Abstract
Background:
GPR120 (FFAR4) is a G-protein coupled receptor implicated in the development of obesity and the antiinflammatory and insulin-sensitizing effects of omega-3 (ω-3) polyunsaturated fatty acids. Increasing central ω-3 polyunsaturated fatty acid levels has been shown to have both anorectic and anxiolytic actions. Despite the strong clinical interest in GPR120, its role in the brain is largely unknown, and thus we sought to determine the impact of central GPR120 pharmacological activation on energy balance, food reward, and anxiety-like behavior.
Methods:
Male C57Bl/6 mice with intracerebroventricular cannulae received a single injection (0.1 or 1 µM) or continuous 2-week infusion (1 µM/d; mini-pump) of a GPR120 agonist or vehicle. Free-feeding intake, operant lever-pressing for palatable food, energy expenditure (indirect calorimetry), and body weight were measured. GPR120 mRNA expression was measured in pertinent brain areas. Anxiety-like behavior was assessed in the elevated-plus maze and open field test.
Results:
GPR120 agonist injections substantially reduced chow intake during 4 hours postinjection, suppressed the rewarding effects of high-fat/-sugar food, and blunted approach-avoidance behavior in the open field. Conversely, prolonged central GPR120 agonist infusions reduced anxiety-like behavior in the elevated-plus maze and open field, yet failed to affect free-feeding intake, energy expenditure, and body weight on a high-fat diet.
Conclusion:
Acute reductions in food intake and food reward suggest that GPR120 could mediate the effects of central ω-3 polyunsaturated fatty acids to inhibit appetite. The anxiolytic effect elicited by GPR120 agonist infusions favors the testing of compounds that can enter the brain to activate GPR120 for the mitigation of anxiety.
Keywords: FFAR4, appetite, mood, GPCR, fatty acids
Introduction
Obesity and mood disorders represent dominant sources of morbidity and disability. Anxiety disorders are the most common psychiatric condition in developed countries, and their prevalence is significantly increased by the incidence of obesity (Gariepy et al., 2010). Due to their persistent nature, anxiety disorders can be particularly debilitating by impairing not only well-being and quality of life but also intensifying the threat of obesity-related complications. Substantial research efforts have focused on reducing the burden of obesity and associated illnesses, but results have been unsatisfying.
The search for treatments has prompted the study of free fatty acids and their signalling actions at G-protein-coupled receptors (GPCRs). Indeed, fatty acids are not only essential nutritional components, but they also play an important role as signalling molecules that regulate metabolism and inflammation. Specific fatty acid receptors considered orphan GPCRs have been the subject of much recent investigation for their therapeutic potential (Cornall et al., 2013a; Yonezawa et al., 2013). Among these are GPR120 (FFAR4) and GPR40 (FFAR1) that are stimulated by long-chain fatty acids, including omega-3 (ω-3) polyunsaturated fatty acids (PUFA) (Itoh et al., 2003; Hirasawa et al., 2005). GPR120 loss-of-function in mice prevents the antiinflammatory and insulin sensitizing actions of ω-3 PUFA (Oh et al., 2010), decreases energy expenditure, and increases high-fat diet (HFD)-induced weight gain (Ichimura et al., 2012), whereas variants in the human gene encoding GPR120 increase the risk of obesity (Ichimura et al., 2012) and fasting plasma glucose levels (Bonnefond et al., 2015). Further, GPR120 agonism improves insulin resistance and inflammation in high-fat-fed obese mice, suggesting GPR120 as a promising antidiabetes and -obesity target (Walenta et al., 2014). GPR120 may affect appetite and food intake by regulating the secretion of gut hormones, such as GLP-1 (Tanaka et al., 2008; Paulsen et al., 2014), glucagon (Suckow et al., 2014), cholecystokinin (Tanaka et al., 2008), and ghrelin (Lu et al., 2012; Engelstoft et al., 2013; Gong et al., 2014). Alternatively, GPR120 could affect feeding and energy balance via direct central actions, although the role of GPR120 in the central nervous system is poorly understood.
Beyond their impact on energy balance, dietary ω-3 PUFA are also well implicated in the regulation of emotional states and pathophysiology of mood disorders. Decreasing brain content of ω-3 PUFA via dietary deficiency enhances anxiety- and depressive-like behaviors (Shaldubina et al., 2002; McNamara et al., 2007; Sontrop et al., 2008), whereas ω-3 dietary supplementation can have anxiolytic actions in rodent and primates (Fedorova and Salem, 2006; Ferraz et al., 2011; Languille et al., 2012). Moreover, saturated high-fat feeding, which can diminish brain ω-3 PUFA concentrations (Phivilay et al., 2009), promotes anxiety-like behavior and greater stress sensitivity in mice (Sharma and Fulton, 2013; Sharma et al., 2013; Zemdeg et al., 2015). The objective of the present study was to determine the role of brain GPR120 in the control of appetite, energy balance, and anxiety-like behavior. Here, we demonstrate that pharmacological activation of central GPR120 can profoundly inhibit food intake and the rewarding effects of high-fat/-sugar food on the short term and sustained actions to reduce anxiety-like behavior in high-fat-fed mice.
Materials and Methods
Animals
All procedures were approved by the Institutional Animal Care Committee of the CRCHUM that adheres to the standards of the Canadian Council on Animal Care. Male C57Bl/6 mice (Charles River, St Constant, Canada) received at 7 to 8 weeks of age were maintained in an environmentally controlled room (22–24ºC) with ad libitum access to standard chow and water. Mice were acclimated to a reverse light/dark cycle for at least 7 days before initiation of experiments and were individually housed following intracerebroventricular (ICV) cannula implantation. All behavioral testing was carried out in the dark phase.
ICV Cannula and Osmotic Mini-Pump Implantation
Mice were anaesthetized with isoflurane and placed in an ultraprecise mouse stereotaxic apparatus (Kopf Instruments). For acute agonist treatment experiments, a single ICV cannula was implanted. With Bregma and Lambda in the same horizontal plane, a cannula (guide cannula: C315GS-5-SP, 5mm, 26 gauge, Plastics One) was lowered into the lateral ventricle (+0.5mm caudal and +1mm lateral to bregma; -2mm ventral), fixed to the skull with cyanoacrylate glue and Cerabond adhesive (Plastics One) and closed with an adapted dust cap (Dummy cannula: C315DCS-5-SPC, 4mm, 33 gauge, Plastics One). Five days following the surgery, the placement of cannulas was verified by the drinking response to an injection of Angiotensin II (10ng/µL; Sigma). For chronic agonist administration, ICV cannulae with an L-shaped adaptor (Alzet, 28 gauge, 3–5mm from skull surface) were attached to a primed 21-day osmotic mini-pump with a flow rate of 0.25 µL/h (Alzet osmotic pump catalog #1002) via polyethylene tubing that was prefilled with vehicle. Fluid movement through the assembly was verified at the cannula tip prior to stereotaxic implantation as described above. The mini-pump was inserted underneath the skin and pushed into place between the scapulae.
Drugs
The GPR120 agonist (GPR120 III) was purchased from Calbiochem (San Diego, CA). GPR120 III is a potent agonist of GPR120 (EC50 =17nM) that exhibits minor affinity for GPR40 (EC50 = 64.6 µM) and no activity toward short chain fatty acid receptors (Shimpukade et al., 2012). Despite the much reduced potency toward GPR40 of the GPR120 agonist, we also tested the central effects of the specific GPR40 agonist, TAK-875 (Selleckchem) (Negoro et al., 2012) on food intake. All drugs were dissolved in saline + 1% dimethylsulfoxide (DMSO)..
Food Intake
The effects of acute ICV injections of GPR120 and GPR40 agonists on food intake were assessed. The 0.1- and 1-µM doses of GPR120 agonist selected correspond to those reported to inhibit formalin-induced pain upon ICV injection (Nakamoto et al., 2012). Via an internal injector cannula attached to a 1-µl Hamilton microsyringe, mice (n=11) received 1 µL of vehicle (saline + 1% DMSO) on day 1 followed by 0.1 μM of the GPR120 agonist on day 3 and then 1 µM of GPR120 agonist on day 5 (1-day recovery period between each injection). Three days later, the same mice were again injected with vehicle and then the GPR40 agonist (1 μM) 2 days later. Single-housed mice were initially habituated to bedding-free cages with absorbent padding (for accurate food intake measurements) prior to testing. ICV injections were performed in fed mice at the beginning of the dark cycle. Following injections, chow intake was measured at 1, 2, 4, 6, 12, and 24 hours postinjection.
Locomotor Activity
Mice were habituated to metabolic cages (Comprehensive Lab Animal Monitoring System CLAMS, Columbus, OH) for 2 days prior to testing. Locomotor activity (X-Y-Z beambreaks) was measured for 12 hours following ICV injection of GPR120 agonist or vehicle.
Real-Time Quantitative PCR
Brain punches of all specified regions were obtained from 250-μM frozen coronal sections. Tissues were further processed for mRNA extraction (TRIzol) and cDNA synthesis to perform quantitative PCR as described (Fernandes et al., 2015) using the following primer sequences: β-Actin, F: TTCTTGGGTATGGAATCCTGTGGCA; R: ACCAGACAGCACTGTGTTGGCATA and GPR120, F: AGAGGCT TACGCTGAGCTTG; R: TGGATCAAGATGAGGATG. Relative gene expression was calculated using the ΔΔCT method using B-actin as the housekeeping gene.
Food-Motivated Behavior
Mice were trained to press an ultra-light retractable lever for food rewards (14mg high-fat, high-sucrose pellets, Bio-Serv) in mouse operant cages (Med Associates, Inc.) on a progressive ratio (PR) schedule of reinforcement as described previously (Sharma et al., 2012). Briefly, mice were mildly food restricted to acquire the lever-pressing task on a fixed-ratio-1 followed by a fixed-ratio-5 and PR schedules of reinforcement. Mice were returned to ad libitum feeding conditions during PR training. Once stable responding on the PR schedule was achieved, mice were first tested in basal conditions without injection (day 1) and then following injection of vehicle (day 2) and the GPR120 agonist (1 µg; day 3). ICV injections were carried out 1 hour before testing. The number of active lever presses, rewards earned, and the breakpoint ratio (last ratio successfully completed) were calculated by MedPC software (Med Associates, Inc).
Anxiety: Elevated Plus Maze
Mice were placed in the middle of the elevated-plus maze (EPM; Med Associates, Inc., St Albans, VT) facing the open arm opposing the experimenter 1 hour following ICV injections. Movement in the maze was recorded and tracked for 5 minutes by an overhead video camera connected to a PC with Ethovision XT software. Mice with mini-pumps followed the same procedure but without injection prior to the test.
Anxiety: Open Field Test
The open field test (OFT) was used as an additional measure of anxiety-like behavior as previously described (Sharma and Fulton, 2013). Briefly, each mouse was placed in the middle of the arena 1 hour after injection and allowed to explore the field. Time spent in the center of the arena, center entries, plus the time and frequency of stretched attend postures (body elongation, an important component of defensive behavior in rodents) during the first 5 minutes of this test were used as measures of exploration that serve as indices of anxiety. Behavior was recorded and tracked by an overhead video camera connected to a PC with Ethovision XT software. Mice with mini-pumps followed the same procedure but without injection prior to the test.
Mini-Pump ICV Infusions
Mini-pumps delivered either vehicle or GPR120 agonist (1 µM/d; ~42nM/h) at a flow rate of 0.25 µL/h into the lateral ventricle. Food intake and body weight measures began 3 days following cannula and mini-pump implantation. Mice initially fed a chow diet were separated into 3 weight-matched groups (n=5–8/group): (1) vehicle infusion on an ingredient-matched, low-fat diet (LFD; 4.07 kcal/gm: 10.5% kcal fat, 16.4% kcal protein, 73.1% kcal carbohydrates, D12328, Research Diets, Inc., New Brunswick, NJ); (2) vehicle infusion on a HFD (5.56 kcal/gm: 58% kcal fat, 16.4% kcal protein, 25.5% kcal carbohydrates, D12331, Research Diets, Inc.); and (3) GPR120 agonist infusion on a HFD. Mice received their respective diet for 3 weeks.
Statistical Analyses
Statistics were calculated using GraphPad Prism 6 (San Diego, CA). The effects of agonists on short-term food intake were analyzed by 1 -way ANOVAs with posthoc Dunnett tests. Anxiety-like behavior following acute injection was assessed by 2-tailed, paired t-tests. A 2-way ANOVA with the Bonferroni posttests was used for food intake, body weight, and energy expenditure data, whereas a 1-way ANOVA plus Bonferonni posttests was used for comparisons of anxiety-like behavior following prolonged pump treatment. Data are presented as mean ± SEM and P ≤ .05 was set as the criteria for statistical significance.
Results
Central GPR120 Agonism Inhibits Food Intake
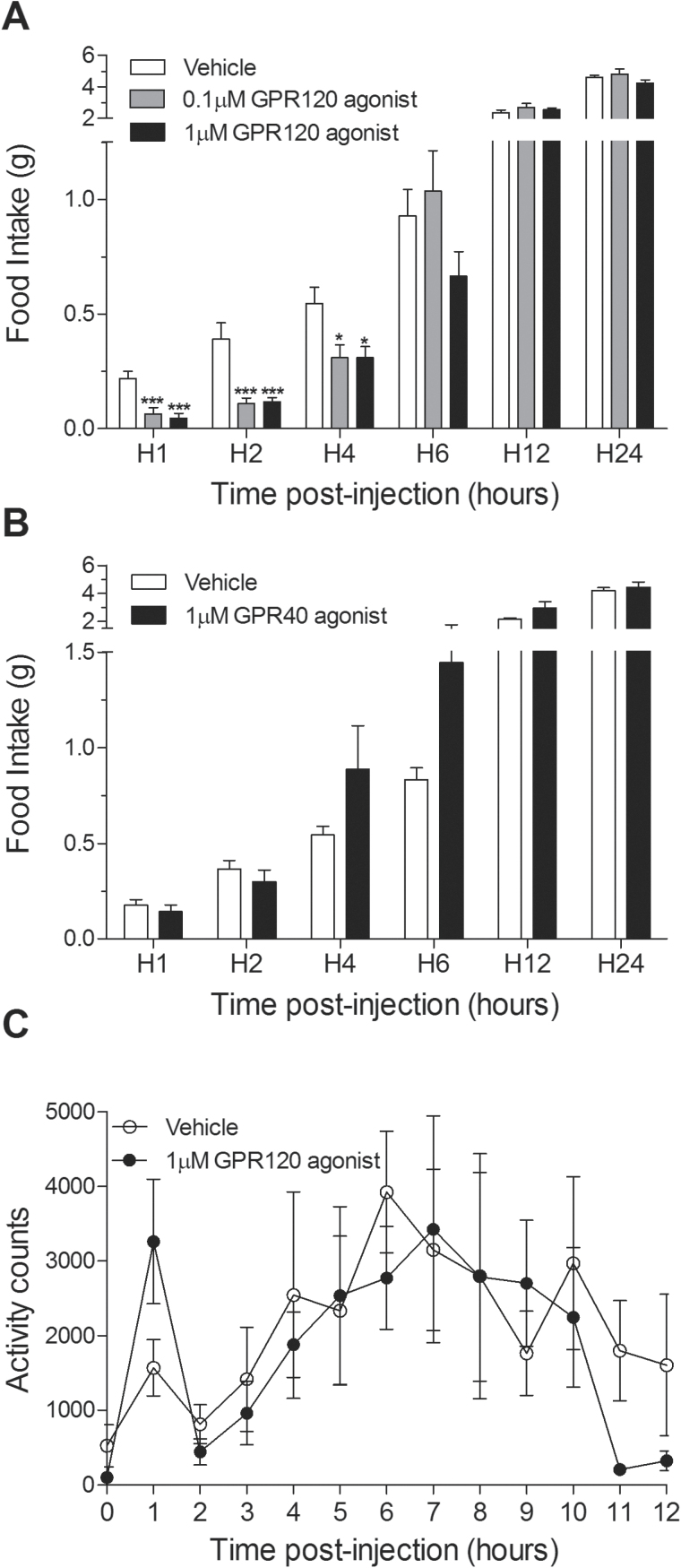
Food intake at 1, 2, and 4 hours postinjection was significantly decreased in chow-fed mice receiving an ICV bolus of the GPR120 agonist at each of the 2 doses compared with controls (Figure 1A). GPR120 agonist treatment elicited up to an 80% and 72% reduction in food intake at the first and second hour postinjection, respectively (Figure 1A) (P<.001). In contrast, the GPR40 agonist did not affect food intake at any time point (Figure 1B). To determine if central GPR120 stimulation affects ambulatory activity, we next examined basal locomotion. Agonist-injected mice exhibited a trend (P=.1) for increased activity only during the first hour postinjection (Figure 1C).
Figure 1.
Central injection of a GPR120 agonist acutely inhibits food intake. (A) Cumulative food intake following acute central agonist injection. GPR120 agonist treatment elicited up to an 80% and 72% reduction in food intake at the first and second hour postinjection, respectively (n=11). (B) A selective GPR40 agonist (1 μM) did not influence food intake (n=9). (C) A single ICV injection of the GPR120 agonist had no overall effect on locomotor activity; however, it produced a trend (P=.1) for increased ambulatory activity during the first hour postinjection (n=5/group). Mean ± SEM; *P<.05, *** P<.001.
GPR120 Is Expressed in Reward-Related Nuclei and Influences Food-Motivated Behavior
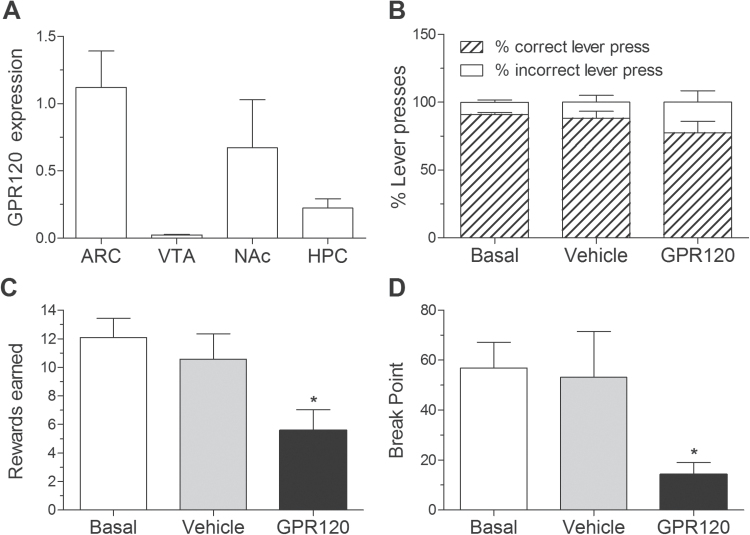
The consumption of rewarding, energy-dense food is a major factor contributing to overeating and the development obesity and associated anxiety-like behavior (Sharma and Fulton, 2013). First, we sought to determine if GPR120 is expressed in brain areas important for the control of food reward and mood in addition to its reported expression in the hypothalamus (Cintra et al., 2012). Consistent with previous reports, we found relatively high expression of GPR120 mRNA in the arcuate nucleus (ARC) of the hypothalamus. GPR120 was also comparatively elevated in the nucleus accumbens (NAc), a brain region well implicated in the control of motivation and mood, with lesser and minimal expression in the hippocampus and ventral tegmental area, respectively (Figure 2A). We next ascertained if central GPR120 agonism decreases the motivational effects of palatable food using an operant conditioning task in which mice press a lever to receive high-fat/-sugar pellets on a PR schedule of reinforcement. Agonist treatment did not affect the proportion of active and inactive lever presses (Figure 2B), suggesting there were no changes in operant learning; however, it substantially reduced the number of rewards earned (Figure 2C; P<.05) and breakpoint response thresholds (Figure 2D; P<.05) relative to vehicle-injected controls.
Figure 2.
GPR120 is expressed in reward-relevant nuclei, and central agonism inhibits the rewarding effect of palatable food. (A) GPR120 mRNA expression in the arcuate nucleus (ARC), ventral tegmental area, hippocampus, and the nucleus accumbens (NAc) (n=4/group). (B) Central injection of the GPR120 agonist (1 μM) did not impair operant learning as reflected by the proportion of correct and incorrect lever responses (n=7). (C) GPR120 agonism reduced the number of high-fat/-sucrose pellet rewards on progressive ratio (PR) schedule of reinforcement as compared to vehicle treatment. Vehicle treatment did not alter rewards earned relative to baseline testing (n=7). (D) Breakpoint response thresholds (maximum response ratio at which point mice will work to obtain the reward) in the PR task were suppressed by GPR120 agonist injection (n=7). Mean ± SEM. *P<.05.
Central GPR120 Agonism Dampens Conflict Behavior
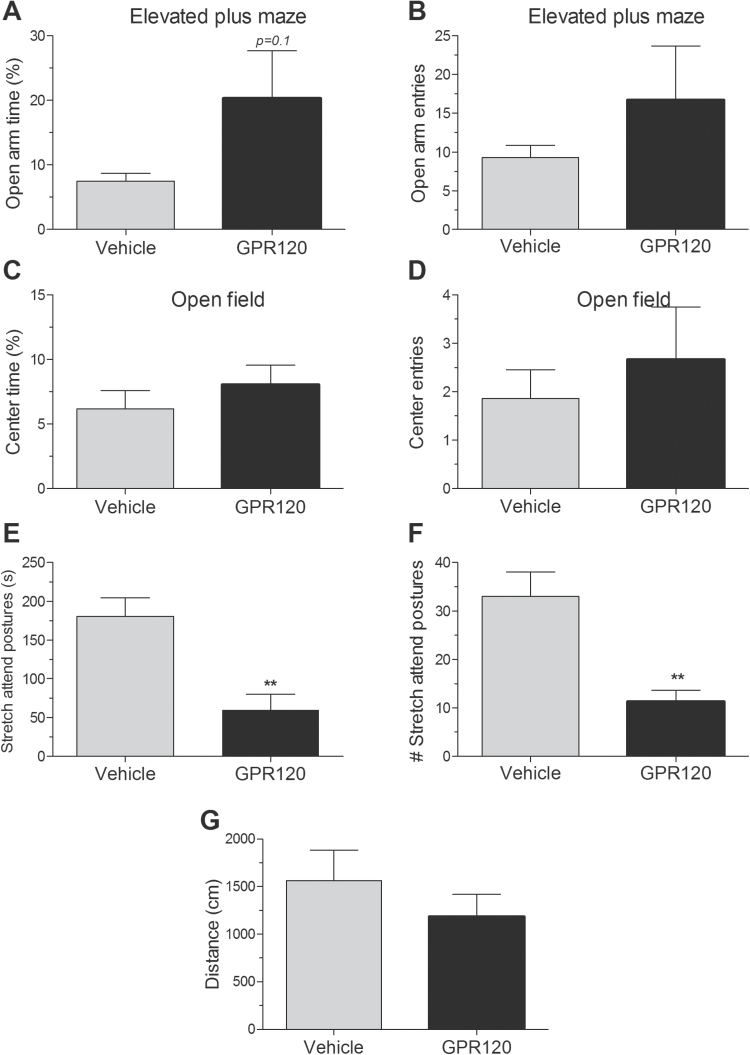
In view of the anxiolytic actions associated with dietary ω-3 PUFA, we sought to determine if central GPR120 stimulation modulates anxiety-like behavior using 2 standard tests of anxiety: the EPM and OFT. Acute agonist injection produced a trend (P=.1) for increased time spent in the open arms of the EPM (Figure 3A) without altering open arm entries (Figure 3B). The amount of time spent in the center of the OFT and the number of center entries were not different between groups (Figure 3C-D); however, the duration (Figure 3E, P<.01) and frequency (Figure 3F, P<.01) of stretch attend postures, classified as an approach-avoidance element of conflict behavior (Grewal et al., 1997), in the OFT were substantially reduced in agonist-injected mice relative to controls. Horizontal locomotor activity on the OFT was not different between groups (Figure 3G).
Figure 3.
GPR120 agonist injection dampens signs of anxiety-like behavior. (A) GPR120 agonist (1 µM) injection produces a trend (P=.1) for increased time spent in the open arms of the elevated-plus maze (EPM) (n=7). (B) GPR120 agonist did not alter open arm entries in the EPM (n=7). (C) The proportion of time spent the center of the open field was unchanged (n=7). (D) The number of center entries in the open field was not different. (E) The duration of stretch attend postures (elongation) was suppressed by the agonist (n=7). (F) The frequency of stretch attend postures was inhibited by the agonist (n=7). (G) Horizontal locomotor activity in the open field test (OFT) was not different between groups. Mean ± SEM. **P<.01.
Prolonged Central GPR120 Agonism Fails to Affect Energy Balance on a HFD
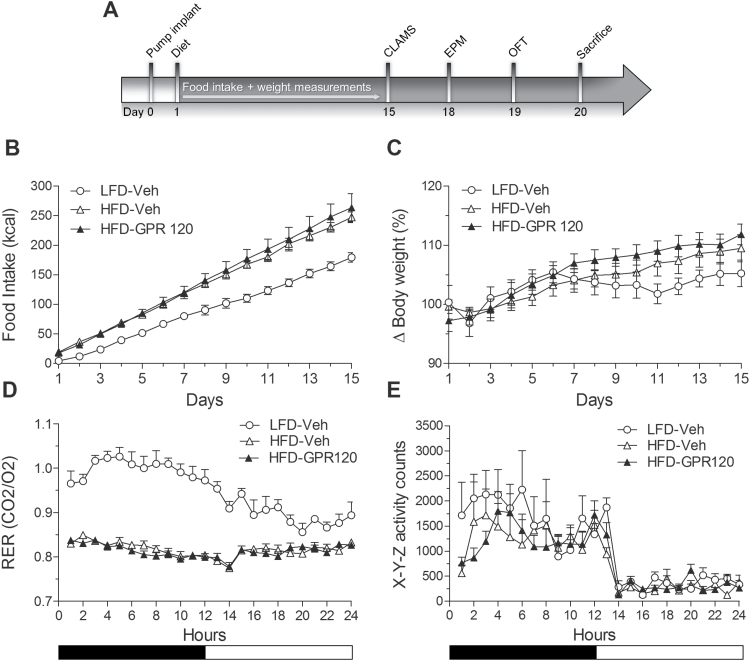
Administration of a GPR120 oral agonist was recently reported to have greater metabolic actions in the context of a HFD (Walenta et al., 2014); thus, we next assessed the effects of prolonged central agonist infusion on energy balance parameters in high-fat-fed mice. Free-feeding intake, body weight, respiratory exchange, and locomotor activity were measured in mice with osmotic mini-pumps connected to ICV cannula that delivered either the GPR120 agonist or vehicle (Figure 4A). As shown in Figure 4B, exposure to the HFD increased caloric intake relative to LFD-fed controls as expected (day 11–15: P<.05); however, food intake on the HFD was similar between agonist and vehicle-treated mice. Likewise, body weight increased on HFD but weight gain was similar between groups (Figure 4C). Consistent with greater utilization of lipids, the respiratory exchange ratio measured 2 weeks following the pump infusions decreased with HFD (P<.01) yet was comparable between agonist- and vehicle-treated HFD mice (Figure 4D). Finally, locomotor activity that was measured in parallel with respiratory exchange ratio was similar across groups (Figure 4E).
Figure 4.
Chronic GPR120 administration does not affect food intake, body weight, or energy expenditure. (A) Graphic timeline illustrating experimental protocol following implant of 21-day osmotic mini-pump. (B) Two-week central GPR120 agonist infusions (1 μM/d) did not affect high-fat diet (HFD) consumption. HFD increased caloric intake relative to low-fat diet (LFD) controls (n=5–8/group). (C) GPR120 agonist infusions did not modulate body weight in high-fat-fed mice. (D) The respiratory exchange ratio was not altered by agonist infusions; however, respiratory exchange ratio was increased by HFD relative to LFD consistent with greater lipid utilization (n=5–8/group). (E) Locomotor activity was unchanged by agonist infusions and HFD (n=5–8/group). Mean ± SEM. CLAMS, comprehensive lab animal monitoring system; EPM, elevated-plus maze; OFT, open field test.
Prolonged Central GPR120 Activation Inhibits Anxiety-Like Behavior on a HFD
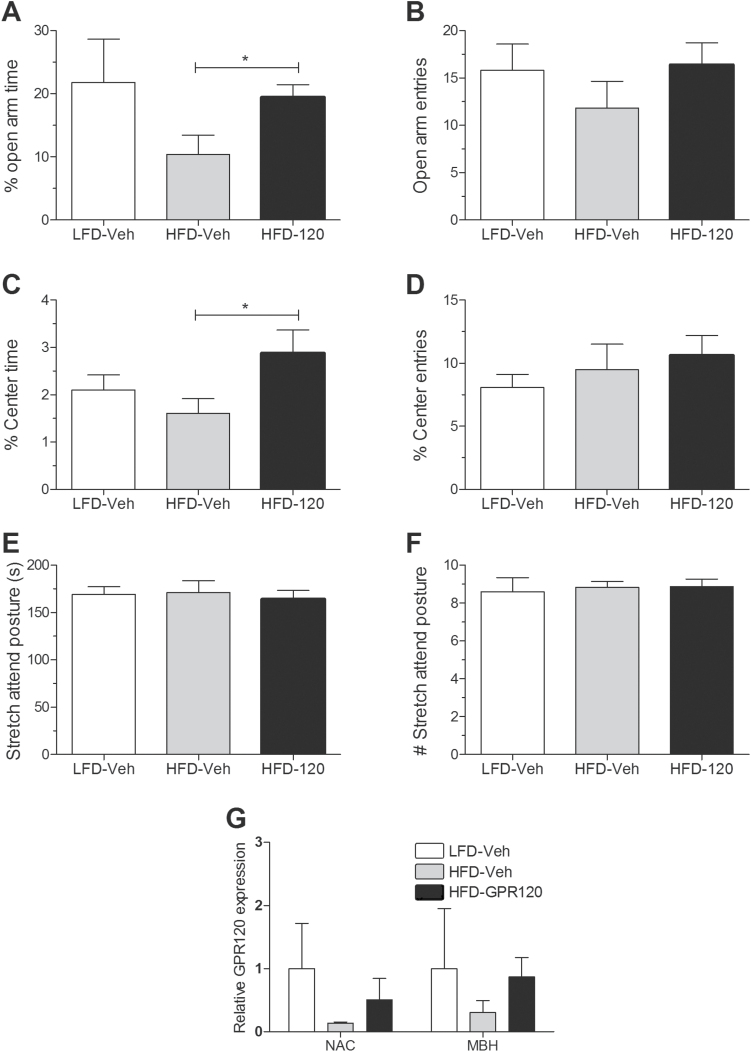
Anxiety-like behavior was assessed in the EPM and OFT following mini-pump infusions. As shown in Figure 5A, GPR120 agonist infusion reduced the proportion of time spent in the open arms of the EPM without changing open arm entries (Figure 5B) (P<.05). Similarly, the amount of time spent in the center of the OFT was significantly decreased in agonist-treated mice (Figure 5C, P<.05) without any changes in center entries (Figure 5D). There were no differences in the stretch attend posture measure of conflict behavior between groups (Figure 5E-F). Finally, to determine if high-fat feeding and prolonged GPR120 agonism alters GPR120 expression, we evaluated mRNA expression in the NAc and mediobasal hypothalamus (region including ARC, ventromedial hypothalamus, and dorsomedial hypothalamus), two brain regions in which we found relatively higher GPR120 expression. No differences were found between group despite a tendency for HFD to decrease GPR120 expression and for the agonist to increase it.
Figure 5.
Prolonged central GPR120 agonist infusion reduces anxiety-like behavior. (A) Mice receiving the GPR120 agonist spent more time in the open arms of the elevated-plus maze (EPM) relative to high-fat diet (HFD) controls (n=5–8/group). (B) Open arm entries in the EPM were not significantly different between groups (n=5–8/group). (C) Proportion of time spent in the center of the open field was increased in HFD mice receiving the GPR120 agonist relative to HFD mice receiving vehicle (n=5–8/group). (D) The number of center entries was unchanged across groups (n=5–8/group). (E) Duration of stretch attends posture was not different between groups (n=5–8/group). (F) Frequency of stretch attends postures was unchanged between groups (n=5–8/group). (G) GPR120 mRNA expression following 21-day agonist or vehicle infusions. MBH, mediobasal hypothalamus; NAc, nucleus accumbens. Mean ± SEM. *P<.05.
Discussion
Several lines of evidence implicate GPR120 in the regulation of energy homeostasis including the control of glycemia and gut hormone secretion (Ulven and Christiansen, 2015). Here, we demonstrate the consequences of acute and chronic central GPR120 stimulation on appetite, energy expenditure, and anxiety. In addition to eliciting a sizeable reduction in food intake, acute stimulation of GPR120 potently decreased the motivational and rewarding effects of palatable food and blunted approach avoidance conflict behavior. Chronic central infusions of the agonist in the context of high-fat feeding produced more robust anxiolytic effects yet failed to affect free-feeding intake, energy expenditure, and body weight on a HFD. Collectively, these results demonstrate the anxiolytic actions of central pharmacological GPR120 stimulation and reveal its central appetite-suppressing effects, which may be limited to transient receptor activation.
A limitation of our study is the use of a GPR120 agonist that weakly binds to GPR40. However, as central administration of a GPR40 specific agonist did not affect food intake, the strong anorectic effects observed are likely attributable to GPR120 activation. The anorectic effect of central GPR120 stimulation is consistent with reports that ω-3 PUFA, including docosahexaenoic acid and eicosapentaenoic acid, but not saturated fatty acids, inhibit food intake upon acute central administration (Schwinkendorf et al., 2011; Cintra et al., 2012). This raises the possibility that ω-3 PUFA inhibit feeding by targeting GPR120 in brain regions important for controlling food intake. Correspondingly, we found GPR120 mRNA to be expressed at relatively high levels in the ARC nucleus of the hypothalamus, a finding that coincides with observations of GPR120 protein expression in ARC neuropeptide-Y neurons (Cintra et al., 2012). Moreover, acute ICV injections of PUFA increased GPR120 association with β-arrestin-2, a GPCR regulatory protein that directly couples to GPR120 (Talukdar et al., 2011) in the hypothalamus (Cintra et al., 2012). We also noted relatively elevated levels of GPR120 in the NAc, a key brain site controlling mood and goal-directed behavior for food. In line with these data, we extended the appetite-suppressing effects of central GPR120 agonist treatment to decreased motivation to obtain palatable high-fat and -sugar food using an operant task. Thus, apart from the implication of GPR120 in the stimulation of gut hormones that can modulate feeding, our data suggest that direct activation of central GPR120 can reduce appetite, in part, by suppressing the rewarding effects of palatable food. Future studies are required to determine if decreased food-motivated behavior by GPR120 occurs via signaling actions in the NAc.
Contrary to the impact of acute GPR120 activation on food intake, we did not detect changes in feeding in high-fat-fed mice receiving prolonged agonist treatment. High-fat feeding increased caloric intake, body weight, and respiratory exchange (consistent with increased lipid utilization) as expected, but continuous central GPR120 infusion did not affect these parameters or locomotor activity. In contrast, chronic GPR120 agonist infusion reduced anxiety-like behavior in two standardized tests, having a greater anxiolytic impact compared with the discrete effect on approach-avoidance behavior observed following acute agonist treatment. Thus, the anxiolytic actions of central GPR120 agonism require sustained or cumulative stimulation of GPR120. Consistent with these anxiolytic actions, we found that GPR120 is expressed in nuclei such as the NAc that are well implicated in the control of emotional processes and mood (Russo and Nestler, 2013). No significant differences were observed in GPR120 gene expression with high-fat feeding and agonist treatment due to highly variable expression levels in the LFD group. Nonetheless, the tendency for GPR120 in the NAc and mediobasal hypothalamus to decrease in HFD mice and approach LFD levels after agonist infusions argues for further analyses of expression patterns under different dietary conditions.
A possible explanation for the lack of maintained anorectic effects with prolonged GPR120 stimulation could be the presence of counter-regulatory mechanisms that offset catabolic conditions to maintain energy homeostasis. An alternative possibility involves GPR120 desensitization. Desensitization of GPCRs and decreased responsiveness to agonists has been noted in several systems and involves reduced binding of β-arrestins (Pitcher et al., 1998). As noted above, the anorectic effects of acute ICV PUFA injections have been linked to GPR120 activation and association with β-arrestin-2 (Cintra et al., 2012); however, long-term treatment and receptor binding may diminish β-arrestin signaling and lead to GPR120 resistance. Indeed, HFD consumption is unchanged in GPR120 knockout mice (Ichimura et al., 2012). Moreover, while body weight on a HFD was found to be elevated in one study of GPR120 knockout mice (associated with decreased energy expenditure; Ichimura et al., 2012), others report no changes in body weight following GPR120 gene deletion (Oh et al., 2010) or with prolonged treatment of a GPR120 oral agonist (Walenta et al., 2014).
The anxiolytic actions of prolonged central GPR120 stimulation suggest that potential GPR120 desensitization does not affect this behavioral function. These findings could suggest that distinct signaling mechanisms may mediate GPR120 action on feeding vs anxiety. The antiinflammatory effects and benefits of the GPR120 activation by ω-3 PUFA involve several mechanisms, including inhibition of the NFkB pathway by β-arrestin (Oh et al., 2010); the activation of phospholipase A2, which releases ω-3 PUFA (docosahexaenoic acid and EPA) esterified in membrane phospholipids (Im, 2012); and induction of enzyme stearoyl-CoA desaturase-1 generating monounsaturated fatty acids (Ichimura et al., 2012). Central inflammatory processes are well implicated in the etiology of mood disorders, and the beneficial influence of ω-3 dietary supplementation on emotional states has been linked to antiinflammatory actions in the CNS (Hayley et al., 2005; Capuron and Miller, 2011; Rosenblat et al., 2014). Indeed, GPR120 was recently identified as having antiinflammatory properties in neural cell lines (Wellhauser and Belsham, 2014). GPR120 is highly expressed in peripheral macrophages and may therefore also localize to brain macrophages (notably microglia) to modulate inflammatory processes. Although purely speculative, it is possible that the anxiolytic actions of chronic GPR120 activation implicate distinct antiinflammatory signaling pathways in brain microglia that are not subject to GPR120 desensitization.
Targeting fatty acid receptors is proclaimed as a promising avenue to pharmaceutically treat metabolic diseases. The present study demonstrates that sustained GPR120 agonism in the brain can decrease anxiety-like behavior and thus suggests GPR120 as potential pathway through which ω-3 PUFA have central anxiolytic actions. An emerging element in the treatment of obesity is its strong link with mood disorders and the advantages of an integrated therapeutic approach. The concept of a multi-target strategy is developing and consists of simultaneously producing beneficial effects on energy balance and mood. A GPR120 agonist that can cross the blood brain barrier offers the advantage of this poly-target approach. Our data favor testing compounds that cross the blood brain barrier to target GPR120 and/or downstream effectors for the treatment of anxiety.
Statement of Interest
None.
Acknowledgments
This project was supported by a Natural Sciences and Engineering Research Council of Canada grant to S.F., a Canadian Institutes of Health Research New Investigator salary award to S.F., a salary award to T.A. from the Fonds de Recherche du Québec Santé, postdoctoral and doctoral fellowships from the Canadian Diabetes Association to A.F. and M.F., respectively, and awards from the International Atomic Energy Association and Department of Nutrition, Université de Montréal to S.A. V.P. holds the Canada Research Chair in Diabetes and Pancreatic Beta-Cell Function.
References
- Bonnefond A, Lamri A, Leloire A, Vaillant E, Roussel R, Lévy-Marchal C, Weill J, Galan P, Hercberg S, Ragot S, Hadjadj S, Charpentier G, Balkau B, Marre M, Fumeron F, Froguel P. (2015) Contribution of the low-frequency, loss-of-function p.R270H mutation in FFAR4 (GPR120) to increased fasting plasma glucose levels. J Med Genet 52595–52598. [DOI] [PubMed] [Google Scholar]
- Capuron and Miller (2011) Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther 130:226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H-F, Su H-M. (2012) Fish oil supplementation of maternal rats on an n-3 fatty acid-deficient diet prevents depletion of maternal brain regional docosahexaenoic acid levels and has a postpartum anxiolytic effect. J Nutr Biochem 23:299–305. [DOI] [PubMed] [Google Scholar]
- Cintra DE, Ropelle ER, Moraes JC, Pauli JR, Morari J, Souza CT, Grimaldi R, Stahl M, Carvalheira JB, Saad MJ, Velloso LA. (2012) Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PloS One 7: e30571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornall LM, Mathai ML, Hryciw DH, McAinch AJ. (2013. a) GPR120 agonism as a countermeasure against metabolic diseases. Drug Discovery Today. 19:670–679. [DOI] [PubMed] [Google Scholar]
- Cornall LM, Mathai ML, Hryciw DH, McAinch AJ. (2013. b) The therapeutic potential of GPR43: a novel role in modulating metabolic health. CMLS 70: 4759–4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelstoft MS, Park WM, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, Piper PK, Walker AK, Pedersen MH, Nøhr MK, Pan J, Sinz CJ, Carrington PE, Akiyama TE, Jones RM, Tang C, Ahmed K, Offermanns S, Egerod KL, Zigman JM, Schwartz TW. (2013) Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab 2:376–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorova I, Salem N., Jr (2006) Omega-3 fatty acids and rodent behavior. Prostagla Leukotr Ess 75:271–289. [DOI] [PubMed] [Google Scholar]
- Fernandes M, Matthys D, Hryhorczuk C, Sharma S, Mogra S, Alquier T, Fulton S. (2015) Leptin supresses the rewarding effects of running via STAT3 signaling in dopamine neurons. Cell Metab 22:741–749. [DOI] [PubMed] [Google Scholar]
- Ferraz AC, Delattre AM, Almendra RG, Sonagli M, Borges C, Araujo P, Andersen ML, Tufik S, Lima MM. (2011) Chronic ω-3 fatty acids supplementation promotes beneficial effects on anxiety, cognitive and depressive-like behaviors in rats subjected to a restraint stress protocol. Behav Brain Res 219:116–122. [DOI] [PubMed] [Google Scholar]
- Gariepy G, Nitka D, Schmitz N. (2010) The association between obesity and anxiety disorders in the population: a systematic review and meta-analysis. Int J Obes 34:407–419. [DOI] [PubMed] [Google Scholar]
- Gong Z, Yoshimura M, Aizawa S, Kurotani R, Zigman JM, Sakai T, Sakata I. (2014) G protein-coupled receptor 120 signaling regulates ghrelin secretion in vivo and in vitro. Am J Physiol-Endoc M 306:28–35. [DOI] [PubMed] [Google Scholar]
- Grewal SS, Shepherd JK, Bill DJ, Fletcher A, Dourish CT. (1997) Behavioral and pharmacological characterisation of the canopy stretched attend posture test as a model of anxiety in mice and rats. Psychopharmacology 133:29–38. [DOI] [PubMed] [Google Scholar]
- Hayley S, Poulter MO, Merali Z, Anisman H. (2005) The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience 135:659–678. [DOI] [PubMed] [Google Scholar]
- Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. (2005) Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11:90–94. [DOI] [PubMed] [Google Scholar]
- Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, Kimura I, Leloire A, Liu N, Iida K, Choquet H, Besnard P, Lecoeur C, Vivequin S, Ayukawa K, Takeuchi M, Ozawa K, Tauber M, Maffeis C, Morandi A, et al. (2012) Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 483:350–354. [DOI] [PubMed] [Google Scholar]
- Im DS. (2012) Omega-3 fatty acids in anti-inflammation (pro-resolution) and GPCRs. Prog Lipid Res 51:232–237. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. (2003) Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422:173–176. [DOI] [PubMed] [Google Scholar]
- Languille S, Aujard F, Pifferi F. (2012) Effect of dietary fish oil supplementation on the exploratory activity, emotional status and spatial memory of the aged mouse lemur, a non-human primate. Behav Brain Res 235:280–286. [DOI] [PubMed] [Google Scholar]
- Lu X, Zhao X, Feng J, Liou AP, Anthony S, Pechhold S, Sun Y, Lu H, Wank S. (2012) Postprandial inhibition of gastric ghrelin secretion by long-chain fatty acid through GPR120 in isolated gastric ghrelin cells and mice. Am J Physiol-Gastr L 303:367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Hahn C-G, Jandacek R, Rider T, Tso P, Stanford KE, Richtand NM. (2007) Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry 62:17–124. [DOI] [PubMed] [Google Scholar]
- Nakamoto K, Nishinaka T, Matsumoto K, Kasuya F, Mankura M, Koyama Y, Tokuyama S. (2012) Involvement of the long-chain fatty acid receptor GPR40 as a novel pain regulatory system. Brain Res 1432:74–83. [DOI] [PubMed] [Google Scholar]
- Negoro N, Sasaki S, Mikami S, Ito M, Tsujihata Y, Ito R, Suzuki M, Takeuchi K, Suzuki N, Miyazaki J, Santou T, Odani T, Kanzaki N, Funami M, Morohashi A, Nonaka M, Matsunaga S, Yasuma T, Momose Y. (2012) Optimization of (2,3-dihydro-1-benzofuran-3-yl)acetic acids: discovery of a non-free fatty acid-like, highly bioavailable G protein-coupled receptor 40/free fatty acid receptor 1 agonist as a glucose-dependent insulinotropic agent. J Med Chem 55:3960–3974. [DOI] [PubMed] [Google Scholar]
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. (2010) GPR120 Is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142:687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen SJ, Larsen LK, Hansen G, Chelur S, Larsen PJ, Vrang N. (2014) Expression of the fatty acid receptor GPR120 in the gut of diet-induced-obese rats and its role in GLP-1 secretion. PloS One 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phivilay A, Julien C, Tremblay C, Berthiaume L, Julien P, Giguère Y, Calon F. (2009) High dietary consumption of trans fatty acids decreases brain docosahexaenoic acid but does not alter amyloid-beta and tau pathologies in the 3xTg-AD model of Alzheimer’s disease. Neuroscience 159:296–307. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, Lefkowitz RJ. (1998) G protein-coupled receptor kinases. Annu Rev Biochem 67:653–692. [DOI] [PubMed] [Google Scholar]
- Rosenblat JD, Cha DS, Mansur RB, McIntyre RS. (2014) Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog Neuropsychopharmacol Biol Psychiatry 53:23–34. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. (2013) The brain reward circuitry in mood disorders. Nat Rev Neurosci 14:609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwinkendorf DR, Tsatsos NG, Gosnell BA, Mashek DG. (2011) Effects of central administration of distinct fatty acids on hypothalamic neuropeptide expression and energy metabolism. Int J Obes 35:336–344. [DOI] [PubMed] [Google Scholar]
- Shaldubina A, Nemets B, Bersudsky Y. (2002) Lack of effect of eicosapentaenoic acid in the Porsolt forced swimming test model of depression. Acta Neuropsychiatrica 14:203–206. [DOI] [PubMed] [Google Scholar]
- Sharma S, Fernandes MF, Fulton S. (2013) Adaptations in brain reward circuitry underlie food cravings and anxiety induced by high-fat diet withdrawal. Int J Obes 37:1183–1191. [DOI] [PubMed] [Google Scholar]
- Sharma S, Fulton S. (2013) Diet-induced obesity promotes depressive-like behavior that is associated with neural adaptations in brain reward circuitry. Int J Obes 48. [DOI] [PubMed] [Google Scholar]
- Sharma S, Hryhorczuk C, Fulton S. (2012) Progressive-ratio responding for palatable high-fat and high-sugar food in mice. J Visual Exper 63:3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimpukade B, Hudson BD, Hovgaard CK, Milligan G, Ulven T. (2012) Discovery of a potent and selective GPR120 agonist. J Med Chem 55:4511–4515. [DOI] [PubMed] [Google Scholar]
- Sontrop J, Avison WR, Evers SE, Speechley KN, Campbell MK. (2008) Depressive symptoms during pregnancy in relation to fish consumption and intake of n-3 polyunsaturated fatty acids. Paed Perinatal Epidemiol 22:389–399. [DOI] [PubMed] [Google Scholar]
- Suckow AT, Polidori D, Yan W, Chon S, Ma JY, Leonard J, Briscoe CP. (2014) Alteration of the glucagon axis in GPR120 (FFAR4) knockout mice: a role for GPR120 in glucagon secretion. J Biol Chem 289:15751–15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Olefsky JM, Osborn O. (2011) Targeting GPR120 and other fatty acid-sensing GPCRs ameliorates insulin resistance and inflammatory diseases. Trends in pharmacological sciences 32:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Katsuma S, Adachi T, Koshimizu TA, Hirasawa A, Tsujimoto G. (2008) Free fatty acids induce cholecystokinin secretion through GPR120. N-S Arch Pharmacol 377:523–527. [DOI] [PubMed] [Google Scholar]
- Ulven T, Christiansen E. (2015) Dietary fatty acids and their potential for controlling metabolic diseases through activation of FFA4/GPR120. Annu Rev Nutr 35:239–263. [DOI] [PubMed] [Google Scholar]
- Walenta E, Akiyama TE, Lagakos WS, Lackey D, Pessentheiner AR, Sasik R, Hah N, Chi TJ, Cox JM, Powels MA, Di Salvo J, Sinz C, Watkins SM, Armando AM, Chung H, Evans RM, Quehenberger O, McNelis J, Bogner-Strauss JG, Olefsky JM. (2014) A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat Med 20:942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellhauser L, Belsham D. (2014) Activation of the omega-3 fatty acid receptor GPR120 mediates anti-inflammatory actions in immortalized hypothalamic neurons. J Neuroinflammation 11:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa T, Kurata R, Yoshida K, Murayama MA, Cui X, Hasegawa A. (2013) Free fatty acids-sensing G protein-coupled receptors in drug targeting and therapeutics. Curr Med Chem 20:3855–3871. [DOI] [PubMed] [Google Scholar]
- Zemdegs J, Quesseveur G, Jarriault D, Pénicaud L, Fioramonti X, Guiard BP. (2015) High fat diet-induced metabolic disorders impairs serotonergic function and anxiety-like behaviors in mice. Br J Pharmacol. Oct 16. doi: 10.1111/bph.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]