Abstract
Background:
In utero exposure to maternal viral infections is associated with a higher incidence of psychiatric disorders with a supposed neurodevelopmental origin, including schizophrenia. Hence, immune response factors exert a negative impact on brain maturation that predisposes the offspring to the emergence of pathological phenotypes later in life. Although ventral tegmental area dopamine neurons and their target regions play essential roles in the pathophysiology of psychoses, it remains to be fully elucidated how dopamine activity and functionality are disrupted in maternal immune activation models of schizophrenia.
Methods:
Here, we used an immune-mediated neurodevelopmental disruption model based on prenatal administration of the polyriboinosinic-polyribocytidilic acid in rats, which mimics a viral infection and recapitulates behavioral abnormalities relevant to psychiatric disorders in the offspring. Extracellular dopamine levels were measured by brain microdialysis in both the nucleus accumbens shell and the medial prefrontal cortex, whereas dopamine neurons in ventral tegmental area were studied by in vivo electrophysiology.
Results:
Polyriboinosinic-polyribocytidilic acid-treated animals, at adulthood, displayed deficits in sensorimotor gating, memory, and social interaction and increased baseline extracellular dopamine levels in the nucleus accumbens, but not in the prefrontal cortex. In polyriboinosinic-polyribocytidilic acid rats, dopamine neurons showed reduced spontaneously firing rate and population activity.
Conclusions:
These results confirm that maternal immune activation severely impairs dopamine system and that the polyriboinosinic-polyribocytidilic acid model can be considered a proper animal model of a psychiatric condition that fulfills a multidimensional set of validity criteria predictive of a human pathology.
Keywords: Schizophrenia, dopamine, electrophysiology, microdialysis
Introduction
In the last decades, an association between in utero insults and enhanced risks for psychosis during adulthood has been underscored by both clinical and preclinical observations (Boksa, 2010). In particular, among the vast number of environmental factors deemed responsible of prenatal insults, the exposure to maternal infections in utero, and specifically to maternal immune response products, has attracted special interest. In fact, epidemiological studies show increased risk for schizophrenia and related disorders following prenatal maternal exposure to infection or inflammation (Brown and Derkits, 2010). In line with this notion, several studies have demonstrated that by mimicking a viral or bacterial infection in pregnant dams at specific ages of pregnancy (mainly comparable with the second trimester of human pregnancy), the progeny displays a wide array of psychotic-like anatomical, behavioral, and neurochemical aberrations (Patterson, 2002; Meyer et al., 2005; Patterson, 2009; Boksa, 2010).
One of the most studied rodent models of neurodevelopmental psychosis induced by maternal infection is obtained by exposing fetuses to the administration to the mother of a proinflammatory cytokine inductor, a double stranded RNA named polyriboinosinic-polyribocytidilic acid [poly(I:C)]), during gestational day (GD) 14 to 16 (Zuckerman et al., 2003). Poly(I:C) mimics the acute phase of viral infection by inducing a robust inflammatory response leading to the synthesis of several proinflammatory cytokines, such as interleukin-1β, interleukin-6, and tumor necrosis factor-α (Zuckerman et al., 2003; Meyer et al., 2006). Interestingly, this particular type of prenatal immune activation elicits in rodent offspring a broad spectrum of behavioral impairments commonly described in schizophrenic patients, such as deficits in sensorimotor gating, latent inhibition, and social interaction (Zuckerman et al., 2003).
Furthermore, poly(I:C) rats show hypersensitivity to the effects of amphetamine and phencyclidine, compounds that are known to produce positive, negative, and cognitive schizophrenia-like symptoms in healthy humans (Meyer et al., 2006; Ozawa et al., 2006). In addition, alongside of disrupted behavior, studies have also reported alterations in many neurotransmitter systems such as dopamine (Meyer et al., 2008; Vuillermot et al., 2010), acetylcholine (Wu et al., 2015), and GABA (Richetto et al., 2015).
In particular, dopaminergic abnormalities are considered a final common pathway in the neuropathogenesis of schizophrenia and remain central in both treatment and etiology (see Eyles et al., 2012 and references therein). Consistently, previous studies on maternal immune activation have found abnormalities in dopamine system in the offspring, such as increased numbers of tyrosine hydroxylase (TH) immunoreactive cells in the VTA and TH-positive terminals in the striatum (Meyer et al., 2008; Winter et al., 2009; Vuillermot et al., 2010) or dopamine output in striatal slices (Zuckerman et al., 2003) in the lateral globus pallidus and prefrontal cortex (Winter et al., 2009).
Although evidence is mounting that dopamine transmission is indeed impaired in several neurodevelopmental models of psychosis, including the poly(I:C) model, no study to our knowledge has yet combined behavioral, neurochemical, and electrophysiological analysis to assess and characterize this disruption.
To this aim, in the present study, we used the poly(I:C) neurodevelopmental model in rats. Male adult offspring were behaviorally assessed for deficits in sensorimotor gating, short-term memory impairment, and social interaction. In these animals, mesolimbic dopamine neuron activity was examined by in vivo electrophysiology, and extracellular dopamine levels were measured by brain microdialysis.
Materials and Methods
Prenatal Treatment and Subjects
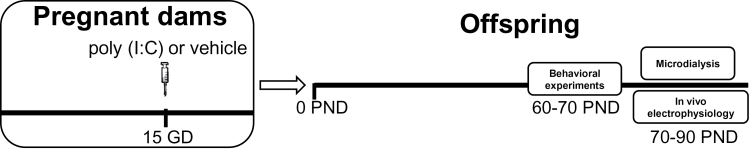
Female Sprague Dawley rats (Harlan, Italy) were mated at the age of 3 months, and the first day after the copulation was defined as GD 1. We set our protocol basically following that already published by Zuckerman et al. (2003). At GD 15, pregnant dams were given either a single dose of poly(I:C) (4.0mg/kg, i.v.; InvivoGen, San Diego, CA) or an equivalent volume of endotoxin free saline solution in the lateral vein of the tail. To minimize the animal discomfort, we induced pregnant dams with isoflurane (2%) anesthesia, and then we kept the anesthesia during the whole injection with an infusion system. Pregnant dams were weighed for the first 3 days after the administration of either poly(I:C) or saline to evaluate weight loss as reported by previous investigations (Zuckerman et al., 2003). Consistent with previous studies (Wolff and Bilkey, 2010), poly(I:C)-treated dams lost 2.6±2.5g (n=11) 24 hours after the injection, whereas saline-treated dams increased their weight by 8.3±2.1g (n=7). This difference is statistically significant (P<.01). The offspring were randomly assigned according to postnatal age to different experimental protocols (see Figure 1 for a schematic description).
Figure 1.
Schematic representation of the experimental protocol. Polyriboinosinic-polyribocytidilic acid [poly(I:C)] treatment during pregnancy consisted in a single i.v. injection of poly(I:C) (4mg/kg) or vehicle (sterile pyrogen-free saline) at the 15th gestational day (GD). Behavioral experiments were performed between postnatal day (PND) 60 and 70, whereas in vivo electrophysiology and microdialysis between 70 and 90 PND.
Adult (60–90 days) Sprague Dawley rats, offspring of poly(I:C)- or vehicle-treated dams, were bred in our facilities and were used for the experiments described below. We housed animals in groups of 3 to 6 in standard conditions of temperature and humidity under a 12-h-light/-dark cycle (with lights on at 7:00 am) with food and water available ad libitum.
All experiments were approved by the University of Cagliari Committee on Animal Use and Care and performed in strict accordance with the EEC Council Directive of 24 November 1986 (86/609). We made all efforts to minimize animal discomfort and to reduce the number of animals used.
Behavioral Experiments
Prepulse Inhibition (PPI) of Startle Reflex
Startle and PPI were performed as previously described by Frau et al. (2014) with slight modifications. The apparatus used for detection of startle reflexes (Med Associates, St Albans, VT) consisted of 4 standard cages placed in sound-attenuated chambers with fan ventilation. Each cage consisted of a Plexiglas cylinder of 9-cm diameter mounted on a piezoelectric accelerometric platform connected to an analogue-digital converter. Two separate speakers conveyed background noise and acoustic bursts, each one properly placed to produce a variation of sound within 1 dB across the startle cage. Both speakers and startle cages were connected to a main PC, which detected and analyzed all chamber variables with specific software. Before each testing session, acoustic stimuli and mechanical responses were calibrated via specific devices supplied by Med Associates.
On the testing day, each rat was placed in the cage for a 5-minute acclimatization period consisting of 70-dB white noise background, which continued for the remainder of the session. Each session consisted of 3 consecutive sequences of trials (blocks). During the first and third blocks, rats were presented with only 5 pulse-alone trials of 115 dB. In the second block was delivered a pseudorandom sequence of 50 trials, including 12 pulse-alone trials; 30 trials of pulse preceded by 74-, 78-, or 86-dB prepulses (10 for each level of prepulse loudness); and 8 no-stimulus trials, where only the background noise was delivered. Inter-trial intervals were selected randomly between 10 and 15 seconds, while the inter-stimulus intervals were set at 100 milliseconds.
Novel Object Recognition (NOR) Test
NOR test was performed in adult rats (60–70 postnatal days [PND]) according to Spano et al. (2010). Each rat, after a habituation trial (10 minutes) on the arena (60×60cm), was placed into the arena and allowed to explore 2 identical objects for 10 minutes (familiarization phase or T1). The second trial (choice phase or T2) started after an interval of 1 hour and lasted 3 minutes, with 2 objects placed in the same positions: one novel and the other a third copy of the object used in T1. The objects to be discriminated were made of glass, plastic, or metal, devoid of any natural significance, and were carefully cleaned with H2O2 between each trial to avoid olfactory cues. Exploratory behavior was defined as the animal directing its nose toward the object at a distance ≤2cm and/or touching it with the nose. Conversely, turning around, climbing over, or sitting on the object was not considered as exploratory behavior. The following parameters were examined: (1) total time spent exploring the objects during T1 and T2; (2) latency of first approaches, that is, time taken by animal to approach any of the 2 objects when it is placed into the experimental arena; and (3) frequency of approaches, that is, numbers of approaching the 2 objects. A recognition index was calculated for each animal using the formula N/(N+F)×100 (N=time spent exploring the novel object; F=time spent exploring the familiar one).
Social Interaction (SI) Test
SI test was performed in adult rats (60–70 PND) according to Spano et al. (2010). The experimental animal was placed into the arena (60×60cm) with a novel unfamiliar conspecific rat of the same sex and of similar weight. Each test was performed with a novel rat that was exposed to neither the same test nor to any pharmacological treatment. Behavior was recorded for 10 minutes, and time spent by the experimental rat in social interactions (sniffing, following or grooming the partner, boxing, and wrestling) was monitored. The box was cleaned with H2O2 after each experimental session.
In Vivo Electrophysiological Experiments
Extracellular single unit recordings from VTA dopamine neurons in anesthetized male Sprague Dawley rats were carried out as described previously (Melis et al., 2008, 2009). We anesthetized animals (70–90 PND) with urethane (1.3g/kg, i.p.), cannulated their femoral vein for i.v. administration of supplemental doses of the anesthetic, and placed them in the stereotaxic apparatus (Kopf, Tujunga, CA) with their body temperature maintained at 37±1°C by a heating pad. The scalp was retracted and one burr hole was drilled above the parabrachial nucleus of the VTA (6.0mm posterior from bregma, 0.3–0.6mm lateral from midline) for the placement of a recording electrode. We localized structures according to the stereotaxic atlas of Paxinos and Watson (2007). Single unit activity of neurons located in the VTA (V 7.0–8.0mm from the cortical surface) was recorded extracellularly with glass micropipettes filled with 2% pontamine sky blue dissolved in 0.5M sodium acetate (impedance 2–5 MΩ). Single unit activity was filtered (bandpass 500–5000 Hz), and individual spikes were isolated by means of a window discriminator (Neurolog Instruments, Digitimer), displayed on a digital storage oscilloscope (TDS 3012, Tektronics), and digitally recorded. We sampled experiments on line and off line with Spike2 software (Cambridge Electronic Design, Cambridge, UK) by a computer connected to CED 1401 interface (Cambridge Electronic Design). The electrophysiological properties of spontaneously active VTA dopamine neurons were sampled by making 9 stereotaxic descents separated from each other by 200 µm, the sequence which was kept constant from animal to animal. Single units were identified according to already published criteria (Grace and Bunney, 1983; Ungless et al., 2004). We selected VTA dopamine neurons when all criteria for identification were satisfied, including: firing rate ≤10 Hz, duration of action potential ≥2.5ms, and inhibitory responses to hindpaw pinching. Each neuron was recorded for 2 to 3 minutes. Different electrophysiological parameters were evaluated, the first being the number of spontaneously active dopamine cells per electrode track (ie, population activity). The other measurements were: (1) basal firing rate, (2) CV (SD of interspike intervals divided by the mean interspike interval; a measure of firing regularity), and (3) bursting activity. The onset of a burst was identified by 2 consecutive spikes with an interspike interval <80 milliseconds, and the termination of a burst was defined as an interspike interval exceeding 160 milliseconds (Grace and Bunney, 1983). The degree and mode of bursting activity were measured by the percentage of spikes that occurred in bursts (percentage of spikes in burst), mean spikes per burst, and burst duration.
At the end of each recording section, negative DC (10 mA for 15 minutes) was passed through the recording electrode to eject Pontamine sky blue, which allowed the identification of the recorded cells. Brains were removed and fixed in 8% formalin solution. We identified the position of the electrodes microscopically on serial sections (60 µm) stained with Neutral red.
Microdialysis Experiments
Extracellular dopamine levels were evaluated by microdialysis in freely moving animals as previously described (Devoto et al., 2015). Rats (70–90 days, 300–400g) were anesthetized with Equithesin (0.97g pentobarbital, 2.1g MgSO4, 4.25g chloral hydrate, 42.8mL propylene glycol, 11.5mL 90% ethanol, distilled water up to 100mL, 5ml/kg, i.p.) and placed in a stereotaxic apparatus (Kopf). Animals were implanted with vertical microdialysis probes (membrane AN 69-HF, Hospal-Dasco, Bologna, Italy; cut-off 40000 Dalton) in the shell of the nucleus accumbens (NAc; AP 1.8, L ± 0.7, V -8.5mm from bregma, 2mm active membrane length) or in the medial prefrontal cortex (mPFC; AP 3.0, L ± 0.6, V -6.5mm from bregma, 4-mm active membrane length) according to the coordinates of the atlas by Paxinos and Watson (1997).
The day after probe implantation, an artificial cerebrospinal fluid (147mM NaCl, 4mM KCl, 1.5mM CaCl2, 1mM MgCl2, pH 6–6.5) was pumped through the dialysis probes at a constant rate of 1.1 µL/min via a CMA/100 microinjection pump (Carnegie Medicine, Stockholm, Sweden). Samples were collected every 20 minutes and dopamine and 3,4-Dihydroxyphenylacetic acid (DOPAC) simultaneously analyzed by HPLC with electrochemical detection. The HPLC systems were equipped with 3.0- x 150-mm C18 (3.5µ) Symmetry columns (Waters, Milan, Italy) kept at 30°C by Series 1100 thermostats (Agilent Technologies, Waldbronn, Germany) and ESA Coulochem II detectors (Chelmsford, MA). The mobile phase consisted of 80mM Na2HPO4, 0.27mM EDTA, 0.58mM sodium octyl sulfate, 10% methanol, 4% acetonitrile, pH 2.8 with H3PO4, delivered at 0.35mL/min; the Coulochem analytical cell first electrode was set at +200 mV, the second at -300 mV. In these conditions, the detection limit (signal to noise ratio 3:1) was 0.3 pg of dopamine on column.
Basal extracellular values are expressed as pg/20 µL dialysate injected on column immediately after collection. Changes produced by drug treatments were calculated as percent of mean basal value obtained from 3 consecutive samples with a variance not exceeding 10%.
At the end of experiment, rats were killed by decapitation, and the brain was frozen and dissected with a cryostat for visual inspection of dialysis probe position.
Statistical Analysis
Startle response was based on the first positive wave that meets the minimum wave criteria and determined as mean startle amplitude of the pulse-alone trials relative to second block. Startle habituation across the 2 halves of the second block was evaluated as percent inter-block ratio using the following formula: (mean startle amplitude for first half of the second block/mean startle amplitude for second half of the second block) ×100. Latency to startle was based on the first peak value across pulse-alone trials of the second block. “Arbitrary units” were calculated by the Med Associates apparatus software by proportionally converting the analog voltage signal recorded by the startle sensor (ranging from -10 to +10V) to a digital unit, within a range of values between –2048 and +2048. The % PPI was calculated only on the values relative to the second block using the following formula: ([mean startle amplitude pulse alone trials-mean startle amplitude prepulse+pulse trials]/mean startle amplitude for pulse alone trials)×100.
According to normality and homoscedasticity assumptions verified by Kolmogorov-Smirnov and Bartlett’s tests, data were compared across groups by Student’s t test (startle parameters) and 2-way ANOVA for repeated measures (PPI values). Significance threshold was set at 0.05.
For the NOR test, the time spent in exploring objects during T1 was calculated by summating the time spent exploring each identical object to produce a single score. For the SI test, the amount of time spent sniffing, following the partner, wrestling/boxing, or grooming were summated for each rat to produce a single score. The unpaired Student’s t test was used to compare differences between experimental groups. In all tests statistical significance was set at P < .05.
For electrophysiological and microdialysis studies, averaged data from different experiments are presented as mean±SEM. Statistical significance was assessed using 1- or 2-way ANOVA for repeated measures followed by either Dunnett’s or t test where appropriate.
Results
The Offspring of poly(I:C)-Treated Dams Display Abnormal PPI, NOR, and SI
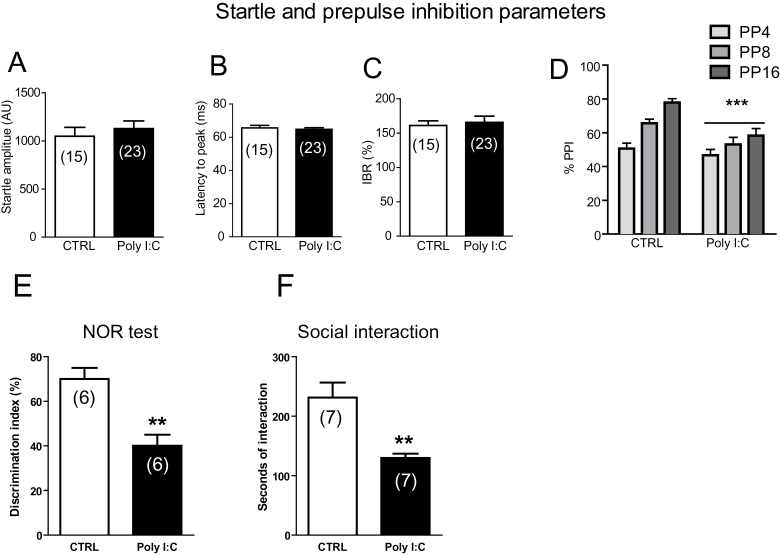
To validate our model, we first carried out behavioral experiments in poly(I:C) rats and controls at adulthood (PND 60–70). The acoustic startle response was measured at PND 60 to 70. As shown in Figure 2A-C, poly(I:C) treatment did not affect overall startle reflex values. Accordingly, no significant effects for mean startle amplitude (t(36)=0.64, n=15–23, P=.52, Student’s t test) (Figure 2A), latency to peak (t(36)=0.49, n=15–23, P=.62, Student’s t test) (Figure 2B), and startle habituation (t(36)=0.35, n=15–23, P=.72, Student’s t test) (Figure 2C) between control and poly(I:C) groups were found. Subsequently, a 2-way ANOVA (with treatment as independent factor and prepulse levels as repeated measures) assessed that the maternal infection with poly(I:C) significantly reduced PPI (main effect of treatment: F(1,108)=14.92, P<.001; main effect of prepulse level: F(2,108)=12.99, P<.001; interaction treatment ×prepulse level: F(2,108)=2.1, P=.12, NS) (Figure 2D).
Figure 2.
Behavioral abnormalities in polyriboinosinic-polyribocytidilic acid [poly(I:C)] offspring. Effects of poly(I:C) on startle reflex (A-C) and prepulse inhibition (PPI) (D) parameters. Graphs show that offspring from poly(I:C)-treated mothers exhibited impairments in PPI but not in startle indices. Values are expressed as mean±SEM. ***P<.001 vs rats treated with vehicle (main effect of treatment). Prepulses are indicated by the intensity corresponding to decibels above background noise. AU, arbitrary units; %IBR, percent inter-block ratio; ms, milliseconds. For further details, see text. (E) Graphs show that offspring of poly(I:C)-treated mothers exhibited reduced discrimination index toward a novel object and (F) reduced social interactions. Data are expressed as means±SEM. *P<.01 vs rats treated with vehicle (CTRL group).
Next, we explored the presence of memory deficits and social withdrawal by means of the NOR test and SI test, respectively. During the familiarization phase (ie, T1), no difference was observed in the time of interaction with the objects between poly(I:C) and control rats (time of interaction with left and right objects: controls: 24.5±6.0 and 25±5.9 seconds; poly(I:C) 42.3±16.3 and 44.8±17.7; n=6, P>.05, Student’s t test). During the choice phase (ie, T2), the poly(I:C) offspring exhibited a significantly lower preference for the novel object than control animals, as indicated by the ratio between the time spent with the novel and the familiar object, respectively (controls: 70±5.6% and poly(I:C) 42.4±3.65%, t(10)=4.129, P<.01, n=6) (Figure 2E). Thus, the poly(I:C) offspring expressed abnormally low sensitivity to a novel object, which is indicative of poor short-term memory.
A separate group of animals, poly(I:C) and controls, underwent the SI test. Poly(I:C)-exposed animals displayed a reduced time of interaction with the conspecific rats compared with controls (129.7±7.3 vs 231±25.4 seconds, n=7, t(12)=3.83, P<.01, Student’s t test) (Figure 2F), while no differences were found in the number of contact (data not shown).
Taken together, these results suggest that maternal immune activation induces several behavioral alterations in the offspring that have been associated with schizophrenia-like symptoms in humans and support previous literature on the validity of poly(I:C) administration during pregnancy as a model for schizophrenia and related psychoses.
The Offspring of poly(I:C)-Treated Mothers Display Elevated Levels of Dopamine in the Shell of the NAc but Not in the mPFC
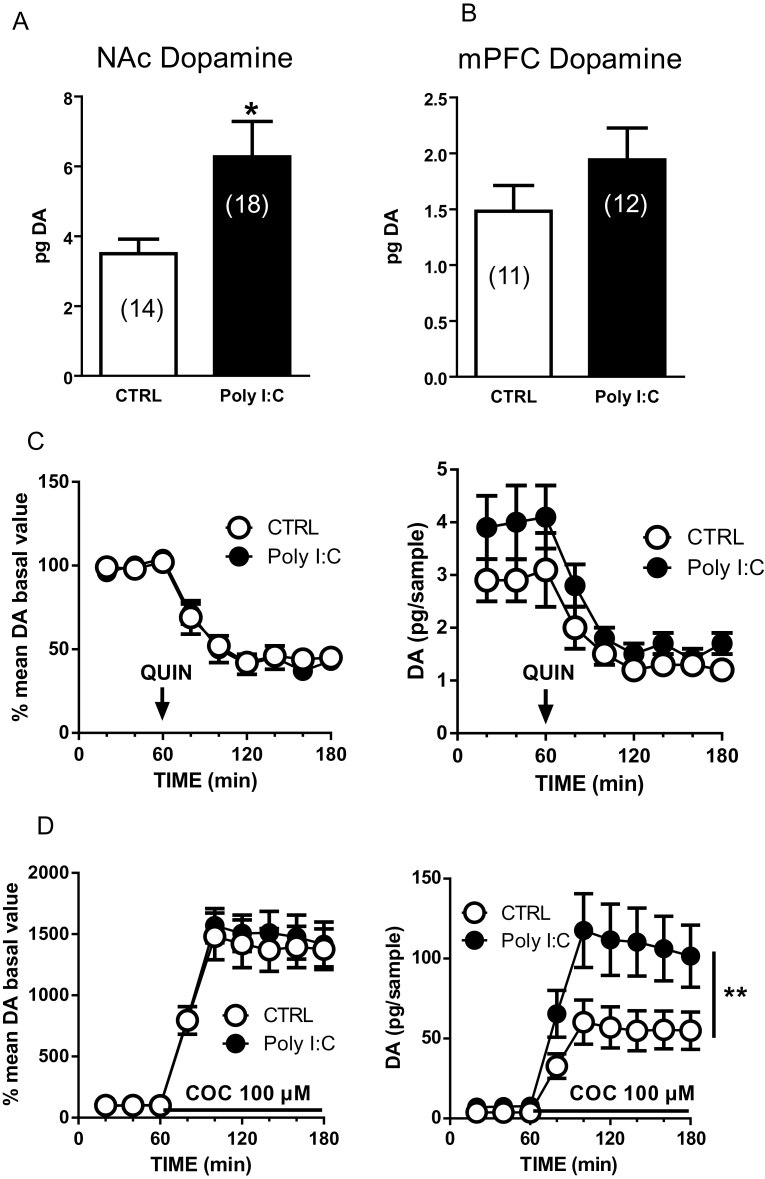
As dopamine imbalances are a hallmark of schizophrenia, we assessed whether offspring of poly(I:C)-treated dams display changes in baseline dopamine levels measured in the shell of the NAc and in the mPFC by brain microdialysis in behaving animals. For experiments in the NAc, 14 control and 18 poly(I:C) rats were used. Basal values (mean±SEM, expressed as pg/20 µL dialysate) are shown in Figure 3A. According to the hypothesis of enhanced dopamine transmission in poly(I:C) animals, extracellular dopamine levels were significantly higher in poly(I:C)- vs controls (+79%, t(22.49)=2.536, P=.0186, Student’s t test with Welch’s correction) (Figure 3A). No difference was found in DOPAC concentration (controls=1627±236, n=9, Poly(I:C)=2015±274 pg/20 µL, n=12, t(19)=1.0.72, P=.297, Student’s t test with Welch’s correction; data not shown).
Figure 3.
Enhanced extracellular dopamine (DA) levels in the nucleus accumbens (NAc) shell of polyriboinosinic-polyribocytidilic acid [poly(I:C)]-treated rats and effects of quinpirole and cocaine. Extracellular dopamine concentrations in the NAc shell (A) and medial prefrontal cortex (mPFC) (B) of control (CTRL) and poly(I:C) rats. (C) These graphs show the effect of systemically administered quinpirole (QUIN, 0.2mg/kg, s.c., n=5–6) on extracellular dopamine levels in the NAc shell expressed as percent of baseline (left) or in absolute values (pg/sample, right). (D) Locally perfused cocaine (COC, 100 µM, n=9–12) enhances extracellular dopamine levels in the NAc shell. Data are expressed as percent of baseline (left) or in absolute values (pg/sample, right). The arrow represents the time of quinpirole administration, whereas the solid line represents the time of cocaine perfusion. Values are the mean±SEM and are expressed as pg/20 µL dialysate. *P<.05, unpaired t test with Welch’s correction.
To test the functionality of D2 dopamine receptors in controlling extracellular dopamine, we administered the selective D2 agonist quinpirole. Quinpirole (0.2mg/kg, s.c.) decreased extracellular dopamine levels to about 40% of baseline level in both control and poly(I:C) rats (Figure 3C). Two-way ANOVA revealed a significant effect of time (F(5, 45)=20.27; P<.0001) but no effect of treatment (F(1, 9)=0.08199; P=.7811) or time x treatment interaction (F(5, 45)=0.3689; P=.8672). The functionality of dopamine transporter (DAT) was tested by local perfusion of cocaine (100 μM) into the NAc through the microdialysis probe. Cocaine perfusion increased extracellular dopamine levels to a maximum of about 1500% in both groups. Again, ANOVA analysis yielded a significant value for time (F(5, 95)=29.57; P<.0001) but no effect for treatment (F(1, 19)=0.1234; P=.7292) or time x treatment interaction (F(5, 95)=0.2001; P=.9617). However, following cocaine perfusion, extracellular dopamine levels expressed as absolute values were significantly higher in poly(I:C) rats compared with controls (controls: 60.2±13.8 pg/sample, n=9; poly(I:C)=117.50±23 pg/sample, n=12; effect of poly(I:C) treatment: F(1, 19)=12.98, P<.01, 2-way ANOVA). Experiments in the mPFC did not show differences in baseline dopamine and DOPAC levels between poly(I:C) and controls. For these experiments, 11 controls and 12 poly(I:C) rats were used. Basal values are shown in Figure 3B. Dopamine levels did not differ in poly(I:C)- vs controls (Student’s t test with Welch’s correction, t(21)=1.237, P=.22) (Figure 3B). No difference was found in DOPAC concentration (controls=115.4±15.5, poly(I:C)=123.7±12.7 pg/20 µL, n, t(21)=0.4174, P=.68, Student’s t test with Welch’s correction; data not shown).
Effect of Prenatal poly(I:C) on Dopamine Cell Activity and Functionality in Vivo
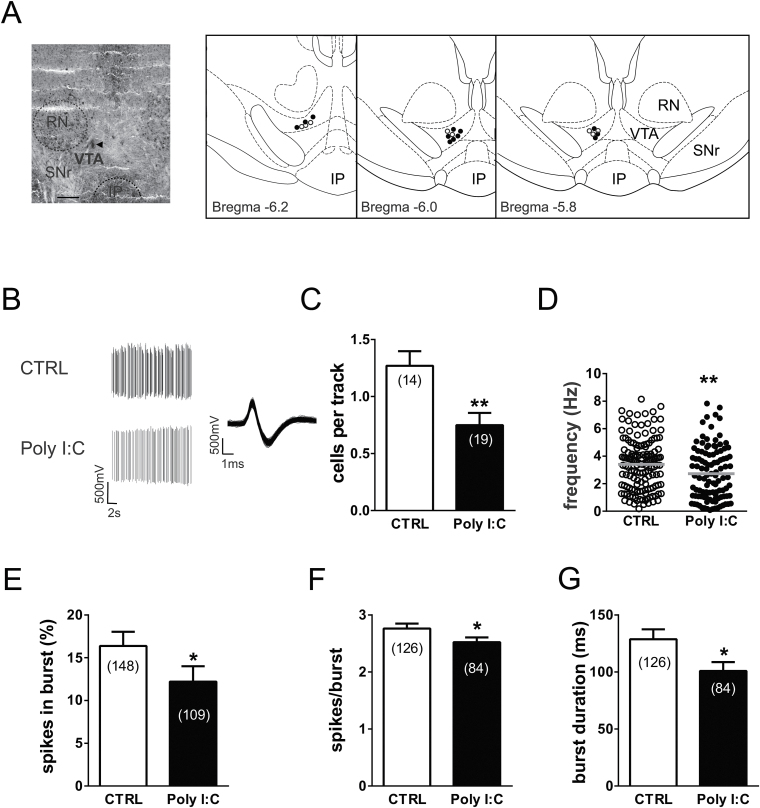
The increase in extracellular dopamine in the shell of the NAc prompted us to study dopamine cell activity in the VTA. To address this question, a population sampling of dopamine cells was performed in the parabrachial nucleus of the VTA (Figure 4A). Although the VTA shows a degree of heterogeneity, the subregion where we recorded from contains the largest density of dopamine neurons projecting to the NAc compared with the more medial levels of the posterior VTA (Yamaguchi et al., 2011; Lammel et al., 2015). A total of 257 dopamine cells was recorded from urethane anesthetized adult rats, 109 from rats exposed to poly(I:C) (n=19), and 148 from controls (n=14). Baseline activity of each cell was recorded for 120 to 180 seconds (Figure 4B).
Figure 4.
Prenatal polyriboinosinic-polyribocytidilic acid [poly(I:C)] treatment dysregulates dopamine neuron firing activity in adulthood. (A) Example of a recording location for a ventral tegmental area (VTA) dopamine neuron in a poly(I:C)-treated rat (the triangle indicates the pontamine sky blue dye). The diagrams at right show samples of localizations of recording sites in poly(I:C) rats (black dots) and controls (white dots) as verified by histological sections. IP, interpeduncular nucleus; RN, red nucleus; SNr, substantia nigra pars reticulata. Scale bar = 0.5mm. (B) This panel shows traces illustrating representative extracellular recordings of a putative dopamine neuron in the VTA of anesthetized rats belonging to the control group (CTRL, above) and poly(I:C) group (below). Dopamine neurons recorded from poly(I:C) rats typically show slower firing activity and a reduced bursting compared with CTRL. The left trace shows the typical broad spike waveform of a dopamine neuron. (C) The bar graph shows the number of spontaneously active VTA dopamine neurons, which was different between the experimental groups. The scatter plot in (D) displays individual dopamine neuron firing rate in CTRL and poly(I:C) rats. The horizontal lines represent average values that are significantly different between the 2 groups. Graph histograms represent the percentage of spikes in bursts (E), the mean number of spikes per each burst (F), and the mean burst duration (G). Data are expressed as percentage or mean±SEM. *P<.05, **P<.01.
Poly(I:C) rats showed a reduced number of spontaneously active VTA dopamine neurons compared with controls as measured by the analysis of cells per track (1.27±0.13 vs 0.75±0.11 control vs poly(I:C); t(31)=3.12, P<.01; Student’s t test) (Figure 4C).
In line with previous results in urethane-anesthetized rats (Kelland et al., 1990; Lecca et al., 2012), dopamine neurons recorded from control rats fired at 3.4±0.15 Hz (n=148) with a mean CV of interspike intervals of 64.1±2.8% and presented 16.38±1.67% of spikes in bursts. On the other hand, as depicted in Figure 4D, putative dopamine neurons (n=109) recorded from poly(I:C) rats exhibited a lower discharge activity (2.73 ±0.18 Hz; t(255)=2.874, P<.01; Student’s t test) than controls.
We carried out a more detailed analysis of burst episodes, which included parameters as the mean spikes per burst and mean burst duration. Poly(I:C) showed significant differences in the percentage of spikes in burst compared with controls (16.38±1.67% vs 12.21±1.79%; controls vs poly(I:C); t(255)=1.68, P<.05; Student’s t test) (Figure 4E). Additionally, values of mean spikes per burst and mean burst duration (n=84) of VTA dopamine neurons were significantly altered compared with those recorded from vehicle-injected littermates (n=126). In poly(I:C)-treated rats, burst episodes were shorter (duration: 128.8±8.64 milliseconds vs 100.9±7.77 milliseconds; controls vs poly(I:C); t(208)=2.26, P<.05, Student’s t test) (Figure 4G) and included fewer spikes on average (spikes per burst: 2.76 ±0.09 vs 2.52±0. 08; controls vs Poly(I:C); t(208)=1.90, P<.05, Student’s t test) (Figure 4F).
Discussion
Our study, the first to combine electrophysiological and microdialysis experiments on the dopamine system in a maternal immune activation model of schizophrenia, reveals that poly(I:C) treatment during pregnancy induces a disruption in dopamine functions in the offspring. Specifically, we found increased basal levels of dopamine in the NAc but, surprisingly, a reduction in both the number and the firing rate of spontaneously active dopamine cells in the VTA. As expected, electrophysiological and neurochemical changes were paralleled by behavioral abnormalities, as revealed by deficits in PPI, SI, and NOR tests.
One of the hallmarks of positive symptoms of schizophrenia and related psychoses is an increased dopamine transmission in the mesolimbic system (Abi-Dargham et al., 2000). Accordingly, we found increased baseline levels of dopamine in the NAc, but not in the mPFC, in poly(I:C)-treated rats compared with controls. This finding is in agreement with previous studies showing that poly(I:C) animals exhibited hyperactivity of the dopamine system (Zuckerman et al., 2003; Hadar et al., 2015). In addition, it has been reported that poly(I:C) treatment increases the number of TH immunoreactive cells in the VTA and TH-positive terminals in the striatum (Meyer et al., 2008; Winter et al., 2009; Vuillermot et al., 2010). Other studies found increases in evoked striatal dopamine release ex vivo (Zuckerman et al., 2003) as well as augmented levels of dopamine and DOPAC in the lateral globus pallidus and PFC (Winter et al., 2009) or only DOPAC in the striatum (Ozawa et al., 2006). Conversely, our electrophysiological sampling of dopamine neurons in the VTA yielded a reduced number of spontaneously active cells together with a reduced firing activity. This finding is in contrast with previous studies that utilized the mitotoxin methyl azoxymethanol acetate (MAM) model of schizophrenia (Lodge and Grace, 2007; Du and Grace, 2013). The reason for this discrepancy might be due to the profound differences in the neuropathological mechanisms between the 2 animal models [MAM vs poly(I:C)]. Administration of MAM, a DNA chelating agent, to pregnant dams at GD 15 or earlier disrupts normal fetal brain development and induces gross neurodevelopmental abnormalities both in the macro- and microstructure, particularly in cortical regions, and microcephaly (Singh, 1980; Jongen-Relo et al., 2004). When MAM is administered at a later stage during pregnancy (GD 17), less severe abnormalities with hyperdopaminergia were reported (Lodge and Grace, 2007; Du and Grace, 2013). On the other hand, maternal immune activation models recapitulate the effects of a maternal viral infection by activating microglia and cytokines production. These effects induce severe functional changes but are believed to induce minor anatomical abnormalities in the brain of offspring (Meyer, 2014).
A decrease in spontaneously active dopamine neurons, and in their frequency of discharge, is apparently difficult to reconcile with an augmented dopamine output in terminal regions, but it confirms previous reports that firing rate and release might not always be correlated (Manta et al., 2013) and that dopamine release can be controlled efficiently at terminal regions without increased impulse activity of dopamine cells. We tested the hypothesis that a reduced dopamine cell activity is an adaptation to augmented synaptic dopamine, likely paralleled by a dysregulation in dopamine D2 autoreceptor sensitivity, as these receptors are key in determining the amount of released dopamine (Anzalone et al., 2012). However, our microdialysis experiments indicated that D2 receptor sensitivity did not change between poly(I:C) and controls, as quinpirole was equally effective in decreasing extracellular dopamine. We also tested the possibility that baseline increase in NAc dopamine was due to a deficient clearance of this neurotransmitter from the synaptic cleft. Hence, a study with lipopolysaccharide as a maternal immune activator showed reduced binding levels for the DAT in the NAc of the offspring of treated dams (Baharnoori et al., 2013). When cocaine was administered locally via the microdialysis fiber, dopamine levels were further markedly enhanced in poly(I:C) rats. This finding indicates that the effects of cocaine are not occluded and that dopamine clearance by DAT is similarly efficient in both groups of animals. However, this further increase in dopamine in spite of high baseline levels provides an explanation for the enhanced motor stimulating effect of amphetamine in poly(I:C) animals observed in other studies (Zuckerman et al., 2003; Ozawa et al., 2006).
Synchronized, neural network activity in the neocortex, termed cortical oscillations, has been known to impact the pattern of neuronal firing of dopamine neurons (Gao et al., 2007), and this functional coupling allows a bidirectional control of dopamine neurons by the mPFC. Cortical oscillations and neural synchrony are abnormal in patients with schizophrenia and in animal models of this disease (Uhlhaas and Singer, 2010; Dickerson and Bilkey, 2013; Gonzalez-Burgos et al., 2015). The disruption of dopamine neuron function could be due to a different cortical state resulting from poly(I:C) exposure. In fact, maternal immune activation has been shown to disrupt oscillatory activity in the hippocampus and the neocortex (Dickerson et al., 2010; Ducharme et al., 2012, 2014; Dickerson and Bilkey, 2013). Irrespective of the mechanism involved, firing rate of dopamine neurons might be reduced or suppressed through an inhibitory synaptic feedback loop arising from the NAc (Paladini et al., 2003; Watabe-Uchida et al., 2012).
Consistent with previous studies, adult poly(I:C) offspring exhibited significant PPI deficits. PPI provides an operational measure of sensorimotor gating, a neurophysiological mechanism that filters or “gates out” relevant stimuli from its surroundings, allowing the brain to focus attention on the most salient aspects of a stimulus (Braff and Geyer, 1990). The value of this experimental measure is based on its face, predictive, construct validity for schizophrenia and other psychiatric disorders (Wolff and Bilkey, 2008). In fact, PPI is regulated by the same forebrain regions involved in schizophrenia, patients with schizophrenia show PPI dysfunctions, and PPI deficits are efficiently reversed by the benchmark antipsychotics (Braff et al., 2001). Nevertheless, PPI alterations have not constantly been reported in MIA model, and even some recent studies reported no PPI disruptions by poly(I:C) administration (Fortier et al., 2007; Van den Eynde et al., 2014). While the reasons for these apparent discrepancies remain unknown, they may reflect subtle differences in the timing of prenatal infections, the rat strain used, and the setting of the PPI protocol.
Unlike PPI parameters, poly(I:C) exposure failed to induce significant changes in startle reflex. Indeed, although our protocol is not specifically designed for assessing startle parameters, poly(I:C) offspring did not display significant differences in the average of startle reflex as well as in the startle habituation and latency. Of note, since PPI is extrapolated by startle values and PPI deficits occurred without concomitant alterations in the startle parameters, the reductions of PPI observed in poly(I:C) offspring likely reflect an effective impairment of sensorimotor gating.
One question that arises from our findings and other studies is whether maternal immune activation models schizophrenia or other neurodevelopmental disorders, such as autism spectrum disorders. In fact, several epidemiological studies have demonstrated an association between infection or inflammation during pregnancy and increased risk of autism (Atladottir et al., 2010). Prenatal infection and notably prenatal poly(I:C) and influenza virus administration can cause autism-relevant behaviors in the offspring and have been considered models of autism (Patterson, 2009; Schwartzer et al., 2013). This is not surprising when considering the similarities between schizophrenia and autism-related disorders, which might also share a neuroinflammatory pathogenesis during early fetal development (Meyer et al., 2011). Indeed, psychoses, ranging from autism to schizophrenia and bipolar disorders, might represent a continuum in both human pathology and animal models of these diseases.
Statement of Interest
None.
Acknowledgments
We thank Stefano Aramo, Maria Collu, and Barbara Tuveri for their skillful assistance. We are grateful to Pierluigi Saba for his competent technical assistance in the execution of microdialysis experiments.
This research was supported by the Italian Ministry of University (Grant PRIN 2009-200928EEX4) and Fondazione Banco di Sardegna (Grant 2014) to M.P. We thank Regione Autonoma della Sardegna for bursaries for young researchers awarded to A.L and S.L. The authors declare no competing financial interests.
References
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M. (2000) Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A 97:8104–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzalone A, Lizardi-Ortiz JE, Ramos M, De Mei C, Hopf FW, Iaccarino C, Halbout B, Jacobsen J, Kinoshita C, Welter M, Caron MG, Bonci A, Sulzer D, Borrelli E. (2012) Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci 32:9023–9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET. (2010) Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 40:1423–1430. [DOI] [PubMed] [Google Scholar]
- Baharnoori M, Bhardwaj SK, Srivastava LK. (2013) Effect of maternal lipopolysaccharide administration on the development of dopaminergic receptors and transporter in the rat offspring. PloS one 8:e54439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksa P. (2010) Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun 24:881–897. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. (1990) Sensorimotor gating and schizophrenia.Human and animal model studies. Arch Gen Psychiatry 47:181–188. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. (2001) Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 156:234–258. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. (2010) Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. AJ Psychiatry 167:261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto P, Flore G, Saba P, Frau R, Gessa GL. (2015) Selective inhibition of dopamine-beta-hydroxylase enhances dopamine release from noradrenergic terminals in the medial prefrontal cortex. Brain Behav 5:e00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson DD, Wolff AR, Bilkey DK. (2010) Abnormal long-range neural synchrony in a maternal immune activation animal model of schizophrenia. J Neurosci 30:12424–12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson DD, Bilkey DK. (2013) Aberrant neural synchrony in the maternal immune activation model: using translatable measures to explore targeted interventions. Front Behav Neurosci 7:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson DD, Overeem KA, Wolff AR, Williams JM, Abraham WC, Bilkey DK. (2014) Association of aberrant neural synchrony and altered GAD67 expression following exposure to maternal immune activation, a risk factor for schizophrenia. Transl Psychiatry 4:e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Grace AA. (2013) Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology 38:1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme G, Lowe GC, Goutagny R, Williams S. (2012) Early alterations in hippocampal circuitry and theta rhythm generation in a mouse model of prenatal infection: implications for schizophrenia. PLoS One 7:e29754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles D, Feldon J, Meyer U. (2012) Schizophrenia: do all roads lead to dopamine or is this where they start? Evidence from two epidemiologically informed developmental rodent models. Transl Psychiatry 2:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier ME, Luheshi GN, Boksa P. (2007) Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res 181:270–277. [DOI] [PubMed] [Google Scholar]
- Frau R, Bini V, Pes R, Pillolla G, Saba P, Devoto P, Bortolato M. (2014) Inhibition of 17alpha-hydroxylase/C17,20 lyase reduces gating deficits consequent to dopaminergic activation. Psychoneuroendocrinology 39:204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Liu CL, Yang S, Jin GZ, Bunney BS, Shi WX. (2007) Functional coupling between the prefrontal cortex and dopamine neurons in the ventral tegmental area. J Neurosci 27:5414–5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Cho RY, Lewis DA. (2015) Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry 77:1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. (1983) Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1.Identification and characterization. Neuroscience 10:301–315. [DOI] [PubMed] [Google Scholar]
- Hadar R, Soto-Montenegro ML, Gotz T, Wieske F, Sohr R, Desco M, Hamani C, Weiner I, Pascau J, Winter C. (2015) Using a maternal immune stimulation model of schizophrenia to study behavioral and neurobiological alterations over the developmental course. Schizophr Res 166:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen-Relo AL, Leng A, Luber M, Pothuizen HH, Weber L, Feldon J. (2004) The prenatal methylazoxymethanol acetate treatment: a neurodevelopmental animal model for schizophrenia? Behav Brain Res 149:159–181. [DOI] [PubMed] [Google Scholar]
- Kelland MD, Chiodo LA, Freeman AS. (1990) Anesthetic influences on the basal activity and pharmacological responsiveness of nigrostriatal dopamine neurons. Synapse 6:207–209. [DOI] [PubMed] [Google Scholar]
- Lammel S, Steinberg EE, Foldy C, Wall NR, Beier K, Luo L, Malenka RC. (2015) Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron 85:429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Muntoni AL, Pistis M. (2012) Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology 37:1164–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. (2007) Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci 27:11424–11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manta S, El Mansari M, Debonnel G, Blier P. (2013) Electrophysiological and neurochemical effects of long-term vagus nerve stimulation on the rat monoaminergic systems. Int J Neuropsychopharmacol 16:459–470. [DOI] [PubMed] [Google Scholar]
- Melis M, Pillolla G, Luchicchi A, Muntoni AL, Yasar S, Goldberg SR, Pistis M. (2008) Endogenous fatty acid ethanolamides suppress nicotine-induced activation of mesolimbic dopamine neurons through nuclear receptors. J Neurosci 28:13985–13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Pillolla G, Perra S, Colombo G, Muntoni AL, Pistis M. (2009) Electrophysiological properties of dopamine neurons in the ventral tegmental area of Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 201:471–481. [DOI] [PubMed] [Google Scholar]
- Meyer U. (2014) Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry 75:307–315. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. (2005) Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev 29:913–947. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. (2006) Immunological stress at the maternal-foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav Immun 20:378–388. [DOI] [PubMed] [Google Scholar]
- Meyer U, Engler A, Weber L, Schedlowski M, Feldon J. (2008) Preliminary evidence for a modulation of fetal dopaminergic development by maternal immune activation during pregnancy. Neuroscience 154:701–709. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Dammann O. (2011) Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res 69:26R–33R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. (2006) Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry 59:546–554. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Robinson S, Morikawa H, Williams JT, Palmiter RD. (2003) Dopamine controls the firing pattern of dopamine neurons via a network feedback mechanism. Proc Natl Acad Sci U S A 100:2866–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH. (2002) Maternal infection: window on neuroimmune interactions in fetal brain development and mental illness. Curr Opin Neurobiol 12:115–118. [DOI] [PubMed] [Google Scholar]
- Patterson PH. (2009) Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res 204:313–321. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (2007) The rat brain in stereotaxic coordinates. 7th edition. London: Elsevier Academic Press,. [Google Scholar]
- Richetto J, Labouesse MA, Poe MM, Cook JM, Grace AA, Riva MA, Meyer U. (2015) Behavioral effects of the benzodiazepine-positive allosteric modulator SH-053-2’F-S-CH3 in an immune-mediated neurodevelopmental disruption model. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzer JJ, Careaga M, Onore CE, Rushakoff JA, Berman RF, Ashwood P. (2013) Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl Psychiatry 3:e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SC. (1980) Deformed dendrites and reduced spine numbers on ectopic neurones in the hippocampus of rats exposed to methylazoxymethanol-acetate.A Golgi-Cox study. Acta Neuropathol 49:193–198. [DOI] [PubMed] [Google Scholar]
- Spano MS, Fadda P, Frau R, Fattore L, Fratta W. (2010) Cannabinoid self-administration attenuates PCP-induced schizophrenia-like symptoms in adult rats. Eur Neuropsychopharmacol 20:25–36. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. (2010) Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 11:100–113. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. (2004) Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 303:2040–2042. [DOI] [PubMed] [Google Scholar]
- Van den Eynde K, Missault S, Fransen E, Raeymaekers L, Willems R, Drinkenburg W, Timmermans JP, Kumar-Singh S, Dedeurwaerdere S. (2014) Hypolocomotive behaviour associated with increased microglia in a prenatal immune activation model with relevance to schizophrenia. Behav Brain Res 258:179–186. [DOI] [PubMed] [Google Scholar]
- Vuillermot S, Weber L, Feldon J, Meyer U. (2010) A longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia. J Neurosci 30:1270–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. (2012) Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74:858–873. [DOI] [PubMed] [Google Scholar]
- Winter C, Djodari-Irani A, Sohr R, Morgenstern R, Feldon J, Juckel G, Meyer U. (2009) Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: implications for brain disorders of neurodevelopmental origin such as schizophrenia. Int J Neuropsychopharmacol 12:513–524. [DOI] [PubMed] [Google Scholar]
- Wolff AR, Bilkey DK. (2008) Immune activation during mid-gestation disrupts sensorimotor gating in rat offspring. Behav Brain Res 190:156–159. [DOI] [PubMed] [Google Scholar]
- Wolff AR, Bilkey DK. (2010) The maternal immune activation (MIA) model of schizophrenia produces pre-pulse inhibition (PPI) deficits in both juvenile and adult rats but these effects are not associated with maternal weight loss. Behav Brain Res 213:323–327. [DOI] [PubMed] [Google Scholar]
- Wu WL, Adams CE, Stevens KE, Chow KH, Freedman R, Patterson PH. (2015) The interaction between maternal immune activation and alpha 7 nicotinic acetylcholine receptor in regulating behaviors in the offspring. Brain Behav Immun 46:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Li X, Ng TH, Morales M. (2011) Mesocorticolimbic glutamatergic pathway. J Neurosci 31:8476–8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. (2003) Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology 28:1778–1789. [DOI] [PubMed] [Google Scholar]