Abstract
Background:
A difficult problem in treating opioid addicts is the maintenance of a drug-free state because of the negative emotional symptoms associated with withdrawal, which may trigger relapse. Several lines of evidence suggest a role for the metabotropic glutamate receptor 5 in opioid addiction; however, its involvement during opioid withdrawal is not clear.
Methods:
Mice were treated with a 7-day escalating-dose morphine administration paradigm. Following withdrawal, the development of affective behaviors was assessed using the 3-chambered box, open-field, elevated plus-maze and forced-swim tests. Metabotropic glutamate receptor 5 autoradiographic binding was performed in mouse brains undergoing chronic morphine treatment and 7 days withdrawal. Moreover, since there is evidence showing direct effects of opioid drugs on the metabotropic glutamate receptor 5 system, the presence of an metabotropic glutamate receptor 5/μ-opioid receptor interaction was assessed by performing metabotropic glutamate receptor 5 autoradiographic binding in brains of mice lacking the μ-opioid receptor gene.
Results:
Withdrawal from chronic morphine administration induced anxiety-like, depressive-like, and impaired sociability behaviors concomitant with a marked upregulation of metabotropic glutamate receptor 5 binding. Administration of the metabotropic glutamate receptor 5 antagonist, 3-((2-Methyl-4-thiazolyl)ethynyl)pyridine, reversed morphine abstinence-induced depressive-like behaviors. A brain region-specific increase in metabotropic glutamate receptor 5 binding was observed in the nucleus accumbens shell, thalamus, hypothalamus, and amygdala of μ-opioid receptor knockout mice compared with controls.
Conclusions:
These results suggest an association between metabotropic glutamate receptor 5 alterations and the emergence of opioid withdrawal-related affective behaviors. This study supports metabotropic glutamate receptor 5 system as a target for the development of pharmacotherapies for the treatment of opioid addiction. Moreover, our data show direct effects of μ-opioid receptor system manipulation on metabotropic glutamate receptor 5 binding in the brain.
Keywords: Morphine, withdrawal, μ-opioid receptor, mGlu5R, opioids
Introduction
Opioid addiction is a relapsing brain disorder characterized by compulsion to seek and take the drug, despite the negative physical and emotional consequences following abstinence (Le Moal and Koob, 2007). Symptoms associated with affective emotional impairment, including anxiety, depression, stress, and anhedonia, during drug withdrawal act as a motivational trigger to relapse (Martin and Jasinski, 1969; Jaffe, 1990; Nunes et al., 2004; Peles et al., 2007), which is the primary problem for the treatment of opioid addiction (Stotts et al., 2009). In fact, there is 30% to 40% comorbidity between substance use disorders and illicit drug abuse with major depression (Davis et al., 2008). Addressing the mechanisms underlying the emotional impairment associated with opioid abstinence is essential given this high comorbidity prevalence, along with the ambiguous efficacy of classic antidepressant treatment in this patient population (Nunes et al., 2004).
While opioid drugs, including morphine and heroin, primarily act on the μ-opioid receptor (MOPr) to exert their neurochemical and behavioral effects (Matthes et al., 1996), evidence also suggests a role for the glutamatergic system to underlie several opioid addiction processes (Kalivas, 2009). A role for metabotropic glutamate receptor 5 (mGlu5R) in drug self-administration as well as reinstatement of drug-seeking behavior has been reported (Gass and Olive, 2008). With respect to opioids, pharmacological blockade of the mGlu5R attenuated the development of tolerance to morphine (Kozela et al., 2003; Gabra et al., 2008), inhibited the expression of morphine locomotor sensitisation (Kotlinska and Bochenski, 2007), prevented the acquisition (Roohi et al., 2014) and expression of morphine place-preference (Popik and Wrobel, 2002; Aoki et al., 2004; Herzig and Schmidt, 2004; Veeneman et al., 2011), and decreased morphine self-administration (Brown et al., 2012) in rodents. In addition, administration of the mGlu5R antagonist, 3-((2-Methyl-4-thiazolyl)ethynyl)pyridine (MTEP), decreased cue-induced reinstatement of morphine seeking in mice (Brown et al., 2012), and intra-nucleus accumbens shell (AcbSh) administration of the mGlu5R antagonist 2-Methyl-6-(phenylethynyl)pyridine (MPEP) decreased heroin-seeking induced by context, cues, or heroin priming in rats (Lou et al., 2014). Moreover, mGlu5R antagonism has been found to potentiate the antinociceptive effects of morphine in rats (Picker et al., 2011). However, the role of the mGlu5R on the modulation of morphine withdrawal-induced emotional impairment has not yet been investigated.
Therefore, in the present study, we first aimed to characterize the behavioral/emotional consequences of morphine withdrawal and investigate the effects of chronic morphine administration and withdrawal on mGlu5R binding in the brain of mice. We found that morphine abstinence induced anxiety-like, depressive-like, and impaired sociability behaviors concomitant with a marked region-specific upregulation of mGlu5R binding, possibly suggesting the presence of MOPr-mGlu5R interaction in the brain. We also assessed the effects of mGlu5R antagonism on the reversal of morphine withdrawal-induced depressive-like behaviors to directly explore the possible involvement of central mGlu5R system in the emotional component of morphine withdrawal. To further assess the effects of MOPr system manipulation on the mGlu5R system, we performed mGlu5R autoradiographic binding in brains of mice lacking the MOPr gene (MOPr knockout mice). Our findings provide further evidence for a key role of the central mGlu5R system in the modulation of the emotional consequences of opioid abstinence and suggest the existence of an mGlu5R-MOPr interaction to be localized in the nucleus accumbens, thalamus, hypothalamus, and amygdala, regions highly associated with reward and emotions.
Methods
Animals
Chronic Morphine and Withdrawal Experiments—Male C57BL/6J mice (7 weeks old, 20–25g, Charles River Laboratories, Kingston, UK) were housed individually in a temperature-controlled environment with a 12-hour-light/-dark cycle (lights on 6:00 am). Food and water were available ad libitum. Mice acclimatized in their new environment for at least 7 days prior to experiments and were handled daily. All experimental procedures were conducted in accordance with the UK Animal Scientific Procedures Act (1986).
Mu Opioid Receptor Knockout Study—The brains from 8-week-old MOPr knockout mice were provided by Professor Brigitte Kieffer’s laboratory (IGBMC, Strasbourg, France). The experimental methodology for the generation of MOPr knockout mice used in the current study has been previously described elsewhere (Matthes et al., 1996).
Chronic “Intermittent” Escalating-Dose Morphine Administration Paradigm and Withdrawal
Mice were randomly divided into chronic saline- and morphine-treated groups (n=6/group). Mice were injected (i.p.) with saline (4mL/kg) or morphine-HCl (Sigma-Aldrich, Poole, UK) with a chronic, “intermittent” escalating-dose administration paradigm (20mg/kg on day 1, 40mg/kg on days 2 and 3, 80mg/kg on days 4 and 5, and 100mg/kg on days 6 and 7) twice per day at 9:00 am and 5:00 pm in accordance with previously published protocols (Muller and Unterwald, 2004; Zhou et al., 2006; Goeldner et al., 2011; Zanos et al., 2014). Both groups of animals were left to spontaneously withdraw in their home cages without injections. Different cohorts of mice were assessed for withdrawal-associated affective behaviors and mGlu5R binding. Body weight was measured daily.
Characterization of a Mouse Model of Opioid Withdrawal
To characterize the behavioral effects of morphine withdrawal, mice were treated with 7 days of escalating-dose morphine paradigm and spontaneous withdrawal for a period of 6 to 8 days. Mice were tested for sociability (6 days withdrawal; Crawley’s 3-chambered social approach test), anxiety-like behavior (7 days withdrawal; open-field and elevated-plus maze [EPM] tests), and depression-like behaviors (8 days withdrawal; forced-swim test [FST]). The order of testing was determined by the degree of stress-inducing properties of each test, with the least stressful conducted first and the most potentially distressing test last (Clemens et al., 2007). The Crawley’s 3-chambered social approach test, EPM, and FST were performed as previously described (Zanos et al., 2014) (see supplementary Materials and Methods). To further assess opioid abstinence-associated anxiety-related behaviors, mice were placed in open-field arenas (40cm x 20cm x 20cm) 7 days following the last treatment injection, and time spent in the center of the arena was scored for 10 minutes by a trained observer blind to the treatment groups. Twenty-four hours following the last behavioral test (ie, FST), mice were euthanized by 30-second exposure to CO2 followed by decapitation and spleen was weighed for peripheral assessment of stress (Kyo et al., 1999; Dominguez-Gerpe and Rey-Mendez, 2001).
To assess the role of mGlu5R on morphine withdrawal-induced depressive-like behaviors, we used a different cohort of mice undergoing the same morphine administration and withdrawal paradigm as described above. On day 8 (24 hours following a 15-minute FST pretest) of withdrawal, we administered saline (vehicle) or MTEP (3mg/kg, i.p.), and 30 minutes later we assessed for depressive-like behaviors in the FST, as described by Zanos et al. (2014). Dose and timing of administration of MTEP was determined based on previous studies assessing the effects of MTEP in the FST paradigm (Palucha et al., 2005; Belozertseva et al., 2007; Pomierny-Chamiolo et al., 2010; Domin et al., 2014).
Autoradiographic Binding of mGlu5R in the Brain of Mice
A separate cohort of mice underwent the same administration paradigm for biochemical assessments. For this cohort of animals, mice were not assessed in any other behavioral test. One hour following the final morphine/saline injection for the chronic administration group and 7 days postfinal injection for the withdrawal groups, mice were euthanized by 30-second exposure to CO2 followed by decapitation and brains were collected and immediately frozen in isopentane solution (-25oC). Coronal brain sections were cut (20 μm thick; 300 μm apart) using a cryostat (Zeiss Microm 505E, Hertfordshire, UK), thaw-mounted onto gelatin subbed ice-cold microscope slides, and processed for autoradiography as described previously (Kitchen et al., 1997).
Quantitative mGlu5R autoradiography was performed in brain sections of all treatment groups and both genotypes as described previously (Georgiou et al., 2015; Wright et al., 2015). For the determination of total binding, slides were incubated for 60 minutes in 10nM [3H]-MPEP (American Radiolabeled Chemical, 2.22 TBq/mmol) in Tris-HCl buffer (pH 7.4, 4oC). Adjacent brain section were incubated with [3H]-MPEP (10nM) in the presence of 10 μM fenobam (Tocris Bioscience, Bristol, UK) to determine the nonspecific binding.
Slides with radioligand bound sections were apposed to films (Kodak BioMax MR-1 films; Sigma-Aldrich, Gillingham, UK) for 3 weeks along with appropriate 3H microscale standards (Amersham Pharmacia Biotech) to allow quantification. Films were then developed and analyzed in parallel in a complete paired protocol as described by Kitchen et al. (1997). All structures were identified by reference to the mouse brain atlas of Franklin & Paxinos (2007) and analyzed using an image analyzer (MCID; Image Research, Linton, UK).
Statistical Analysis
All the values are expressed as mean±SEM. The effects of morphine withdrawal on anxiety-like and depressive-like behaviors were analyzed by Student’s t test. For analysis of sociability deficits, 2-way repeated-measures ANOVA was performed with factors “treatment” and “chamber (ie, empty and stranger – repeated factor).” The effects of morphine administration and withdrawal on spleen weight were analyzed by 2-way ANOVA for factors “treatment” (ie, saline and morphine) and “experimental phase” (ie, chronic and withdrawal). For analysis of mGlu5R autoradiographic binding following chronic morphine administration and withdrawal, 2-way ANOVA with factors “treatment” (ie, saline and morphine) and “experimental phase” (ie, chronic and withdrawal) was performed in each brain region. The effects of MTEP pretreatment on depressive-like behaviors in the FST were assessed using 2-way ANOVA for factors “pretreatment” (ie, saline and MTEP) and “treatment” (ie, saline and morphine). For assessing the effects of MOPr gene deletion on mGlu5R binding, unpaired Student’s t test was used in each individual region. For the regression analysis, Pearson correlation coefficient test was performed. ANOVAs were followed by a Bonferoni post-hoc test when significant interaction effect was reached (ie, P <.05). All statistical analyses were performed using SigmaPlot v11.0 (Systat Software, London, UK).
Results
Effect of Morphine Withdrawal on Anxiety-Like, Depressive-Like, and Sociability Behaviors
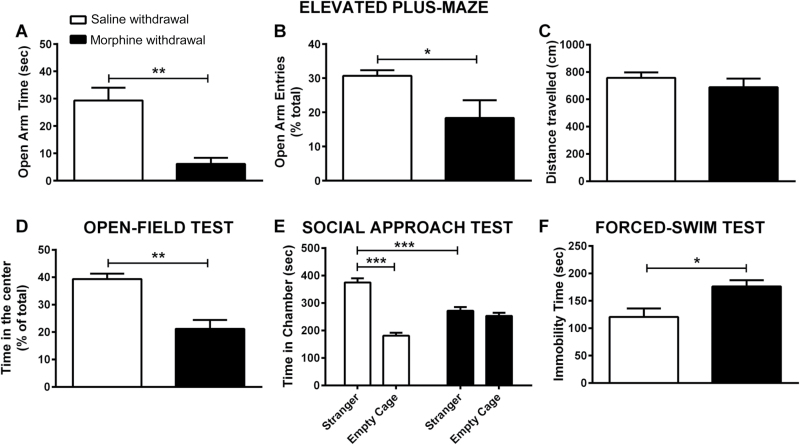
Anxiety-Like Behaviors—We assessed anxiety-like behavior following the 7-day withdrawal from morphine using the EPM and open-field tests. Morphine-withdrawn animals spent significantly less time in the open arms (P<.01; Figure 1a) and showed decreased time in the centre of the open-field arena (P<.01; Figure 1b) compared with saline-withdrawn animals.
Figure 1.
Depressive-like, anxiety-like behaviors, and sociability deficits following chronic protracted morphine withdrawal. Male C57BL/6J mice were treated with either saline or an escalating-dose morphine paradigm (2 x 20–100mg/kg/d, i.p.) followed by 6 to 8 days withdrawal. (A) Open-arm time. (B) Open-arm entries. (C) Distance travelled in the elevated plus-maze (EPM) and (D) time in the center (percent of total) of an open field (OF) were measured to assess anxiety-like behaviors following 6 and 7 days of morphine withdrawal, respectively. (E) Sociability of morphine- and saline-withdrawn mice was also assessed using Crawley’s 3-chambered social approach test. (F) Immobility time was assessed in the forced-swim test (FST) following 7 days morphine withdrawal. Data are expressed as the mean±SEM (n=6/group). *P<.05, **P<.01, ***P<.001 (EPM, OF, and FST behaviors were scored using the Student’s t test; sociability behavior was analyzed using repeated-measures 2-way ANOVA followed by Bonferroni post-hoc test).
Depressive-Like Behavior—Depressive-like behavior following the 8-day withdrawal from morphine was assessed using the FST. Morphine-withdrawn animals showed significantly increased immobility time compared with saline-withdrawn animals (P<.05) (Figure 1c). Morphine-withdrawn mice needed significantly less time to reach the first immobility compared to saline-withdrawn mice (P<.01) (Figure 1d).
Sociability—The social preference in morphine-withdrawn mice was assessed using the 3-chambered sociability test following 6 days of withdrawal. Saline-withdrawn mice exhibited a significant preference for the chamber containing stranger mouse vs the empty chamber (P<.001) (Figure 1e). However, morphine-withdrawn animals did not show any preference between the chamber containing the stranger mouse and the empty chamber (P>.05; Figure 1e), demonstrating a lack of social preference. To directly demonstrate a lack of social preference of morphine withdrawn mice, we further analyzed the ratio of time spent in the “stranger” side vs time spent in the chamber containing the empty cage (social interaction ratio=time in stranger side/time in empty cage chamber). This analysis revealed a strong preference for saline control mice to spend their time in the social chamber (2.14±0.23 vs threshold of 1; 1-sample t test; P<.001), whereas morphine withdrawn mice did not show preference for either of the 2 compartments (1.09±0.09 vs threshold of 1; 1-sample t test; P>.05).
Effect of Chronic Morphine Administration and Withdrawal on Body Mass and Relative Spleen Weight
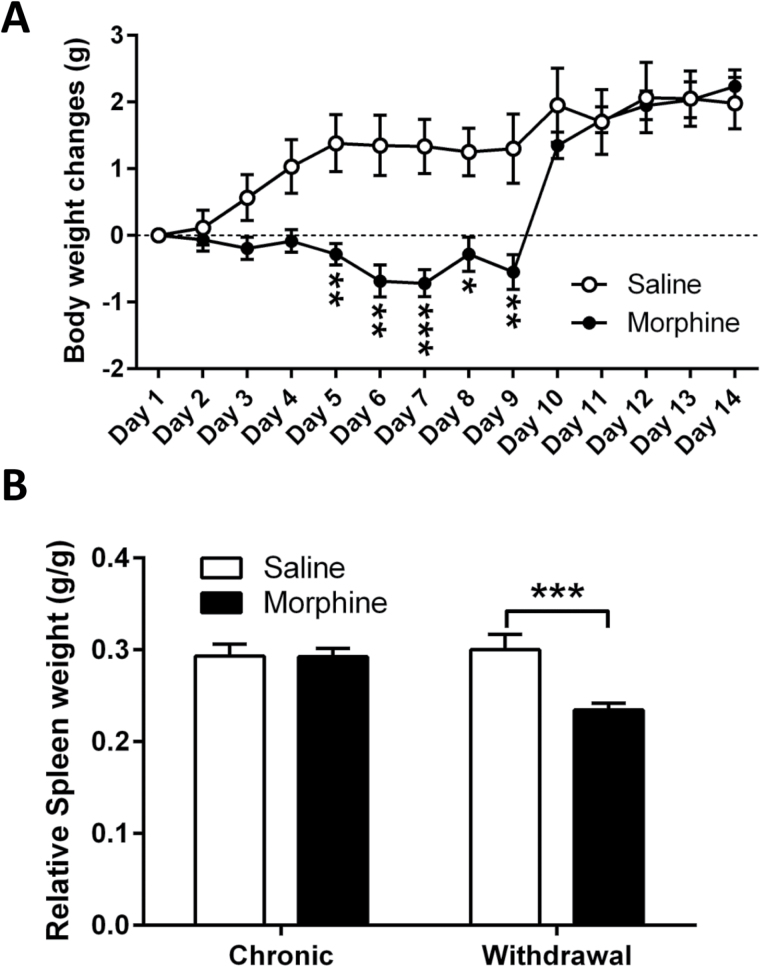
Body weight was measured daily throughout the chronic morphine administration and withdrawal paradigm. Chronic administration of morphine resulted in a lack of body weight gain compared with saline-treated animals, which reached statistical significance on day 5 (P<.01) (Figure 2a). Decreased weight gain persisted up to the second day of withdrawal (ie, day 9) (day 6, P<.01; day 7, P<.001; day 8, P<.05; day 9, P<.01 vs saline controls) (Figure 2a).
Figure 2.
Effects of chronic morphine administration and withdrawal on body weight and relative spleen weight. Male C57BL/6J mice were treated with either saline or escalating-dose morphine paradigm (2 x 20–100mg/kg/d, i.p.) followed by a 7-day withdrawal period. (A) Changes in body weight and (B) relative spleen weight during chronic morphine or saline administration and withdrawal. Data are expressed as the mean ± SEM (n=6–12/group). *P<.05, **P<.01, ***P<.001 vs saline control (repeated-measures 2-way ANOVA followed by Bonferroni post-hoc test).
We measured spleen weight in animals undergoing chronic morphine administration as well as withdrawal from morphine as an indicator of stress state of the animal. Indeed, decreased spleen weight has been previously associated with stress in rodents (Kyo et al., 1999; Dominguez-Gerpe and Rey-Mendez, 2001). Seven days of morphine administration did not change the relative spleen weight compared with the saline-treated animals. However, the 7-day withdrawal period from morphine administration induced a significant decrease in relative spleen weight compared with the saline-withdrawn controls (P<.001) (Figure 2b).
Effect of Chronic Morphine Administration and Withdrawal on mGlu5R Binding
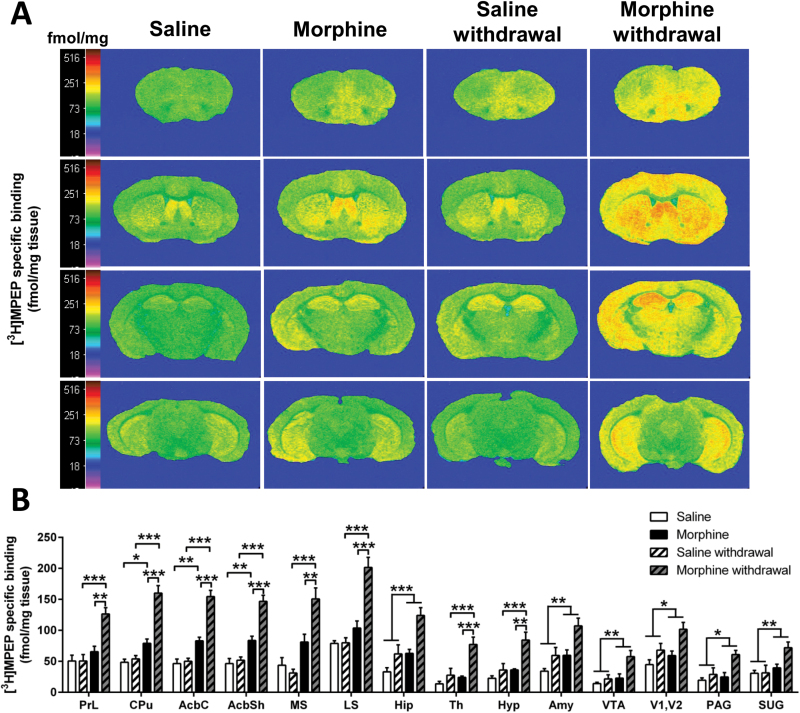
Quantitative analysis of mGlu5R binding of morphine treated and withdrawal animals revealed a significant treatment effect in all the brain regions analyzed, including prelimbic cortex (F(1,20)= 21.370; P<.001), AcbC (F(1,22)= 94.810; P<.001), AcbSh (F(1,22)= 74.450; P<.001), lateral septum (F(1,20)= 37.160; P<.001), medial septum (F(1,22)= 37.310; P<.001), caudate putamen (F(1,20)= 61.160; P<.001), hippocampus (F(1,23)= 19.850; P<.001), amygdala (F(1,23)= 13.540; P<.01), thalamus (F(1,23)= 13.170; P<.01), hypothalamus (F(1,23)= 13.60; P<.01), visual cortex (F(1,24)= 6.498; P<.05), ventral tegmental area (F(1,24)= 10.490; P<.01), superficial grey (F(1,24)= 8.356; P<.01), and periaqueductal grey (F(1,24)= 6.188; P<.05). A significant treatment x experimental phase interaction effect was observed in prelimbic cortex (F(1,20)=9.612; P<.001), AcbC (F(1,22)=21.760; P<.001), AcbSh (F(1,22)=14.180; P<.01), lateral septum (F(1,20)=20.114; P<.001), medial septum (F(1,22)=10.120; p<0.01), caudate putamen (F(1,20)=16.280; P<.001), thalamus (F(1,23)=5.625; P<.05) and hypothalamus (F(1,23)=4.330; P<.05). Following the 7-day morphine administration, Bonferroni post-hoc analysis showed a significant increase in mGlu5R binding in the AcbC (P<.001), AcbSh (P<.01), and caudate putamen (P<.01) compared with the respective saline control (Figure 3a-b). Notably, the increase in mGlu5R binding observed in these regions was further enhanced following the 7-day withdrawal period (chronic morphine compared with morphine withdrawal; P<.001) (Figure 3a-b). A morphine-withdrawal effect was observed in all the brain regions analyzed (Figure 3a-b; prelimbic cortex, P<.001; lateral septum, P<.001; medial septum, P<.001; thalamus, P<.001; hypothalamus, P<.001) compared with the respective saline-withdrawn controls. Compared with chronic morphine treatment, morphine withdrawal induced an upregulation of the mGlu5R binding in the prelimbic cortex (P<.01), medial septum (P<.01), lateral septum (P<.001), thalamus (P<.001), and hypothalamus (P<.01).
Figure 3.
Morphine withdrawal increases metabotropic glutamate receptor 5 (mGlu5R) binding in the brain. (A) Representative autoradiograms of 10 μΜ [3H]-2-methyl-6-([3,5-3H] phenylethynl) pyridine ([3H]MPEP) binding to mGlu5R in coronal brain sections of mice undergoing chronic saline or morphine administration and 7-day withdrawal. Autoradiograms of brain sections were taken at the level of prefrontal cortex (Bregma: 2.34mm; first row), striatum (Bregma: 0.62mm; second row), thalamus (Bregma: -1.82mm; third row), and ventral tegmental area (Bregma: -3.40mm; fourth row). Binding levels are represented using a pseudocolor interpretation of black and white film images in fmol/mg of tissue equivalent. (B) Quantitative mGlu5R binding in the brain of mice subjected to morphine administration and withdrawal. Data are expressed as the mean±SEM (n=6–7/group). *P<.05, **P<.01, ***P<.001 (2-way ANOVA followed by Bonferroni post-hoc test per brain region).
Effect of Acute Administration of the mGlu5R Antagonist MTEP on Morphine Withdrawal-Induced Depressive-Like Behaviors
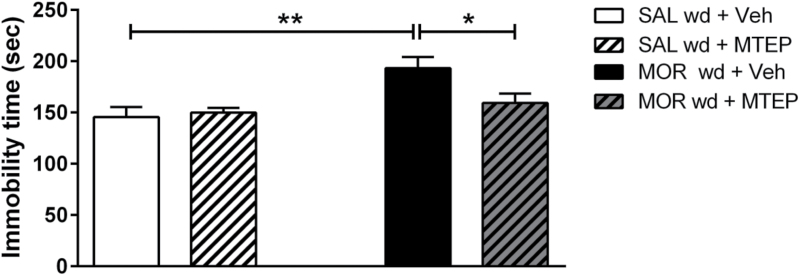
Administration of MTEP 30 minutes prior to the FST reversed the increased immobility time induced by 8 days of morphine withdrawal in mice (Figure 4). Two-way ANOVA revealed a significant main effect of treatment (F(1,19)= 10.230; P<.01) and pre-treatment x treatment interaction (F(1,23)=4.66; P<.05). Morphine-withdrawn animals manifested increased immobility time in the FST, indicating the emergence of a depressive-like phenotype, while MTEP pretreatment abolished this behavioral deficit induced by morphine abstinence. MTEP administration did not alter immobility time of control/saline-treated animals (Figure 4).
Figure 4.
Pretreatment with the metabotropic glutamate receptor 5 (mGlu5R), 3-((2-Methyl-4-thiazolyl)ethynyl)pyridine (MTEP), prevents morphine abstinence-induced depressive-like behaviors. Male C57BL/6J mice were treated with either saline or escalating-dose morphine paradigm (2 x 20–100mg/kg/d, i.p.) followed by a 7-day withdrawal period. Administration of the mGlu5R antagonist, MTEP, abolished the depressive-like behaviors induced by morphine withdrawal in the forced-swim test (FST). Data are expressed as the mean±SEM (n=5–6/group). *P<.05, **P<.01 (2-way ANOVA followed by Bonferroni post-hoc test).
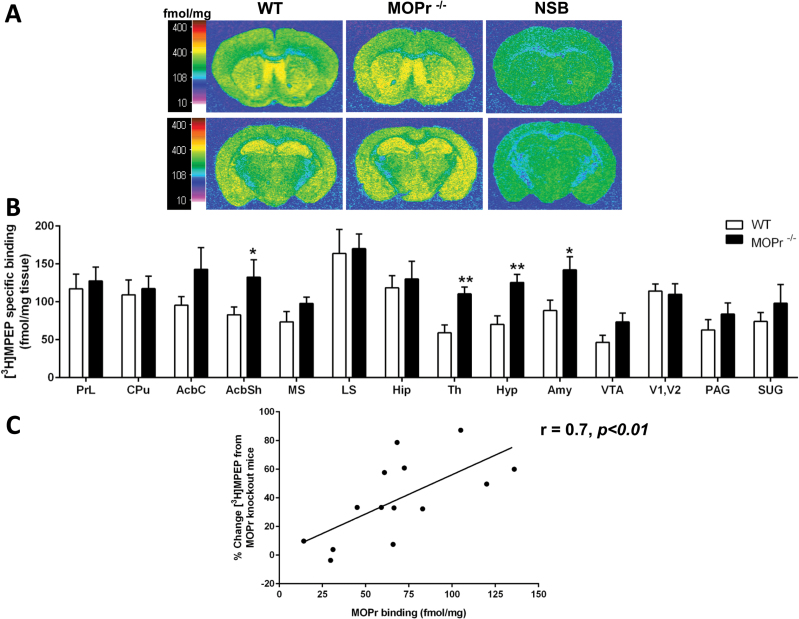
Effects of MOPr Gene Deletion on mGlu5R Binding
Quantitative autoradiography of mGlu5 receptors from coronal sections of fore-, mid-, and hind-brain mice showed a widespread distribution. The pattern of distribution of mGlu5 receptors on MOPr knockout mice brains was identical to the wild-type mouse brains (Figure 5a). MOPr knockout brains had significantly increased mGlu5R binding in the AcbSh (P<.05), thalamus (P<.01), hypothalamus (P<.01), and amygdala (P<.05) (Figure 5b).
Figure 5.
Metabotropic glutamate receptor 5 (mGlu5R) binding in the brain of mice lacking the μ-opioid receptor (MOPr) gene. (A) Representative autoradiograms of 10 μΜ [3H]-2-methyl-6-([3,5-3H] phenylethynl) pyridine ([3H]MPEP) binding to mGlu5R in coronal brain sections of wild-type (WT) and MOPr knockout (MOPr-/-) mice. Autoradiograms of brain sections were taken at the level of striatum (Bregma: 0.62mm; first row) and thalamus (Bregma: -1.82mm; second row). Representative autoradiograms for nonspecific binding (NSB) are shown (far right column). Binding levels are represented using a pseudocolor interpretation of black and white film images in fmol/mg of tissue equivalent. (B) Quantitative mGlu5R binding. Data are expressed as the mean±SEM (n=7/group). *P<.05, **P<.01, ***P<.001 vs wild-type control (Student’s t-test per brain region). (C) Correlation analysis between percentage change in mGlu5R binding in MOPr knockout mice and MOPr binding in wild-type mice. (n=14; r=0.7; P<.01).
Regression analysis was carried out to determine if there was a correlation between the MOPr binding levels in regions of wild-type brains and percent change in mGlu5R binding in the regions analyzed of MOPr knockout mouse brains compared with MOPr binding levels derived from wild-type mice (Kitchen et al., 1997). Regression analysis revealed a significant correlation (r=0.7, n=14, P<.01) (Figure 5c).
Discussion
The present study is the first, to our knowledge, to demonstrate a significant upregulation of mGlu5R throughout the whole brain in a mouse model of opioid-withdrawal characterized by emotional impairment. We showed that a 6- to 8-day withdrawal period from chronic morphine administration induced anxiety-like and depressive-like behaviors, and impaired sociability. Moreover, we provide evidence for direct effects of a MOPr system manipulation on the mGlu5R in the AcbSh, thalamus, hypothalamus, and amygdala, which are brain regions important in drug addiction and emotional regulation (Bossert et al., 2007; Chaudhri et al., 2010; Zanos et al., 2014).
We first characterized a mouse model of opioid withdrawal, which mimics the negative affective state displayed by opioid addicts undergoing abstinence following detoxification (Martin and Jasinski, 1969; Jaffe, 1990; Nunes et al., 2004; Peles et al., 2007; Veilleux et al., 2010). We have previously shown, using the same chronic morphine administration and withdrawal protocol, that physical withdrawal symptoms associated with acute morphine withdrawal (ie, withdrawal jumping, increased defecation, and changes in body weight) disappear following a 7-day abstinence period (Goeldner et al., 2011; Zanos et al., 2014); however, emotional impairment is evident, highlighting the translational validity of our mouse model. This impairment has been reported to be even more evident after 4 weeks of abstinence (Goeldner et al., 2011) and was accompanied by significant alterations of gene expression in the extended amygdala (Le Merrer et al., 2012). In the present study, decrease in relative spleen weight was observed in 7-day-withdrawn animals, further supporting a stress-related phenotype. Decreased spleen weight has been previously correlated with higher levels of emotional stress in rodents (Kyo et al., 1999; Dominguez-Gerpe and Rey-Mendez, 2001).
The emotional impairment observed in the present study was accompanied by an abundant increase in mGlu5R binding in the brain. These findings are supported by previous data showing that chronic morphine administration induces a dose-dependent upregulation of mGlu5R immunoreactivity in the spinal cord in mice (Narita et al., 2005). An increase in the expression of mGlu5R protein was also found in the spinal cord of morphine-tolerant rats (Liu et al., 2009) and the ventral pallidum of morphine-dependent rats (Herrold et al., 2013). Interestingly, administration of the mGlu5R antagonist MPEP prevented the development of morphine tolerance by specifically preventing the increased expression of mGlu5R protein in the spinal cord (Liu et al., 2009), supporting a biological/behavioral relevance of these mGlu5R alterations. However, this is first study to demonstrate profound alterations of mGlu5R binding in the brain following opioid withdrawal.
Although the molecular mechanism underlining the chronic morphine/withdrawal induced upregulation of mGlu5R is not clear, one can speculate on various potential mechanisms. First, there is evidence to suggest that mGlu5R upregulation may be a result of a compensatory neuroadaptive response to a suppression of glutamate neurotransmission during morphine withdrawal. Indeed there is evidence showing that chronic opioid administration suppresses glutamate neurotransmission in the central nervous system (Manzoni and Williams, 1999; Xu et al., 2003). This hypoglutamatergic state may thus in turn induce a compensatory increase in mGlu5R to enhance glutamatergic synaptic transmission. It has been postulated that such a mechanism may at least partly be responsible for the suppression of tolerance to spinal antinociception induced by morphine treatment (Suzuki et al., 2006). Another potential mechanism may involve chronic morphine-induced allosteric modulation of mGlu5R. Indeed, Schroder et al. (2009) provided compelling evidence that this may be the case in vitro. They found that MPEP binding site affinity and Bmax for mGlu5R is significantly increased in MOPr- mGlu5R coexpressing cells vs mGlu5R-only expressing cells, suggesting that the interaction between MOPr and mGlu5R can profoundly alter the conformation of mGlu5R. In our study, it cannot be determined if chronic morphine/withdrawal alters the affinity or Bmax of mGlu5R or both, but based on the evidence of Schroder et al. (2009), it is tempting to speculate that the profound upregulation of mGlu5R in response to chronic morphine treatment could be caused by a change in mGlu5R affinity and/or decrease in receptor internalization. Finally, there is increasing evidence suggesting the existence of MOPr-mGlu5R heterodimerization that may at least partly explain the profound changes of mGlu5R following chronic morphine treatment/withdrawal. Indeed, studies showing that desensitization, phosphorylation and internalization of MOPr are influenced by mGlu5R modulation (Schroder et al., 2009), further suggest the presence of MOPr-mGlu5R heterodimers. Furthermore, it has been demonstrated that targeting the putative MOPr-mGlu5R heteromer using a bivalent ligand containing MOPr agonist and mGlu5R antagonist is effective in decreasing inflammatory and chronic cancer pain (Akgun et al., 2013; Smeester et al., 2014).
To directly assess the relation between the observed upregulation of the mGlu5R and the behavioral deficits of morphine-withdrawn mice, we tested the effect of an mGlu5R receptor antagonist, MTEP, on morphine abstinence-induced depressive-like behavior in the FST. The complete reversal of morphine abstinence-associated depressive-like behavior following administration of MTEP clearly indicates a key role for mGlu5R in the manifestation of the emotional consequences of opioid abstinence and is highly suggestive of a causal relationship between the evident mGlu5R upregulation in the brain of morphine-withdrawn mice and the observed emotional deficits. This finding is consistent with the antidepressant effect of mGlu5R antagonists in rodent stress models (Li et al., 2006; Belozertseva et al., 2007). It is also likely that mGlu5R may also be involved in the anxiogenic and social deficit consequences of opioid abstinence, as mGlu5R antagonism was shown to reverse ethanol withdrawal-induced anxiety (Kumar et al., 2013) and rescue social deficits observed in a mouse model of autism (Silverman et al., 2012).
To further explore possible effects of a manipulated MOPr system on the mGlu5R in the brain, we assessed the effect of MOPr gene deletion on mGlu5R binding with the use of MOPr knockout mice, which are characterized by certain behavioral phenotypic and MOPr signalling characteristics that are reminiscent of our model of morphine abstinence. Notably, similar to our morphine-withdrawal mouse model, MOPr knockout mice display deficits in social behavior and emotional impairment (Becker et al., 2014). Furthermore, consistent with the MOPr KO mouse model, where MOPr are completely absent, repeated administration of morphine, which primarily acts on MOPr, is well known to induce a significant reduction in MOPr activation of G-protein in the brain of rats (Sim et al., 1996) and thus lead to MOPr desensitization that persists during withdrawal (Ingram et al., 2007). Therefore, it is not perhaps surprising that similar alterations of mGlu5R binding in the same direction are observed in brain regions of MOPr KO mice and chronically morphine-treated/-withdrawn mice, both of which are models of reduced MOPr activity.
Our data demonstrate a marked mGlu5R upregulation in MOPr-null mice in brain regions that are well known to be rich in MOPr, such as the AcbSh, thalamus, hypothalamus, and amygdala, supporting the plausible existence of brain region-specific mGlu5R-MOPr interactions; however, in the present study, direct receptor interactions have not been investigated. These brain regions are involved in the regulation of drug-induced reward (Bosser et al., 2007; Chaudhri et al., 2010) and withdrawal-induced emotional deficits (Zanos et al., 2014), as well as reinstatement (Georgiou et al., 2015). Our data add to the existing evidence for an association between the central mGlu5R and MOP systems (Schroder et al., 2009; Akgun et al., 2013; Smeester et al., 2014). Indirect evidence further suggests a possible modulatory inter-play between mGlu5R and MOPr systems in the striatum to regulate opioid-related behaviors, since intra-Acb administration of an mGlu5R antagonist attenuated the acquisition of morphine CPP (Roohi et al., 2014), and intra-AcbSh, but not intra-AcbC, administration of an mGlu5R antagonist attenuated both cue- and priming-induced reinstatement of heroin-seeking behavior in rats (Lou et al., 2014). The exact neurobiological mechanism underlying the observed mGlu5R upregulation in the MOPr knockout mice remains unclear, since glutamate content and/or release in the brain of MOPr knockout mice have not been evaluated so far. If glutamatergic neurotransmission in these mice is generally reduced, an upregulation of the mGlu5R may represent a compensatory mechanism to counteract the neuronal responses to decreased glutamate levels; however, in the present study we observed region-specific upregulation of mGlu5R in these mice and not a global effect, which indicates that the possibility of a direct molecular interaction between mGlu5R and MOPr in specific brain regions is more likely to exist. Indeed, GPCRs, including mGlu5R and MOPr, are capable of forming heterodimers, and it has been shown that this physical association can modulate receptor binding and function (Schroder et al., 2009; Smeester et al., 2014). If dimeric interactions exist between these 2 systems, the lack of MOPr may result in alterations of mGlu5R pharmacology in brain regions where the 2 receptors physically interact. Although this possibility is only speculative at present, it deserves further investigation.
Taken together, our findings showed that 6 to 8 days of morphine withdrawal induces emotional deficits in mice, which is concomitant with marked neuroadaptations in the mGlu5R system in the brain. These results suggest a possible involvement of the mGlu5R in the modulation of comorbid behavioral impairment during opioid abstinence and point toward targeting this system for the development of novel pharmacotherapies for opioid addiction. Moreover, our data suggest a direct inter-play between mGlu5R and MOPr in brain regions associated with drug addiction, reward, and emotions.
Statement of Interest
None
Supplementary Material
Acknowledgments
The authors thank Michaella Georgiou for her contribution in brain sectioning and binding experiments.
This study was supported by a RCUK academic fellowship, a Royal Society Research grant (RG120556), and the European Commission (Contact Number: LSHM-CT2004-005166). The sponsors had no involvement in the design of the study and in the collection, analyses, and interpretation of the data, or in the writing of the report and the decision to submit this article for publication.
References
- Akgun E, Javed MI, Lunzer MM, Smeester BA, Beitz AJ, Portoghese PS. (2013) Ligands that interact with putative MOR-mGluR5 heteromer in mice with inflammatory pain produce potent antinociception. Proc Natl Acad Sci U S A 110:11595–11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T, Narita M, Shibasaki M, Suzuki T. (2004) Metabotropic glutamate receptor 5 localized in the limbic forebrain is critical for the development of morphine-induced rewarding effect in mice. Eur J Neurosci 20:1633–1638. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Kupferschmidt DA, Lovinger DM. (2014) Opioids induce dissociable forms of long-term depression of excitatory inputs to the dorsal striatum. Nat Neurosci 17:540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JA, Clesse D, Spiegelhalter C, Schwab Y, Le Merrer J, Kieffer BL. (2014) Autistic-like syndrome in mu opioid receptor null mice is relieved by facilitated mGluR4 activity. Neuropsychopharmacology 39:2049–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belozertseva IV, Kos T, Popik P, Danysz W, Bespalov AY. (2007) Antidepressant-like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests. Eur Neuropsychopharmacol 17:172–179. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. (2007) Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci 27:12655–12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Stagnitti MR, Duncan JR, Lawrence AJ. (2012) The mGlu5 receptor antagonist MTEP attenuates opiate self-administration and cue-induced opiate-seeking behaviour in mice. Drug Alcohol Depend 123:264–268. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, Janak PH. (2010) Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology 35:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens KJ, Cornish JL, Hunt GE, McGregor IS. (2007) Repeated weekly exposure to MDMA, methamphetamine or their combination: long-term behavioural and neurochemical effects in rats. Drug Alcohol Depend 86:183–190. [DOI] [PubMed] [Google Scholar]
- Davis L, Uezato A, Newell JM, Frazier E. (2008) Major depression and comorbid substance use disorders. Curr Opin Psychiatry 21:14–18. [DOI] [PubMed] [Google Scholar]
- Domin H, Szewczyk B, Wozniak M, Wawrzak-Wlecial A, Smialowska M. (2014) Antidepressant-like effect of the mGluR5 antagonist MTEP in an astroglial degeneration model of depression. Behav Brain Res 273:23–33. [DOI] [PubMed] [Google Scholar]
- Dominguez-Gerpe L, Rey-Mendez M. (2001) Alterations induced by chronic stress in lymphocyte subsets of blood and primary and secondary immune organs of mice. BMC Immunol 2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabra BH, Smith FL, Navarro HA, Carroll FI, Dewey WL. (2008) mGluR5 antagonists that block calcium mobilization in vitro also reverse (S)-3,5-DHPG-induced hyperalgesia and morphine antinociceptive tolerance in vivo. Brain Res 1187:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. (2008) Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol 75:218–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou P, Zanos P, Ehteramyan M, Hourani S, Kitchen I, Maldonado R, Bailey A. (2015) Differential regulation of mGlu5R and ΜOPr by priming- and cue-induced reinstatement of cocaine-seeking behaviour in mice. Addiction Biology 20:902–912. [DOI] [PubMed] [Google Scholar]
- Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM, Kieffer BL. (2011) Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry 69:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou P, Zanos P, Garcia-Carmona J-A, Hourani S, Kitchen I, Kieffer BL, Laorden M-L, Bailey A. (2015) The oxytocin analogue carbetocin prevents priming-induced reinstatement of morphine-seeking: Involvement of dopaminergic, noradrenergic and MOPr systems. Eur Neuropsychopharmacol 25:2459–2464. [DOI] [PubMed] [Google Scholar]
- Herrold AA, Persons AL, Napier TC. (2013) Cellular distribution of AMPA receptor subunits and mGlu5 following acute and repeated administration of morphine or methamphetamine. J Neurochem 126:503–517. [DOI] [PubMed] [Google Scholar]
- Herzig V, Schmidt WJ. (2004) Effects of MPEP on locomotion, sensitization and conditioned reward induced by cocaine or morphine. Neuropharmacology 47:973–984. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Fossum EN, Morgan MM. (2007) Behavioral and electrophysiological evidence for opioid tolerance in adolescent rats. Neuropsychopharmacology 32:600–606. [DOI] [PubMed] [Google Scholar]
- Jaffe JH. (1990) Trivializing dependence. 85:1425–1427; discussion 1429–1431. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10:561–572. [DOI] [PubMed] [Google Scholar]
- Kitchen I, Slowe SJ, Matthes HW, Kieffer B. (1997) Quantitative autoradiographic mapping of mu-, delta- and kappa-opioid receptors in knockout mice lacking the mu-opioid receptor gene. Brain Res 778:73–88. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Bochenski M. (2007) Comparison of the effects of mGluR1 and mGluR5 antagonists on the expression of behavioral sensitization to the locomotor effect of morphine and the morphine withdrawal jumping in mice. Eur J Pharmacol 558:113–118. [DOI] [PubMed] [Google Scholar]
- Kozela E, Pilc A, Popik P. (2003) Inhibitory effects of MPEP, an mGluR5 antagonist, and memantine, an N-methyl-D-aspartate receptor antagonist, on morphine antinociceptive tolerance in mice. Psychopharmacology 165:245–251. [DOI] [PubMed] [Google Scholar]
- Kumar J, Hapidin H, Bee YT, Ismail Z. (2013) Effects of the mGluR5 antagonist MPEP on ethanol withdrawal induced anxiety-like syndrome in rats. Behav Brain Funct 9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyo E, Uda N, Ushijima M, Kasuga S, Itakura Y. (1999) Prevention of psychological stress-induced immune suppression by aged garlic extract. Phytomedicine 6:325–330. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Befort K, Gardon O, Filliol D, Darcq E, Dembele D, Becker JA, Kieffer BL. (2012) Protracted abstinence from distinct drugs of abuse shows regulation of a common gene network. Addict Biol 17:1–12. [DOI] [PubMed] [Google Scholar]
- Le Moal M, Koob GF. (2007) Drug addiction: pathways to the disease and pathophysiological perspectives. Eur Neuropsychopharmacol 17:377–393. [DOI] [PubMed] [Google Scholar]
- Li X, Need AB, Baez M, Witkin JM. (2006) Metabotropic glutamate 5 receptor antagonism is associated with antidepressant-like effects in mice. J Pharmacol Exp Ther 319:254–259. [DOI] [PubMed] [Google Scholar]
- Liu JB, Jiang W, Wang AZ. (2009) [Expression of metabotropic glutamate receptor 5 in spinal cord of morphine tolerant rats]. Zhonghua Yi Xue Za Zhi 89:2221–2224. [PubMed] [Google Scholar]
- Lou ZZ, Chen LH, Liu HF, Ruan LM, Zhou WH. (2014) Blockade of mGluR5 in the nucleus accumbens shell but not core attenuates heroin seeking behavior in rats. Acta Pharmacol Sin 35:1485–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni OJ, Williams JT. (1999) Presynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawal. J Neurosci 19:6629–6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Jasinski DR. (1969) Physiological parameters of morphine dependence in man--tolerance, early abstinence, protracted abstinence. J Psychiatr Res 7:9–17. [DOI] [PubMed] [Google Scholar]
- Matthes HW Maldonado R Simonin F Valverde O Slowe S Kitchen I Befort K Dierich A, Le Meur M Dolle P Tzavara E Hanoune J Roques BP Kieffer BL (1996) Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 383:819–823. [DOI] [PubMed] [Google Scholar]
- Muller DL, Unterwald EM. (2004) In vivo regulation of extracellular signal-regulated protein kinase (ERK) and protein kinase B (Akt) phosphorylation by acute and chronic morphine. J Pharmacol Exp Ther 310:774–782. [DOI] [PubMed] [Google Scholar]
- Narita M, Suzuki M, Niikura K, Nakamura A, Miyatake M, Aoki T, Yajima Y, Suzuki T. (2005) Involvement of spinal metabotropic glutamate receptor 5 in the development of tolerance to morphine-induced antinociception. J Neurochem 94:1297–1305. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Sullivan MA, Levin FR. (2004) Treatment of depression in patients with opiate dependence. Biol Psychiatry 56:793–802. [DOI] [PubMed] [Google Scholar]
- Palucha A, Branski P, Szewczyk B, Wieronska JM, Klak K, Pilc A. (2005) Potential antidepressant-like effect of MTEP, a potent and highly selective mGluR5 antagonist. Pharmacol Biochem Behav 81:901–906. [DOI] [PubMed] [Google Scholar]
- Peles E, Schreiber S, Naumovsky Y, Adelson M. (2007) Depression in methadone maintenance treatment patients: rate and risk factors. J Affect Disord 99:213–220. [DOI] [PubMed] [Google Scholar]
- Picker MJ, Daugherty D, Henry FE, Miller LL, Dykstra LA. (2011) Metabotropic glutamate antagonists alone and in combination with morphine: comparison across two models of acute pain and a model of persistent, inflammatory pain. Behav Pharmacol 22:785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomierny-Chamiolo L, Poleszak E, Pilc A, Nowak G. (2010) NMDA but not AMPA glutamatergic receptors are involved in the antidepressant-like activity of MTEP during the forced swim test in mice. Pharmacol Rep 62:1186–1190. [DOI] [PubMed] [Google Scholar]
- Popik P, Wrobel M. (2002) Morphine conditioned reward is inhibited by MPEP, the mGluR5 antagonist. Neuropharmacology 43:1210–1217. [DOI] [PubMed] [Google Scholar]
- Roohi N, Sarihi A, Shahidi S, Zarei M, Haghparast A. (2014) Microinjection of the mGluR5 antagonist MTEP into the nucleus accumbens attenuates the acquisition but not expression of morphine-induced conditioned place preference in rats. Pharmacol Biochem Behav 126:109–115. [DOI] [PubMed] [Google Scholar]
- Schroder H, Wu DF, Seifert A, Rankovic M, Schulz S, Hollt V, Koch T. (2009) Allosteric modulation of metabotropic glutamate receptor 5 affects phosphorylation, internalization, and desensitization of the micro-opioid receptor. Neuropharmacology 56:768–778. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Smith DG, Rizzo SJ, Karras MN, Turner SM, Tolu SS, Bryce DK, Smith DL, Fonseca K, Ring RH, Crawley JN. (2012) Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism. Sci Transl Med 4:131ra151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Dworkin SI, Childers SR. (1996) Effects of chronic morphine administration on mu opioid receptor-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci 16:2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeester BA, Lunzer MM, Akgun E, Beitz AJ, Portoghese PS. (2014) Targeting putative mu opioid/metabotropic glutamate receptor-5 heteromers produces potent antinociception in a chronic murine bone cancer model. Eur J Pharmacol 743:48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotts AL, Dodrill CL, Kosten TR. (2009) Opioid dependence treatment: options in pharmacotherapy. Expert Opin pharmacother 10:1727–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Narita M, Niikura K, Suzuki T. (2006) Chronic morphine treatment increases the expression of the neural cell adhesion molecule in the dorsal horn of the mouse spinal cord. Neurosci Lett 399:202–205. [DOI] [PubMed] [Google Scholar]
- Veeneman MM, Boleij H, Broekhoven MH, Snoeren EM, Guitart Masip M, Cousijn J, Spooren W, Vanderschuren LJ. (2011) Dissociable roles of mGlu5 and dopamine receptors in the rewarding and sensitizing properties of morphine and cocaine. Psychopharmacology (Berl) 214:863–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veilleux JC, Colvin PJ, Anderson J, York C, Heinz AJ. (2010) A review of opioid dependence treatment: pharmacological and psychosocial interventions to treat opioid addiction. Clin Psychol Rev 30:155–166. [DOI] [PubMed] [Google Scholar]
- Wright SR, Zanos P, Georgiou P, Yoo JH, Ledent C, Hourani SM, Kitchen I, Winsky-Sommerer R, Bailey A. (2015) A critical role of striatal A2AR-mGlu5R interactions in modulating the psychomotor and drug-seeking effects of methamphetamine. Addict Biol. doi: 10.1111/adb.12259. [DOI] [PubMed] [Google Scholar]
- Xu NJ, Bao L, Fan HP, Bao GB, Pu L, Lu YJ, Wu CF, Zhang X, Pei G. (2003) Morphine withdrawal increases glutamate uptake and surface expression of glutamate transporter GLT1 at hippocampal synapses. J Neurosci 23:4775–4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Georgiou P, Wright SR, Hourani SM, Kitchen I, Winsky-Sommerer R, Bailey A. (2014) The oxytocin analogue carbetocin prevents emotional impairment and stress-induced reinstatement of opioid-seeking in morphine-abstinent mice. Neuropsychopharmacology 39:855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ. (2006) Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol 191:137–145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.