized trial. BMJ. 2015;350:h1019. doi: 10.1136/bmj.h1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moon S, Pérez Casas C, Kindermans JM, de Smet M, von Schoen-Angerer T. Focusing on quality patient care in the new global subsidy for malaria medicines. PLoS Med. 2009;6:e1000106. doi: 10.1371/journal.pmed.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyce RM, Muiru A, Reyes R, Ntaro M, Mulogo E, Matte M, et al. Impact of rapid diagnostic tests for the diagnosis and treatment of malaria at a peripheral health facility in Western Uganda: An interrupted time series analysis. Malar J. 2015;14:203. doi: 10.1186/s12936-015-0725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh N, Sharma RK. Improving diagnosis and treatment of uncomplicated malaria. Lancet Glob Health. 2014;2:e304–5. doi: 10.1016/S2214-109X(14)70222-0. [DOI] [PubMed] [Google Scholar]
- 5.Nicastri E, Bevilacqua N, Sañé Schepisi M, Paglia MG, Meschi S, Ame SM, et al. Accuracy of malaria diagnosis by microscopy, rapid diagnostic test, and PCR methods and evidence of antimalarial overprescription in non-severe febrile patients in two Tanzanian hospitals. Am J Trop Med Hyg. 2009;80:712–7. [PubMed] [Google Scholar]
- 6.Gatton ML, Rees-Channer RR, Glenn J, Barnwell JW, Cheng Q, Chiodini PL, et al. Pan-Plasmodium band sensitivity for Plasmodium falciparum detection in combination malaria rapid diagnostic tests and implications for clinical management. Malar J. 2015;14:115. doi: 10.1186/s12936-015-0629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen KS, Grieve E, Mikhail A, Mayan I, Mohammed N, Anwar M, et al. Cost-effectiveness of malaria diagnosis using rapid diagnostic tests compared to microscopy or clinical symptoms alone in Afghanistan. Malar J. 2015;14:217. doi: 10.1186/s12936-015-0696-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rauf A, Anto BP, Koffuor GA, Buabeng KO, Abdul-Kabir M. Assessing the diagnosis of uncomplicated malaria after introduction of malaria rapid diagnostic tests. Br J Med Med Res. 2014;4:3167–78. [Google Scholar]
- 9.Bastiaens GJ, Schaftenaar E, Ndaro A, Keuter M, Bousema T, Shekalaghe SA. Malaria diagnostic testing and treatment practices in three different Plasmodium falciparum transmission settings in Tanzania: Before and after a government policy change. Malar J. 2011;10:76. doi: 10.1186/1475-2875-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Causer L, Abdulla S, Williams H, Shebuge H, Magonyozi D, Msuya H, et al. Malaria diagnosis and role of diagnostics – implications for malaria drug policy. American Society of Tropical Medicine and Hygiene Conference Miami, FL; 2004 [Google Scholar]
- 11.Chanda P, Hamainza B, Moonga HB, Chalwe V, Pagnoni F. Community case management of malaria using ACT and RDT in two districts in Zambia: Achieving high adherence to test results using community health workers. Malar J. 2011;10:158. doi: 10.1186/1475-2875-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratsimbasoa A, Randriamanantena A, Raherinjafy R, Rasoarilalao N, Ménard D. Which malaria rapid test for Madagascar. Field and laboratory evaluation of three tests and expert microscopy of samples from suspected malaria patients in Madagascar? Am J Trop Med Hyg. 2007;76:481–5. [PubMed] [Google Scholar]
- 13.van den Broek I, Kitz C, Al Attas S, Libama F, Balasegaram M, Guthmann JP. Efficacy of three artemisinin combination therapies for the treatment of uncomplicated Plasmodium falciparum malaria in the Republic of Congo. Malar J. 2006;5:113. doi: 10.1186/1475-2875-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariette N, Barnadas C, Bouchier C, Tichit M, Ménard D. Country-wide assessment of the genetic polymorphism in Plasmodium falciparum and Plasmodium vivax antigens detected with rapid diagnostic tests for malaria. Malar J. 2008;7:219. doi: 10.1186/1475-2875-7-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishengoma DS, Francis F, Mmbando BP, Lusingu JP, Magistrado P, Alifrangis M, et al. Accuracy of malaria rapid diagnostic tests in community studies and their impact on treatment of malaria in an area with declining malaria burden in North-Eastern Tanzania. Malar J. 2011;10:176. doi: 10.1186/1475-2875-10-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemma H, San Sebastian M, Löfgren C, Barnabas G. Cost-effectiveness of three malaria treatment strategies in rural Tigray, Ethiopia where both Plasmodium falciparum and Plasmodium vivax co-dominate. Cost Eff Resour Alloc. 2011;9:2. doi: 10.1186/1478-7547-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batwala V, Magnussen P, Nuwaha F. Are rapid diagnostic tests more accurate in diagnosis of Plasmodium falciparum malaria compared to microscopy at rural health centres? Malar J. 2010;9:349. doi: 10.1186/1475-2875-9-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B. 1995;57:289–300. [Google Scholar]
- 19.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: A single indicator of test performance. J Clin Epidemiol. 2003;56:1129–35. doi: 10.1016/s0895-4356(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 20.Kyabayinze DJ, Asiimwe C, Nakanjako D, Nabakooza J, Counihan H, Tibenderana JK. Use of RDTs to improve malaria diagnosis and fever case management at primary health care facilities in Uganda. Malar J. 2010;9:200. doi: 10.1186/1475-2875-9-200. [DOI] [PMC free article] [PubMed] [Google Scholar]


