Abstract
Background:
Salvinorin-A is a terpene found in the leaves of the plant Salvia divinorum. When administered to humans, salvinorin-A induces an intense but short-lasting modified state of awareness, sharing features with those induced by the classical serotonin-2A receptor agonist psychedelics. However, unlike substances such as psilocybin or mescaline, salvinorin-A shows agonist activity at the kappa-opioid receptor rather than at the serotonin-2A receptor. Here, we assessed the involvement of kappa-opioid receptor and serotonin-2A agonism in the subjective, cardiovascular, and neuroendocrine effects of salvinorin-A in humans.
Methods:
We conducted a placebo-controlled, randomized, double-blind study with 2 groups of 12 healthy volunteers with experience with psychedelic drugs. There were 4 experimental sessions. In group 1, participants received the following treatment combinations: placebo+placebo, placebo+salvinorin-A, naltrexone+placebo, and naltrexone+salvinorin-A. Naltrexone, a nonspecific opioid receptor antagonist, was administered at a dose of 50mg orally. In group 2, participants received the treatment combinations: placebo+placebo, placebo+salvinorin-A, ketanserin+placebo, and ketanserin+salvinorin-A. Ketanserin, a selective serotonin-2A antagonist, was administered at a dose of 40mg orally.
Results:
Inhalation of 1mg of vaporized salvinorin-A led to maximum plasma concentrations at 1 and 2 minutes after dosing. When administered alone, salvinorin-A severely reduced external sensory perception and induced intense visual and auditory modifications, increased systolic blood pressure, and cortisol and prolactin release. These effects were effectively blocked by naltrexone, but not by ketanserin.
Conclusions:
Results support kappa opioid receptor agonism as the mechanism of action underlying the subjective and physiological effects of salvinorin-A in humans and rule out the involvement of a serotonin-2A-mediated mechanism.
Keywords: Salvinorin-A, naltrexone, ketanserin, kappa opioid receptor antagonism, serotonin-2A antagonism, human pharmacology
Introduction
Salvinorin-A is a terpene compound thought to be the main psychoactive component present in the leaves of the plant Salvia divinorum (Labiatae), a mint endemic to the Sierra Madre Oriental of Oaxaca, Mexico. The plant has been used for centuries by the Mazatec people who inhabit the region in the treatment of various medical conditions and for spiritual purposes, divination, and shamanic healing (Valdés et al., 1983; Ott, 1995).
S. divinorum preparations have attracted the interest of users of psychoactive drugs worldwide, leading to widespread experimentation with the plant. The dried leaves and fortified extracts can be smoked or administered sublingually, inducing brief but intense psychotropic effects (González et al., 2006). Ortega et al. (1982) and later Valdés et al. (1984) isolated salvinorin-A from S. divinorum, which was shown to be psychoactive in a series of laboratory studies. The effects induced by the drug in humans include prominent modifications in audio-visual perception and, at higher doses, intense dissociation with disconnection from external reality and loss of contact with the body (Johnson et al., 2011; MacLean et al., 2013; Addy et al., 2015; Maqueda et al., 2015).
Salvinorin-A shows remarkable pharmacological characteristics. Although its profile of effects in humans has analogies to that of classical serotonergic psychedelics, salvinorin-A does not show affinity in vitro for the serotonin-2A (5-HT2A) receptor. Unlike drugs such as LSD, mescaline, or psilocybin, salvinorin-A instead shows high affinity for the kappa opioid receptor (KOR) (Roth et al., 2002; Prisinzano, 2005). Salvinorin-A is also an unusual KOR agonist. In contrast with drugs such as pentazocine and enadoline, the salvinorin-A molecule does not contain nitrogen. Additionally, in contrast with these nitrogenated compounds, salvinorin-A displays perception-modifying rather than somato-dysphoric effects (Walsh et al., 2001), although salvinorin-A has not been compared directly with synthetic kappa agonists within one study.
Animal studies further suggest the KOR-specific profile of salvinorin-A effects. In adult rhesus monkeys, salvinorin-A induces prolactin release (Butelman et al., 2007), facial relaxation, and ptosis (Butelman et al., 2009) as well as discriminative stimulus effects (Butelman et al., 2010). All these effects are blocked by pretreatment with the KOR partial agonist nalmefene (Butelman et al., 2007, 2009) and the antagonist quadazocine (Butelman et al., 2010), but not by pretreatment with the 5-HT2A antagonist ketanserin (Butelman et al., 2007, 2009, 2010) or the cannabinoid antagonist rimonibant (Butelman et al., 2009). Analogous results have been obtained in mice (Walentiny et al., 2010). However, despite the evidence from in vitro assays and animal studies, to date no studies have been published demonstrating in vivo that the pharmacological effects of salvinorin-A in humans are indeed mediated by the KOR.
In the present study, we sought to investigate further the pharmacology of salvinorin-A in humans by assessing the involvement of KOR and 5-HT2A agonism in the subjective, cardiovascular, and neuroendocrine effects induced by the drug. To do so, we conducted a study involving salvinorin-A administration to experienced psychedelic drug users following pretreatment with the opioid antagonist naltrexone or the 5-HT2A antagonist ketanserin. In addition to subjective effects measures, we utilized several outcomes known to be sensitive to KOR agonists in humans, including neuroendocrine variables such as plasma cortisol and prolactin (sensitive to salvinorin-A; Ranganathan et al., 2012) and growth hormone (GH) (sensitive to the synthetic KOR agonist spiradoline; Ur et al., 1997).
Materials and Methods
Ethics
The study was conducted in accordance with the Declarations of Helsinki and its updates concerning experimentation on humans and was approved by the hospital’s ethics committee and the Spanish Ministry of Health. All participants gave their written informed consent prior to participation.
Participants
The study included 24 volunteers with previous experience in the use of psychedelics. The final participant sample had at least 10 previous experiences with psychedelics and no history of adverse effects from their use. Exclusion criteria included a current or past history of psychiatric disorders, alcohol or other substance dependence, evidence of significant illness, and pregnancy. Participants underwent a complete physical examination that included a medical history, laboratory tests, ECG, and urinalysis. Cannabis users were requested to abstain from cannabis use since enrollment and until the end of the study. This was verified by urinalysis (see below). Twelve participants were allocated to group 1 involving the administration of naltrexone and 12 to group 2 involving the administration of ketanserin. The first 12 participants were allocated to group 1 (naltrexone pretreatment group) and the subsequent 12 to group 2 (ketanserin pretreatment group). Additional details on the study participants are provided in the supplementary information file.
Drugs
A fully psychoactive dose of 1mg vaporized pure (>99%) salvinorin-A was chosen for the study based on results from a previous trial (Maqueda et al, 2015). Oral capsules were prepared containing either 50mg naltrexone, 40mg ketanserin, or lactose placebo. The chosen naltrexone dose is the standard clinical daily dose. The ketanserin dose was chosen based on a previous study that showed that 40mg blocked the subjective effects of a high dose of psilocybin (Vollenweider et al., 1998). These capsules were administered 1 hour prior to salvinorin-A vaporization and inhalation (see study design below). Additional information is provided in the supplementary file.
Study Design
The study was carried out in a double-blind, randomized, crossover fashion. It involved 4 experimental sessions 1 week apart. Two weeks before the beginning of the experimental sessions, volunteers were instructed to abstain from all medications (including prescription drugs) and illicit drugs and remain drug-free throughout the study. Upon arrival in the morning to the research unit, breath analysis for alcohol, urinalysis for illicit drug use, and a urine pregnancy test (for women only) were administered. An i.v. catheter was placed in a vein of the left arm for drawing blood samples. Pretreatment capsules were administered, and 1 hour later, salvinorin-A or placebo was administered by vaporization and inhalation.
Participants received the following treatment combinations: oral placebo+vaporized placebo (placebo+placebo), oral placebo+vaporized salvinorin-A (placebo+salvinorin), oral study drug+ vaporized placebo (antagonist+placebo), and oral study drug+vaporized salvinorin-A (antagonist+ salvinorin). Participants received either naltrexone (group 1) or ketanserin (group 2) as their study drug. The order in which the different treatment combinations were administered was counterbalanced between subjects according to a randomization table. For further details on study design, see the supplementary information file.
Outcome Measures
Psychological effects were captured using the Hallucinogen Rating Scale (HRS) (Strassman et al., 1994), the Altered States of Consciousness questionnaire (Aussergewöhnliche Psychische Zustände [APZ]) (Dittrich, 1998), and the state version of the State-Trait Anxiety Inventory (STAI) (Spielberger et al., 1970). Finally, self-administered Visual Analogue Scales (VAS) were used to retrospectively rate the following peak effects during the session: any effect, good effects, bad effects, sudden start of effects, fear, time, changes in dimensionality, changes in external reality, loss of contact with external reality, and visions. See the supplementary information file for further details. All questionnaires and VAS items were administered in Spanish.
Cardiovascular effects were captured by measuring systolic and diastolic blood pressure (SBP and DBP, respectively) and heart rate (HR) while volunteers were in a recumbent position.
Neuroendocrine effects were captured by measuring cortisol, prolactin and GH. Salvinorin-A plasma levels were also assessed and pharmacokinetic parameters calculated. The limits of quantification were 1 μg/dL for cortisol, 0.6ng/mL for prolactin, 0.05ng/mL for GH, and 0.035mg/mL for salvinorin A.
Additional information on outcome measures is provided in the supplement.
Statistical Analyses
The statistical analyses were conducted using the SPSS software. Descriptive and inferential statistics were used on all measures. Scores on each questionnaire subscale and VAS item were calculated for each participant and dosing condition. Means and standard errors of the mean were used in the figures. Data were analyzed using repeated-measures ANOVAs with treatment as a repeated factor (placebo+placebo, placebo+salvinorin-A, antagonist+placebo, antagonist+salvinorin-A). When a significant effect of treatment was found, posthoc pair-wise comparisons between treatments were conducted using the Bonferroni correction as implemented in SPSS.
For cardiovascular and neuroendocrine data, preadministration (baseline) values were subtracted from postadministration measures. Subsequently, peak effect (maximum absolute change from baseline values) and area under the curve of effect vs time were calculated: from 0 to 120min for cardiovascular measures; and from 0 to 240 minutes for hormone concentrations. The obtained values were analyzed using the aforementioned repeated-measures ANOVA followed by posthoc comparisons between treatments using the Bonferroni correction.
Pharmacokinetic parameters were expressed for each group (group 1 and group 2) and pretreatment (placebo, naltrexone, ketanserin) as mean and standard deviations. To examine any possible pharmacokinetic interaction between salvinorin-A and the active pretreatments, pharmacokinetic parameters for salvinorin-A were compared in the absence and presence of the antagonists (naltrexone and ketanserin). Comparisons were conducted for each pharmacokinetic parameter using Student’s t tests followed.
Results for the ANOVAs are given following Greenhouse-Geisser correction. Results were considered significant for P<.05.
Results
All 24 participants completed the 4 experimental sessions, and there were no drop-outs in the course of the study. Pharmacokinetic and neuroendocrine data from one volunteer in group 1 (naltrexone) could not be obtained. This was due to malfunction of the i.v. catheter in the experimental session in which the participant received salvinorin-A alone.
Psychological Effects
HRS
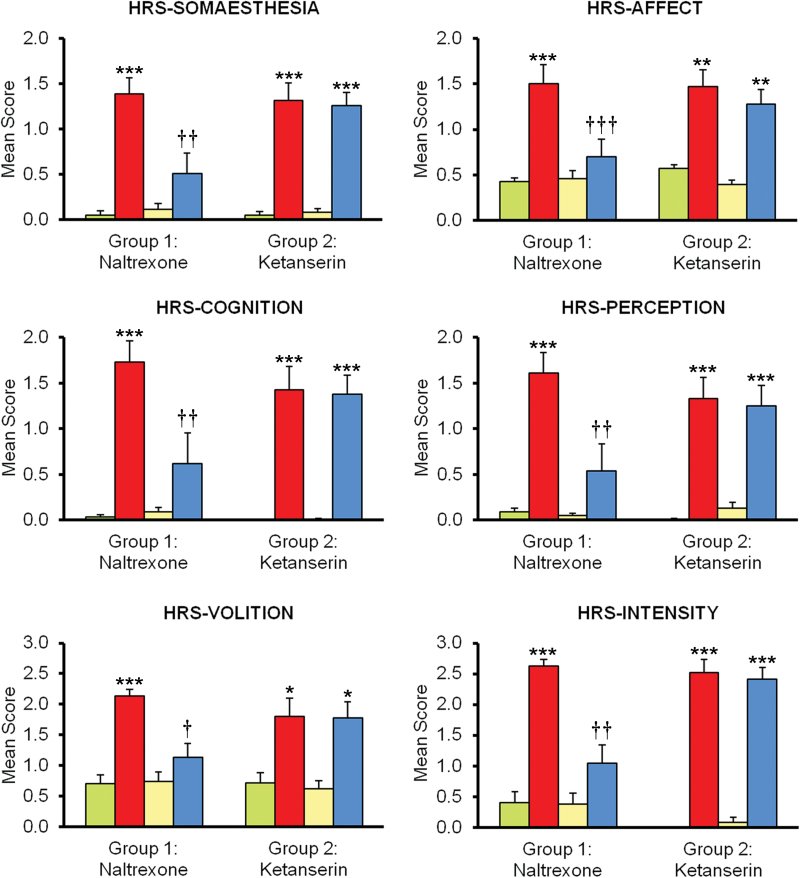
Mean scores on all subscales of the HRS for each group and treatment are shown in Figure 1. In group 1 (naltrexone), significant effects of treatment were observed in all subscales of the HRS: somaesthesia F(3,33) = 24.68, P<.001; affect F(3,33) = 21.25, P<.001; perception F(3,33) = 22.39, P<.001; cognition F(3,33) = 21.33, P<.001; volition F(3,33) = 29.74, P<.001; and intensity F(3,33) = 28.59, P<.001. While salvinorin-A increased scores in all subscales, naltrexone effectively blocked these effects. Posthoc comparisons using the Bonferroni correction showed significant naltrexone-induced reductions of the effects of salvinorin-A in all subscales: somaesthesia P<.01; affect P<.001; perception P<.01; cognition P<.01; volition P<.01; and intensity P<.01. Scores for the combination were not different from placebo (Figure 1).
Figure 1.
Mean scores on the 6 Hallucinogen Rating Scale (HRS) subscales. Participants in group 1 received placebo (green), 1mg salvinorin-A (red), 50mg naltrexone (yellow), and the combination naltrexone+salvinorin-A (blue). Participants in group 2 received placebo (green), 1mg salvinorin-A (red), 40mg ketanserin (yellow), and the combination ketanserin+salvinorin-A (blue). Error bars denote 1 SEM (n=12 in each group). Significant differences from placebo are denoted as *P<.05, **P<.01, and ***P<.001. Significant differences between salvinorin-A alone and salvinorin-A after pretreatment with an antagonist (naltrexone or ketanserin) are denoted as †P<.05, ††P<.01, and †††P<.001.
In group 2 (ketanserin), a significant effect of treatment was again observed in all subscales: somaesthesia F(3,33) = 42.10, P<.001; affect F(3,33) = 21.08, P<.001; perception F(3,33) = 22.05, P<.001; cognition F(3,33) = 30.26, P<.001; volition F(3,33) = 16.99, P<.001; and intensity F(3,33) = 110.74, P<.001. Salvinorin-A increased scores on the HRS, and ketanserin coadministration did not modify this effect. The posthoc comparisons using the Bonferroni correction showed no significant variations in any subscale. To the contrary, scores for the combination were significantly different from placebo in all cases (Figure 1).
APZ
Mean scores on all subscales of the APZ are shown in Figure 2. In group 1 (naltrexone), significant effects of treatment were observed in the 3 subscales: Oceanic Boundlessness (OSE) F(3,33) = 18.40, P<.001; Dread of Ego Dissolution (AIA) F(3,33) = 10.72, P<.01; Visionary Restructuralization (VUS) F(3,33) = 28.61, P<.001. Salvinorin-A led to significant increases in the scores of the 3 subscales. Naltrexone again blocked these effects. Posthoc comparisons using the Bonferroni correction showed naltrexone-induced significant reductions: OSE P<.01; AIA P<.05; VUS P<.001.
Figure 2.
Mean scores on the Altered States of Consciousness (Aussergewöhnliche Psychische Zustände [APZ]) questionnaire. Participants in group 1 received placebo (green), 1mg salvinorin-A (red), 50mg naltrexone (yellow), and the combination naltrexone+salvinorin-A (blue). Participants in group 2 received placebo (green), 1mg salvinorin-A (red), 40mg ketanserin (yellow), and the combination ketanserin+salvinorin-A (blue). Error bars denote 1 SEM (n=12/group). Significant differences from placebo are denoted as *P < .1, *P<.05, **P<.01, and ***P<.001. Significant differences between salvinorin-A alone and salvinorin-A after pretreatment with an antagonist (naltrexone or ketanserin) are denoted as †P<.05, ††P<.01, and †††P<.001.
In group 2 (ketanserin), a significant effect of treatment was also pobserved in all subscales: OSE F(3,33) = 34.75, P<.001; AIA F(3,33) = 10.71, P<.01; VUS F(3,33) = 19.34, P<.001. Ketanserin had no effect when it was administered in combination with salvinorin-A. The posthoc comparisons using the Bonferroni correction showed no significant variations in any subscale. Scores for the combination remained significantly different from placebo for the OSE and VUS subscales and showed a trend for AIA (Figure 2).
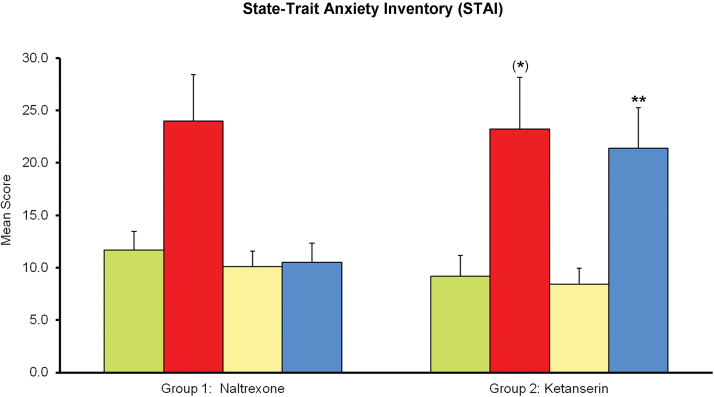
State STAI
Mean scores on the state STAI are shown in Figure 3. In group 1 (naltrexone), the ANOVA showed a main effect of treatment F(3,33) = 5.90, P<.05. Mean anxiety scores were highest after salvinorin-A. However, the posthoc comparisons using the Bonferroni correction did not find differences with placebo for any treatment, and neither did the comparison between salvinorin-A alone and the salvinorin-A plus naltrexone combination.
Figure 3.
Mean scores on the state State-Trait Anxiety Inventory (STAI) questionnaire. Participants in group 1 received placebo (green), 1mg salvinorin-A (red), 50mg naltrexone (yellow), and the combination naltrexone+salvinorin-A (blue). Participants in group 2 received placebo (green), 1mg salvinorin-A (red), 40mg ketanserin (yellow), and the combination ketanserin+salvinorin-A (blue). Error bars denote 1 SEM (n=12/group). Significant differences from placebo are denoted as *P < .1, *P<.05, **P<.01, and ***P<.001. Significant differences between salvinorin-A alone and salvinorin-A after pretreatment with an antagonist (naltrexone or ketanserin) are denoted as †P<.05, ††P<.01, and †††P<.001.
In group 2 (ketanserin), a significant effect of treatment was observed F(3,33) = 10.47, P<.01. In this sample, the salvinorin-induced increases in STAI mean values were marginally significant (P=.051). Ketanserin coadministration had no effect on the anxiogenic effects of salvinorin-A. On the contrary, scores for the combination showed less inter-subject variability and were significantly higher than placebo (Figure 3).
VAS
Mean scores on all VAS items are shown in Figure 4. In group 1 (naltrexone), significant effects of treatment were observed in all but one item: any effect, F(3,33) = 67.11, P<.001; good effects, F(3,33) = 10.78, P<.01; sudden start of effects, F(3,33) = 81.23, P<.001; fear, F(3,33) = 4.82, P<.05; altered time perception, F(3,33) = 68.81, P<.001; altered body dimensionality, F(3,33) = 43.13, P<.001; altered external reality, F(3,33) = 43.51, P<.001; lost contact with external reality, F(3,33) = 124.88, P<.001; and visual effects, F(3,33) = 43.01, P<.001. The item “bad effects” only showed a trend [F(3,33)=4.44, P=.059]. Salvinorin-A increased scores in all subscales, whereas naltrexone effectively blocked these effects. Posthoc comparisons using the Bonferroni correction showed significant score reductions in the 7 subscales showing the highest elevations after salvinorin: any effect, P<.001; sudden start of effects, P<.001; altered time perception, P<.001; altered body dimensionality, P<.001; altered external reality, P<.001; lost contact with external reality, P<.001; and visual effects, P<.001. Nonsignificant reductions were found for good effects, bad effects, and fear, the scales showing the smallest effects when salvinorin-A was administered alone. Scores for the naltrexone+salvinorin-A combination on all subscales were not different from placebo (Figure 4).
Figure 4.
Mean scores on the self-administered visual analogue scales (VAS) items. Participants in group 1 received placebo (green), 1mg salvinorin-A (red), 50mg naltrexone (yellow), and the combination naltrexone+ salvinorin-A (blue). Participants in group 2 received placebo (green), 1mg salvinorin-A (red), 40mg ketanserin (yellow), and the combination ketanserin+salvinorin-A (blue). Error bars denote 1 SEM (n=12/group). Significant differences from placebo are denoted as *P < .1, *P<.05, **P<.01, and ***P<.001. Significant differences between salvinorin-A alone and salvinorin-A after pretreatment with an antagonist (naltrexone or ketanserin) are denoted as †P<.05, ††P<.01, and †††P<.001.
In group 2 (ketanserin), a significant effect of treatment was observed in all VAS items: any effect, F(3,33) = 133.98, P<.001; good effects, F(3,33) = 20.04, P<.01; bad effects, F(3,33)= 6.54, P<.001; sudden start of effects, F(3,33) = 64.82, P<.001; fear, F(3,33) = 7.37, P<.01; altered time perception, F(3,33) = 27.18, P<.001; altered body dimensionality, F(3,33) = 42.43, P<.001; altered external reality, F(3,33) = 28.99, P<.001; lost contact with external reality, F(3,33) = 31.98, P<.001; visual effects, F(3,33) = 23.17, P<.001. Ketanserin administration had no effect on the salvinorin-induced increases. The posthoc comparisons using the Bonferroni correction found no significant variations in any subscale. Scores for the combination were significantly different from placebo in all cases, except for bad effects and fear, which showed only trends (Figure 4).
Cardiovascular Effects
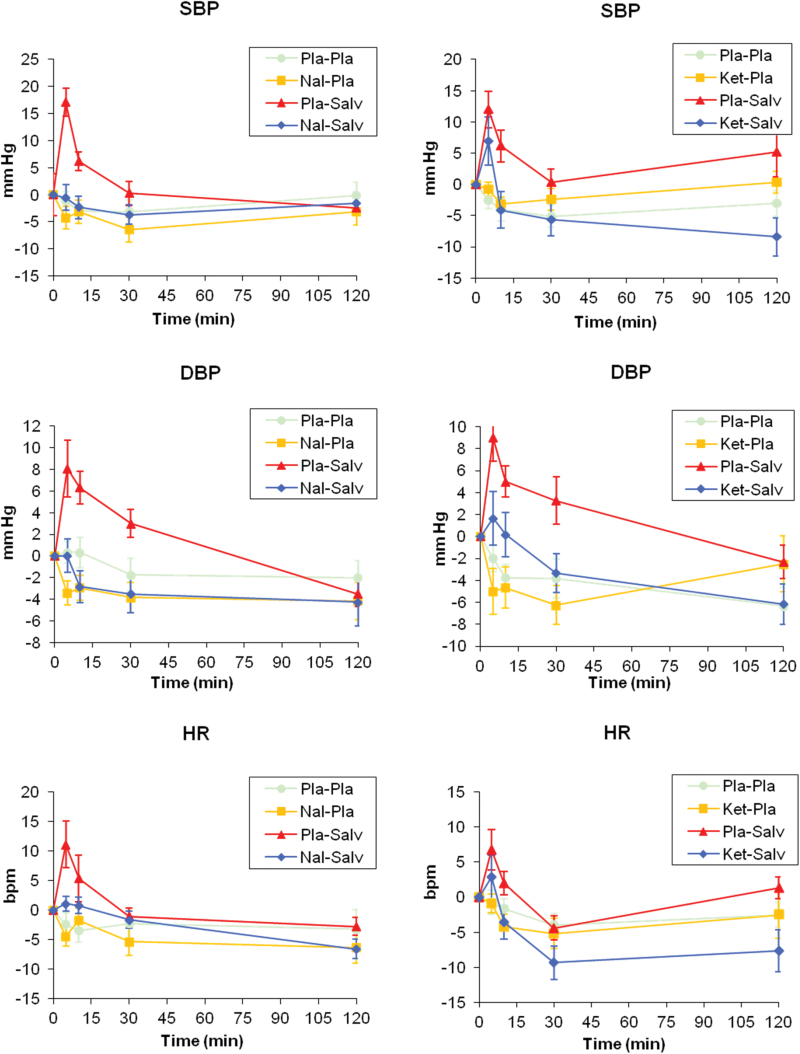
The time course of cardiovascular measures is shown in Figure 5. In both groups, SBP, DBP, and HR values increased above the preadministration baseline following salvinorin-A administration. This increase was very sharp and short lived, peaking after the first measurement point and decreasing thereafter. The results of the statistical analyses are shown in Tables 1 and 2. SBP increases in peak values reached statistical significance in both groups, whereas DBP increases were only significant in group 2. The brief nature of these increases is highlighted by the fact that the AUC values between 0 and 2 hours were not significantly different from placebo in either group; neither were changes in HR peak and AUC after salvinorin-A.
Figure 5.
Time course of cardiovascular measures. Panels on the left show mean data for SBP, DBP, and heart rate (HR) for group 1 (naltrexone, n=12). Panels on the right show mean data for SBP, DBP, and HR for group 2 (ketanserin, n=12). The plots show data following placebo+placebo (circle), antagonist (naltrexone or ketanserin)+placebo (square), placebo+salvinorin (triangle), and antagonist+salvinorin (diamond). Error bars denote ±1 SEM.
Table 1.
Cardiovascular and Hormone Data from Group 1
| Cardiovascular | PL | SA | NA | NASA | F(3,33) | P |
Pairwise Comparisons (Bonferroni) P Value |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PL:SA | PL:NA | PL:NASA | SA:NA | SA:NASA | |||||||
| SBP_pk (mm Hg) | -1.17 (6.19) | 17.08 (14.41) | -4.25 (7.34) | -0.50 (8.24) | 15.10 | <.001 | .006 | NS | NS | .003 | .001 |
| SBP_AUC (mm Hg·min) | -123.75 (365.36) | 133.54 (308.24) | -270.83 (406.70) | -147.08 (335.64) | 2.69 | .096 | NS | NS | NS | NS | NS |
| DBP_pk (mm Hg) | 0.33 (3.50) | 8.08 (9.06) | -3.42 (3.96) | 0.00 (5.34) | 8.64 | .002 | NS | NS | NS | .019 | .016 |
| DBP_AUC (mm Hg·min) | -67.92 (256.38) | 142.08 (217.08) | -211.88 (234.13) | -186.67 (304.83) | 4.48 | .020 | NS | NS | NS | .030 | .036 |
| HR_pk (bpm) | -2.42 (6.84) | 11.08 (13.59) | -4.42 (5.90) | 1.08 (4.27) | 6.97 | .009 | NS | NS | NS | .047 | NS |
| HR_AUC (bpm·min) | -159.79 (470.13) | 53.75 (275.17) | -272.29 (418.65) | -125.63 (211.35) | 1.68 | .205 | NS | NS | NS | NS | NS |
| Hormones | F(3,30) | ||||||||||
| Cort_pk (μg/dL) | -0.65 (3.01) | 8.73 (2.29) | 1.65 (7.05) | 5.25 (3.36) | 9.02 | .002 | <.001 | NS | .023 | .037 | NS |
| Cort_AUC (μg/dL·min) | -66.4 (419.4) | 539.1 (405.9) | 4.48 (774.7) | 324.6 (540.9) | 3.02 | .075 | .012 | NS | NS | NS | NS |
| GH_pk (ng/mL) | 2.96 (3.49) | 7.62 (7.51) | 2.88 (3.00) | 6.81 (6.48) | 2.88 | .082 | NS | NS | .081 | NS | NS |
| GH_AUC (ng/mL·min) | 221.6 (231.7) | 513.7 (521.4) | 207.5 (243.8) | 569.5 (520.2) | 2.75 | .093 | NS | NS | .040 | NS | NS |
| Prol_pk (ng/mL) | 3.55 (2.22) | 27.09 (19.84) | 3.49 (5.54) | 7.24 (12.61) | 12.63 | .001 | .05 | NS | NS | .021 | .003 |
| Prol_AUC (ng/mL·min) | 192.7 (314.4) | 1848.6 (1169.0) | 142.9 (585.6) | 390.3 (892.1) | 14.74 | <.001 | .03 | NS | NS | .011 | .007 |
Abbreviations: AUC, area under the curve; Cort, cortisol; DBP, diastolic blood pressure; GH, growth hormone; HR, heart rate; NA, naltrexone+placebo; NASA, naltrexone+salvinorin A; pk, peak value; PL, placebo+placebo, SA, placebo+salvinorin-A; SBP, systolic blood pressure; Prol, prolactin.
Effects induced by placebo (placebo+placebo), salvinorin-A (placebo+salvinorin-A), naltrexone (naltrexone+placebo), and naltrexone+salvinorin-A on cardiovascular and neuroendocrine measures. Means (SD) of values obtained (n=12 for cardiovascular measures and n=11 for hormones) and results of the statistical analyses performed. The comparison naltrexone vs naltrexone+salvinorin-A has been omitted.
Table 2.
Cardiovascular and Hormone Data from Group-2
| Cardiovascular | PL | SA | KET | KETSA | F(3,33) | P |
Pairwise Comparisons (Bonferroni) P value |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PL:SA | PL:KET | PL:KETSA | SA:KET | SA:KETSA | |||||||
| SBP_pk (mm Hg) | -2.50 (5.00) | 12.00 (10.14) | -0.75 (3.82) | 6.92 (13.38) | 8.13 | .003 | .013 | NS | NS | .011 | NS |
| SBP_AUC (mm Hg·min) | -236.88 (288.58) | 222.92 (504.39) | -98.75 (259.67) | -281.88 (496.15) | 4.59 | .012 | NS | NS | NS | NS | .070 |
| DBP_pk (mm Hg) | -2.00 (4.20) | 9.00 (7.48) | -5.00 (7.19) | 1.67 (8.49) | 12.25 | <.001 | .004 | NS | NS | .001 | .062 |
| DBP_AUC (mm Hg·min) | -247.71 (266.43) | 153.75 (280.70) | -277.08 (357.59) | -165.83 (281.18) | 5.58 | .007 | .058 | NS | NS | .008 | .039 |
| HR_pk (bpm) | -0.75 (5.99) | 6.75 (10.01) | -0.92 (4.62) | 2.83 (10.88) | 2.71 | .083 | NS | NS | NS | NS | NS |
| HR_AUC (bpm·min) | -162.08 (277.20) | -31.67 (268.53) | -223.33 (383.41) | -371.88 (437.46) | 2.03 | .144 | NS | NS | NS | NS | NS |
| Hormones | |||||||||||
| Cort_pk (μg/dL) | -0.66 (5.62) | 6.06 (7.44) | -3.74 (5.61) | 4.75 (6.43) | 7.44 | .004 | .009 | NS | .081 | .083 | NS |
| Cort_AUC (μg/dL·min) | -269.1 (600.9) | 425.0 (691.3) | -750.0 (569.8) | 83.7 (562.5) | 11.98 | .001 | .005 | NS | NS | .013 | .078 |
| GH_pk (ng/mL) | 1.60 (1.66) | 4.71 (3.80) | 1.83 (2.77) | 3.67 (3.23) | 3.55 | .032 | NS | NS | NS | NS | NS |
| GH_AUC (ng/mL·min) | 162.2 (213.2) | 304.9 (278.4) | 135.4 (184.7) | 206.9 (225.3) | 1.51 | .233 | NS | NS | NS | NS | NS |
| Prol_pk (ng/mL) | 0.78 (4.45) | 20.78 (14.81) | 1.48 (5.69) | 28.18 (18.10) | 20.79 | <.001 | .007 | NS | .002 | .012 | NS |
| Prol_AUC (ng/mL·min) | -26.2 (547.5) | 1231.1 (903.8) | 182.8 (776.4) | 1698.9 (1216.4) | 18.48 | <.001 | .002 | NS | .001 | .034 | NS |
Abbreviations: AUC, area under the curve; Cort, cortisol; DBP, diastolic blood pressure; GH, growth hormone; HR, heart rate; KET, ketanserin+placebo; KETSA, ketanserin+salvinorinA; pk, peak value; PL, placebo+placebo; Prol, prolactin; SA, placebo+salvinorin-A; SBP, systolic blood pressure.
Effects induced by placebo (placebo+placebo), salvinorin-A (placebo+salvinorin-A), ketanserin (ketanserin+placebo), and ketanserin+salvinorin-A on cardiovascular and neuroendocrine measures. Means (SD) of values obtained (n=12), and results of the statistical analyses performed. The comparison ketanserin vs ketanserin+salvinorin-A has been omitted.
In group 1, naltrexone blocked the increases in SBP and DBP peak value, significantly reducing both values when it was administered together with salvinorin-A compared with when salvinorin-A was administered alone.
In group 2, mean peak values after ketanserin+salvinorin-A were lower than those obtained after salvinorin-A alone. However, in contrast with the findings in group 1, these reductions were not statistically significant.
Neuroendocrine Effects
The concentration-time curves for cortisol, GH, and prolactin are shown in Figure 6 and the results of the statistical analyses in Tables 1 and 2. In group 1, the i.v. catheter did not work properly, and blood samples could not be obtained from one of the participants during the experimental session in which salvinorin-A was administered alone. Thus, hormone levels could not be assessed for this participant, and results are shown for only 11 volunteers.
Figure 6.
Time course of neuroendocrine measures. Panels on the left show mean data for cortisol, growth hormone (GH), and prolactin for group 1 (naltrexone, n=11). Panels on the right show mean data for cortisol, GH, and prolactin for group 2 (ketanserin, n=11). The plots show data following placebo+placebo (circle), antagonist (naltrexone or ketanserin)+placebo (square), placebo+salvinorin (triangle), and antagonist+salvinorin (diamond). Error bars denote ±1 SEM.
In both groups, hormone levels increased above preadministration values following salvinorin-A administration. However, statistical significance was attained only for cortisol and prolactin.
In group 1, naltrexone reduced cortisol and prolactin levels when it was administered together with salvinorin-A compared with when salvinorin-A was administered alone. However, statistical significance was only attained for prolactin.
In group 2, cortisol levels were lower for the ketanserin+salvinorin-A combination than for salvinorin-A alone. However, this reduction was not significant for peak values and showed a trend only for the AUC. In the case of prolactin, ketanserin did not inhibit its release. In fact, mean values were higher for the combination ketanserin+ salvinorin-A than for salvinorin-A alone. However, these differences were not statistically significant.
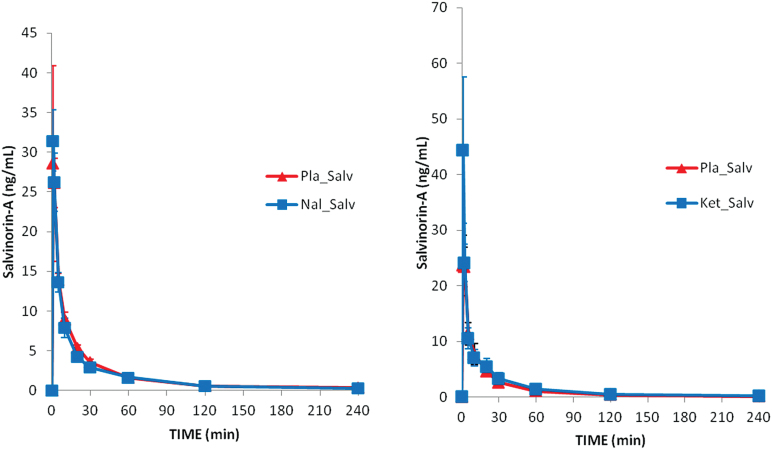
Pharmacokinetics
The time course of salvinorin-A plasma concentrations and the calculated pharmacokinetic parameters are shown in Figure 7 and Table 3, respectively. As indicated above, blood samples could not be obtained from one of the participants in group 1 when salvinorin-A was administered alone. Results are shown for 11 volunteers only.
Figure 7.
Time course of salvinorin-A concentrations. The left panel shows mean data for group 1 (salvinorin-A and salvinorin-A+naltrexone, n=11). The right panel shows mean data for group 2 (salvinorin-A and salvinorin-A+ketanserin, n=12).
Table 3.
Pharmacokinetic Parameters for Salvinorin-A for Each Group (1 and 2) and Experimental Session in which It Was Administered (Placebo+Salvinorin-A and Antagonist+Salvinorin-A)
| Group 1 (n=11) | ||||
|---|---|---|---|---|
| Parameter | Placebo+Salvinorin-A | Naltrexone+ Salvinorin-A | t(10) | P |
| Tmax (min) | 1.36 (0.50) | 1.36 (0.50) | 0.00 | NS |
| Cmax (ng/mL) | 31.28 (13.38) | 40.34 (36.95) | -1.06 | NS |
| AUC0-240 min (ng/mL·min) | 463.61 (132.77) | 436.76 (106.58) | 0.71 | NS |
| AUC0-∞ (ng/mL·min) | 487.36 (142.24) | 457.06 (113.82) | 0.76 | NS |
| t1/2λz (min) | 50.44 (14.79) | 56.96 (7.77) | -2.12 | NS |
| Cl/F (L/min) | 2.33 (1.17) | 2.32 (0.62) | 0.03 | NS |
| Vz/F (L) | 166.32 (77.47) | 188.80 (46.00) | -1.06 | NS |
| Group 2 (n=12) | ||||
| Parameter | Placebo+Salvinorin-A | Ketanserin +Salvinorin-A | t(11) | P |
| Tmax (min) | 1.50 (0.52) | 1.42 (0.51) | 0.432 | NS |
| Cmax (ng/mL) | 31.25 (15.4) | 50.04 (37.64) | -1.89 | NS |
| AUC0-240 min (ng/mL·min) | 358.29 (162.69) | 423.82 (215.71) | -0.90 | NS |
| AUC0-∞ (ng/mL·min) | 371.14 (168.91) | 440.40 (219.39) | 0.93 | NS |
| t1/2λz (min) | 49.21 (15.58) | 56.24 (16.80) | -1.28 | NS |
| Cl/F (L/min) | 3.32 (1.60) | 2.83 (1.51) | 0.95 | NS |
| Vz/F (L) | 237.08 (133.90) | 223.00 (100.75) | 0.41 | NS |
Abbreviations: Tmax: time taken to reach the maximum concentration; Cmax: maximum concentration; AUC0-240: area under the concentration-time curve from 0 to 240 min; AUC0-∞: area under the concentration-time curve from 0 to infinity; t1/2λz: terminal half-life; Cl/F: clearance; Vz/F: apparent volume of distribution.
Values indicate mean (SD). In group 1, values were calculated for 11 volunteers only. Pairwise comparisons were conducted using within-subjects Student’s t tests.
As shown in the graphs and tables, drug levels were highest in the first and second measurement points after the start of the inhalation. Values decreased rapidly thereafter, falling below 1ng/mL at 2 hours after dosing. The statistical comparison of pharmacokinetic parameters in each group did not show any significant differences between the placebo+salvinorin-A and the antagonist+salvinorin-A sessions.
Discussion
Our results confirm the involvement of opioid receptors in the human pharmacology of salvinorin-A. The administration of naltrexone blocked the modified state of awareness induced by salvinorin-A in humans. On the contrary, pretreatment with the 5-HT2A antagonist ketanserin had no effect on the nature and intensity of the subjective experience.
The 1-mg dose of salvinorin-A and the route of administration chosen here induced a pattern of subjective effects of fast onset and short duration that replicated previous research conducted by our group in 2 different laboratories (Johnson et al., 2011; MacLean et al., 2013; Maqueda et al., 2015). The effects induced were reflected as significant increases in all 3 subscales of the APZ questionnaire, in the 6 subscales of the HRS, and in an extensive battery of VAS items previously shown to be sensitive to salvinorin-A (Maqueda et al., 2015). The efficacy of vaporization followed by inhalation was further evidenced by the measurable levels of salvinorin-A found in plasma. The drug was rapidly absorbed reaching its maximum concentrations in the first 2 measurement time points at 1 and 2 minutes after dosing, in line with previous data in humans (Johnson et al., 2016). This peak was followed by a rapid decrease. Assuming a parallel between plasma and CNS, these results suggest a rapid clearance from the brain, in line with observations in monkeys using nuclear medicine techniques (Hooker et al., 2008). This concentration-time pattern paralleled the time course of subjective effects described in previous studies using the same administration route (Johnson et al., 2011; MacLean et al., 2013; Maqueda et al., 2015).
The present findings support the involvement of the KOR in the subjective effects induced by salvinorin-A. The KOR and the dynorphins, its endogenous ligands, are broadly distributed throughout the central nervous system (Simonin et al., 1995). An agonist-mediated inhibitory effect of salvinorin-A on KOR-rich nuclei of the thalamus (Le Merrer et al., 2009) could explain the characteristic blockade of external audiovisual stimuli observed. On the other hand, a study in rodents has shown that salvinorin-A increases fluorodeoxyglucose uptake in the sensory cortex (Hooker et al., 2009). Activation of neocortical areas of the temporal lobe could underlie the vivid visual imagery and auditory phenomena reported here. As hypothesized, naltrexone pretreatment blocked most aspects of the subjective experience (HRS, APZ, VAS, STAI), whereas ketanserin had no effect on any of these measures. Thus, salvinorin-A was shown to exert its effects on human perception, cognition, and emotion via opioidergic processes, without the involvement of serotonergic processes. Naltrexone is a nonspecific opioid receptor antagonist with the highest affinity for the mu and kappa receptors (μ:κ relative affinity of 1.7) and the lowest for the delta receptor (μ:δ relative affinity of 550) (Wentland et al., 2009). Naltrexone was administered at the 50-mg standard clinical dose. Doses of 25 to 30mg had proved effective in previous studies to block the kappa-related effects of the opiates butorphanol and pentazocine (Preston and Bigelow, 1993; Walsh et al., 2008).
In addition to the thalamus and the neocortex, high KOR levels are also found in more primitive regions of the brain such as the hypothalamus, the ventral tegmental area, and the nucleus accumbens (Simonin et al., 1995), associated with homeostasis, reward, and motivation. In fact, besides its characteristic perceptual-cognitive effects, salvinorin-A also induced a series of cardiovascular and neuroendocrine effects when given alone. At the 1-mg dose, salvinorin-A consistently increased SBP, cortisol, and prolactin levels in the 2 participant groups. While previous studies had not found effects of salvinorin-A on blood pressure and HR (Johnson et al., 2011; Addy, 2012; MacLean et al., 2013), increases in cortisol and prolactin had been reported (Johnson et al., 2016; Ranganathan et al., 2012). The absence of significant results on cardiovascular measures in the previous studies may be due to the small size of 2 of the studies (Johnson et al., 2011; MacLean et al., 2013), the length of time between measurements (Addy, 2012), and differences in the vaporization method used that may have led to lower absorption and drug levels in plasma (Ranganathan et al., 2012).
The increased blood pressure and cortisol release observed could be a direct effect of salvinorin-A at the KOR sites. Synthetic KOR agonists increase cortisol in humans (Ur et al., 1997) and adrenocorticotropic and cortisol levels in monkeys (Pascoe et al., 2008). However, despite this evidence of a direct effect, a nonspecific stress reaction cannot be entirely ruled out, as these increases have also been reported for a broad range of psychoactive drugs with diverse mechanisms of action. They have been described, for instance, for the psychostimulant d-amphetamine, which increases noradrenergic and dopaminergic neurotransmission, and for 5-HT2A agonist psychedelics such as ayahuasca (Dos Santos et al., 2011) and psilocybin (Hasler et al., 2004). Agonism at the KOR is able on its own to stimulate the hypothalamus (Hooker et al., 2009), potentially facilitating a direct drug effect on cortisol levels. Whereas KOR antagonists attenuate the physiological reactions to stress in rats (Fassini et al., 2015), agonists have been shown to induce large increases in vasopressin release in some but not all human subjects (Pfeiffer et al., 1986). This inter-subject variability in vasopressin response could provide another potential explanation for the disparity of results observed between laboratories regarding salvinorin-A effects on blood pressure.
Salvinorin-A administered alone increased prolactin plasma concentration, as previously reported for this drug (Johnson et al., 2016) and other KOR agonists such as spiradoline (Ur et al., 1997). Prolactin release is physiologically under inhibitory control by dopaminergic neurotransmission, with amphetamine and other pro-dopaminergic drugs effectively blocking its release (Samuels et al., 2007; Dos Santos et al., 2011). The increase in prolactin concentrations induced by salvinorin-A could be secondary to the inhibition of dopamine release in the tuberoinfundibular pathway. KORs are localized on dopaminergic neurons where they exert a tonic inhibitory effects (Chefer et al., 2005). Several studies have shown that kappa agonists decrease dopamine release in the NAcc (Spanagel et al., 1992; Maisonneuve et al., 1994), the ventral tegmental area (Margolis et al., 2003), and the prefrontal cortex (Heijna et al., 1990; Margolis et al., 2006). Additionally, repeated administration of kappa agonists leads to reductions in the levels of dopamine-2 receptors in the ventral striatum (Izenwasser et al., 1998). Animal research has shown that naltrexone administered alone reduces basal prolactin levels (Enjalbert et al, 1979), and here it effectively reduced prolactin release. By contrast, ketanserin pretreatment had no effect on this measure.
Mean GH plasma levels increased after salvinorin-A, but these were not statistically significant. In humans, GH may be regulated by KOR, as levels increase following the administration of the synthetic KOR agonist spiradoline (Ur et al., 1997). Possibly the effect is not robust enough following salvinorin-A administration to be detected with the sample size used in the present study.
The global pattern of effects emerging from the present study is in accordance with animal studies that support the involvement of the KOR in salvinorin-A effects. Salvinorin-A induces hypolocomotion and antinociception effects that can be blocked by a selective KOR antagonist, but not by rimonabant (Walentiny et al., 2010). In rhesus monkeys, salvinorin-A stimulates prolactin release (Butelman et al., 2007), facial relaxation and ptosis (Butelman et al., 2009), as well as discriminative stimulus effects (Butelman et al., 2010). All these effects can be prevented by the KOR partial agonist nalmefene (Butelman et al., 2007, 2009) and the antagonist quadazocine (Butelman et al., 2010). However, they cannot be blocked by ketanserin (Butelman et al., 2007, 2009, 2010) or rimonibant (Butelman et al., 2009).
To sum up, naltrexone but not ketanserin effectively blocked the subjective, cardiovascular, and neuroendocrine effects of salvinorin-A in humans. The 2 pharmacological interaction studies conducted demonstrate the involvement of opioidergic, rather than serotonergic neurotransmission in the effects of salvinorin-A in humans. These results are consistent with agonist actions of salvinorin-A at the KOR.
Statement of Interest
Dr Griffiths is member of the Board of Directors of the Heffter Research Institute, which also supports some of his research. Dr Griffiths and the rest of the authors declare no biomedical financial interests or potential conflicts of interest.
Supplementary Material
Acknowledgements
The authors thank Laboratorio Echevarne for the neuroendocrine determinations and the study volunteers for their participation.
This work was supported by grant PI12/02758 from the Instituto de Salud Carlos III of the Spanish Government, which is cofunded by FEDER. Marta Valle is supported by the Fondo de Investigación Sanitaria through grant CP04/00121 from the Spanish Ministry of Health in collaboration with Institut de Recerca de l’Hospital de la Santa Creu i Sant Pau, Barcelona. Support for Dr. Griffiths was provided by NIDA Grant R01DA003889.
References
- Addy PH. (2012) Acute and post-acute behavioral and psychological effects of salvinorin A in humans. Psychopharmacology (Berl) 220:195–204. [DOI] [PubMed] [Google Scholar]
- Addy PH, Garcia-Romeu A, Metzger M, Wade J. (2015) The subjective experience of acute, experimentally-induced Salvia divinorum inebriation. J Psychopharmacol (Oxf) 29:426–435. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Mandau M, Tidgewell K, Prisinzano TE, Yuferov V, Kreek MJ. (2007) Effects of salvinorin A, a kappa-opioid hallucinogen, on a neuroendocrine biomarker assay in nonhuman primates with high kappa-receptor homology to humans. J Pharmacol Exp Ther 320:300–306. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Prisinzano TE, Deng H, Rus S, Kreek MJ. (2009) Unconditioned behavioral effects of the powerful kappa-opioid hallucinogen salvinorin A in nonhuman primates: fast onset and entry into cerebrospinal fluid. J Pharmacol Exp Ther 328:588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Rus S, Prisinzano TE, Kreek MJ. (2010) The discriminative effects of the kappa-opioid hallucinogen salvinorin A in nonhuman primates: dissociation from classic hallucinogen effects. Psychopharmacology (Berl) 210:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Czyzyk T, Bolan EA, Moron J, Pintar JE, Shippenberg TS. (2005) Endogenous kappa-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine. J Neurosci 25:5029–5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich A. (1998) The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry 31 Suppl 2:80–84. [DOI] [PubMed] [Google Scholar]
- Dos Santos RG, Valle M, Bouso JC, Nomdedéu JF, Rodríguez-Espinosa J, McIlhenny EH, Barker SA, Barbanoj MJ, Riba J. (2011) Autonomic, neuroendocrine, and immunological effects of ayahuasca: a comparative study with d-amphetamine. J Clin Psychopharmacol 31:717–726. [DOI] [PubMed] [Google Scholar]
- Fassini A, Scopinho AA, Resstel LBM, Corrêa FMA. (2015) κ-opioid receptors in the infralimbic cortex modulate the cardiovascular responses to acute stress. Exp Physiol 100:377–387. [DOI] [PubMed] [Google Scholar]
- González D, Riba J, Bouso JC, Gómez-Jarabo G, Barbanoj MJ. (2006) Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug Alcohol Depend 85:157–162. [DOI] [PubMed] [Google Scholar]
- Hasler F, Grimberg U, Benz MA, Huber T, Vollenweider FX. (2004) Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology (Berl) 172:145–156. [DOI] [PubMed] [Google Scholar]
- Heijna MH, Padt M, Hogenboom F, Portoghese PS, Mulder AH, Schoffelmeer AN. (1990) Opioid receptor-mediated inhibition of dopamine and acetylcholine release from slices of rat nucleus accumbens, olfactory tubercle and frontal cortex. Eur J Pharmacol 181:267–278. [DOI] [PubMed] [Google Scholar]
- Hooker JM, Xu Y, Schiffer W, Shea C, Carter P, Fowler JS. (2008) Pharmacokinetics of the potent hallucinogen, salvinorin A in primates parallels the rapid onset and short duration of effects in humans. NeuroImage 41:1044–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker JM, Patel V, Kothari S, Schiffer WK. (2009) Metabolic changes in the rodent brain after acute administration of salvinorin A. Mol Imaging Biol MIB Off Publ Acad Mol Imaging 11:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izenwasser S, Acri JB, Kunko PM, Shippenberg T. (1998) Repeated treatment with the selective kappa opioid agonist U-69593 produces a marked depletion of dopamine D2 receptors. Synapse 30:275–283. [DOI] [PubMed] [Google Scholar]
- Johnson MW, MacLean KA, Reissig CJ, Prisinzano TE, Griffiths RR. (2011) Human psychopharmacology and dose-effects of salvinorin A, a kappa opioid agonist hallucinogen present in the plant Salvia divinorum. Drug Alcohol Depend 115:150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, MacLean KA, Caspers MJ, Prisinzano TE, Griffiths RR. (2016) Time course of pharmacokinetic and hormonal effects of inhaled high-dose salvinorin A in humans. J Psychopharmacol (Oxf). In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Becker JAJ, Befort K, Kieffer BL. (2009) Reward processing by the opioid system in the brain. Physiol Rev 89:1379–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean KA, Johnson MW, Reissig CJ, Prisinzano TE, Griffiths RR. (2013) Dose-related effects of salvinorin A in humans: dissociative, hallucinogenic, and memory effects. Psychopharmacology (Berl) 226:381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve IM, Archer S, Glick SD. (1994) U50,488, a kappa opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats. Neurosci Lett 181:57–60. [DOI] [PubMed] [Google Scholar]
- Maqueda AE, Valle M, Addy PH, Antonijoan RM, Puntes M, Coimbra J, Ballester MR, Garrido M, González M, Claramunt J, Barker S, Johnson MW, Griffiths RR, Riba J. (2015) Salvinorin-A induces intense dissociative effects, blocking external sensory perception and modulating interoception and sense of body ownership in humans. Int J Neuropsychopharmacol 18 pii: pyv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. (2003) Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci 23:9981–9986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. (2006) Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci U S A 103:2938–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega A, Blount JF, Manchand PS. (1982) Salvinorin, a new trans-neoclerodane diterpene from Salvia divinorum (Labiatae). J Chem Soc [Perkin 1]:2505–2508. [Google Scholar]
- Ott J. (1995) Ethnopharmacognosy and human pharmacology of Salvia divinorum and salvinorin A. Curare 18:103–129. [Google Scholar]
- Pascoe JE, Williams KL, Mukhopadhyay P, Rice KC, Woods JH, Ko M-C. (2008) Effects of mu, kappa, and delta opioid receptor agonists on the function of hypothalamic-pituitary-adrenal axis in monkeys. Psychoneuroendocrinology 33:478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Knepel W, Braun S, Meyer HD, Lohmann H, Brantl V. (1986) Effects of a kappa-opioid agonist on adrenocorticotropic and diuretic function in man. Horm Metab Res 18:842–848. [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE. (1993) Differential naltrexone antagonism of hydromorphone and pentazocine effects in human volunteers. J Pharmacol Exp Ther 264:813–823. [PubMed] [Google Scholar]
- Prisinzano TE. (2005) Psychopharmacology of the hallucinogenic sage Salvia divinorum. Life Sci 78:527–531. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, Schnakenberg A, Skosnik PD, Cohen BM, Pittman B, Sewell RA, D’Souza DC. (2012) Dose-related behavioral, subjective, endocrine, and psychophysiological effects of the κ opioid agonist Salvinorin A in humans. Biol Psychiatry 72:871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. (2002) Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci U S A 99:11934–11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels ER, Hou RH, Langley RW, Szabadi E, Bradshaw CM. (2007) Comparison of pramipexole with and without domperidone co-administration on alertness, autonomic, and endocrine functions in healthy volunteers. Br J Clin Pharmacol 64:591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin F, Gaveriaux-Ruff C, Befort K, Matthes H, Lannes B, Micheletti G, Mattei MG, Charron G, Bloch B, Kieffer B. (1995) kappa-Opioid receptor in humans: cDNA and genomic cloning, chromosomal assignment, functional expression, pharmacology, and expression pattern in the central nervous system. Proc Natl Acad Sci U S A 92:7006–7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. (1992) Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A 89:2046–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. (1970) Manual for the State-Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologists. [Google Scholar]
- Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. (1994) Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry 51:98–108. [DOI] [PubMed] [Google Scholar]
- Ur E, Wright DM, Bouloux PM, Grossman A. (1997) The effects of spiradoline (U-62066E), a kappa-opioid receptor agonist, on neuroendocrine function in man. Br J Pharmacol 120:781–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes LJ, Butler WM, Hatfield GM, Paul AG, Koreeda M. (1984) Divinorin A, a psychotropic terpenoid, and divinorin B from the hallucinogenic Mexican mint, Salvia divinorum. J Org Chem 49:4716–4720. [Google Scholar]
- Valdés LJ, Díaz JL, Paul AG. (1983) Ethnopharmacology of ska María Pastora (Salvia divinorum, Epling and Játiva-M.). J Ethnopharmacol 7:287–312. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D. (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9:3897–3902. [DOI] [PubMed] [Google Scholar]
- Walentiny DM, Vann RE, Warner JA, King LS, Seltzman HH, Navarro HA, Twine CE, Thomas BF, Gilliam AF, Gilmour BP, Carroll FI, Wiley JL. (2010) Kappa opioid mediation of cannabinoid effects of the potent hallucinogen, salvinorin A, in rodents. Psychopharmacology (Berl) 210:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Chausmer AE, Strain EC, Bigelow GE. (2008) Evaluation of the mu and kappa opioid actions of butorphanol in humans through differential naltrexone blockade. Psychopharmacology (Berl) 196:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Strain EC, Abreu ME, Bigelow GE. (2001) Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans. Psychopharmacology (Berl) 157:151–162. [DOI] [PubMed] [Google Scholar]
- Wentland MP, Lou R, Lu Q, Bu Y, Denhardt C, Jin J, Ganorkar R, VanAlstine MA, Guo C, Cohen DJ, Bidlack JM. (2009) Syntheses of novel high affinity ligands for opioid receptors. Bioorg Med Chem Lett 19:2289–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.