Abstract
Background:
As a modulator of dopaminergic system, trace amine-associated receptor 1 has been shown to play a critical role in regulating the rewarding properties of additive drugs. It has been demonstrated that activation of trace amine-associated receptor 1 decreased the abuse-related behaviors of cocaine in rats. However, the role of trace amine-associated receptor 1 in specific stages of cocaine reward memory is still unclear.
Methods:
Here, using a cocaine-induced conditioned place preference model, we tested the effects of a selective trace amine-associated receptor 1 agonist RO5166017 on the expression, reconsolidation, and extinction of cocaine reward memory.
Results:
We found that RO5166017 inhibited the expression but not retention of cocaine-induced conditioned place preference. RO5166017 had no effect on the reconsolidation of cocaine reward memory. Pretreatment with RO5166017 before extinction hindered the formation of extinction long-term memory. RO5166017 did not affect the movement during the conditioned place preference test, indicating the inhibitory effect of RO5166017 on the expression of cocaine-induced conditioned place preference was not caused by locomotion inhibition. Using a cocaine i.v. self-administration model, we found that the combined trace amine-associated receptor 1 partial agonist RO5263397 with extinction had no effect on the following cue- and drug-induced reinstatement of cocaine-seeking behavior. Repeated administration of the trace amine-associated receptor 1 agonist during extinction showed a continually inhibitory effect on the expression of cocaine reward memory both in cocaine-induced conditioned place preference and cocaine self-administration models.
Conclusions:
Taken together, these results indicate that activation of trace amine-associated receptor 1 specifically inhibited the expression of cocaine reward memory. The inhibitory effect of trace amine-associated receptor 1 agonists on cocaine reward memory suggests that trace amine-associated receptor 1 agonists could be a promising agent to prevent cocaine relapse.
Keywords: TAAR1, cocaine reward memory, expression, reconsolidation, extinction
Introduction
Trace amine-associated receptor 1 (TAAR1) is one of the most studied receptors of trace amines, a less well-characterized group of low-concentration amines derived from the metabolism of amino acids in the central and peripheral systems (Zucchi et al., 2006; Sotnikova et al., 2009). TAAR1 is a G protein-coupled receptor expressed broadly throughout the brain (Zucchi et al., 2006). In the rat, TAAR1 mRNA was found with the highest expression in the olfactory bulb, nucleus accumbens (NAc)/olfactory tubercle, cortical regions, substantia nigra, ventral tegmental area, cerebellum, and pons/medulla. Recently, TAAR1 has been demonstrated to be an important modulator of the dopaminergic, serotonergic, and glutamatergic activity (Xie and Miller, 2007; Miller, 2011; Espinoza et al., 2015). Modulation of TAAR1 activity by TAAR1 partial or full agonist would affect several psychiatric diseases, for example, schizophrenia, depression, and anxiety, suggesting a promising novel paradigm for neuropsychiatric therapeutics (Revel et al., 2012, 2013).
As a modulator of the dopaminergic system, TAAR1 has been shown to play a critical role in regulating the rewarding effects of drugs, especially the psychostimulant amphetamine (Lindemann et al., 2008). Amphetamine shares a similar chemical structure with the endogenous trace amine β-phenylethylamine. It is demonstrated that amphetamine and several amphetamine derivatives can activate TAAR1 directly (Bunzow et al., 2001). TAAR1 knockout mice showed higher sensitivity to amphetamine, d-amphetamine, and methamphetamine in the locomotor activity test (Wolinsky et al., 2007; Lindemann et al., 2008; Achat-Mendes et al., 2012). Interestingly, TAAR1 can also modulate the effects of another psychostimulant, cocaine, which does not directly activate TAAR1. Our recent results and others’ showed that TAAR1 partial agonists and full agonists decreased the abuse-related behaviors of cocaine in rats (Li, 2014; Pei et al., 2014; Thorn et al., 2014a, 2014d; Jing and Li, 2015; Pei et al., 2015). According to the previous results, it is suggested that TAAR1 agonists decrease the rewarding properties of cocaine. However, TAAR1 agonist RO5263397 had no effect on the induction but inhibited the expression of cocaine induced-conditioned place preference (CPP) (Thorn et al., 2014d). As CPP is a conditioned Pavlovian memory model (Sorg, 2012; Luo et al., 2013), TAAR1 may modulate the rewarding memory system to influence drug-related behaviors.
Persistent conditioned memories between the drug-paired cues and drug-induced effects play a critical role in drug addiction (Robbins et al., 2008; Milton and Everitt, 2012). It is suggested that the drug-associated learning hijacks the normal memory system in the brain to form a persistent reward memory. Drug memory has the same processes as the classic conditioned memory, that is, acquisition, consolidation, reconsolidation, extinction, and storage (Peters et al., 2009; Li et al., 2010; Alaghband and Marshall, 2013). By reexposure to the drug-paired cues or context, consolidated drug memory can enter into two opposite processes: reconsolidation and extinction. During reconsolidation, memories are labile and can be updated or disrupted (Nader et al., 2000b). Extinction suppresses but does not erase the original memory (Quirk and Mueller, 2008). In specific conditions, the original memory will reemerge after extinction, that is, reinstatement, renewal, and spontaneous recovery. Although previous studies showed that TAAR1 agonists inhibited cocaine-related behavior, it is unclear whether TAAR1 activation affects each reward memory process.
Here, we assessed the role of TAAR1 in cocaine reward memory by using the cocaine-induced CPP and cocaine self-administration models. We tested the effects of a TAAR1 full agonist RO5166017 on the expression, reconsolidation, and extinction of cocaine-induced CPP. Furthermore, we tested the effect of the TAAR 1 partial agonist RO5263397 combined with extinction on the subsequent reinstatement of cocaine-seeking behavior. It has been shown that TAAR 1 agonists with varying efficacy show similar antipsychotic-like properties and that both full and partial TAAR 1 agonists are effective in preventing cocaine-seeking behavior (Revel et al., 2013; Thorn et al., 2014; Pei et al.; 2015). This study used 2 different TAAR 1 agonists to confirm the pharmacological generality.
Materials and Methods
Subject
Adult male Sprague–Dawley rats (initial weight 250–280g; Harlan, Indianapolis, IN) were housed individually on a 12-hour-light/-dark cycle (behavioral experiments were conducted during the light period) with free access to water and food except during experimental sessions. For self-administration reinstatement studies, food access was restricted to 10g/d for 3 days before lever press training for food. Animals were maintained, and surgical and experimental procedures were approved by the Institutional Animal Care and Use Committee, University at Buffalo, the State University of New York, and with the 2011 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences, Washington, DC).
Drugs
Drugs used in this study included cocaine hydrochloride (Research Technology Branch, National Institute of Drug Abuse, Rockville, MD), RO5166017, and RO5263397 (synthesized at Research Triangle Institute, purity >98%). Cocaine hydrochloride was dissolved in 0.9% physiological saline. RO5166017 (10mg/mL/kg, i.p.) and RO5263397 (5.6mg/mL/kg, i.p.) were dissolved in a mixture of 1 part absolute ethanol, 1 part Emulphor-620 (Rhodia), and 18 parts physiologic saline.
Cocaine-Induced CPP
The experimental chambers for automated assessment of CPP consisted of 2 custom-made Perspex compartments of the same size (35cm × 28cm × 32cm) separated by a removable Perspex guillotine door. One compartment consisted of custom-made stainless-steel mesh floor and white-painted wall; the other compartment consisted of custom-made stainless grid rod floor and black-painted wall. Adjustable house lights (ENV221M, Med Associates, St Albans, VT) were mounted on the lids of both compartments. Locomotor activity was monitored by photocell detectors (ENV256, Med Associates). Data recording was controlled by Med-PC IV software (SOF700RA-25, Med Associates).
The procedure for cocaine-induced CPP was the same as our previous study (Thorn et al., 2014d). Briefly, all rats were allowed free access to both compartments for 15minutes to verify the absence of preference for either side (pretest). Six rats in this study spending >75% (>675seconds) or <25% (< 225 seconds) of the total time in a compartment were eliminated from further testing. Group assignment was counterbalanced within each group according to the pretest results such that cocaine was paired with the more-preferred compartment for one-half of the rats and paired with the less-preferred compartment for the other one-half of the rats. Following the pretest phase, rats underwent place conditioning across 8 days, with alternating treatment-compartment pairings. For a conditioning session, saline (1mL/kg, i.p.) or cocaine (10mg/kg, i.p.) was administered, and the rat was confined to the paired compartment for 30minutes. One day later, rats were randomly placed into one compartment and had access to both compartments during the 15-minute test period (posttest), and the time spent in each compartment was recorded. In the study that examined the effect of RO5166017 on the expression of cocaine CPP, RO5166017 was administered once 10minutes before CPP test. The CPP score was defined as the time (in seconds) spent in the cocaine-paired compartment minus the time spent in the saline-paired compartment during the CPP test (Jian et al., 2014; Xue et al., 2014).
Reconsolidation of Cocaine-Induced CPP
In the reconsolidation experiment, 1 day after posttest, rats were reexposed to the drug-paired side for 10 minutes to reactive cocaine reward memory. Rats were administered RO5166017 immediately after reexposure and then placed back in their home cages. CPP was tested 24 hours later (test 1). One day after test 1, the reexposure and RO5166017 treatment was repeated once. CPP was tested again 24 hours later (test 2). One day after test 2, the rats were administered one dosing of cocaine (10mg/kg) followed by a CPP test to examine the reinstatement of cocaine-induced CPP.
Extinction of Cocaine-Induced CPP
In the extinction experiment, rats were tested once daily after posttest for 6 days to extinguish cocaine-induced CPP. RO5166017 was administered 10 minutes before each test during extinction. One day after extinction, rats were tested for extinction long-term memory (LTM) without pretreatment of RO5166017.
I.V. Self-Administration Apparatus
Twelve standard operant chambers (Med Associates) were used for all cocaine self-administration studies. Each chamber was equipped with a house light that signaled the start of the session and was turned off during the timeout periods. On one wall of the chamber were 2 response levers and a food receptacle (5- × 5-cm opening) located 3cm above the chamber floor and midway between the levers. A pellet dispenser delivered 45-mg food pellets. Stimulus lights located above each lever illuminated the chamber during sessions. Infusion pumps were located outside of the sound-attenuating cubicles and delivered drug or vehicle via Tygon tubing through a swivel (Instech, Plymouth Meeting, PA). Stimulus presentations, food delivery, drug infusion, and data recording were controlled by Med-PC IV software and solid state interface equipment (Med Associates).
Food Training and Catheterization Surgery
The procedure for food training was the same as our previous study (Thorn et al., 2014d). For cocaine self-administration reinstatement experiments, rats were first trained to lever press for food reward (45mg; BioServ, Frenchtown, NJ). During the daily 1-hour training sessions, rats could press the right lever (active lever) under a fixed ratio (FR) 1 schedule and earn up to 100 food pellets. The response requirement was gradually increased to FR 5 over a period of 7 days. Responses on the left lever (inactive lever) were recorded but had no programmed consequence. After the food training, rats were implanted with chronic indwelling jugular catheters and allowed 7 days to recover following surgery as previously described (Thorn et al., 2014d). Catheters were flushed daily with 0.2mL solution of enrofloxacin (4mg/mL) mixed in a heparinized saline solution (50IU/mL in 0.9% sterile saline) to preserve catheter patency.
Cocaine Self-Administration
One week after surgery, rats began to self-administer 0.75mg/kg/infusion cocaine. Rats self-administered cocaine for 14 sessions, during which responses to the active lever resulted in i.v. injections of cocaine under a FR 5 schedule of reinforcement followed by a 30-second time-out period. Infusions were accompanied by a 5-secibd illumination of the stimulus light above the active lever, and the house light was extinguished for the duration of the time-out period. Sessions were terminated after either a 2-hour duration or 40 infusions had been earned, whichever occurred first. Following cocaine self-administration sessions, rats then were given 7 daily extinction sessions, during which lever presses had no consequence (no drug or cues). RO5263397 was administered 10 minutes before each session of extinction daily. Cue- or drug (10mg/kg cocaine, i.p.)-induced reinstatement was tested 24hours after the last day of extinction session, during which active lever presses resulted in presentation of light cues in the same manner as during self-administration with no drug delivery.
Data Analysis
All results are presented as mean±SEM. All data were analyzed by 1-way or 2-way repeated-measures ANOVA followed by Bonferroni’s posthoc test to compare the differences among groups treated with vehicle and TAAR1 agonist. Student’s t test was used to compare difference between the 2 groups. P < .05 was considered statistically significant.
Results
RO5166017 Suppressed the Expression but Not the Retention of Cocaine-Induced CPP
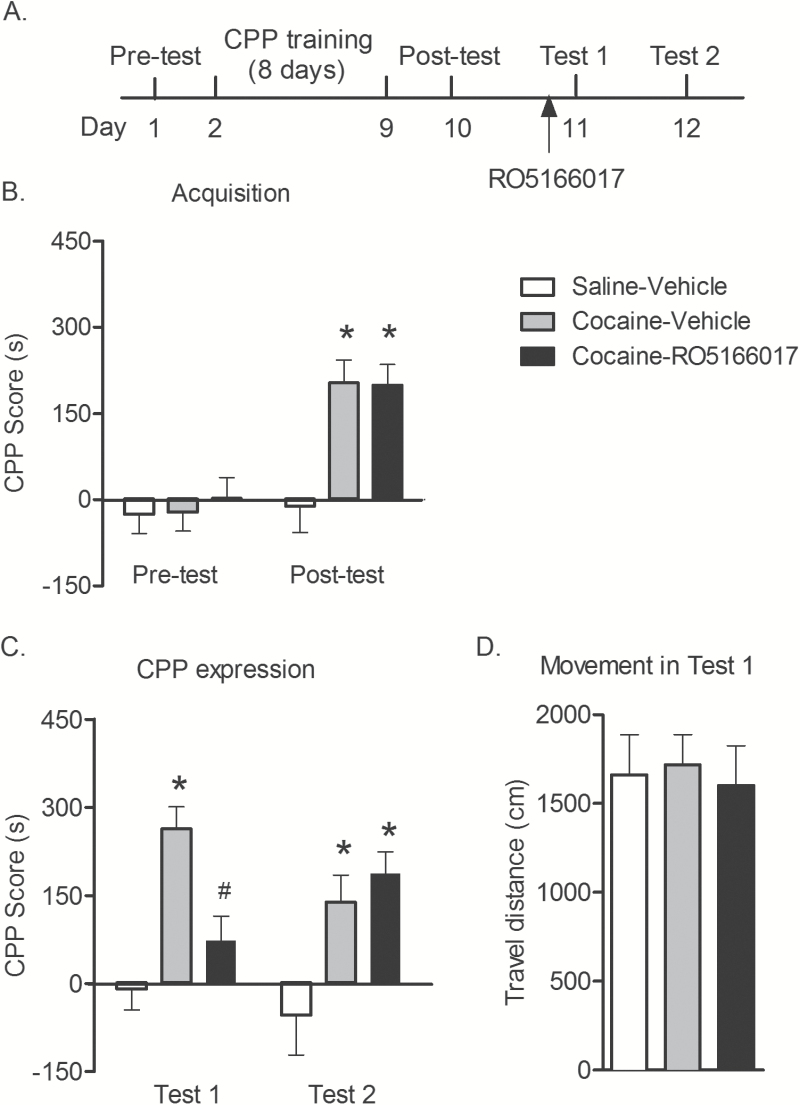
We first examined the effect of the TAAR1 full agonist RO5166017 on the expression of cocaine reward memory. Rats were assigned to different groups according to the baseline test (pretest) and then trained for cocaine-induced CPP (n = 9–10/group). One day after conditioning, rats underwent the postconditioning CPP test (posttest), which was conducted without any pretreatment (Figure 1A). The preference for drug side in posttest was increased in the cocaine-treated group compared with the saline-treated group (F 2,25 = 10.17, P < .05) (Figure 1B). One day after posttest, rats were administered RO5166017 or vehicle followed by CPP tests immediately and 24 hours later. Two-way repeated-measures ANOVA was conducted to analyze the effect of RO5166017 on the expression of cocaine reward memory, indicating an effect of group (F 2,25 = 2.97, P < .05) and interaction of group × test (F 2,25 = 5.42, P < .05) (Figure 1C). Bonferroni’s multiple comparisons test showed that RO5166017 inhibited the expression of cocaine-induced CPP when tested immediately but not 24 hours later.
Figure 1.
RO5166017 suppressed the expression but not the retention of cocaine-induced conditioned place preference (CPP). (A) Experiment design. (B) Rats acquired cocaine-induced CPP after training (both P < .05). (C) Pretreatment of RO5166017 before test 1 inhibited cocaine-induced CPP (P < .05). The inhibitory effect on CPP did not persist when tested again 1 day later. (D) No difference was found in the movement in test 1 (both P > .05). Data are shown as mean ± SEM, n = 9–10/group. *P < .05, compared with saline-vehicle group; #P < .05 compared with cocaine-vehicle group.
Furthermore, RO5166017 did not affect the movement during the CPP test 1 (F 2,25 = 0.12, P > .05) (Figure 1D), indicating the inhibitory effect of RO5166017 on expression of cocaine-induced CPP was not caused by locomotion inhibition. Altogether, these results demonstrated that activation of TAAR1 suppressed the expression but not the retention of cocaine reward memory.
RO5166017 Had No Effect on the Reconsolidation of Cocaine-Induced CPP
After retrieval, memory could enter into the reconsolidation process, in which memory can be updated or disrupted (Nader et al., 2000a; Lee, 2009; Liu et al., 2014). Although pretreatment of RO5166017 before the CPP test inhibited the expression of cocaine-induced CPP, it is unclear whether RO5166017 prevents the reactivation of cocaine reward memory, which is critical for initiation of reconsolidation. We then tested whether treatment of RO5166017 after reactivation of cocaine reward memory would affect the reconsolidation of cocaine-induced CPP.
One day after conditioning, rats underwent the posttest, which was conducted without any pretreatment (Figure 2A). Two groups of rats were trained cocaine-induced CPP (F 1,17 = 0.03, P > .05; n = 9–10/group) (Figure 2B). One day after posttest, rats were placed into the cocaine-paired side of the CPP chamber for 10 minutes to reactivate cocaine reward memory (Li et al., 2010; Jian et al., 2014). Immediately after that, rats were treated with RO5166017 or vehicle and then placed back in their home cages. Cocaine-induced CPP was tested 24 hours later (test 1). Unpaired t test showed no difference (t17 =0.11, P > .05). To confirm this effect, we repeated the reconsolidation procedure once. One day after test 1, rats were exposed to the cocaine-paired side again followed by RO5166107 treatment and underwent the CPP test 24 hours later. No difference was found during the test 2 (t17 = 0.45, P > .05) (Figure 2C). One day after test 2, rats were given an injection of cocaine (10mg/kg, i.p.) to test the reinstatement of cocaine-induced CPP. No difference was found between the 2 groups (t17 = 0.09, P > .05) (Figure 2D), suggesting that the cocaine reward memory was intact. RO5166017 did not affect the movement during all of the CPP tests (data not shown). Altogether, these results indicated that activation of TAAR1 did not affect the reconsolidation of cocaine reward memory.
Figure 2.
RO5166017 had no effect on the reconsolidation of cocaine-induced conditioned place preference (CPP). (A) Experiment design. (B) Rats acquired cocaine-induced CPP after training (both P < .05). (C) RO5166017 after reexposure did not affect following CPP test 1 day later (both P > .05), suggested that RO5166017 had no effect on the reconsolidation of cocaine-induced CPP. (D) No difference was found in the movement in reinstatement test (P > .05). Data are shown as mean ± SEM, n = 9–10/group. *P < .05, compared with pretest.
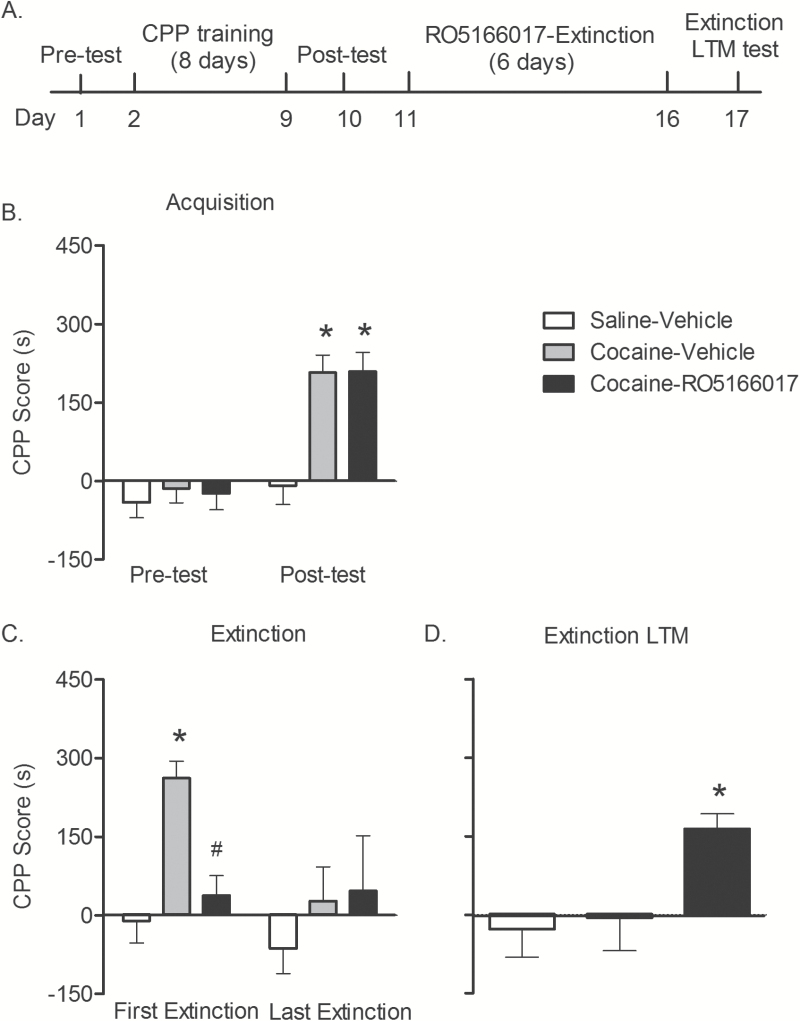
Pretreatment of RO5166017 Prevented the Formation of Cocaine Extinction LTM
Repeated exposure to conditioned stimulus leads to extinction. To test the effect of TAAR1 activation on cocaine reward memory extinction, 2 groups of rats were trained cocaine-induced CPP, and one saline group was set as control. One day after conditioning, rats underwent the posttest, which was conducted without any pretreatment (Figure 3A). Rats trained with cocaine showed significant preference for the drug-paired side in posttest (F2,32 = 5.37, P < .05; n = 11–12/group) (Figure 3B). Rats were then tested CPP for 6 continuous days. RO5166017 was administered 10 minutes before each test. Two-way repeated-measures ANOVA with group (saline-vehicle, cocaine-vehicle, and cocaine-RO5166017) as between subjects and test (first trial and last trial of extinction) as within subjects was conducted to analyze the effect of RO5166017 on extinction, showing an effect of group (F2,32 = 3.83, P < .05) and test (F2,32 = 4.64, P < .05) and a strong trend of interaction of group × test (F2,32 = 2.89, P = .07). Bonferroni’s multiple comparisons test showed that RO5166017 inhibited the expression of cocaine-induced CPP in the first trial of extinction (t32 = 2.65, P < .05) (Figure 3C). RO5166017 did not affect the movement during all of the CPP tests (data not shown). Rats were then tested for extinction LTM 1 day later. One-way ANOVA showed a significant effect (F2,32 = 4.00, P < .05). Posthoc analysis showed that rats treated with RO5166017 but not vehicle showed a robust preference for the cocaine-paired side compared with the control group (t32 = 2.60, P < .05) (Figure 3D), indicating that RO5166017 disrupted the formation of extinction LTM.
Figure 3.
Pretreatment of RO5166017 prevented the formation of cocaine extinction long-term memory (LTM). (A) Experiment design. (B) Rats acquired cocaine-induced conditioned place preference (CPP) after training (both P < .05). (C) RO5166017 inhibited expression of CPP during first extinction (P < .05). No difference was found in the last extinction (both P > .05). (D) Pretreatment of RO5166017 before extinction prevented the formation of extinction LTM (P < .05). Data are shown as mean ± SEM, n = 11–12/group. *P < .05, compared with saline-vehicle group; # P < .05 compared with cocaine-vehicle group.
TAAR1 Partial Agonist RO5263397 Combined with Extinction Did Not Affect the Subsequent Reinstatement of Cocaine-Seeking Behavior
We tested whether activation of TAAR1 before extinction would affect the extinction LTM in rat cocaine self-administration model. Two groups of rats were trained to self-administer cocaine, with a group of rats self-administering saline as control (n = 8–10/group). Rats trained with cocaine showed higher infusions compared with the saline group on the last day of training (F2,24 = 2.96, P < .05) (Figure 4A). One day after training, rats were administered RO5263397 followed by 2-hour extinction test daily for 10 days. Two-way repeated-measures ANOVA with group (saline-vehicle, cocaine-vehicle, and cocaine-RO5263397) as between-subjects factor and test (10 days of extinction) as within-subjects factor was conducted to analyze the effect of RO5263397 on extinction. The analyses showed significant main effects of group (F2,24 = 10.22, P < .05), test (F9,216 = 40.79, P < .05), and interaction of group × test (F18,216 = 9.85, P < .05). Bonferroni’s multiple comparisons test showed that RO5263397 decreased the active responses on days 1, 2, and 4 during extinction (all P < .05) (Figure 4B). One day after the last day of extinction, rats underwent a cue-induced reinstatement test. One-way ANOVA showed a significant effect (F2,24 = 6.94, P < .05). No difference was found between groups of cocaine-vehicle and cocaine-RO5263397 (P > .05) (Figure 4C). One day later, rats were administered a dose of cocaine (10mg/kg, i.p.) to test drug-induced reinstatement. One-way ANOVA showed a significant effect (F2,24 = 20.24, P < .05). No difference was found between the 2 groups of cocaine-vehicle and cocaine-RO5263397. Altogether, these results indicated that activation of TAAR1 combined with extinction had no effect on subsequent reinstatement of cocaine-seeking behavior.
Figure 4.
Trace amine-associated receptor 1 (TAAR1) partial agonist RO5263397 combined with extinction did not affect the subsequent reinstatement of cocaine-seeking behavior. (A) Two groups of rats that were trained to self-administer cocaine showed no difference in cocaine taking on the last day (P > .05). (B) RO5263397 decreased the active responses during extinction (P < .05). (C) Discrete cue reinstated extinguished active responses in both cocaine self-administrated groups of rats (both P < .05). No difference was found between cocaine-vehicle and cocaine-RO5263397 groups (P > .05). (D) Active responses were reinstated after cocaine priming (both P < .05). No difference was found between cocaine-vehicle and cocaine-RO5263397 groups (P > .05). Data are shown as mean ± SEM, n = 9–10/group. *P < .05, compared with saline-vehicle group; # P < .05 compared with cocaine-RO5263397 group.
Discussion
TAAR1 Activation Inhibits Cocaine Reward Memory
Preventing drug relapse by inhibition or disruption of drug related memories showed a promising strategy to treat drug addiction (Koob, 2009; Milton and Everitt, 2012; Taylor and Torregrossa, 2015). Two kinds of drug memory models were used in the present study: a Pavlovian conditioned memory model (cocaine-induced CPP) and an instrumental memory model (cocaine self-administration). In the cocaine-induced CPP model, appetitive context is associated with drug reward effect, which will elicit a conditioned response after conditioning. The conditioned response is strongly dependent on the strength of the LTM of the association between appetitive context and drug effect (Itzhak et al., 2014). Cocaine self-administration is also dependent on reinforcement learning, whereas an operant behavior is involved in this process, which makes it completely different from the CPP behavior (Aguilar et al., 2009; Sorg, 2012). The present study showed that TAAR 1 agonists inhibited expression of both kinds of memory, suggesting that the effect of TAAR 1 agonists on cocaine reward memory were independent of memory types.
Consistent with our previous results that the TAAR1 partial agonist RO5263397 inhibited the expression of cocaine-induced CPP (Thorn et al., 2014d), the present data showed that the TAAR1 full agonist RO5166017 given before test also decreased cocaine-induced CPP, suggesting that the activation of TAAR1 inhibited the retrieval of cocaine reward memory. However, activation of TAAR1 did not erase the drug reward memory, even when combined with reconsolidation and extinction procedure. The failure of TAAR 1 agonist on reconsolidation was not due to the posttest conducted following CPP training, because previous studies have shown that one posttest does not prevent subsequent reexposure-induced reconsolidation in cocaine-induced CPP (Li et al., 2010; Jian et al., 2014). Our previous result indicated that RO5263397 did not affect the development of cocaine-induced CPP (Thorn et al., 2014a), which suggested that TAAR1 is not involved in the acquisition or consolidation of cocaine-induced CPP. To enter into the reconsolidation process, drug memory has to be reactivated. Reexposure to drug-paired context is a process to reactivate memory. However, the present data cannot identify whether pretreatment of RO5263397 prevented the reactivation of drug memory, since memory reactivation could be independent of memory expression (Barreiro et al., 2013). The results that pretreatment of RO5263397 before each trial of extinction inhibited cocaine-induced CPP confirmed the inhibitory role of TAAR1 activation on the expression of cocaine reward memory. Furthermore, RO5166017 prevented the formation of extinction LTM, suggesting that activation of TAAR1 could hamper extinction learning. Nevertheless, recently it is suggested that extinction learning might not be a consequence of retrieval (de Carvalho Myskiw et al., 2015). It is also possible that pretreatment of RO5166017 before extinction did not prevent extinction learning but disrupted the consolidation of extinction, which requires further studies to identify the role of TAAR1 in extinction memory. Taken together, these data indicated that the unique effect of activation of TAAR1 in all memory stages of cocaine-induced CPP is to inhibit the retrieval of cocaine reward memory.
Although erasure of drug memory would be better than inhibition of drug memory to prevent relapse, there is no effective agent to selectively erase specific memory nowadays. The continued inhibition on cocaine reward memory by RO5166017 and RO5263397 when being administered before extinction each day suggested a consistent inhibitory effect of TAAR 1 agonists on drug-seeking behavior. These data suggested that TAAR1 agonist could be a promising candidate to prevent cocaine relapse. However, the major problem in overcoming cocaine relapse after long-term abstinence is craving, and no detailed date showed the effect of TAAR1 agonist on drug craving.
TAAR1 in Drug Memory Model: Drug Reward Properties or Drug Memory
TAAR1 is suggested to be a modulator of dopaminergic system (Xie and Miller, 2007). It has been demonstrated that the firing rate of dopaminergic neurons in the ventral tegmental area was higher in the TAAR1 knockout mice compared with wild-type mice. The endogenous TAAR1 agonist p-tyramine decreased the spike frequency of ventral tegmental area dopaminergic neurons in wild-type mice (Lindemann et al., 2008). RO5166017 also decreased the firing of DA neurons in a manner similar to p-tyramine (Revel et al., 2011). Other studies found that extracellular dopamine was increased in the NAc of TAAR1 knockout mice. Furthermore, RO5166017 reduced the electrically evoked dopamine overflow in the NAc (Leo et al., 2014). It is suggested that TAAR1 activation decreased the drug reward properties of cocaine. Consistent with this, our previous results showed that the TAAR1 agonist RO5263397 increased the elasticity of cocaine demand curve (Thorn et al., 2014d). TAAR1 full agonist and partial agonist both decreased the reinforcing efficacy of cocaine and prevented cocaine-induced changes in brain reward thresholds (Pei et al., 2015).
Our previous study showed that the TAAR1 partial agonist RO5263397 prevented both the development and expression of cocaine-induced behavioral sensitization (Thorn et al., 2014a). However, in the cocaine-induced CPP model, activation of TAAR1 only inhibited the expression but not the development of cocaine-induced CPP (Thorn et al., 2014d), suggesting that the effect of RO5166017 on cocaine-induced CPP could not be attributed to the inhibition of reward properties of cocaine. Moreover, RO5263397 decreased the challenge dose of cocaine-induced hyperactivity only after the rats were sensitized to, but not acutely exposed to, cocaine (Thorn et al., 2014d). TAAR1 knockout mice showed facilitated methamphetamine-induced CPP, but the same level of methamphetamine-induced reinstatement of CPP was observed, suggesting that the effects of TAAR1 in drug-related behaviors are behavior dependent (Achat-Mendes et al., 2012). Consistent with this, although TAAR1 agonists reduce the maintenance of cocaine self-administration (Pei Y et al., 2015; D. A. Thorn, J. Li, unpublished observation), the combination of a TAAR1 agonist with extinction had no effect on the following cue- and drug-induced reinstatement. These results suggested that activation of TAAR1 could not, at least completely, prevent the rewarding effects of cocaine under specific conditions.
The role of TAAR1 in cognition and memory has not been clearly investigated. In the object retrieval paradigm test in monkeys, a TAAR1 full agonist RO5256390 and partial agonist RO5263397 and RO5203648 improved accuracy in the difficult trials, indicating cognition-enhancing properties of these compounds (Revel et al., 2012, 2013). In the attentional set-shifting task in rats, RO5256390 reversed the NMDA receptor blocker PCP-induced deficit in extradimensional attentional set-shifting performance (Revel et al., 2013). These data suggested that TAAR1 activation could improve executive function, which is mainly controlled by the prefrontal cortex. Besides the role of modulating dopaminergic system, TAAR1 has been demonstrated playing an important role in regulating glutamatergic system. RO5166017 reversed a glycin site NMDA receptor antagonist L-687, 414-induced hyperlocomotion (Revel et al., 2011). TAAR1 knockout mice showed a perseverative and impulsive phenotype, which was associated with the dysregulated glutamate transmission in the PFC (Espinoza et al., 2015). As the PFC is a critical brain area for self-control in addiction (Heatherton and Wagner, 2011; Tang et al., 2015), RO5166017 could also improve the executive function of PFC to reduce the motivation to cocaine-related context cue and inhibited the expression of cocaine-induced CPP.
The Effects of TAAR1 Activation on Other Drugs
The role of TAAR1 in specific stages of other drug memories, for example, consolidation, expression, reconsolidation, extinction, and storage, is still unknown. TAAR1 knockout mice showed normal morphine-induced CPP compared with wild-type mice (Achat-Mendes et al., 2012). Although cocaine and morphine are different addictive drugs, they share some common molecular mechanisms. The different roles of TAAR1 in cocaine and morphine would provide us with different mechanisms underlying these 2 kinds of drugs of abuse. As severe withdrawal signs developed after morphine dependence but not cocaine abstinence, we presume that TAAR1 might pay little role in the “dark side” of drug addiction (Koob, 2015; Koob and Mason, 2015). Most studies on TAAR1 in drug addiction are psycho-stimulants, especially amphetamines and cocaine, therefore it is important to test the role of TAAR1 in other drugs of abuse, especially depressants, for example, alcohol (Lynch et al., 2013). To demonstrate the distinct roles of TAAR1 in different kinds of addictive drugs would help us to understand both the function of TAAR1 in addiction and drug addiction itself.
Conclusion
Taken together, our results revealed that 2 TAAR1 agonists RO5166017 and RO5263397 inhibited the expression of cocaine memory. Repeated administration of RO5166017 showed a continually inhibitory effect on the expression of cocaine reward memory. However, RO5166017 had no effect on reconsolidation, extinction, or storage of cocaine reward memory, suggesting that TAAR1 activation did not interfere with all stages of cocaine reward memory. These data suggested that TAAR1 agonist could be a promising agent to prevent cocaine relapse.
Statement of Interest
None.
Acknowledgments
This work was supported by the National Institute of Health grants R01-DA034806 and R21-DA033426. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Achat-Mendes C, Lynch LJ, Sullivan KA, Vallender EJ, Miller GM. (2012) Augmentation of methamphetamine-induced behaviors in transgenic mice lacking the trace amine-associated receptor 1. Pharmacol Biochem Behav 101:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar MA, Rodriguez-Arias M, Minarro J. (2009) Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Res Rev 59:253–277. [DOI] [PubMed] [Google Scholar]
- Alaghband Y, Marshall JF. (2013) Common influences of non-competitive NMDA receptor antagonists on the consolidation and reconsolidation of cocaine-cue memory. Psychopharmacology (Berl) 226:707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro KA, Suarez LD, Lynch VM, Molina VA, Delorenzi A. (2013) Memory expression is independent of memory labilization/reconsolidation. Neurobiol Learn Mem 106:283–291. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, Olson SB, Magenis RE, Amara SG, Grandy DK. (2001) Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol 60:1181–1188. [DOI] [PubMed] [Google Scholar]
- de Carvalho Myskiw J, Furini CR, Schmidt B, Ferreira F, Izquierdo I. (2015) Extinction learning, which consists of the inhibition of retrieval, can be learned without retrieval. Proc Natl Acad Sci U S A 112:E230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza S, Lignani G, Caffino L, Maggi S, Sukhanov I, Leo D, Mus L, Emanuele M, Ronzitti G, Harmeier A, Medrihan L, Sotnikova TD, Chieregatti E, Hoener MC, Benfenati F, Tucci V, Fumagalli F, Gainetdinov RR. (2015) TAAR1 modulates cortical glutamate NMDA receptor function. Neuropsychopharmacology 40:2217–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Wagner DD. (2011) Cognitive neuroscience of self-regulation failure. Trends Cogn Sci 15:132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak Y, Perez-Lanza D, Liddie S. (2014) The strength of aversive and appetitive associations and maladaptive behaviors. IUBMB Life 66:559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian M, Luo YX, Xue YX, Han Y, Shi HS, Liu JF, Yan W, Wu P, Meng SQ, Deng JH, Shen HW, Shi J, Lu L. (2014) eIF2alpha dephosphorylation in basolateral amygdala mediates reconsolidation of drug memory. J Neurosci 34:10010–10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L, Li JX. (2015) Trace amine-associated receptor 1: a promising target for the treatment of psychostimulant addiction. Eur J Pharmacol 761:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. (2009) Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry 1:S32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. (2015) The dark side of emotion: the addiction perspective. Eur J Pharmacol 753:73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Mason BJ. (2016) Existing and future drugs for the treatment of the dark side of addiction. Annu Rev Pharmacol Toxicol 56:299–322. [DOI] [PubMed] [Google Scholar]
- Lee JL. (2009) Reconsolidation: maintaining memory relevance. Trends Neurosci 32:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo D, Mus L, Espinoza S, Hoener MC, Sotnikova TD, Gainetdinov RR. (2014) Taar1-mediated modulation of presynaptic dopaminergic neurotransmission: role of D2 dopamine autoreceptors. Neuropharmacology 81:283–291. [DOI] [PubMed] [Google Scholar]
- Li FQ, Xue YX, Wang JS, Fang Q, Li YQ, Zhu WL, He YY, Liu JF, Xue LF, Shaham Y, Lu L. (2010) Basolateral amygdala cdk5 activity mediates consolidation and reconsolidation of memories for cocaine cues. J Neurosci 30:10351–10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX. (2014) Trace amines and cocaine abuse. ACS Chem Neurosci 5:497–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H, Bettler B, Wettstein JG, Borroni E, Moreau JL, Hoener MC. (2008) Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther 324:948–956. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhao L, Xue Y, Shi J, Suo L, Luo Y, Chai B, Yang C, Fang Q, Zhang Y, Bao Y, Pickens CL, Lu L. (2014) An unconditioned stimulus retrieval extinction procedure to prevent the return of fear memory. Biol Psychiatry 76:895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo YX, Xue YX, Shen HW, Lu L. (2013) Role of amygdala in drug memory. Neurobiol Learn Mem 105:159–173. [DOI] [PubMed] [Google Scholar]
- Lynch LJ, Sullivan KA, Vallender EJ, Rowlett JK, Platt DM, Miller GM. (2013) Trace amine associated receptor 1 modulates behavioral effects of ethanol. Subst Abuse 7:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GM. (2011) The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J Neurochem 116:164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. (2012) The persistence of maladaptive memory: addiction, drug memories and anti-relapse treatments. Neurosci Biobehav Rev 36:1119–1139. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. (2000. a) Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406:722–726. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. (2000. b) The labile nature of consolidation theory. Nat Rev Neurosci 1:216–219. [DOI] [PubMed] [Google Scholar]
- Pei Y, Lee J, Leo D, Gainetdinov RR, Hoener MC, Canales JJ. (2014) Activation of the trace amine-associated receptor 1 prevents relapse to cocaine seeking. Neuropsychopharmacology 39:2299–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Mortas P, Hoener MC, Canales JJ. (2015) Selective activation of the trace amine-associated receptor 1 decreases cocaine’s reinforcing efficacy and prevents cocaine-induced changes in brain reward thresholds. Prog Neuropsychopharmacol Biol Psychiatry 63:70–75. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. (2009) Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem 16:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. (2008) Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33:56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, Durkin S, Zbinden KG, Norcross R, Meyer CA, Metzler V, Chaboz S, Ozmen L, Trube G, Pouzet B, Bettler B, Caron MG, Wettstein JG, Hoener MC. (2011) TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc Natl Acad Sci U S A 108:8485–8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel FG, Moreau JL, Gainetdinov RR, Ferragud A, Velazquez-Sanchez C, Sotnikova TD, Morairty SR, Harmeier A, Groebke Zbinden K, Norcross RD, Bradaia A, Kilduff TS, Biemans B, Pouzet B, Caron MG, Canales JJ, Wallace TL, Wettstein JG, Hoener MC. (2012) Trace amine-associated receptor 1 partial agonism reveals novel paradigm for neuropsychiatric therapeutics. Biol Psychiatry 72:934–942. [DOI] [PubMed] [Google Scholar]
- Revel FG, Moreau JL, Pouzet B, Mory R, Bradaia A, Buchy D, Metzler V, Chaboz S, Groebke Zbinden K, Galley G, Norcross RD, Tuerck D, Bruns A, Morairty SR, Kilduff TS, Wallace TL, Risterucci C, Wettstein JG, Hoener MC. (2013) A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight. Mol Psychiatry 18:543–556. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. (2008) Drug addiction and the memory systems of the brain. Ann N Y Acad Sci 1141:1–21. [DOI] [PubMed] [Google Scholar]
- Sorg BA. (2012) Reconsolidation of drug memories. Neurosci Biobehav Rev 36:1400–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotnikova TD, Caron MG, Gainetdinov RR. (2009) Trace amine-associated receptors as emerging therapeutic targets. Mol Pharmacol 76:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Posner MI, Rothbart MK, Volkow ND. (2015) Circuitry of self-control and its role in reducing addiction. Trends Cogn Sci DOI: 10.1016/j.tics.2015.06.007 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Taylor JR, Torregrossa MM. (2015) Pharmacological disruption of maladaptive memory. Handb Exp Pharmacol 228:381–415. [DOI] [PubMed] [Google Scholar]
- Thorn DA, Zhang C, Zhang Y, Li JX. (2014. a) The trace amine associated receptor 1 agonist RO5263397 attenuates the induction of cocaine behavioral sensitization in rats. Neurosci Lett 566:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, Jing L, Qiu Y, Gancarz-Kausch AM, Galuska CM, Dietz DM, Zhang Y, Li JX. (2014. d) Effects of the trace amine-associated receptor 1 agonist RO5263397 on abuse-related effects of cocaine in rats. Neuropsychopharmacology 39:2309–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky TD, Swanson CJ, Smith KE, Zhong H, Borowsky B, Seeman P, Branchek T, Gerald CP. (2007) The Trace Amine 1 receptor knockout mouse: an animal model with relevance to schizophrenia. Genes Brain Behav 6:628–639. [DOI] [PubMed] [Google Scholar]
- Xie Z, Miller GM. (2007) Trace amine-associated receptor 1 is a modulator of the dopamine transporter. J Pharmacol Exp Ther 321:128–136. [DOI] [PubMed] [Google Scholar]
- Xue YX, Xue LF, Liu JF, He J, Deng JH, Sun SC, Han HB, Luo YX, Xu LZ, Wu P, Lu L. (2014) Depletion of perineuronal nets in the amygdala to enhance the erasure of drug memories. J Neurosci 34:6647–6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi R, Chiellini G, Scanlan TS, Grandy DK. (2006) Trace amine-associated receptors and their ligands. Br J Pharmacol 149:967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]