Abstract
Background:
Inter-individual differences in the monoaminergic systems have been shown to moderate the risk for a lifetime history of anxiety, affective, and alcohol use disorders. A common single nucleotide polymorphism in the vesicular monoamine transporter 1 gene (VMAT1 rs1390938 G/A; Thr136Ile) has been reported as functional in vitro and associated with bipolar disorder and anxiety. We aimed at assessing the association between the VMAT1 genotype, affect, and affect-related psychiatric disorders in a longitudinal population-representative study.
Methods:
We used the database of the Estonian Children Personality Behaviour and Health Study (beginning in 1998). Cohorts of initially 9- (recalled at ages 15 and 18 years, n=579) and 15- (recalled at ages 18 and 25 years; n=654) year-old children provided self-reports on impulsivity, anxiety, depressiveness, neuroticism, and alcohol use. In addition, psychiatric assessment based on DSM-IV was carried out in the older cohort at age 25 years.
Results:
Subjects homozygous for the less prevalent A (136Ile) allele reported lower maladaptive impulsivity, state and trait anxiety, depressiveness, and neuroticism and were less likely to have been diagnosed with an affective, anxiety, and/or alcohol use disorder by young adulthood. While in the younger cohort alcohol use started at younger age, this birth cohort effect was dependent on genotype: only G allele carriers and in particular the GG homozygotes started alcohol use earlier.
Conclusions:
VMAT1 rs1390938/Thr136Ile is associated with mood, personality, and alcohol use in the general population. Subjects homozygous for the “hyperfunction” allele (AA; Ile/Ile) appear to be more resilient to these disorders.
Keywords: VMAT1, affective disorders, anxiety, alcohol use, cohort effect
Introduction
Mental disorders are the leading cause of years lived with disability (Whiteford et al., 2013). Depressive disorders accounted for 40.5% of disability-adjusted life years caused by mental disorders, with anxiety disorders accounting for another 14.6% and alcohol use disorders for 9.6%. Alcohol use is the leading risk factor for global disease burden in Eastern Europe, Andean Latin America, and southern sub-Saharan Africa (Lim et al., 2013), and comorbidity of alcohol use disorders with anxiety and affective disorders has been extensively documented (for review, see Boschloo et al., 2013).
The risk for a lifetime history of anxiety, affective, and alcohol use disorders is strongly influenced by genetic factors. Genetic epidemiological studies report heritability estimates about 30–50% in the case of anxiety disorders (Shimada-Sugimoto et al., 2015) and about 30 to 40% for depression (Craddock and Forty, 2006). Twin and family studies largely provide evidence for the shared etiology of affective and anxiety disorder symptoms (Waszcuk et al., 2014). For a lifetime history of alcohol abuse and dependence, the estimates of heritability range around 30% in adolescents and 45 to 65% in adults (reviewed by van Beek et al., 2012).
Monoamine systems undergo extensive and interdependent functional reorganization as affective disorders develop (eg, Harro and Oreland, 2001). Several functional polymorphisms in the monoaminergic (dopaminergic, serotonergic, and noradrenergic) systems have been reported to moderate anxiety and affective disorders (for review, see Lacerda-Pinheiro et al., 2014) and alcohol use (Guo et al., 2007). Although deviations in monoaminergic function probably vary between disorders, a common source of vulnerability could lie in the vesicular function that controls monoamine storage and homeostasis. Vesicular monoamine transporters (VMATs) carry monoamines such as serotonin, dopamine, adrenaline, noradrenaline, and histamine from the cytoplasm into storage vesicles (Edwards, 1992). Hence, VMATs are an important target for biological research in psychiatric disorders (Lohoff, 2010; Wimalasena, 2011).
Two structurally related but pharmacologically distinct human VMATs have been identified, encoded by separate genes, VMAT1 (SLC18A1) located on chromosome 8p21 and VMAT2 (SLC18A2) on chromosome 10q25 (Peter et al., 1993). It was initially reported that only VMAT2 is expressed in the brain (Peter et al., 1995; Erickson et al., 1996). However, it was later found that VMAT1 is also widely expressed in human brain at the mRNA and protein level (Lohoff et al., 2006). The transporters share common substrates with the exception of histamine, which is believed to be preferentially packaged by VMAT2 (for review, see Bernstein et al., 2014). They also differ in affinities: VMAT1 shows higher affinity for serotonin (Brunk et al., 2006).
As reviewed by Lohoff et al. (2006), studies in vitro show that lithium and valproate, effective pharmacotherapies for bipolar disorder, increase the expression of VMAT1, suggesting that the VMAT1 might be a target for therapeutic drug action. Variations in the VMAT1 gene can affect transporter function and/or expression and therefore be involved in the etiology of neuropsychiatric disorders. Indirect evidence that the VMAT is involved in psychiatric disorders stems from positron emission tomography imaging studies. Binding of radiolabelled dihydrotetrabenazine, a catecholamine depleter with higher VMAT2 than VMAT1 affinity (DaSilva et al., 1993, 1994), was found to be increased in thalamus and brainstem of bipolar patients when compared with controls (Zubieta et al., 2000). Binding in ventral brainstem was higher in bipolar and schizophrenia patients compared with controls (Zubieta et al., 2001). Several recent genetic case-control studies have documented an association between common missense variations in the VMAT1 gene and susceptibility to bipolar disorder (Lohoff et al., 2006) and schizophrenia (Bly, 2005; Chen et al., 2007; Lohoff et al., 2008a).
A common single nucleotide polymorphism in the VMAT1 gene (rs1390938 G/A) that results in threonine or isoleucine at amino acid 136 (Thr136Ile) has recently been shown to be functional in vitro, with the 136Ile variant leading to increased monoamine transport into presynaptic vesicles (Khalifa et al., 2012). Thr136Ile polymorphism is located in the intravesicular loop 1, and the frequency of the hyperfunction allele (A; 136Ile) is ~0.25 in European and Caucasian samples and <0.1 in African samples. It was reported by Lohoff et al. (2014) that carriers of the 136Ile (A) variant show diminished hemodynamic responses to negative emotional words in the medial prefrontal cortex and pregenual anterior cingulate cortex when compared with Thr136 homozygotes, suggesting that the VMAT1 hyperfunction allele may predispose certain individuals to a diminished cortical response to negative stimuli. An association of the 136Thr variant with bipolar disorder (Lohoff et al., 2006) and higher self-report State-Trait Anxiety Inventory (STAI) scores in Thr/Ile heterozygous females (Lohoff et al., 2008b) has been described previously.
Based on these previous associations of the variation in the VMAT1 encoding gene to anxiety-related personality traits and bipolar disorder, we tested the hypothesis that VMAT1 rs1390938/Thr136Ile polymorphism is associated with anxiety and affective disorders, making use of a population-representative sample of young adults. Considering that increased impulsivity and neuroticism are vulnerability markers for bipolar disorder (Jylhä et al., 2010; Wessa et al., 2015) and symptoms of anxiety and depressiveness have been associated with problematic alcohol use (de Abreu Costa et al., 2013; Edwards et al., 2014), we also examined the association between rs1390938 polymorphism and impulsivity, personality traits, and alcohol consumption.
Methods
Study Population
The study was carried out on the sample of the longitudinal Estonian Children Personality Behaviour and Health Study (ECPBHS), initially cohorts of 9- (born in 1988/1989, recalled at ages 15 and 18 years) and 15- (born in 1982/1983, recalled at ages 18 and 25 years) year-old children. The rationale and procedure of sample formation have been described elsewhere (Harro et al., 2001; Tomson et al., 2011). ECPBHS is population representative, while 79.1% of subjects of the randomized regional sample participated in the original sampling. Most of the present analysis is focused on data from the older cohort, but relevant measures in both cohorts were subject to analysis if available. The complete number of subjects for whom all data used in this analysis were available is shown in Table 1. The participants were all of Caucasian descent.
Table 1.
VMAT1 rs1390938 Genotype Frequencies in the Entire Sample
| AA | AG | GG | |
|---|---|---|---|
| Older birth cohort (n=654) | 76 (12%) | 279 (43%) | 299 (45%) |
| Younger birth cohort (n=579) | 52 (9%) | 265 (46%) | 262 (45%) |
The study was approved by the Ethics Review Committee on Human Research of the University of Tartu, and written informed consent was obtained from all the participants, and in case of minors, also from their parents.
Lifetime Prevalence of Affective, Anxiety, and Alcohol Use Disorders
Psychiatric assessment based on DSM-IV was carried out in the older cohort at age 25 by experienced clinical psychologists using the Mini-International Neuropsychiatric Interview (M.I.N.I.5.0.0; Sheehan et al., 1998; Estonian version: Shlik et al., 1999) at age 25 years.
Anxiety
In the younger birth cohort, the Spielberger State Anxiety Inventory (STAI-S; Spielberger et al., 1983) was used at ages 15 and 18 years and the Spielberger Trait Anxiety Inventory (STAI-T) at age 18 years. In the older birth cohort, STAI-S was used only at 25 years and STAI-T at ages 18 and 25 years.
Depressiveness
Beck Depression Inventory (Beck et al., 1961) was used to measure depressiveness in the younger birth cohort at age 15 years. Montgomery–Åsberg Depression Rating Scale MÅDRS (Montgomery and Åsberg, 1979) was used in the younger cohort at age 18 yeras and in the older birth cohort at ages 18 and 25 years.
Impulsivity
Self-reports for different facets of impulsivity were completed at ages 15 and 18 yeras for the younger cohort and at ages 18 and 25 years for the older cohort. The Adaptive and Maladaptive Impulsivity Scale, which follows the concept of functional and dysfunctional impulsivity (Dickman, 1990) and comprises subscales measuring fast decision-making and excitement seeking (functional or adaptive impulsivity) and disinhibition and thoughtlessness (dysfunctional or maladaptive impulsivity), was used (Laas et al., 2010).
Personality
Personality traits of the 5-factor model (Costa and McCrae, 1989) were measured by self-reports with the Estonian version of Revised NEO Personality Inventory (NEO-PI-R) (Kallasmaa et al., 2000) called EPIP-NEO (Mõttus et al., 2006), or Estonian Brief Big Five Inventory (EBBFI) (Laidra et al., 2006; Harro et al., 2009). EPIP-NEO is a semantically simplified full-length version of NEO-PI-R, whereas EBBFI is a shorter and semantically simplified questionnaire. Personality data of the younger cohort were collected at ages 15 (EPIP-NEO) and 18 years (NEO-PI-R) and of the older cohort at age 15 years (EBBFI) and 18 and 25 years (both NEO-PI-R). As the data have been collected with different instruments, all scores were transformed into Z-scores for statistical analysis.
Alcohol Use
Subjects reported the age when they first consumed one-half a unit of alcohol during both follow-up studies. One unit of alcohol was defined as a glass of light wine or champagne (12 cL), a shot of vodka (4 cL), or a bottle (33 cL) of light alcohol (beer, long drink, cider, etc.). In all data collection waves, the participants reported how often they had consumed different types of alcoholic beverages. According to the most frequently consumed type of alcoholic beverage, a 5-point total alcohol use scale was constructed: (1) almost never, (2) less than monthly, (3) monthly, (4) weekly, (5) every day, as previously described in Merenäkk et al. (2003).
Genotyping
Genomic DNA was extracted from whole blood samples using Qiagen QIAamp DNA Blood Midi Kit. Genotyping was performed on the Applied Biosystems ViiA 7 Real-Time PCR System using TaqMan Pre-Designed SNP Genotyping Assay and Solis BioDyne 5x HOT FIREPol Probe qPCR Mix Plus (ROX). All DNA samples were successfully genotyped. Genotype frequencies were in Hardy-Weinberg equilibrium (Table 1).
Statistical Analysis
Categorical measures were compared by Pearson’s chi-square tests. Categorical variable relations to continuous variable were explored with ANOVA and presented as F-statistic, raw P value and eta-squared (η 2) as a measure of effect size. Fisher’s least significance difference method was used in posthoc comparisons. Contrasts were calculated for significant model effects. All P values are reported as 2-tailed, and results are considered significant at the P<.05 level. Statistical analyses were performed using IBM SPSS Statistics, Version 20.
Results
The lifetime prevalence of affective, anxiety, and alcohol use disorders was similar to comparable epidemiological studies (Hasin et al., 2007; de Graaf et al., 2012; WHO, 2014; NIMH, 2015; for a more detailed information of the prevalence of affective and anxiety disorders in the ECPBHS, see Laas et al., 2014). VMAT1 was associated with the prevalence of psychiatric disorders: the likelihood of having been diagnosed with an affective, anxiety, and/or alcohol use disorder at some point of their life was significantly lower for the AA homozygotes compared with G allele carriers (Table 2).
Table 2.
VMAT1 rs1390938 Effects on Lifetime Prevalence of Affective, Anxiety, and Alcohol Use Disorders
| Psychiatric Disorder | Total | Main Statistics (Pearson’s χ 2) | AA | AG | GG |
|---|---|---|---|---|---|
| (n=501) | (n=62) | (n=208) | (n=231) | ||
| Affective disorders | 114 (23%) | χ2=(2, n=501)=4.86, P = .088 | 10 (16%) | 41 (20%) | 63 (27%) |
| Anxiety disorders | 84 (17%) | χ2=(2, n=501)=3.85, P = .146 | 5 (8%) | 37 (18%) | 42 (18%) |
| Affective or anxiety disorder or both | 152 (30%) | χ2=(2, n=501)=4.52, P = .104 | 12 (19%) | 63 (30 %) | 77 (33%) |
| AUD | 95 (19%) | χ2=(2, n=501)=2.15, P = .341 | 8 (13%) | 44 (21%) | 43 (19%) |
| Affective, anxiety, and/or AUD | 214 (43%) | χ 2=(2, n=501)=6.78, P = .034 | 17 (27%) | 94 (45%) | 103 (45%) |
Abbreviations: AUD, alcohol use disorder.
Significant difference in prevalence presented in italics.
STAI-S scores at age 25 years in the older birth cohort were associated with the VMAT1 rs1390938 genotype (Figure 1). AA homozygotes received lower STAI-S scores compared with G allele carrier groups [F(2,472)=3.2, P=.041, η 2=0.013]. Association of the VMAT1 rs1390938 with STAI-T scores at the age of 25 years in the older birth cohort appeared similar, but the genotype main effect did not reach the level of statistical significance [F(2,474)=2.6, P=.078, η 2=0.011]. At age 18 years, no significant VMAT1 rs1390938 genotype effects on anxiety scores were identified in either birth cohort.
Figure 1.
The association of VMAT1 rs1390938 polymorphism and anxiety scores in the older birth cohort at ages 18 and 25 years. Whiskers denote standard errors of mean. Significant differences between groups: *P < .05.
Findings were similar in the case of depressiveness (Figure 2): AA homozygotes reported significantly lower MÅDRS scores than the G allele carrier groups in the older birth cohort at the age of 25 years [F(2,535)=4.6, P=.011, η 2=0.017].
Figure 2.
The association of VMAT1 rs1390938 polymorphism and depressiveness in the older birth cohort at the ages 18 and 25 years. Whiskers denote standard errors of mean. Significant differences between groups: **P < .01.
In the older birth cohort by the age of 25 years, the VMAT1 rs1390938 genotype was also found associated with maladaptive impulsivity (Figure 3), the AA homozygotes reporting lower thoughtlessness [F(2,507)=4.0, P=.02, η 2=0.015], and overall maladaptive impulsivity scores [F(2,507)=3.9, P=.022, η 2=0.015] than G allele carriers. Adaptive impulsivity was not influenced by VMAT1.
Figure 3.
The association of VMAT1 rs1390938 polymorphism and thoughtlessness scores in the older birth cohort at the ages 18 and 25 years. Whiskers denote standard errors of mean. Significant differences between groups denoted as follows: *P < .05, # P = .062.
Of the 5-factor model personality traits, VMAT1 was associated only with neuroticism (Figure 4). In the older birth cohort at age 25 years, AA homozygotes reported lower neuroticism scores than G allele carriers [F(2,492)=3.7, P=.025, η 2=0.015]. At age 18 years, no significant VMAT1 rs1390938 genotype effects on personality scores were identified in either birth cohort.
Figure 4.
The association of VMAT1 rs1390938 polymorphism and neuroticism scores in the older birth cohort at the ages 18 and 25 years. Whiskers denote standard errors of mean. Significant differences between groups denoted as follows: *P < .05, **P < .01.
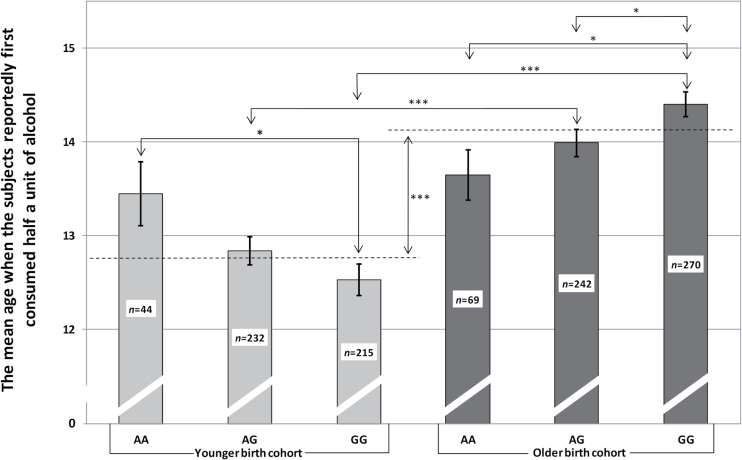
VMAT1 rs1390938 genotype did not affect the self-reported measures of the frequency of alcohol use in different ages (data not shown). However, VMAT1 was associated with the average age when the subjects reportedly first consumed one-half a unit of alcohol in both younger [F(2,488)=3.0, P=.053, η 2=0.012] and older [F(2,578)=4.2, P=.015, η 2=0.014] birth cohorts, but in opposite directions (Figure 5). In the younger birth cohort, GG homozygotes were the first and AA homozygotes the last to start experimenting; in the older cohort, it was the other way around. The younger birth cohort generally started experimenting with alcohol at an earlier age than the older birth cohort [F(1,1070)=96.0, P<.001, η 2 =0.082] with the exception of theVMAT1 rs1390938 AA homozygotes. Consistently, at age 15 years, the younger cohort was using alcohol more frequently than the older cohort. Alcohol use frequency was not, however, statistically significantly different at age 18 years (data not shown). Subjects in the older cohort diagnosed with alcohol use disorder by age 25 years reported experimenting with alcohol at an earlier age (13.4±0.3) than the rest of the sample [14.4±0.1; F(1,488)=13.7, P<.001, η 2=0.027].
Figure 5.
The association of VMAT1 rs1390938 polymorphism in interaction with birth cohort with the average age when the subjects reportedly first consumed one-half a unit of alcohol [F VMAT1 rs1390938 * cohort (2,1066)=6.9, P = .001, η 2 =0.013]. The dashed line indicates the mean age when the subjects from the respective cohort first consumed one-half a unit of alcohol. Whiskers denote standard errors of mean. Significant differences between groups denoted as follows: *P < .05, ***P < .001.
Discussion
In this study, we tested the hypothesis that the VMAT1 rs1390938/Thr136Ile polymorphism is associated with mood disorders, alcohol use, and personality. We found that subjects with the AA (Ile/Ile) genotype were less likely to have been diagnosed with an affective, anxiety, and/or alcohol use disorder at some point of their life and reported lower state and trait anxiety, depressiveness, maladaptive impulsivity, and neuroticism by young adulthood compared with G allele carriers. In addition, while initiation of alcohol use is taking place at progressively younger ages (Currie et al., 2000, 2004, 2008; Pärna et al., 2012; Vaht et al., 2014; present findings), this did not occur in AA homozygotes. All these associations were similar for men and women. Our results are essentially consistent with previous research. It has been found that the gain-of-function A-allele (136Ile) is protective against bipolar disorder (Lohoff et al., 2006), and the AA (Ile/Ile) homozygotes have lower STAI state and trait scores (Lohoff et al., 2008b). Very recently, the A-allele (136Ile) has been found to be associated with reduced connectivity in networks that show a general increased connectivity in alcoholics, indicating a potential protective effect (Zhu et al., 2015).
Genetic variation in plasma membrane transporters (serotonin, noradrenaline, and dopamine transporters) can serve as a basis for inter-individual differences in brain circuits associated with affective behavior (Bevilacqua and Goldman, 2011). These transporters are mainly involved in synaptic neurotransmitter reuptake, which contributes to the duration of signaling. In contrast, variation in the magnitude of signaling may be more closely related to mechanisms regulating synaptic neurotransmitter release (Lohoff et al., 2014). Efficient reuptake of the transmitter from the synaptic cleft through plasma membrane monoamine transporters followed by reaccumulation into synaptic vesicles through the VMATs constitute crucial interlinked steps of monoamine neurotransmission (Wimalasena, 2011).
The common Thr136Ile polymorphism is located in the first luminal domain of the transporter. This region of the protein interacts with inhibitors and substrates (Sievert and Ruoho, 1997) and mediates G-protein-dependent regulation of transmitter uptake (Brunk et al., 2006). 136Thr has been related to decreased monoamine transport in vitro (Khalifa et al., 2012; Lohoff et al., 2014). Reduced storage and release of monoamines in brain regions expressing VMAT1 and in adrenal medulla where VMAT1 is the major type of VMAT have been suggested to alter the balance of monoamine availability both peripherally and centrally (Khalifa et al., 2012). Such presynaptic components are likely part of a shared pathway of vulnerability to a range of neuropsychiatric phenotypes (Lohoff et al., 2014).
While the association of VMAT1 rs1390938/Thr136Ile polymorphism with psychological measures and prevalence of psychiatric disorders in this sample is straighforward and the findings provide a remarkably coherent picture, an interesting observation was the interaction with birth cohort in the mean age when subjects reportedly first consumed one-half a unit of alcohol. Younger age at first use of alcohol has been associated with significantly higher risk of heavy alcohol use (Liang and Chikritzhs, 2015). In the younger birth cohort of the ECPBHS, GG homozygotes were the first and AA homozygotes the last to start experimenting; in the older cohort, this was completely reversed. Unlike in the case of G allele carriers, there was no cohort difference in the mean age when AA homozygotes started experimenting with alcohol. Given that peer drinking serves as a model for alcohol use (Milgram, 2001) and socially anxious youth can be motivated to use alcohol to manage their anxious arousal (Blumenthal et al., 2010), one possible explanation could be that AA homozygotes may be less sensitive to peer pressure in this regard. As described before, it was determined by Lohoff et al. (2014) that carriers of the VMAT1 hyperfunction allele (A) may be predisposed to a diminished cortical response to negative stimuli. Activity of prefrontal regions is a critical component of regulating emotional arousal, particularly those triggered in response to environmental factors.
Belonging to a particular birth cohort can serve as a proxy for the socioeconomic environment experienced by a generation. Alcohol consumption has been shown to be subject to birth cohort effects (Johnson and Gerstein, 1998; Rice et al., 2003; Pabst et al., 2010). Economic fluctuation, political instability, policies and laws, and social norms are group-level exposures that can vary between time periods and countries, potentially impacting particular birth cohorts in ways that affect their risk for higher alcohol consumption and alcohol use disorder (Keyes et al., 2011). In a previous analysis, we found that expression of genetic vulnerability to alcohol use is influenced by birth cohort effects. We demonstrated that the serotonin transporter gene promoter polymorphism (5-HTTLPR) is associated with alcohol consumption in the general population, but the effect is dependent on birth cohort and gender (Vaht et al., 2014). In this instance, the female s/s homozygotes were the most changeable group across the 2 birth cohorts, and this group could be interpreted as the most susceptible to peer pressure. The country of the present study, Estonia, is a representative transition society that moved away from socialism in the late 1980s and became an independent and highly liberal economy since 1991. The Estonian economy was one of the fastest growing in the world until 2007 (World Bank, 2015), bringing about rapid but multifaceted social changes. Therefore, environmental conditions and demands have been rather different for the 2 birth cohorts, bringing about a change in the E component of the G × E formula; in turn, such a change in environment indeed should be reflected in how specific gene variants relate to the behavior in question, in this case, alcohol use initiation (Harro, 2010). Thus, one could speculate that in the condition of increased alcohol availability, the shift toward a younger age of alcohol use mediated by peer pressure is better resisted by the AA homozygotes.
These results should be interpreted in the context of limitations. First, we used self-reports for the majority of measurements. However, the results with the self-reports fall in line with our previous data based on parents’ and teachers’ ratings of personality and are consistent with findings based on clinical interview. Another limitation of the study is the small group sizes in the case of AA homozygous subjects diagnosed with affective, anxiety, or alcohol use disorders. This may lead to insufficient power and type II error when genotype effects on separate diagnosis are analyzed. Nevertheless, the strength of the present study is the longitudinal design, rigorous questionnaire data collection performed in uniform conditions of the laboratory, and the fact that the sample has a strong representation of regional population.
In conclusion, we have found that the VMAT1 rs1390938/Thr136Ile polymorphism is associated with mood, personality, and alcohol use in the general population. Subjects homozygous for the hyperfunction allele (AA; Ile/Ile) appear to have features supporting resiliency to negative emotionality and these disorders.
Statement of Interest
J.H. has received speaker’s fees from Lundbeck and is a Faculty Member of the Lundbeck International Neuroscience Foundation, all of which is not directly related to the subject of this work. Other authors declare no conflict of interest.
Acknowledgments
We are grateful to the participants of the ECPBHS and to the whole ECPBHS Study Team.
This work was supported by grants from the Estonian Ministry of Education and Science (IUT20-40), European Regional Development Fund ERC Program TerVE (ELIKTU 3.2.10002.11-0002) and European Community’s Seventh Framework Programme (FP7/2007– 2013) under grant agreement 602805 (Aggressotype).
References
- Beck AT, Ward C, Mendelson M. (1961) Beck Depression Inventory (BDI). Arch Gen Psychiatry 4:561–571. [DOI] [PubMed] [Google Scholar]
- Bernstein AI, Stout KA, Miller GW. (2014) The vesicular monoamine transporter 2: an underexplored pharmacological target. Neurochem Int 73:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua L, Goldman D. (2011) Genetics of emotion. Trends Cogn Sci 15:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal H, Leen-Feldner EW, Frala JL, Badour CL, Ham LS. (2010) Social anxiety and motives for alcohol use among adolescents. Psychol Addict Behav 24:529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bly M. (2005) Mutation in the vesicular monoamine gene, SLC18A1, associated with schizophrenia. Schizophr Res 78:337–338. [DOI] [PubMed] [Google Scholar]
- Boschloo L, Vogelzangs N, van den Brink W, Smit JH, Veltman DJ, Beekman AT, Penninx BW. (2013) Depressive and anxiety disorders predicting first incidence of alcohol use disorders: results of the Netherlands Study of Depression and Anxiety (NESDA). J Clin Psychiatry 74:1233–1240. [DOI] [PubMed] [Google Scholar]
- Brunk I, Blex C, Rachakonda S, Holtje M, Winter S, Pahner I, Walther DJ, Ahnert-Hilger G. (2006) The first luminal domain of vesicular monoamine transporters mediates G-protein-dependent regulation of transmitter uptake. J Biol Chem 281:33373–33385. [DOI] [PubMed] [Google Scholar]
- Chen SF, Chen CH, Chen JY, Wang YC, Lai IC, Liou YJ, Liao DL. (2007) Support for association of the A277C single nucleotide polymorphism in human vesicular monoamine transporter 1 gene with schizophrenia. Schizophr Res 90:363–365. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. (1989) The NEO-PI/NEO-FFI manual supplement. Odessa (FL): Psychological Assessment Resources. [Google Scholar]
- Craddock N, Liz Forty L. (2006) Genetics of affective (mood) disorders. Eur J Hum Genet 14:660–668. [DOI] [PubMed] [Google Scholar]
- Currie C, Hurrelmann K, Settertobulte W, Smith R, Todd J. (eds) (2000) Health and health behaviour among young people. Health behaviour in school-aged children: a WHO cross-national study (HBSC) international report. Health policy for children and adolescents. No. 1. Copenhagen, Denmark. [Google Scholar]
- Currie C, Roberts C, Morgan A, Smith R, Settertobulte W, Samdal O, Barnekow Rasmussen V. (eds) (2004) Young people’s health in context: international report from the HBSC 2001/02 survey. Health policy for children and adolescents, No.4. Copenhagen, Denmark. [Google Scholar]
- Currie C, Gabhainn SN, Godeau E, Roberts C, Smith R, Currie D, Pickett W, Richter M, Morgan A, Barnekow V. (eds) (2008) Inequalities in young people’s health: International report from the HBSC 2005/06 survey. Health policy for children and adolescents, No. 5. Copenhagen, Denmark. [Google Scholar]
- DaSilva JN, Kilbourn MR, Mangner TJ. (1993) Synthesis of a [11C]methoxy derivative of alpha-dihydrotetrabenazine: a radioligand for studying the vesicular monoamine transporter. Appl Radiat Isot 44:1487–1489. [DOI] [PubMed] [Google Scholar]
- DaSilva JN, Carey JE, Sherman PS, Pisani TJ, Kilbourn MR. (1994) Characterization of [11C]tetrabenazine as an in vivo radioligand for the vesicular monoamine transporter. Nucl Med Biol 21:151–156. [DOI] [PubMed] [Google Scholar]
- de Abreu Costa M Salum GA Jr Isolan LR Acosta JR Jarros RB Blaya C, Von Diemen L Manfro GG (2013) Association between anxiety symptoms and problematic alcohol use in adolescents. Trends Psychiatry Psychother 35:106–110. [DOI] [PubMed] [Google Scholar]
- de Graaf R, ten Have M, van Gool C, van Dorsselaer S. (2012) Prevalence of mental disorders and trends from 1996 to 2009. Results from the Netherlands Mental Health Survey and Incidence Study-2. Soc Psychiatry Psychiatr Epidemiol 47:203–213. [DOI] [PubMed] [Google Scholar]
- Dickman SJ. (1990) Functional and dysfunctional impulsivity: personality and cognitive correlates. J Person Soc Psychol 58:95–102. [DOI] [PubMed] [Google Scholar]
- Edwards AC, Joinson C, Dick DM, Kendler KS, Macleod J, Munafò M, Hickman M, Lewis G, Heron J. (2014) The association between depressive symptoms from early to late adolescence and later use and harmful use of alcohol. Eur Child Adolesc Psychiatry 23:1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RH. (1992) The transport of neurotransmitters into synaptic vesicles. Curr Opin Neurobiol 2:586–594. [DOI] [PubMed] [Google Scholar]
- Erickson JD, Schafer MK, Bonner TI, Eiden LE, Weihe E. (1996) Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci USA 93:5166–5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, Wilhelmsen K, Hamilton N. (2007) Gene–lifecourse interaction for alcohol consumption in adolescence and young adulthood: five monoamine genes. Am J Med Genet Part B 144B:417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harro J. (2010) Inter-individual differences in neurobiology as vulnerability factors for affective disorders: Implications for psychopharmacology. Pharmacol Ther 125:402–422. [DOI] [PubMed] [Google Scholar]
- Harro J, Oreland (2001) Depression as a spreading adjustment disorder of monoaminergic neurons: a case for primary implication of the locus coeruleus. Brain Res Rev 38:79–128. [DOI] [PubMed] [Google Scholar]
- Harro J, Merenäkk L, Nordquist N, Konstabel K, Comasco E, Oreland L. (2009) Personality and the serotonin transporter gene: associations in a longitudinal population based study. Biol Psychol 81:9–13. [DOI] [PubMed] [Google Scholar]
- Harro M, Eensoo D, Kiive E, Merenäkk L, Alep J, Oreland L, Harro J. (2001) Platelet monoamine oxidase in healthy 9- and 15-yr old children: the effect of gender, smoking and puberty. Prog Neuropsychopharmacol Biol Psychiatry 25:1497–1551. [DOI] [PubMed] [Google Scholar]
- Hasin DS Stinson FS Ogburn E, MS; Grant BF (2007) Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States. Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 64:830–842. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Gerstein DR. (1998) Initiation of use of alcohol, cigarettes, marijuana, cocaine, and other substances in US birth cohorts since 1919. Am J Public Health 88:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jylhä P, Mantere O, Melartin T, Suominen K, Vuorilehto M, Arvilommi P, Leppämäki S, Valtonen H, Rytsälä H, Isometsä E. (2010) Differences in neuroticism and extraversion between patients with bipolar I or II and general population subjects or major depressive disorder patients. J Affect Disord 125:42–52. [DOI] [PubMed] [Google Scholar]
- Kallasmaa T, Allik J, Realo A, McCrae RR. (2000) The Estonian version of the NEOPI-R: an examination of universal and culture-specific aspects of the five-factor model. Eur J Pers 14:265–278. [Google Scholar]
- Keyes KM, Li G, Hasin DS. (2011) Birth cohort effects and gender differences in alcohol epidemiology: a review and synthesis. Alcohol Clin Exp Res 35:2101–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifa AM, Watson-Siriboe A, Shukry SG, Chiu WL, Nelson ME, Geng Y, Fischer-Stenger K, Porter JH, Jennifer K., Stewart JK. (2012) Thr136Ile polymorphism of human vesicular monoamine transporter-1 (SLC18A1 gene) influences its transport activity in vitro . Neuroendocrinol Lett 33:546–551. [PubMed] [Google Scholar]
- Laas K, Reif A, Herterich S, Eensoo D, Lesch KP, Harro J. (2010) The effect of a functional NOS1 promoter polymorphism on impulsivity is moderated by platelet MAO activity. Psychopharmacology 209:255–261. [DOI] [PubMed] [Google Scholar]
- Laas K, Reif A, Akkermann K, Kiive E, Domschke K, Lesch KP, Veidebaum T, Harro J. (2014) Interaction of the neuropeptide S receptor gene Asn107Ile variant and environment: contribution to affective and anxiety disorders, and suicidal behaviour. Int J Neuropsychopharmacol 17:541–552. [DOI] [PubMed] [Google Scholar]
- Lacerda-Pinheiro SF, Pinheiro Junior RF, Pereira de Lima MA, Lima da Silva CG, Vieira dos Santos Mdo S, Teixeira Júnior AG, Lima de Oliveira PN, Ribeiro KD, Rolim-Neto ML, Bianco BA. (2014) Are there depression and anxiety genetic markers and mutations? A systematic review. J Affect Disord 168:387–398. [DOI] [PubMed] [Google Scholar]
- Laidra K, Allik J, Harro M, Merenäkk L, Harro J. (2006) Agreement among adolescents, parents and teachers on adolescent personality. Assessment 13:187–196. [DOI] [PubMed] [Google Scholar]
- Liang W, Chikritzhs T. (2015) Age at first use of alcohol predicts the risk of heavy alcohol use in early adulthood: a longitudinal study in the United States. Int J Drug Policy 26:131–134. [DOI] [PubMed] [Google Scholar]
- Lim SS. et al. (2013) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohoff FW. (2010) Genetic variants in the vesicular monoamine transporter 1 (VMAT1/SLC18A1) and neuropsychiatric disorders. Methods Mol Biol 637:165–180. [DOI] [PubMed] [Google Scholar]
- Lohoff FW, Dahl JP, Ferraro TN, Arnold SE, Gallinat J, Sander T, Berrettini WH. (2006) Variations in the vesicular monoamine transporter 1 gene (VMAT1/SLC18A1) are associated with bipolar I disorder. Neuropsychopharmacology 31:2739–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohoff FW, Weller AE, Bloch PJ, Buono RJ, Doyle GA, Ferraro TN, Berrettini WH. (2008. a) Association between polymorphisms in the vesicular monoamine transporter 1 gene (VMAT1/SLC18A1) on chromosome 8p and schizophrenia. Neuropsychobiology 57:55–60. [DOI] [PubMed] [Google Scholar]
- Lohoff FW, Lautenschlager M, Mohr J, Ferraro TN, Sander T, Gallinat J. (2008. b) Association between variation in the vesicular monoamine transporter 1 gene on chromosome 8p and anxiety-related personality traits. Neurosci Lett 434:41–45. [DOI] [PubMed] [Google Scholar]
- Lohoff FW, Hodge R, Narasimhan S, Nall A, Ferraro TN, Mickey BJ, Heitzeg MM, Langenecker SA, Zubieta JK, Bogdan R, Nikolova YS, Drabant E, Hariri AR, Bevilacqua L, Goldman D, Doyle GA. (2014) Functional genetic variants in the vesicular monoamine transporter 1 modulate emotion processing. Mol Psychiatry 19:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merenäkk L, Harro M, Kiive E, Laidra K, Eensoo D, Allik J, Oreland L, Harro J. (2003) Association between substance use, personality traits, and platelet MAO activity in preadolescents and adolescents. Addict Behav 28:1507–1514. [DOI] [PubMed] [Google Scholar]
- Milgram GG. (2001) Alcohol influences: the role of family and peers. In: Learning about drinking (Houghton E, Roche AM, eds), pp 85–407. New York: Brunner-Routledge. [Google Scholar]
- Montgomery SA, Åsberg M. (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- Mõttus R, Pullmann H, Allik J. (2006) Toward more readable Big Five personality inventories. Eur J Psychol Assess 22:149–157. [Google Scholar]
- National Institute of Mental Health (NIMH) , USA. http://www.nimh.nih.gov/statistics/index.shtml. Accessed 20 Jan 2015.
- Pabst A, Kraus L, Piontek D, Mueller S. (2010) Age, period, and cohort effects on time trends in alcohol consumption in the German adult population. SUCHT 56:349–359. [DOI] [PubMed] [Google Scholar]
- Pärna K, Tael M, Ringmets I, Aasvee K. (2012) Alcohol consumption among adolescents in Estonia 1994 – 2010. In: Public Health - Social and Behavioural Health (Maddock J, ed), pp187–204. Rijeka: InTech. [Google Scholar]
- Peter D, Finn JP, Klisak I, Liu Y, Kojis T, Heinzmann C, Roghani A, Sparkes RS, Edwards RH. (1993) Chromosomal localization of the human vesicular amine transporter genes. Genomics 18:720–723. [DOI] [PubMed] [Google Scholar]
- Peter D, Liu Y, Sternini C, de Giorgio R, Brecha N, Edwards RH. (1995) Differential expression of two vesicular monoamine transporters. J Neurosci 15:6179–6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Neuman RJ, Saccone NL, Corbett J, Rochberg N, Hesselbrock V, Bucholz KK, McGuffin P, Reich T. (2003) Age and birth cohort effects on rates of alcohol dependence. Alcohol Clin Exp Res 27:93–99. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta E, Baker T, Dunbar GC. (1998) The Mini International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-VI and ICD-10. J Clin Psychiatry 59:22–33. [PubMed] [Google Scholar]
- Shimada-Sugimoto M, Otowa T, Hettema JM. (2015) Genetics of anxiety disorders: genetic epidemiological and molecular studies in humans. Psychiatry Clin Neurosci 69:388–401. [DOI] [PubMed] [Google Scholar]
- Shlik J, Aluoja A, Kihl E. (1999) MINI 5.0.0. Mini rahvusvaheline neuropsühhiaatriline intervjuu DSM –IV. Estonian version of MINI international neuropsychiatric interview. [Google Scholar]
- Sievert MK, Ruoho AE. (1997) Peptide mapping of the [125I]Iodoazidoketanserin and [125I]2-N-[(3_-iodo-4_-azidophenyl)propionyl]tetrabenazine binding sites for the synaptic vesicle monoamine transporter. J Biol Chem 272:26049–26055. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. (1983) Manual for the State-Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychology Press. [Google Scholar]
- Tomson K, Merenäkk L, Loit H-M, Mäestu J, Harro J. (2011) The relationship between serotonin transporter gene promoter polymorphism and serum lipid levels at young age in a longitudinal population-representative study. Progr Neuropsychopharmacol Biol Psychiatry 35:1857–1862. [DOI] [PubMed] [Google Scholar]
- Vaht M, Merenäkk L, Mäestu J, Veidebaum V, Harro J. (2014) Serotonin transporter gene promoter polymorphism (5-HTTLPR) and alcohol use in general population: interaction effect with birth cohort. Psychopharmacology 231:2587–2594. [DOI] [PubMed] [Google Scholar]
- van Beek JH, Kendler KS, de Moor MH, Geels LM, Bartels M, Vink JM, van den Berg SM, Willemsen G, Boomsma DI. (2012) Stable genetic effects on symptoms of alcohol abuse and dependence from adolescence into early adulthood. Behav Genet 42:40–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszczuk MA, Zavos HM, Gregory AM, Eley TC. (2014) The phenotypic and genetic structure of depression and anxiety disorder symptoms in childhood, adolescence, and young adulthood. JAMA Psychiatry 71:905–916. [DOI] [PubMed] [Google Scholar]
- Wessa M, Kollmann B, Linke J, Schönfelder S, Kanske P. (2015) Increased impulsivity as a vulnerability marker for bipolar disorder: Evidence from self-report and experimental measures in two high-risk populations. J Affect Disord 178:18–24. [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T. (2013) Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382:1575–1586. [DOI] [PubMed] [Google Scholar]
- Wimalasena K. (2011) Vesicular monoamine transporters: structure-function, pharmacology, and medicinal chemistry. Med Res Rev 31:483–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank (2015) GDP growth rate. http://data.worldbank.org/indicator/NY.GDP.MKTP.KD.ZG/countries/1W-EE?display=graph. Accessed 30 March 2015. [Google Scholar]
- Zhu X, Dutta N, Helton SG, Schwandt M, Yan J, Hodgkinson CA, Cortes CR, Kerich M, Hall S, Sun H, Phillips M, Momenan R, Lohoff FW. (2015) Resting-state functional connectivity and presynaptic monoamine signaling in alcohol dependence. Hum Brain Mapp 36:4808–4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Huguelet P, Ohl LE, Koeppe RA, Kilbourn MR, Carr JM, Giordani BJ, Frey KA. (2000) High vesicular monoamine transporter binding in asymptomatic bipolar I disorder: sex differences and cognitive correlates. Am J Psychiatry 157:1619–1628. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Taylor SF, Huguelet P, Koeppe RA, Kilbourn MR, Frey KA. (2001) Vesicular monoamine transporter concentrations in bipolar disorder type I, schizophrenia, and healthy subjects. Biol Psychiatry 49:110–116. [DOI] [PubMed] [Google Scholar]