Abstract
Background:
Abundant evidence at the anatomical, electrophysiological, and molecular levels implicates metabotropic glutamate receptor subtype 5 (mGluR5) in addiction. Consistently, the effects of a wide range of doses of different mGluR5 negative allosteric modulators (NAMs) have been tested in various animal models of addiction. Here, these studies were subjected to a systematic review to find out if mGluR5 NAMs have a therapeutic potential that can be translated to the clinic.
Methods:
Literature on consumption/self-administration and reinstatement of drug seeking as outcomes of interest published up to April 2015 was retrieved via PubMed. The review focused on the effects of systemic (i.p., i.v., s.c.) administration of the mGluR5 NAMs 3-((2-Methyl-4-thiazolyl)ethynyl)pyridine (MTEP) and 2-Methyl-6-(phenylethynyl)pyridine (MPEP) on paradigms with cocaine, ethanol, nicotine, and food in rats.
Results:
MTEP and MPEP were found to reduce self-administration of cocaine, ethanol, and nicotine at doses ≥1mg/kg and 2.5mg/kg, respectively. Dose-response relationship resembled a sigmoidal curve, with low doses not reaching statistical significance and high doses reliably inhibiting self-administration of drugs of abuse. Importantly, self-administration of cocaine, ethanol, and nicotine, but not food, was reduced by MTEP and MPEP in the dose range of 1 to 2mg/kg and 2.5 to 3.2mg/kg, respectively. This dose range corresponds to approximately 50% to 80% mGluR5 occupancy. Interestingly, the limited data found in mice and monkeys showed a similar therapeutic window.
Conclusion:
Altogether, this review suggests a therapeutic window for mGluR5 NAMs that can be translated to the treatment of substance-related and addictive disorders.
Keywords: glutamate, mGluR5, addiction, MPEP, MTEP
Introduction
The significance of metabotropic glutamate receptor subtype 5 (mGluR5) for psychiatry is predetermined by its distribution and function. In the brain, mGluR5 density (Shigemoto et al., 1993) peaks in structures involved in motor coordination (Conn et al., 2005), reward-guided behavior (Russo and Nestler, 2013; Schultz, 2015), and substance-related and addictive disorders (Everitt and Robbins, 2005; Volkow et al., 2012). Furthermore, mGluR5 is critically implicated in normal and aberrant neuroplasticity (Kalivas, 2009; Luscher and Huber, 2010; Kalivas and Volkow, 2011) via structural and functional interactions with dopamine D1, D2, NMDA, adenosine A2, and GABA receptors (Conn et al., 2005; Bonsi et al., 2008). Its pharmacological properties have been thoroughly described (Conn and Pin, 1997; Ferraguti and Shigemoto, 2006), and selective pharmacological agents targeting the mGluR5 have been developed (Gasparini et al., 1999; Anderson et al., 2002). Preclinical research with these agents suggests that this receptor is a candidate target for the treatment of MDD (Markou, 2007; Pilc et al., 2008; Palucha-Poniewiera et al., 2013), Parkinson’s disease (Marino et al., 2003; Johnson et al., 2009), schizophrenia (Conn et al., 2009; Herman et al., 2012), and addiction (Markou, 2007; Bird and Lawrence, 2009; Olive, 2009; Holmes et al., 2013; Pomierny-Chamiolo et al., 2014). The development of highly selective mGluR5 radiotracers such as [11C]ABP688 (Ametamey et al., 2006, 2007) has enabled the in vivo assessment of mGluR5 via positron emission tomography (PET) in humans (Terbeck et al., 2015). ABP PET-studies demonstrated altered mGluR5 binding in subjects with MDD (Deschwanden et al., 2011) and to a lesser extent in OCD (Akkus et al., 2014). However, the largest alteration in mGluR5 binding so far was found in smoking addiction (Akkus et al., 2013). It was replicated (Hulka et al., 2014) and extended by evidence for normalization of mGluR5 binding after prolonged smoking cessation (Akkus et al., 2015). Reduced mGluR5 binding was also found in cocaine addicts (Milella et al., 2014) and to a lesser extent in occasional cocaine users (Hulka et al., 2014), indicating a critical role for mGluR5 in human addiction and, consequently, in its treatment. Indeed, the introduction of selective and potent mGluR5 NAMs, such as 2-Methyl-6-(phenylethynyl)pyridine (MPEP) (Gasparini et al., 1999) and 3-((2-Methyl-4-thiazolyl)ethynyl)pyridine (MTEP) (Anderson et al., 2002), has inspired a large and further growing number of experiments testing the effects of a wide dose range of different mGluR5 NAMs on various addiction models in mice, rats, and monkeys. From a clinical point of view, however, innovation stemming from preclinical research needs to be systematically examined for its translational potential (Markou et al., 2009). Here, we suggest 3 incremental requirements to be fulfilled to demonstrate a therapeutic potential for mGluR5 NAMs in substance-related and addictive disorders, based on animal model-studies: (1) addiction-like animal behavior should be reliably suppressed by mGluR5 NAMs; (2) moreover, there should be a clear-cut dose-response relationship allowing a prediction of which dose range and corresponding mGluR5 occupancy range is needed to reduce addiction-like behavior; and (3) finally, there should be a “therapeutic window” within which addiction-like animal behavior is suppressed without affecting responding to natural reinforcers. Previous reviews (Markou, 2007; Bird and Lawrence, 2009; Olive, 2009; Holmes et al., 2013; Pomierny-Chamiolo et al., 2014) have demonstrated the high efficacy of mGluR5 NAMs in reducing addiction-like behavior, as required by the first criterion. However, systematic reviews on the second and third requirements are still missing. To this end, we examined the literature on the effects of mGluR5 NAMs on self-administration of substances of abuse and food.
Methods
Literature was collected using PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) and the terms “mglur5” or “mglu5” followed by “self-administration” and either “cocaine,” “nicotine,” “ethanol,” or “food.” For food, this search scope was extended by entering only “mglur5” or “mglu5” and “food” to account for studies in which animals had free access to food, without the need to perform an operant response. Only publications in English within the scope of PubMed until April 2015 were considered. Search results were narrowed down in 2 steps. First, only studies measuring consumption, self-administration, or reinstatement of food/drug seeking were included. In a second step, only reports on systemic, that is, i.p., s.c., or i.v. administration of mGluR5 NAMs were selected. Studies employing direct intracranial mGluR5 NAM administration to specific brain areas were excluded, since they cannot readily be translated to an established clinical administration routine and use an entirely different dose range.
In studies with direct access, animals could consume food or ethanol without the need to perform operant responses to gain access to it, and consumption was measured as the outcome of interest after administration of mGluR5 NAMs or placebo. In self-administration studies, animals were trained to perform operant responses (eg, lever pressing or nose poking) to gain access to the reinforcer, which was delivered in a receptacle or intravenously, through an implanted catheter. In reinstatement studies, self-administration training was followed by extinction and, subsequently, reinstatement of drug seeking by either a priming administration of the reinforcer (substance-induced reinstatement) or a response-contingent administration of the conditioned cues that had been delivered together with the reinforcer during self-administration training (cue-induced reinstatement). Thus, the main experimental paradigms were consumption under direct access to the reinforcer, self-administration maintenance under a fixed reinforcement schedule, and substance- or cue-induced reinstatement of drug seeking.
A total of 125 reports (Chiamulera et al., 2001; Paterson et al., 2003; Backstrom et al., 2004; Tessari et al., 2004; Bespalov et al., 2005; Bradbury et al., 2005; Cowen et al., 2005; Kenny et al., 2005; Lee et al., 2005; McMillen et al., 2005; Olive et al., 2005; Paterson and Markou, 2005; Schroeder et al., 2005; Varty et al., 2005; Backstrom and Hyytia, 2006; Hodge et al., 2006; Iso et al., 2006; Lominac et al., 2006; Cowen et al., 2007; Liechti and Markou, 2007; Semenova and Markou, 2007; van der Kam et al., 2007; Adams et al., 2008; Besheer et al., 2008; Gupta et al., 2008; Osborne and Olive, 2008; Palmatier et al., 2008; Platt et al., 2008; Schroeder et al., 2008; Gass et al., 2009; Kumaresan et al., 2009; Martin-Fardon et al., 2009; Moussawi et al., 2009; Hao et al., 2010; Ploj et al., 2010; Sidhpura et al., 2010; Tronci et al., 2010; Eiler et al., 2011; Popik et al., 2011; Tronci and Balfour, 2011; Martin-Fardon and Weiss, 2012; Varga et al., 2012; Keck et al., 2013; Watterson et al., 2013; Keck et al., 2014) on the effects of mGluR5 NAMs were extracted and classified with respect to the following parameters: publication (source of the report), mGluR5 NAM (MPEP, MTEP, fenobam, or MFZ 10–7), species (rats, mice, or monkeys), administration route (i.p., s.c., i.m., or i.v.), administered dose (mg/kg), and experimental paradigm (supplementary Figures 1–4). Furthermore, the alpha error correction method reported was extracted for each study (supplementary Table 1). A focus on investigations administering MTEP or MPEP in rats was chosen, since the number of studies carried out in other species or with other mGluR5 NAMs was too low. To address criteria 1 and 2, as formulated above, 87 reports were extracted from 34 studies (supplementary Methods; supplementary Table 1). To address criterion 3, individual doses were aggregated in 3 dose ranges occupying <50%, 50% to 80%, or 80% to 100% of mGluR5. Dose ranges were chosen based on evidence that MTEP produces 50% to 80% mGluR5 occupancy when administered i.p. at 1.1 to 2mg/kg and 100% occupancy at doses of 3mg/kg or more, while MPEP produces 50% to 80% mGluR5 occupancy at 2.3 to 3.2mg/kg i.p. and 100% occupancy at doses of 10mg/kg or higher (Anderson et al., 2003; Urban et al., 2003; Busse et al., 2004; Steckler et al., 2005).
Results
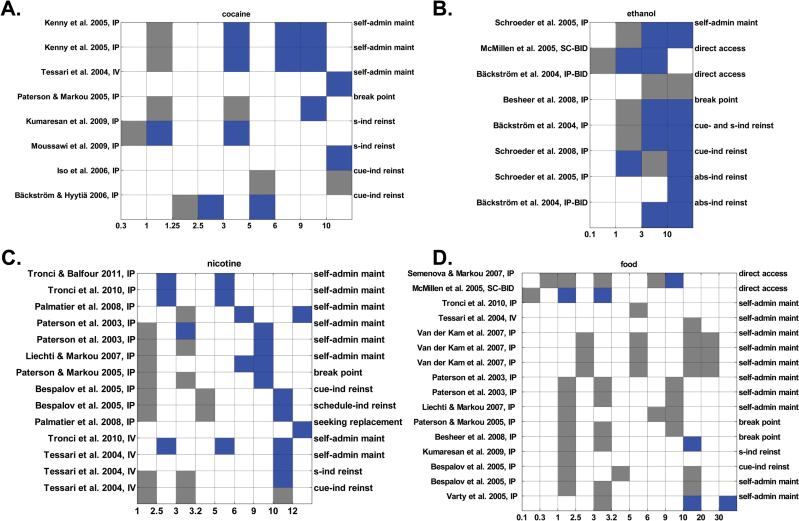
Robust evidence was found that both MTEP and MPEP suppress addiction-like behaviors for cocaine, ethanol, and nicotine (criterion 1) across various experimental models of addiction (Figures 1 and 2, A-C). In 47 of 52 reports (90%), a significant inhibiting effect of at least one of the administered mGluR5 NAM doses on addiction-like behavior in rats was found. This cannot be explained by “false positives” due to alpha error inflation caused by multiple comparisons for 2 reasons. First, statistical correction for multiple comparisons was applied in 30 of 34 studies (88%) (supplementary Table 1). Second, not a single report of statistically enhanced addiction-like behavior by treatment with any NAM dose was found. The graphical summary of these effects revealed a sigmoidal dose response-relationship (criterion 2): for each individual report there was a threshold below which the effect of no tested mGluR5 NAM dose reached statistical significance, whereas for all doses above this threshold, significant effects were observed. There was only one exception, where 1 and 10 but not 3mg/kg MPEP i.p. inhibited cue-induced reinstatement of ethanol seeking in rats (Schroeder et al., 2008) (Figure 2B). Notably, the authors pointed out that this finding was due to a single outlier in the 3-mg/kg treatment group (Schroeder et al., 2008). To explore this dose-response relationship with regard to receptor occupancy levels, all reports on cocaine, ethanol, and nicotine were pooled together in one larger group, substances of abuse, and individual doses were grouped in dose ranges according to mGluR5 occupancy levels (Figure 3). Only 21% of MTEP and 15% of MPEP doses producing <50% mGluR5 occupancy significantly inhibited addiction-like behavior (Figure 3A-B). In the dose range producing 50% to 80% mGluR5 occupancy, 71% of MTEP doses and 57% of MPEP doses significantly attenuated addiction-like behavior. This percentage grew further, reaching 84% for MTEP and 88% for MPEP in the dose range producing 80% to 100% mGluR5 occupancy. This dose-response relationship was similar yet not identical for different substances of abuse (supplementary Figures 5 and 6). MTEP in the dose range 1 to 2mg/kg inhibited addiction-like behavior for cocaine and ethanol for 60% and 88% of doses, respectively (supplementary Figure 5). While 80% of MPEP doses in the range 2.5 to 3.2mg/kg significantly inhibited addiction-like behavior for cocaine, 71% reduced addiction-like behavior in ethanol paradigms and 36% in nicotine paradigms (supplementary Figure 6). Apparently, the effectiveness threshold for MPEP in nicotine paradigms is higher, around 5mg/kg (Figure 2C).
Figure 1.
The effects of 3-((2-Methyl-4-thiazolyl)ethynyl)pyridine (MTEP) on consumption, self-administration, and reinstatement of seeking of cocaine (A), ethanol (B), nicotine (C), and food (D). Left Y-axis labels indicate study citation and administration route. Right Y-axis labels indicate the experimental paradigm. X-Axis labels indicate the MTEP doses administered in mg/kg body weight. Blue squares indicate a significant reduction of the outcome measure and grey squares indicate nonsignificant effects, as reported by the authors. Empty (white) squares indicate that the respective dose has not been tested. Abbreviations: Abs-ind reinst, abstinence-induced reinstatement; Break point, analysis of break point under progressive reinforcement schedule; Cont cue-ind reinst, context- and cue-induced reinstatement; Cue- and s-ind reins, simultaneous cue- and substance priming-induced reinstatement; Cue-ind reinst, cue-induced reinstatement of food/drug seeking; D-r curve, dose-response curve; direct access, direct access to food/drug, with no operant responding needed; Schedule-ind reinst, schedule-induced reinstatement of food/drug seeking; seeking replacement, reinstatement by operant-response noncontingent experimenter-delivered reinforcers; self-admin maint, maintenance of food/drug self-administration; S-ind reinst, substance-induced reinstatement of food/drug seeking; Stress-ind reinst, stress-induced reinstatement of food/drug seeking.
Figure 2.
Effects of 2-Methyl-6-(phenylethynyl)pyridine (MPEP) on experimental paradigms employing cocaine (A), ethanol (B), nicotine (C), and food (D) as reinforcers. Color coding as in Figure 1.
Figure 3.
Dose dependency of the effects of 3-((2-Methyl-4-thiazolyl)ethynyl)pyridine (MTEP) in experimental paradigms employing cocaine, ethanol, or nicotine (A) as well as in food paradigms (C). Dose dependency of the effects of 2-Methyl-6-(phenylethynyl)pyridine (MPEP) in experimental paradigms employing cocaine, ethanol, and nicotine (B) as well as in food paradigms (D). Dose ranges have been chosen to reflect <50% mGluR5 occupancy (<1mg/kg MTEP, <2.5mg/kg MPEP), 50% to 80% mGluR5 occupancy (1–2mg/kg MTEP, 2.5–3.2mg/kg MPEP), and up to 100% mGluR5 occupancy (>2mg/kg MTEP or >3.2mg/kg MPEP).
Strikingly, both MTEP and MPEP showed much weaker effects in experimental paradigms employing food as a reinforcer (Figure 3C-D): significant effects for any of the mGluR5 NAM dose tested were found in 9 of 19 reports for MTEP (47%) and 3 of 16 reports for MPEP (19%) (Figures 1D and 2D). When taking the dose response-relationship into account, MTEP reliably inhibited food intake only at 10mg/kg mGluR5. When considering only the dose range producing 50% to 80% receptor occupancy (1–2mg/kg MTEP and 2.5–3.2mg/kg MPEP), significant effects were found in 2 of 10 reports for MTEP and 1 of 12 reports for MPEP. In sum, MTEP and MPEP inhibit addiction-like behavior for cocaine, ethanol, and nicotine across different experimental paradigms without impairing food self-administration and consumption at doses producing 50% to 80% mGluR5 occupancy (criterion 3). Does this general finding hold when restricted to one outcome of interest? Analysis of reports on the effective doses of MTEP (1mg/kg or more) and MPEP (2.5mg/kg or more) showed that maintenance of self-administration was measured by far most frequently (supplementary Figures 7 and 8). Therefore, further analysis focused on this outcome of interest. The dose range 1 to 2mg/kg MTEP significantly reduced self-administration maintenance in 5 of 9 cases (56%) for substances of abuse (0/2 cocaine, 4/5 ethanol, 1/2 nicotine) and 1 of the 5 cases (20%) for food (Figure 1). The dose range 2.5 to 3.2mg/kg MPEP significantly reduced this outcome in 7 of 9 cases (78%) for substances of abuse (2/2 cocaine, 1/1 ethanol, 4/6 nicotine) and in none of 7 cases (0%) for food (Figure 2D). Taken together, 1 to 2mg/kg MTEP and 2.5 to 3.2mg/kg MPEP significantly inhibited self-administration maintenance for drugs of abuse in 12 of 18 cases (67%) and 1 of 12 (8%) cases for food self-administration (criterion 3). These effects are not altered by food restriction, which can increase glutamate receptor-mediated dopamine activity and is widely used in experimental protocols to enhance operant responding for drugs of abuse or food (Pothos et al., 1995; Avena et al., 2008; Branch et al., 2013). mGluR5 NAMs inhibited maintenance of self-administration of cocaine, nicotine, or alcohol in 5 of 8 reports (63%) in which access to food was restricted (Paterson et al., 2003; Liechti and Markou, 2007; Palmatier et al., 2008; Tronci et al., 2010; Tronci and Balfour, 2011). Similarly, mGluR5 NAMs reduced self-administration maintenance for cocaine, nicotine, or alcohol in 7 of 10 reports (70%) in which animals had ad libitum access to food (Cowen et al., 2005; Kenny et al., 2005; Schroeder et al., 2005; Martin-Fardon et al., 2009; Sidhpura et al., 2010; Keck et al., 2014). Furthermore, mGluR5 NAMs did not impact self-administration of food, regardless of whether access to food was restricted or not (Figures 1D and 2D; supplementary Table 2). Based on these reports, we conclude that criterion 3 regarding the “therapeutic window” is met for maintenance of self-administration in rats.
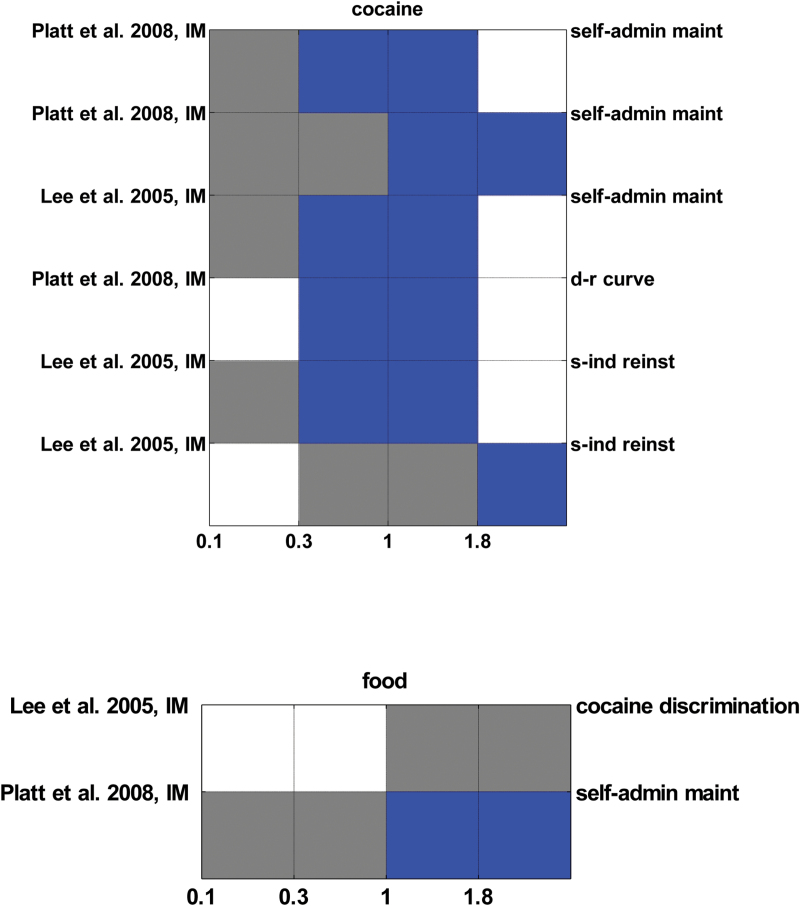
Importantly, the limited evidence from studies in mice and monkeys supports the findings in rats (Figures 4 and 5). In mice, doses of 10 and 20mg/kg MPEP reduced self-administration and consumption of ethanol without a reliable impact on self-administration and consumption of food (Figure 4B). Interestingly, this therapeutic range begins at 10mg/kg, which produces 50% mGluR5 occupancy over 1 hour in mice and thus closely corresponds to the therapeutic dose range identified for MTEP and MPEP in rats (Anderson et al., 2003). Reports on the effects of MTEP on ethanol self-administration are less consistent but also limited in number, which warrants caution in their interpretation (Figure 4A). In monkeys, limited evidence suggests that 0.3mg/kg MPEP i.m. suppress self-administration of cocaine but not food (Figure 5). Although these results have to be interpreted with caution due to the low number of reports available (supplementary Methods), they also suggest a therapeutic dose range for MPEP.
Figure 4.
Effects of 3-((2-Methyl-4-thiazolyl)ethynyl)pyridine (MTEP) (A) and 2-Methyl-6-(phenylethynyl)pyridine (MPEP) (B) on self-administration and consumption of ethanol and food in mice. Color coding and axis labelling as in Figures 1 and 2. The red square indicates increased food intake after MPEP administration.
Figure 5.
Effects of 2-Methyl-6-(phenylethynyl)pyridine (MPEP) on cocaine and food paradigms in monkeys. Color coding and axis labelling as in Figures 1 and 2.
Finally, reports on the action of mGluR5 NAMs on methamphetamine and opiates is scarce and insufficient to draw firm conclusions. In rats, methamphetamine self-administration maintenance was reduced by i.p. 1 and 3mg/kg, but not by 0.3mg/kg MTEP (Osborne and Olive, 2008). Another methamphetamine study in rats showed reduction of both substance-induced and cue-induced methamphetamine seeking by i.p. 1 and 3mg/kg, but not by 0.3mg/kg MTEP, while breaking point and self-administration maintenance were reduced by 3mg/kg, but not by 0.3 and 1mg/kg and MTEP (Gass et al., 2009). These results follow the same sigmoidal dose-response relationship and suggest that mGluR5 NAMs might reduce addiction-like behavior for methamphetamine in the therapeutic window outlined above. In mice, self-administration maintenance and cue-induced seeking for morphine were inhibited by a single i.p. dose of 20mg/kg MTEP (Brown et al., 2012). In rats, heroine self-administration maintenance was inhibited by i.p. 20 but not 1.25, 2.5, 5, or 10mg/kg MPEP (van der Kam et al., 2007). The last report suggests that generally higher mGluR5 doses might be needed to suppress heroin self-administration but should be interpreted with caution until corroborated and extended by further studies.
Conclusions
This is the first systematic review to show that MTEP and MPEP reduce self-administration of cocaine, ethanol, and nicotine (criterion 1) at doses producing 50% to 80% mGluR5 occupancy (criterion 2) without impairing food self-administration (criterion 3). These results indicate a therapeutic potential for mGluR5 NAMs in the treatment of substance-related and addictive disorders.
Aggregating heterogeneous outcome measures, proverbially referred to as “comparing apples with oranges,” is a major methodological issue in systematic reviews (Leucht et al., 2009). Indeed, different experimental paradigms, such as cue-induced reinstatement of drug seeking and maintenance of drug self-administration, can reflect different aspects of substance abuse disorders (Robinson, 2004; Koob et al., 2009) that warrant caution when making general statements about the effects of mGluR5 NAMs on addiction behavior. However, within the wide spectrum of the outcome measures reviewed here, evidence for the effects of mGluR5 NAMs on the maintenance of self-administration still holds when restricted to studies on self-administration of food or drugs of abuse. Self-administration paradigms are considered a model of binge-intoxication in human substance-related and addictive disorders (Koob et al., 2009) with high etiological and construct validity (Markou et al., 2009; O’Connor et al., 2011; Robbins, 2012). Furthermore, self-administration is a key behavioral element of substance abuse and addictive disorders (American Psychiatric et al., 2013). Therefore, the effects of mGluR5 NAMs on self-administration paradigms indicate a significant clinical impact if translated to the treatment of substance-related and addictive disorders.
The literature reviewed here clearly shows that MTEP reduces self-administration of cocaine, ethanol, and nicotine at lower doses (1–2mg/kg) than MPEP (2.5–3.2mg/kg). This finding is consistent with the higher mGluR5 occupancy rates produced by MTEP when administered at equal doses as MPEP (Anderson et al., 2003; Busse et al., 2004). A therapeutic window for MTEP and MPEP was identified at doses reported to produce approximately 50% to 80% mGluR5 occupancy (Anderson et al., 2003; Urban et al., 2003; Busse et al., 2004; Steckler et al., 2005), although a more recent investigation employing a different assessment method suggested lower and almost identical ED50 for these substances (Nagel et al., 2015). It is hard to identify the exact mGluR5 occupancy rate for an optimal ratio of reduction in substance of abuse self-administration to impairment in food consumption. However, the literature reviewed here suggests this occupancy rate is in the range 50% to 80%, as reportedly produced by i.p. administrations of 1 to 2mg/kg MTEP or 2.5 to 3.2mg/kg MPEP. Food consumption can be inhibited by MTEP but only when administered at doses more than 3 times higher than needed to block 100% mGluR5 for 1 hour (Anderson et al., 2003). Interestingly, MPEP can also inhibit food self-administration when administered at comparably high doses, that is, 10 to 30mg/kg (Varty et al., 2005). It is important to point out that the dose ranges required for robust impact on food self-administration lie well outside of the therapeutic window and are likely irrelevant for the translation of mGluR5 NAMs to the treatment of substance-related and addictive disorders.
Self-administration of cocaine, ethanol, and nicotine, which greatly differ with respect to their pharmacodynamics, was reduced by similar levels of mGluR5 negative allosteric modulation. This finding indicates that mGluR5 NAMs act on a molecular final common pathway affected by these substances of abuse (Everitt and Robbins, 2005; Nestler, 2005; Koob and Volkow, 2010) rather than at their primary binding sites. According to the glutamate homeostasis hypothesis (Kalivas, 2009; Kalivas and Volkow, 2011), synaptic glutamate overflow activating mGluR5 drives together with reduced function of mGluR2/3 and glutamate/cysteine transporter pathological neuroplastic changes in the ventral striatum in addiction. The reduction in mGluR5 binding observed in human addiction (Akkus et al., 2013, 2015; Hulka et al., 2014; Martinez et al., 2014; Milella et al., 2014) can be thought of as a compensatory reaction (Kalivas, 2009).
This review focused on MTEP and MPEP because of the scarce literature on other mGluR5 NAMs. Both substances, however, have off-target effects that hinder their clinical application. MPEP is a competitive NMDA antagonist (O’Leary et al., 2000; Movsesyan et al., 2001), which may cause potentially severe side effects, such as hallucinations. Both MTEP and MPEP act as competitive inhibitors of the hepatic enzyme CYP1A2 and can cause clinically important interactions with substances metabolized by this enzyme, such as theophylline, caffeine, fluvoxamine, and olanzapine (Green et al., 2004). Both MTEP and MPEP are rapidly metabolized after administration (Keck et al., 2013). Other highly potent and selective mGluR5 NAMs, such as fenobam (Pecknold et al., 1982; Porter et al., 2005; Berry-Kravis et al., 2009), mavoglurant (Kumar et al., 2013; Stocchi et al., 2013; Reilmann et al., 2015), ADX10059, AZD2066 (Keywood et al., 2009; Zerbib et al., 2010, 2011; Rohof et al., 2012), and AZD9272 (Kalliomaki et al., 2013), have been investigated in humans for different indications and could find application in the treatment of addiction if their pharmacokinetics and side effect profiles prove favorable. Moreover, the industry continuously develops new compounds (Felts et al., 2009; Emmitte, 2011; Kaae et al., 2012; Keck et al., 2012; Molck et al., 2012, 2014; Anighoro et al., 2015; Jaeschke et al., 2015; Lindemann et al., 2015) and new mGluR5-specific PET tracers (Yu, 2007; Mu et al., 2010; Sobrio, 2013), such as [18F]PSS232 (Sephton et al., 2015) and [18F]FPEB (Lim et al., 2014). These developments will inspire new preclinical and clinical research, which can build on the findings reported here by focusing on dose / receptor occupancy ranges that could more directly be translated to the clinic (Markou et al., 2009). On the one hand, different behavioral outcomes with incremental construct validity should be employed to investigate the action of mGluR5 on different aspects of human pathology (Koob et al., 2009). On the other hand, pharmacological small animal PET studies are needed to show the longitudinal impact of substances of abuse and their pharmacological treatment on mGluR5.
Furthermore, clinical studies with mGluR5 NAMs conducted in accordance with the National Institute of Mental Health Research Domain Criteria (Insel et al., 2010, 2014) should consider cutting across disorders characterized by action-to-habit devolution (Fineberg et al., 2010; Robbins, 2012). These include substance abuse disorders (Everitt and Robbins, 2005) but also binge eating disorder (Smith and Robbins, 2013), bulimia nervosa (Calero-Elvira et al., 2009), and pathological gambling (Petry, 2006; Potenza, 2006; Leeman and Potenza, 2012). PET studies are needed to investigate the role for mGluR5 in these disorders (Akkus et al., 2013, 2014, 2015; Hulka et al., 2014; Milella et al., 2014), while clinical trials are needed to probe the therapeutic potential of mGluR5 agents. The results of this systematic review corroborate the feasibility of such pharmacological treatment by showing a therapeutic window for mGluR5 NAMs and suggest that research in this field should be further stimulated.
Statement of Interest
None.
Supplementary Material
Acknowledgments
This work was supported by the University of Bern, Switzerland.
References
- Adams CL, Cowen MS, Short JL, Lawrence AJ. (2008) Combined antagonism of glutamate mGlu5 and adenosine A2A receptors interact to regulate alcohol-seeking in rats. Int J Neuropsychocol 11:229–241. [DOI] [PubMed] [Google Scholar]
- Akkus F, Ametamey SM, Treyer V, Burger C, Johayem A, Umbricht D, Gomez Mancilla B, Sovago J, Buck A, Hasler G. (2013) Marked global reduction in mGluR5 receptor binding in smokers and ex-smokers determined by [11C]ABP688 positron emission tomography. Proc Natl Acad Sci 110:737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkus F, Terbeck S, Ametamey SM, Rufer M, Treyer V, Burger C, Johayem A, Mancilla BG, Sovago J, Buck A, Hasler G. (2014) Metabotropic glutamate receptor 5 binding in patients with obsessive-compulsive disorder. Int J Psychop 17:1915–1922. [DOI] [PubMed] [Google Scholar]
- Akkus F, Treyer V, Johayem A, Ametamey SM, Mancilla BG, Sovago J, Buck A, Hasler G. (2015) Association of long-term nicotine abstinence with normal metabotropic glutamate receptor-5 binding. Biol Psychiatry. In press. [DOI] [PubMed] [Google Scholar]
- American Psychiatric A, American Psychiatric A, Force DSMT (2013) Diagnostic and statistical manual of mental disorders: DSM-5. [Google Scholar]
- Ametamey SM, Kessler LJ, Honer M, Wyss MT, Buck A, Hintermann S, Auberson YP, Gasparini F, Schubiger PA. (2006) Radiosynthesis and preclinical evaluation of 11C-ABP688 as a probe for imaging the metabotropic glutamate receptor subtype 5. J Nucl Med 47:698–705. [PubMed] [Google Scholar]
- Ametamey SM, Treyer V, Streffer J, Wyss MT, Schmidt M, Blagoev M, Hintermann S, Auberson Y, Gasparini F, Fischer UC, Buck A. (2007) Human PET studies of metabotropic glutamate receptor subtype 5 with 11C-ABP688. J Nucl Med 48:247–252. [PubMed] [Google Scholar]
- Anderson JJ, Rao SP, Rowe B, Giracello DR, Holtz G, Chapman DF, Tehrani L, Bradbury MJ, Cosford ND, Varney MA. (2002) [3H]Methoxymethyl-3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine binding to metabotropic glutamate receptor subtype 5 in rodent brain: in vitro and in vivo characterization. J Pharmacol Exp Ther 303:1044–1051. [DOI] [PubMed] [Google Scholar]
- Anderson JJ, Bradbury MJ, Giracello DR, Chapman DF, Holtz G, Roppe J, King C, Cosford ND, Varney MA. (2003) In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligand [3H]3-methoxy-5-(pyridin-2-ylethynyl)pyridine). Eur J Pharmacol 473:35–40. [DOI] [PubMed] [Google Scholar]
- Anighoro A, Graziani D, Bettinelli I, Cilia A, De Toma C, Longhi M, Mangiarotti F, Menegon S, Pirona L, Poggesi E, Riva C, Rastelli G. (2015) Insights into the interaction of negative allosteric modulators with the metabotropic glutamate receptor 5: discovery and computational modeling of a new series of ligands with nanomolar affinity. Bioorg Med Chem 23:3040–3058. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. (2008) Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neuroscience 156:865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. (2006) Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology 31:778–786. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. (2004) mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology 29:921–928. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, Hutchison J, Snape M, Tranfaglia M, Nguyen DV, Hagerman R. (2009) A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. J Med Genet 46:266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Faccidomo S, Grondin JJ, Hodge CW. (2008) Regulation of motivation to self-administer ethanol by mGluR5 in alcohol-preferring (P) rats. Alcohol Clin Exp Res 32:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov AY, Dravolina OA, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, Danysz W, van Heeke G, Markou A. (2005) Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology 49:167–178. [DOI] [PubMed] [Google Scholar]
- Bird MK, Lawrence AJ. (2009) The promiscuous mGlu5 receptor--a range of partners for therapeutic possibilities? Trends Pharmacol Sci 30:617–623. [DOI] [PubMed] [Google Scholar]
- Bonsi P, Platania P, Martella G, Madeo G, Vita D, Tassone A, Bernardi G, Pisani A. (2008) Distinct roles of group I mGlu receptors in striatal function. Neuropharmacology 55:392–395. [DOI] [PubMed] [Google Scholar]
- Bradbury MJ, Campbell U, Giracello D, Chapman D, King C, Tehrani L, Cosford ND, Anderson J, Varney MA, Strack AM. (2005) Metabotropic glutamate receptor mGlu5 is a mediator of appetite and energy balance in rats and mice. J Pharmacol Exp Ther 313:395–402. [DOI] [PubMed] [Google Scholar]
- Branch SY, Goertz RB, Sharpe AL, Pierce J, Roy S, Ko D, Paladini CA, Beckstead MJ. (2013) Food restriction increases glutamate receptor-mediated burst firing of dopamine neurons. J Neurosci 33:13861–13872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Stagnitti MR, Duncan JR, Lawrence AJ. (2012) The mGlu5 receptor antagonist MTEP attenuates opiate self-administration and cue-induced opiate-seeking behaviour in mice. Drug Alcohol Depend 123:264–268. [DOI] [PubMed] [Google Scholar]
- Busse CS, Brodkin J, Tattersall D, Anderson JJ, Warren N, Tehrani L, Bristow LJ, Varney MA, Cosford ND. (2004) The behavioral profile of the potent and selective mGlu5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) in rodent models of anxiety. Neuropsychopharmacology 29:1971–1979. [DOI] [PubMed] [Google Scholar]
- Calero-Elvira A, Krug I, Davis K, Lopez C, Fernandez-Aranda F, Treasure J. (2009) Meta-analysis on drugs in people with eating disorders. Eur Eat Disord Rev 17:243–259. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. (2001) Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci 4:873–874. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37:205–237. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Battaglia G, Marino MJ, Nicoletti F. (2005) Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat Rev Neurosci 6:787–798. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CK. (2009) Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci 30:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen MS, Djouma E, Lawrence AJ. (2005) The metabotropic glutamate 5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems. J Pharmacol Exp Ther 315:590–600. [DOI] [PubMed] [Google Scholar]
- Cowen MS, Krstew E, Lawrence AJ. (2007) Assessing appetitive and consummatory phases of ethanol self-administration in C57BL/6J mice under operant conditions: regulation by mGlu5 receptor antagonism. Psychopharmacology (Berl) 190:21–29. [DOI] [PubMed] [Google Scholar]
- Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A, Burger C, Auberson YP, Sovago J, Stockmeier CA, Buck A, Hasler G. (2011) Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. Am J Psych 168:727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiler WJ, 2nd, Baez M, Yu J, Witkin JM. (2011) mGlu5 receptor deletion reduces relapse to food-seeking and prevents the anti-relapse effects of mGlu5 receptor blockade in mice. Life Sci 89:862–867. [DOI] [PubMed] [Google Scholar]
- Emmitte KA. (2011) Recent advances in the design and development of novel negative allosteric modulators of mGlu(5). ACS Chem Neurosci 2:411–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8:1481–1489. [DOI] [PubMed] [Google Scholar]
- Felts AS, Saleh SA, Le U, Rodriguez AL, Weaver CD, Conn PJ, Lindsley CW, Emmitte KA. (2009) Discovery and SAR of 6-substituted-4-anilinoquinazolines as non-competitive antagonists of mGlu5. Bioorg Med Chem Lett 19:6623–6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraguti F, Shigemoto R. (2006) Metabotropic glutamate receptors. Cell and tissue research 326:483–504. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, Sahakian BJ, Robbins TW, Bullmore ET, Hollander E. (2010) Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology 35:591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini F, Lingenhohl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, Biollaz M, Allgeier H, Heckendorn R, Urwyler S, Varney MA, Johnson EC, Hess SD, Rao SP, Sacaan AI, Santori EM, Velicelebi G, Kuhn R. (1999) 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology 38:1493–1503. [DOI] [PubMed] [Google Scholar]
- Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF. (2009) mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology 34:820–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MD, Jiang X, King CD. (2004) Inhibition of human hepatic CYP isoforms by mGluR5 antagonists. Life Sci 75:947–953. [DOI] [PubMed] [Google Scholar]
- Gupta T, Syed YM, Revis AA, Miller SA, Martinez M, Cohn KA, Demeyer MR, Patel KY, Brzezinska WJ, Rhodes JS. (2008) Acute effects of acamprosate and MPEP on ethanol Drinking-in-the-Dark in male C57BL/6J mice. Alcohol Clin Exp Res 32:1992–1998. [DOI] [PubMed] [Google Scholar]
- Hao Y, Martin-Fardon R, Weiss F. (2010) Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol Psychiatry 68:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman EJ, Bubser M, Conn PJ, Jones CK. (2012) Metabotropic glutamate receptors for new treatments in schizophrenia. Handbook of experimental pharmacology:297–365. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, Schroeder JP. (2006) The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology (Berl) 183:429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Spanagel R, Krystal JH. (2013) Glutamatergic targets for new alcohol medications. Psychopharmacology (Berl) 229:539–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulka LM, Treyer V, Scheidegger M, Preller KH, Vonmoos M, Baumgartner MR, Johayem A, Ametamey SM, Buck A, Seifritz E, Quednow BB. (2014) Smoking but not cocaine use is associated with lower cerebral metabotropic glutamate receptor 5 density in humans. Molec Psychiatry 19:625–632. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. (2010) Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psych 167:748–751. [DOI] [PubMed] [Google Scholar]
- Insel TR. (2014) The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psych 171:395–397. [DOI] [PubMed] [Google Scholar]
- Iso Y, Grajkowska E, Wroblewski JT, Davis J, Goeders NE, Johnson KM, Sanker S, Roth BL, Tueckmantel W, Kozikowski AP. (2006) Synthesis and structure-activity relationships of 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine analogues as potent, noncompetitive metabotropic glutamate receptor subtype 5 antagonists; search for cocaine medications. J Med Chem 49:1080–1100. [DOI] [PubMed] [Google Scholar]
- Jaeschke G, et al. (2015) Metabotropic glutamate receptor 5 negative allosteric modulators: discovery of 2-chloro-4-[1-(4-fluorophenyl)-2,5-dimethyl-1H-imidazol-4-ylethynyl]pyridine (basimglurant, RO4917523), a promising novel medicine for psychiatric diseases. J Med Chem 58:1358–1371. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Conn PJ, Niswender CM. (2009) Glutamate receptors as therapeutic targets for Parkinson’s disease. CNS and neurological disorders drug targets 8:475–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaae BH, Harpsoe K, Kvist T, Mathiesen JM, Molck C, Gloriam D, Jimenez HN, Uberti MA, Nielsen SM, Nielsen B, Brauner-Osborne H, Sauerberg P, Clausen RP, Madsen U. (2012) Structure-activity relationships for negative allosteric mGluR5 modulators. ChemMedChem 7:440–451. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10:561–572. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. (2011) New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry 16:974–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliomaki J, Huizar K, Kagedal M, Hagglof B, Schmelz M. (2013) Evaluation of the effects of a metabotropic glutamate receptor 5-antagonist on electrically induced pain and central sensitization in healthy human volunteers. Eur J Pain 17:1465–1471. [DOI] [PubMed] [Google Scholar]
- Keck TM, Zou MF, Zhang P, Rutledge RP, Newman AH. (2012) Metabotropic glutamate receptor 5 negative allosteric modulators as novel tools for in vivo investigation. ACS Med Chem Lett 3:544–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck TM, Yang HJ, Bi GH, Huang Y, Zhang HY, Srivastava R, Gardner EL, Newman AH, Xi ZX. (2013) Fenobam sulfate inhibits cocaine-taking and cocaine-seeking behavior in rats: implications for addiction treatment in humans. Psychopharmacology (Berl) 229:253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck TM, Zou MF, Bi GH, Zhang HY, Wang XF, Yang HJ, Srivastava R, Gardner EL, Xi ZX, Newman AH. (2014) A novel mGluR5 antagonist, MFZ 10–7, inhibits cocaine-taking and cocaine-seeking behavior in rats. Addict Biol 19:195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. (2005) Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology (Berl) 179:247–254. [DOI] [PubMed] [Google Scholar]
- Keywood C, Wakefield M, Tack J. (2009) A proof-of-concept study evaluating the effect of ADX10059, a metabotropic glutamate receptor-5 negative allosteric modulator, on acid exposure and symptoms in gastro-oesophageal reflux disease. Gut 58:1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Kenneth Lloyd G, Mason BJ. (2009) Development of pharmacotherapies for drug addiction: a Rosetta stone approach. Nat Rev Drug Discov 8:500–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Hauser RA, Mostillo J, Dronamraju N, Graf A, Merschhemke M, Kenney C. (2013) Mavoglurant (AFQ056) in combination with increased levodopa dosages in Parkinson’s disease patients. Int J Neurosci. [DOI] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. (2009) Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res 202:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD. (2005) Attenuation of behavioral effects of cocaine by the Metabotropic Glutamate Receptor 5 Antagonist 2-Methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: comparison with dizocilpine. J Pharmacol Exp Ther 312:1232–1240. [DOI] [PubMed] [Google Scholar]
- Leeman RF, Potenza MN. (2012) Similarities and differences between pathological gambling and substance use disorders: a focus on impulsivity and compulsivity. Psychopharmacology (Berl) 219:469–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Kissling W, Davis JM. (2009) How to read and understand and use systematic reviews and meta-analyses. Acta Psychiatr Scand 119:443–450. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Markou A. (2007) Interactive effects of the mGlu5 receptor antagonist MPEP and the mGlu2/3 receptor antagonist LY341495 on nicotine self-administration and reward deficits associated with nicotine withdrawal in rats. Eur J Pharmacol 554:164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K, Labaree D, Li S, Huang Y. (2014) Preparation of the metabotropic glutamate receptor 5 (mGluR5) PET tracer [(18)F]FPEB for human use: An automated radiosynthesis and a novel one-pot synthesis of its radiolabeling precursor. Appl Radiat Isot 94:349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann L, et al. (2015) Pharmacology of basimglurant (RO4917523, RG7090), a unique metabotropic glutamate receptor 5 negative allosteric modulator in clinical development for depression. J Pharmacol Exp Ther 353:213–233. [DOI] [PubMed] [Google Scholar]
- Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. (2006) Behavioral and neurochemical interactions between Group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Depend 85:142–156. [DOI] [PubMed] [Google Scholar]
- Luscher C, Huber KM. (2010) Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron 65:445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MJ, Valenti O, Conn PJ. (2003) Glutamate receptors and Parkinson’s disease: opportunities for intervention. Drug Aging 20:377–397. [DOI] [PubMed] [Google Scholar]
- Markou A. (2007) Metabotropic glutamate receptor antagonists: novel therapeutics for nicotine dependence and depression? Biol Psychiatry 61:17–22. [DOI] [PubMed] [Google Scholar]
- Markou A, Chiamulera C, Geyer MA, Tricklebank M, Steckler T. (2009) Removing obstacles in neuroscience drug discovery: the future path for animal models. Neuropsychopharmacology 34:74–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F. (2012) (-)-2-oxa-4-aminobicylco[3.1.0]hexane-4,6-dicarboxylic acid (LY379268) and 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine (MTEP) similarly attenuate stress-induced reinstatement of cocaine seeking. Addict Biol 17:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Baptista MA, Dayas CV, Weiss F. (2009) Dissociation of the effects of MTEP [3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer. J Pharmacol Exp Ther 329:1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Nabulsi N, Grassetti A, Urban NB, Perez A, Liu F, Lin SF, Ropchan J, Mao X, Kegeles LS, Shungu DC, Carson RE, Huang Y. (2014) Imaging glutamate homeostasis in cocaine addiction with the metabotropic glutamate receptor 5 positron emission tomography radiotracer [(11)C]ABP688 and magnetic resonance spectroscopy. Biol Psychiatry. doi:10.1016/j.biopsych.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen BA, Crawford MS, Kulers CM, Williams HL. (2005) Effects of a metabotropic, mglu5, glutamate receptor antagonist on ethanol consumption by genetic drinking rats. Alcohol Alcohol 40:494–497. [DOI] [PubMed] [Google Scholar]
- Milella MS, Marengo L, Larcher K, Fotros A, Dagher A, Rosa-Neto P, Benkelfat C, Leyton M. (2014) Limbic system mGluR5 availability in cocaine dependent subjects: a high-resolution PET [(11)C]ABP688 study. NeuroImage 98:195–202. [DOI] [PubMed] [Google Scholar]
- Molck C, Harpsoe K, Gloriam DE, Clausen RP, Madsen U, Pedersen LO, Jimenez HN, Nielsen SM, Mathiesen JM, Brauner-Osborne H. (2012) Pharmacological characterization and modeling of the binding sites of novel 1,3-bis(pyridinylethynyl)benzenes as metabotropic glutamate receptor 5-selective negative allosteric modulators. Mol Pharmacol 82:929–937. [DOI] [PubMed] [Google Scholar]
- Molck C, Harpsoe K, Gloriam DE, Mathiesen JM, Nielsen SM, Brauner-Osborne H. (2014) mGluR5: exploration of orthosteric and allosteric ligand binding pockets and their applications to drug discovery. Neurochemical research 39:1862–1875. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. (2009) N-Acetylcysteine reverses cocaine-induced metaplasticity. Nature neuroscience 12:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movsesyan VA, O’Leary DM, Fan L, Bao W, Mullins PG, Knoblach SM, Faden AI. (2001) mGluR5 antagonists 2-methyl-6-(phenylethynyl)-pyridine and (E)-2-methyl-6-(2-phenylethenyl)-pyridine reduce traumatic neuronal injury in vitro and in vivo by antagonizing N-methyl-D-aspartate receptors. J Pharmacol Exp Ther 296:41–47. [PubMed] [Google Scholar]
- Mu L, Schubiger PA, Ametamey SM. (2010) Radioligands for the PET imaging of metabotropic glutamate receptor subtype 5 (mGluR5). Current topics in medicinal chemistry 10:1558–1568. [DOI] [PubMed] [Google Scholar]
- Nagel J, Greco S, Parsons CG, Flik G, Tober C, Klein KU, Danysz W. (2015) Brain concentrations of mGluR5 negative allosteric modulator MTEP in relation to receptor occupancy: comparison to MPEP. Pharmacol Rep 67:624–630. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. (2005) Is there a common molecular pathway for addiction? Nature neuroscience 8:1445–1449. [DOI] [PubMed] [Google Scholar]
- O’Connor EC, Chapman K, Butler P, Mead AN. (2011) The predictive validity of the rat self-administration model for abuse liability. Neurosci Biobehav Rev 35:912–938. [DOI] [PubMed] [Google Scholar]
- O’Leary DM, Movsesyan V, Vicini S, Faden AI. (2000) Selective mGluR5 antagonists MPEP and SIB-1893 decrease NMDA or glutamate-mediated neuronal toxicity through actions that reflect NMDA receptor antagonism. Br J Pharmacol 131:1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. (2009) Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev 2:83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, McGeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, Messing RO. (2005) The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C epsilon-dependent mechanism. Mol Pharmacol 67:349–355. [DOI] [PubMed] [Google Scholar]
- Osborne MP, Olive MF. (2008) A role for mGluR5 receptors in intravenous methamphetamine self-administration. Ann N Y Acad Sci 1139:206–211. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Liu X, Donny EC, Caggiula AR, Sved AF. (2008) Metabotropic glutamate 5 receptor (mGluR5) antagonists decrease nicotine seeking, but do not affect the reinforcement enhancing effects of nicotine. Neuropsychopharmacology 33:2139–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucha-Poniewiera A, Wieronska JM, Branski P, Burnat G, Chruscicka B, Pilc A. (2013) Is the mGlu5 receptor a possible target for new antidepressant drugs? Pharmacol Rep 65:1506–1511. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. (2005) The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology (Berl) 179:255–261. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Semenova S, Gasparini F, Markou A. (2003) The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 167:257–264. [DOI] [PubMed] [Google Scholar]
- Pecknold JC, McClure DJ, Appeltauer L, Wrzesinski L, Allan T. (1982) Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double-blind standard (diazepam) placebo-controlled study. J Clin Psychopharmacol 2:129–133. [PubMed] [Google Scholar]
- Petry NM. (2006) Should the scope of addictive behaviors be broadened to include pathological gambling? Addiction 101:152–160. [DOI] [PubMed] [Google Scholar]
- Pilc A, Chaki S, Nowak G, Witkin JM. (2008) Mood disorders: regulation by metabotropic glutamate receptors. Biochem Pharmacol 75:997–1006. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. (2008) Attenuation of cocaine self-administration in squirrel monkeys following repeated administration of the mGluR5 antagonist MPEP: comparison with dizocilpine. Psychopharmacology (Berl) 200:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploj K, Albery-Larsdotter S, Arlbrandt S, Kjaer MB, Skantze PM, Storlien LH. (2010) The metabotropic glutamate mGluR5 receptor agonist CHPG stimulates food intake. Neuroreport 21:704–708. [DOI] [PubMed] [Google Scholar]
- Pomierny-Chamiolo L, Rup K, Pomierny B, Niedzielska E, Kalivas PW, Filip M. (2014) Metabotropic glutamatergic receptors and their ligands in drug addiction. Pharmacol Ther 142:281–305. [DOI] [PubMed] [Google Scholar]
- Popik P, Kos T, Zhang Y, Bisaga A. (2011) Memantine reduces consumption of highly palatable food in a rat model of binge eating. Amino Acids 40:477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RH, Jaeschke G, Spooren W, Ballard TM, Buttelmann B, Kolczewski S, Peters JU, Prinssen E, Wichmann J, Vieira E, Muhlemann A, Gatti S, Mutel V, Malherbe P. (2005) Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J Pharmacol Exp Ther 315:711–721. [DOI] [PubMed] [Google Scholar]
- Potenza MN. (2006) Should addictive disorders include non-substance-related conditions? Addiction 101:142–151. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Creese I, Hoebel BG. (1995) Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci 15:6640–6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilmann R, Rouzade-Dominguez ML, Saft C, Sussmuth SD, Priller J, Rosser A, Rickards H, Schols L, Pezous N, Gasparini F, Johns D, Landwehrmeyer GB, Gomez-Mancilla B. (2015) A randomized, placebo-controlled trial of AFQ056 for the treatment of chorea in Huntington’s disease. Mov Disord 30:427–431. [DOI] [PubMed] [Google Scholar]
- Robbins TW. (2012) Animal models of neuropsychiatry revisited: a personal tribute to Teitelbaum. Behav Brain Res 231:337–342. [DOI] [PubMed] [Google Scholar]
- Robinson TE. (2004) Neuroscience. Addicted rats. Science 305:951–953. [DOI] [PubMed] [Google Scholar]
- Rohof WO, Lei A, Hirsch DP, Ny L, Astrand M, Hansen MB, Boeckxstaens GE. (2012) The effects of a novel metabotropic glutamate receptor 5 antagonist (AZD2066) on transient lower oesophageal sphincter relaxations and reflux episodes in healthy volunteers. Alim Pharmacol Ther 35:1231–1242. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. (2013) The brain reward circuitry in mood disorders. Nat Rev Neurosci 14:609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW. (2005) The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology (Berl) 179:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW. (2008) Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology 55:546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. (2015) Neuronal reward and decision signals: from theories to data. Physiol Rev 95:853–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova S, Markou A. (2007) The effects of the mGluR5 antagonist MPEP and the mGluR2/3 antagonist LY341495 on rats’ performance in the 5-choice serial reaction time task. Neuropharmacology 52:863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton SM, Herde AM, Mu L, Keller C, Rudisuhli S, Auberson Y, Schibli R, Kramer SD, Ametamey SM. (2015) Preclinical evaluation and test-retest studies of [(18)F]PSS232, a novel radioligand for targeting metabotropic glutamate receptor 5 (mGlu5). Eur J Nucl Med Mol Imaging 42:128–137. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. (1993) Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett 163:53–57. [DOI] [PubMed] [Google Scholar]
- Sidhpura N, Weiss F, Martin-Fardon R. (2010) Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biol Psychiatry 67:804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DG, Robbins TW. (2013) The neurobiological underpinnings of obesity and binge eating: a rationale for adopting the food addiction model. Biol Psychiatry 73:804–810. [DOI] [PubMed] [Google Scholar]
- Sobrio F. (2013) Radiosynthesis of carbon-11 and fluorine-18 labelled radiotracers to image the ionotropic and metabotropic glutamate receptors. J Labelled Compd Rad 56:180–186. [DOI] [PubMed] [Google Scholar]
- Steckler T, Oliveira AF, Van Dyck C, Van Craenendonck H, Mateus AM, Langlois X, Lesage AS, Prickaerts J. (2005) Metabotropic glutamate receptor 1 blockade impairs acquisition and retention in a spatial Water maze task. Behav Brain Res 164:52–60. [DOI] [PubMed] [Google Scholar]
- Stocchi F, Rascol O, Destee A, Hattori N, Hauser RA, Lang AE, Poewe W, Stacy M, Tolosa E, Gao H, Nagel J, Merschhemke M, Graf A, Kenney C, Trenkwalder C. (2013) AFQ056 in Parkinson patients with levodopa-induced dyskinesia: 13-week, randomized, dose-finding study. Movement Disord 28:1838–1846. [DOI] [PubMed] [Google Scholar]
- Terbeck S, Akkus F, Chesterman LP, Hasler G. (2015) The role of metabotropic glutamate receptor 5 in the pathogenesis of mood disorders and addiction: combining preclinical evidence with human Positron Emission Tomography (PET) studies. Front Neurosci 9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. (2004) Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol 499:121–133. [DOI] [PubMed] [Google Scholar]
- Tronci V, Balfour DJ. (2011) The effects of the mGluR5 receptor antagonist 6-methyl-2-(phenylethynyl)-pyridine (MPEP) on the stimulation of dopamine release evoked by nicotine in the rat brain. Behav Brain Res 219:354–357. [DOI] [PubMed] [Google Scholar]
- Tronci V, Vronskaya S, Montgomery N, Mura D, Balfour DJ. (2010) The effects of the mGluR5 receptor antagonist 6-methyl-2-(phenylethynyl)-pyridine (MPEP) on behavioural responses to nicotine. Psychopharmacology (Berl) 211:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban MO, Hama AT, Bradbury M, Anderson J, Varney MA, Bristow L. (2003) Role of metabotropic glutamate receptor subtype 5 (mGluR5) in the maintenance of cold hypersensitivity following a peripheral mononeuropathy in the rat. Neuropharmacology 44:983–993. [DOI] [PubMed] [Google Scholar]
- van der Kam EL, de Vry J, Tzschentke TM. (2007) Effect of 2-methyl-6-(phenylethynyl) pyridine on intravenous self-administration of ketamine and heroin in the rat. Behav Pharmacol 18:717–724. [DOI] [PubMed] [Google Scholar]
- Varga B, Kassai F, Gyertyan I. (2012) Interactions of CB1 and mGlu5 receptor antagonists in food intake, anxiety and memory models in rats. Pharmacol Biochem Behav 103:425–430. [DOI] [PubMed] [Google Scholar]
- Varty GB, Grilli M, Forlani A, Fredduzzi S, Grzelak ME, Guthrie DH, Hodgson RA, Lu SX, Nicolussi E, Pond AJ, Parker EM, Hunter JC, Higgins GA, Reggiani A, Bertorelli R. (2005) The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists, MPEP and MTEP, and the mGluR1 antagonist, LY456236, in rodents: a comparison of efficacy and side-effect profiles. Psychopharmacology (Berl) 179:207–217. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. (2012) Addiction circuitry in the human brain. Ann Rev Pharmacol Toxicol 52:321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Hood LE, Olive MF. (2013) Attenuation of reinstatement of methamphetamine-, sucrose-, and food-seeking behavior in rats by fenobam, a metabotropic glutamate receptor 5 negative allosteric modulator. Psychopharmacology (Berl) 225:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M. (2007) Recent developments of the PET imaging agents for metabotropic glutamate receptor subtype 5. Curr Top Med Chem 7:1800–1805. [DOI] [PubMed] [Google Scholar]
- Zerbib F, Keywood C, Strabach G. (2010) Efficacy, tolerability and pharmacokinetics of a modified release formulation of ADX10059, a negative allosteric modulator of metabotropic glutamate receptor 5: an esophageal pH-impedance study in healthy subjects. Gastroint Motil 22:859–865, e231. [DOI] [PubMed] [Google Scholar]
- Zerbib F, Bruley des Varannes S, Roman S, Tutuian R, Galmiche JP, Mion F, Tack J, Malfertheiner P, Keywood C. (2011) Randomised clinical trial: effects of monotherapy with ADX10059, a mGluR5 inhibitor, on symptoms and reflux events in patients with gastro-oesophageal reflux disease. Aliment Pharm Ther 33:911–921. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.