Abstract
Background:
This double-blind, parallel-group, multicenter, phase-3 study was designed to test the noninferiority of paliperidone palmitate 3-month formulation (PP3M) to the currently marketed 1-month formulation (PP1M) in patients (age 18–70 years) with schizophrenia, previously stabilized on PP1M.
Methods:
After screening (≤3 weeks) and a 17-week, flexible-dosed, open-label phase (PP1M: day 1 [150mg eq. deltoid], day 8 [100mg eq. deltoid.], weeks 5, 9, and 13 [50, 75, 100, or 150mg eq., deltoid/gluteal]), clinically stable patients were randomized (1:1) to PP3M (fixed-dose, 175, 263, 350, or 525mg eq. deltoid/gluteal) or PP1M (fixed-dose, 50, 75, 100, or 150mg eq. deltoid/gluteal) for a 48-week double-blind phase.
Results:
Overall, 1016/1429 open-label patients entered the double-blind phase (PP3M: n=504; PP1M: n=512) and 842 completed it (including patients with relapse). PP3M was noninferior to PP1M: relapse rates were similar in both groups (PP3M: n=37, 8%; PP1M: n=45, 9%; difference in relapse-free rate: 1.2% [95% CI:-2.7%; 5.1%]) based on Kaplan-Meier estimates (primary efficacy). Secondary endpoint results (changes from double-blind baseline in positive and negative symptom score total and subscale scores, Clinical Global Impression-Severity, and Personal and Social Performance scores) were consistent with primary endpoint results. No clinically relevant differences were observed in pharmacokinetic exposures between PP3M and PP1M. Both groups had similar tolerability profiles; increased weight was the most common treatment-emergent adverse event (double-blind phase; 21% each). No new safety signals were detected.
Conclusion:
Taken together, PP3M with its 3-month dosing interval is a unique option for relapse prevention in schizophrenia.
Keywords: long-acting injectable, paliperidone palmitate 1-month, paliperidone palmitate 3-month, relapse-free, schizophrenia
Introduction
The partial- and total nonadherence to oral antipsychotic therapy that is common among patients with schizophrenia has significant impacts on treatment outcomes and healthcare resources (Higashi et al., 2013; Fleischhacker et al., 2014). Long acting-injectable (LAI) antipsychotics eliminate the need for daily dosing, typically ensure sustained plasma levels for several weeks, and help to reliably monitor adherence (Rauch and Fleischhacker, 2013). Depot preparations of new generation antipsychotics have extended the range of long-term treatment options for patients suffering from schizophrenia. However, they remain underutilized for a variety of reasons, including patient and physician preferences and lack of access (Heres et al., 2006).
Paliperidone palmitate 1-month (PP1M), a LAI formulation designed to be administered once monthly, is approved in multiple countries for the treatment of schizophrenia, and in the US and Canada, also for schizoaffective disorders (Invega Sustenna Prescribing Information, 2015). A new formulation of paliperidone palmitate (PP3M), recently approved in the US for the maintenance treatment of schizophrenia (Invega Trinza Prescribing Information, 2015), offers a substantially longer dosing interval of once every 3 months than is available for typical or new generation atypical LAI formulations (one monthly). Interim analysis results of a long-term maintenance trial of PP3M demonstrated that 93% of patients who were adequately stabilized with PP1M for at least 4 months and subsequently treated with PP3M (doses 175, 263, 350, or 525mg eq.) did not experience a significant return of schizophrenia symptoms (Berwaerts et al., 2015). The current study was designed to demonstrate that the efficacy of PP3M in treating the symptoms of schizophrenia in patients stabilized on PP1M was not less effective (noninferior) than PP1M in these patients.
Methods
The study protocol and amendments were reviewed by an Independent Ethics Committee or Institutional Review Board, as appropriate, for each site. The study was conducted in compliance with the Declaration of Helsinki consistent with Good Clinical Practices and applicable regulatory requirements. Written informed consent was obtained from all patients before enrollment.
Adult patients (men and women, age 18–70 years) with a diagnosis of schizophrenia (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, DSM-IV), a total Positive and Negative Syndrome Scale (PANSS) score between 70 and 120 at screening and baseline, and worsening of symptoms were enrolled. Patients who discontinued other antipsychotics due to insufficient efficacy, safety or tolerability issues with current therapy, or with preferences for injectable medications were eligible. Women included in the study were postmenopausal, surgically sterile, or used adequate contraception, and eligible men used adequate contraception. Major exclusion criteria were: active DSM-IV diagnosis other than schizophrenia; significant risk of suicidal behavior; history of substance dependence within 6 months before screening; involuntary status in a psychiatric hospital at screening; or history of neuroleptic malignant syndrome, tardive dyskinesia, any unstable or significant medical or neurological illness, morbid obesity (BMI >40kg/m2), or other systemic disease, mental retardation, risk factors for prolonged QT interval, torsade de pointes, or sudden death. Patients with a history of intolerability, hypersensitivity, or lack of response to risperidone or paliperidone were also excluded from the study. Additionally, patients taking any LAI antipsychotics within 4 weeks before screening were excluded.
Mood stabilizers (including lithium, valproate, carbamazepine, dilantin, gabapentin, and other antiepileptics), nonselective/irreversible monoamine oxidase inhibitors, herbal, or over-the-counter agents with psychotropic actions, antidepressants started within 4 weeks of screening, and oral antipsychotic drugs including paliperidone extended release had to be tapered and washed out during the screening phase. Stable doses of antidepressants started 30 days before the study could be continued throughout the study. Oral lorazepam or other short-acting benzodiazepines were permissible for treating agitation or anxiety. Antiparkinsonian therapy, beta-blockers, zolpidem, zaleplon, zopiclone, and all forms of psychosocial therapy and education were allowed during the study.
Study Design
This randomized, double-blind (DB), parallel-group, multicenter, noninferiority study was conducted from April 2012 to March 2015 at 199 sites in 26 countries (Argentina, Australia, Austria, Belgium, Brazil, Bulgaria, Canada, China, Czech Republic, France, Germany, Greece, Hungary, Japan, Mexico, Poland, Portugal, Romania, Russian Federation, Slovakia, South Korea, Spain, Sweden, Taiwan, Ukraine, United States). Patients came predominantly from Europe (46%), China (21%), the United States (12%), and Japan (12%). The study consisted of 4 phases: screening (up to 3 weeks), open label (OL) stabilization (17 weeks, flexible doses), DB (48 weeks, fixed doses), and a follow-up phase.
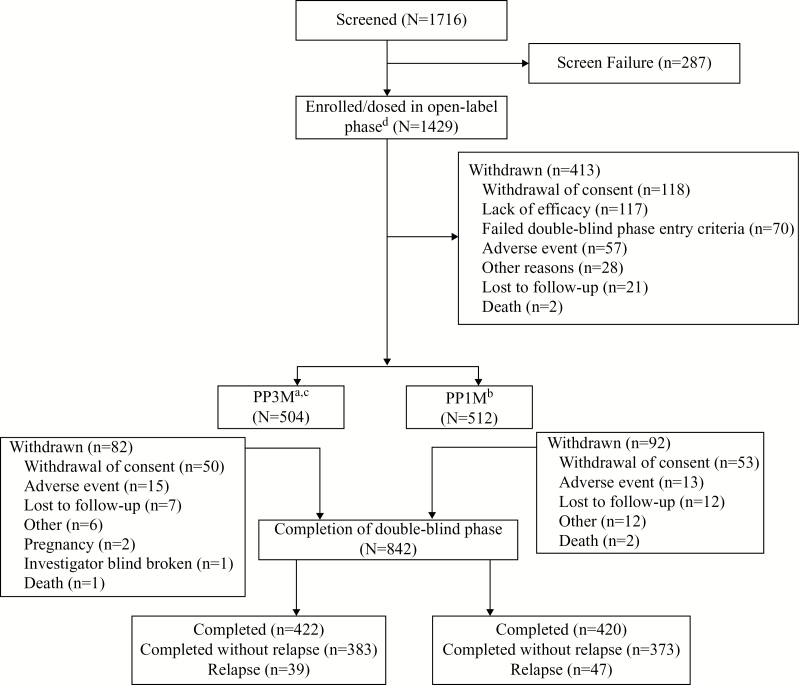
During screening, patients underwent a washout of disallowed psychotropic medications and oral tolerability testing (patients without documented previous exposure to oral risperidone, oral paliperidone, or those patients who were not currently receiving another antipsychotic were administered paliperidone extended release 6mg/d for 4 to 6 consecutive days). Patients not requiring a washout or oral tolerability testing entered directly into the OL stabilization phase after results of screening labs and ECGs were available. In the OL phase, all patients received PP1M for 17 weeks (day 1: 150mg eq. [deltoid]; day 8: 100mg eq. [deltoid]; weeks 5 and 9: flexibly dosed [50, 75, 100, or 150mg eq., deltoid or gluteal]; week 13: same dose of PP1M as at week 9). Clinically stable patients (defined as PANSS total score <70, PANSS item [P1, P2, P3, P6, P7, G8, G14] scores ≤4, reduction in Clinical Global Impression-Severity (CGI-S) score by ≥1 from OL baseline) at weeks 14 and 17 then entered the DB phase, where they were randomized (1:1) to receive fixed doses of PP3M (175, 263, 350, or 525mg eq.) or PP1M (50, 75, 100, or 150mg eq.) (Figure 1; supplementary Table 1).
Figure 1.
Patient disposition. aPaliperidone palmitate 3-month formulation (PP3M) doses: 175, 263, 350, or 525mg eq., ie, 273, 410, 546, or 819mg. bPaliperidone palmitate 1-month formulation (PP1M) doses: 50, 75, 100, or 150mg eq. (78, 117, 156, or 234mg. c21 patients were excluded from the efficacy analyses due to a manufacturing issue with a small lot of PP3M. dAll patients were to receive the first PP1M injection of 150mg eq. (234mg) on day 1 and the second injection of 100mg eq. (156mg) on day 8, both in the deltoid muscle.
Randomization was performed using a computer-generated randomization scheme administered by an interactive web response system, balanced using permuted blocks across the 2 groups, and stratified by study center. Patients in the PP3M group received a fixed dose (3.5-fold multiple of the PP1M dose administered at week 9) at weeks 17, 29, 41, and 53. These patients received active medication every 3 months and, to maintain the blinding, received matched placebo injections (20% intralipid) monthly when they did not receive active medication. All medications (PP3M, PP1M, and placebo) were administered by a study drug administrator who was not involved in any safety or efficacy assessments during the DB phase.
Pharmacokinetic Assessments
Concentrations of paliperidone in blood were measured during the DB phase predose and every 4 weeks thereafter. Pharmacokinetic analysis was performed by Kinesis Pharma B.V., Breda, The Netherlands, using the validated computer program Phoenix™ WinNonlin® (version 6.2.1). Noncompartmental analysis model 200 (extravascular input, plasma data) was applied for the pharmacokinetic analysis. Furthermore, Microsoft Excel (version 2007; Microsoft, Redmond, WA) and/or SAS (version 9.3, SAS Institute Inc., Cary, NC) were used. Plasma concentrations of paliperidone were determined using a lower limit of quantification of 0.1ng/mL.
Pharmacokinetic analysis included calculation of Cpredose (plasma concentration measured immediately before intramuscular injection at week 53); Cmax (observed maximum plasma concentration after the study drug injection at week 53); Tmax (time to reach the maximum plasma concentration after the study drug injection at week 53); AUCτ (area under the plasma concentration-time curve after the study drug injection at week 53); Cavg (average plasma concentration, calculated as the AUCτ divided by the actual dosing interval; and peak-to-trough ratio (defined by Cmax/Cpredose). For PP3M, this was calculated as week 33/week 29 (peak concentrations) and week 57/week 53 (trough concentrations), while for PP1M it was calculated as week 14/week 13 (peak concentrations) and week 54/week 53 (trough concentrations). Dose normalization was done to 350mg eq. for the (relevant) PP3M PK parameters and to 100mg eq. for the (relevant) PP1M PK parameters.
Efficacy Assessments
The primary efficacy endpoint was the percentage of patients who remained relapse free (as defined by Csernansky et al., 2002 and used in previous PP1M [Hough et al., 2010] and PP3M [Berwaerts et al., 2015] studies) (based on the Kaplan-Meier cumulative estimate of survival) at the end of the 48-week DB phase. Relapse was defined as ≥1 of the following: (1) hospitalization for schizophrenia symptoms (involuntary or voluntary admission); (2) 25% increase in PANSS total score from randomization for 2 consecutive assessments between 3 and 7 days apart for patients scoring >40 at randomization, or a 10-point increase for patients scoring ≤40 at randomization; (3) increase in distinct PANSS item scores (P1, P2, P3, P6, P7, or G8) for 2 consecutive assessments between 3 and 7 days apart; (4) clinically significant, deliberate self-injury or violent behavior resulting in suicide, injury, or significant damage; or (5) suicidal or homicidal ideation and aggressive behavior. For relapses based on PANSS ratings, the relapse was determined to occur at the time of the first presentation for increased symptoms and was confirmed by a second assessment 3 to 7 days apart after the first assessment.
Secondary efficacy endpoints included change from DB baseline to endpoint in: PANSS total score, PANSS subscale (positive subscale, negative subscale, general pathophysiology subscale), and Marder factor scores (positive symptoms, negative symptoms, disorganized thoughts, uncontrolled hostility/excitement, anxiety/depression), CGI-S score, and Personal and Social Performance (PSP) scores, assessment of clinical response (≥20% reduction in PANSS total scores from DB baseline to end of DB phase), and symptomatic remission (defined as meeting the Andreasen remission criteria during the 6 months before the end of DB phase, with one excursion allowed) achieved following treatment with PP3M vs PP1M. Remission criterion was defined as having a simultaneous score of mild or less on all selected PANSS items (P1, P2, P3, N1, N4, N6, G5, and G9).
Safety Assessments
Safety evaluations included clinical assessment of treatment-emergent adverse events (TEAEs), extrapyramidal symptoms (EPS) scales (Guy 1976) (Abnormal Involuntary Movement Scale, Barnes Akathisia Rating Scale, and Simpson and Angus Rating Scale), suicidal ideation and behavior using Columbia Suicide Severity Rating Scale, clinical laboratory evaluations (including measurement of prolactin levels and glucose levels), measurement of vital signs and body weight, electrocardiograms, and injection-site evaluations.
Statistical Methods
Sample Size Determination
The primary hypothesis was that PP3M was noninferior to PP1M for the percentage of patients who remained relapse free, as defined by the lower limit of the 95% CI of the difference in relapse-free rates between PP3M and PP1M exceeding -15% over a 48-week period. Assuming that the relapse-free survival rate in PP1M was 70%, a true difference in survival between PP1M and PP3M of 4% in favor of PP1M, and a 1-sided significance level of 2.5%, 380 patients per treatment group were required to demonstrate with 90% power that PP3M was no worse than PP1M by a noninferiority margin of 15% in survival rates. The noninferiority margin was determined based on published literature (Leucht et al., 2003; Hough et al., 2010; Kramer et al., 2010) and clinical recommendations from a panel of experts in the field of schizophrenia relapse prevention studies. Considering that 74% of patients enrolled would be randomized, 1388 patients were planned to be enrolled in the study to provide 380 patients per group, evaluable for the primary efficacy analysis utilizing the per protocol analysis set.
Statistical Analyses
All efficacy and safety analyses for the OL phase were conducted using the intent-to-treat OL analysis set (ITT[OL] all patients who had received at least 1 dose of study drug during the OL phase). The primary efficacy analysis was conducted using per protocol (PP) analysis set (defined as all randomized patients who received at least 1 dose of study drug during the DB phase and did not have major protocol violations that may impact efficacy such as violations of intended study population, errors in treatment assignment or use of excluded medication). All secondary efficacy analyses for the DB phase were conducted using the modified ITT (DB) analysis set (defined as all patients who received at least 1 dose of study drug during the DB phase and had no errors in the delivery of active treatment due to the manufacturing of the investigational product). All safety analyses for the DB phase were conducted using the safety analysis set (defined as all patients who received at least 1 dose of study drug during the DB phase).
The null hypothesis was tested using a 1-sided α=0.025 level such that H0: p3–p1≤-δ vs H1: p3-p1>-δ, where p1 and p3 referred to the percentage of patients in PP1M and PP3M groups, respectively, who remained relapse-free at week 48 (end of DB phase). The Kaplan-Meier method was used to estimate the 48-week cumulative estimate of survival (percentage of patients who remained relapse free). The standard error estimates were based upon Greenwood’s formula. Noninferiority of PP3M to PP1M was concluded if the lower limit of the 2-sided 95% CI of the difference in relapse-free rates between PP3M and PP1M exceeded -15%. PP3M was declared superior to PP1M if the lower limit of the 2-sided 95% CI of the difference in the relapse-free rates between PP3M and PP1M exceeded 0%. Hazard ratio estimates and its 95% CI were based on the Cox proportional hazards model with treatment as the only factor. Treatment comparisons between PP3M and PP1M for the changes from DB baseline to the end of DB phase (last observation carried forward) in PANSS total and subscale, CGI-S, and PSP scores were performed using an ANCOVA model with treatment and country as factors and baseline as a covariate. Least-square estimates of the treatment differences and 95% CI were presented. The proportion of patients who achieved symptomatic remission (Andreasen et al., 2005) was analyzed using the Cochran-Mantel-Haenszel test controlling for country. The point estimate and 95% CI for the relative risk were presented. For each treatment group, the number and percent of responders were tabulated at each time point during the DB phase. At DB endpoint, the point estimate and 95% CI were provided for the relative risk using a Cochran-Mantel-Haenszel test controlling for country. Safety results were analyzed descriptively.
Results
Overall, 1429 patients were enrolled and dosed in the OL phase, and 1016 (71%) were randomized (PP3M: n=504; PP1M: n=512) to the DB phase. The most common reasons for discontinuation during the OL phase were withdrawal of consent (n=118, 8%) and lack of efficacy (n=117, 8%). Out of 1016 randomized patients, 948 patients were included in the PP analysis set (PP3M: n=458; PP1M: n=490), while 995 patients were included in the mITT (DB) analysis set (PP3M: n=483; PP1M: n=512). In total, 842 (83%) of the randomized patients completed the study (including patients with relapse); similar percentages of patients in both groups completed the DB phase (PP3M: 84%; PP1M: 82%). Withdrawal of consent was the most common reason for discontinuation in the DB phase (Figure 1). In the PP3M group, 21 patients received approximately only 75% of the intended dose due to a manufacturing issue (insertion of a plunger into the syringe barrel that was too short to inject all of the content) and were hence excluded from the mITT (DB) and PP analysis sets.
Demographics and Baseline Characteristics
Overall, 782 (55%) of 1429 patients enrolled in the OL phase were men; the majority of the patients were white (55%) with a mean (SD) age of 38.4 (11.86) years. The demographics and baseline characteristics were similar between both groups in the DB phase (Table 1). The patients’ mean (SD) PANSS total score at OL baseline was 85.7 (10.73). Approximately 61% of patients were hospitalized at least once due to psychosis within 24 months before enrollment; the median (range) duration of hospitalization was 48 (1, 5145) days. At DB baseline, the psychiatric characteristics of patients in the PP3M and PP1M groups were generally similar, except for the duration of the most recent psychiatric hospitalization at study entry; patients in the PP3M group had a longer mean (SD) stay compared with PP1M group (96.4 [328.58] vs 88.5 [123.54] days) (Table 1). Though worsening of symptoms were required for study entry, 39% of the enrolled patients had not been hospitalized in the previous 24 months, which was similar to the frequency seen in the previous PP3M study (Berwaerts et al, 2015) but more than in the PP1M vs risperidone CONSTA noninferiority study where only 10% of the patients were not hospitalized in the previous 24 months (Alphs et al, 2013).
Table 1.
Demographic and Baseline Characteristics, and Diagnosis and Psychiatric History (ITT [OL] Analysis Set and mITT [DB] Analysis Set)
| ITT (OL) | mITT (DB) | ||||
|---|---|---|---|---|---|
| OL PP1M (N=1429) | Not Randomized to DB (N=413) | PP3M (N=483) | PP1M (N=512) | Total (N=995) | |
| Age, mean (SD), (y) | 38.4 (11.86) | 37.9 (11.35) | 39.2 (11.90) | 38.3 (12.24) | 38.7 (12.08) |
| Sex, n (%) | |||||
| Men | 782 (55) | 243 (59) | 247 (51) | 281 (55) | 528 (53) |
| Race, n (%) | |||||
| White | 780 (55) | 188 (46) | 280 (58) | 296 (58) | 576 (58) |
| Black or African American | 113 (8) | 49 (12) | 25 (5) | 36 (7) | 61 (6) |
| Asian | 513 (36) | 166 (40) | 172 (36) | 175 (34) | 347 (35) |
| American Indian or Alaska native | 3 (<1) | 0 | 1 (<1) | 1 (<1) | 2 (<1) |
| Not reported | 7 (<1) | 3 (1) | 2 (<1) | 2 (<1) | 4 (<1) |
| Other | 10 (1) | 5 (1) | 2 (<1) | 2 (<1) | 4 (<1) |
| Multiple | 2 (<1) | 1 (<1) | 1 (<1) | 0 | 1 (<1) |
| Unknown | 1 (<1) | 1 (<1) | 0 | 0 | 0 |
| Ethnicity, n (%) | |||||
| Hispanic or Latino | 114 (8) | 25 (6) | 43 (9) | 42 (8) | 85 (9) |
| Not Hispanic or Latino | 1299 (91) | 381 (92) | 435 (90) | 466 (91) | 901 (91) |
| Not reported | 9 (1) | 4 (1) | 2 (<1) | 3 (1) | 5 (1) |
| Unknown | 7 (<1) | 3 (1) | 3 (1) | 1 (<1) | 4 (<1) |
| Weight-baseline (OL), mean (SD), (kg) | 75.89 (17.78) | 75.75 (18.24) | 75.96 (17.33) | 75.66 (17.80) | 75.81 (17.56) |
| BMI-baseline (OL), mean (SD), (kg/m2) | 26.48 (5.10) | 26.35 (5.23) | 26.55 (4.93) | 26.44 (5.17) | 26.50 (5.05) |
| Age at schizophrenia diagnosis, mean (SD), (y) | 27.5 (9.19) | 26.5 (8.89) | 28.8 (9.44) | 27.2 (9.06) | 28.0 (9.27) |
| Prior hospitalizations,a n (%) | |||||
| N | 1146 | 341 | 373 | 414 | 787 |
| None | 450 (39) | 118 (35) | 147 (39) | 179 (43) | 326 (41) |
| Once | 426 (37) | 127 (37) | 147 (39) | 144 (35) | 291 (37) |
| Twice | 192 (17) | 61 (18) | 61 (16) | 67 (16) | 128 (16) |
| Three times | 42 (4) | 16 (5) | 16 (4) | 10 (2) | 26 (3) |
| Four times or more | 36 (3) | 19 (6) | 2 (1) | 14 (3) | 16 (2) |
| PANSS total | |||||
| Baseline (OL), mean (SD) | 85.7 (10.73) | 87.4 (11.75) | 84.8 (10.43) | 85.2 (10.05) | 85.0 (10.23) |
| Baseline (DB), mean (SD) | 57.3 (8.57) | 58.2 (9.07) | 57.8 (8.83) | ||
| PSP | |||||
| Baseline (OL), mean (SD) | 52.7 (12.39) | 50.9 (12.64) | 53.8 (12.03) | 53.3 (12.37) | 53.5 (12.20) |
| Baseline (DB), mean (SD) | 65.5 (10.40) | 64.8 (11.16) | 65.2 (10.80) | ||
| CGI | |||||
| Baseline (OL), mean (SD) | 4.4 (0.69) | 4.5 (0.74) | 4.4 (0.66) | 4.4 (0.66) | 4.4 (0.66) |
| Baseline (DB), mean (SD) | 2.9 (0.57) | 2.9 (0.67) | 2.9 (0.62) | ||
Abbreviations: BMI, Body Mass Index; CGI-S, Clinical Global Impression-Severity; DB, double-blind; mITT, modified intent-to-treat; OL, open-label; PANSS, Positive and Negative Symptom Scale; PP1M, paliperidone palmitate 1-month formulation; PP3M, paliperidone palmitate 3-month formulation; PSP, Personal and Social Performance.
a Number of hospitalizations for psychosis within 24 months prior to study start.
Prior and Concomitant Medications
A majority of the patients (n=1291, 90%) received ≥1 psychotropic medications before study entry. Previous antipsychotic medication use generally reflected the current usage in the schizophrenia population in participating countries. The most commonly used psychotropic medications included new-generation antipsychotics (n=1084, 76%; oral risperidone: n=492, 34%). In total, 325 patients (23%) received first-generation antipsychotics (haloperidol: n=160 [11%]), 312 patients (22%) received anti-EPS medications, 25 patients (2%) received antihistamines, and 44 patients (3%) were on beta blockers before study entry. The percentage of patients who received psychotropic medications before study entry was similar in the PP3M and PP1M groups.
Overall, 457 (32%) of the 1429 patients received benzodiazepines in the OL phase (lorazepam: 21%; clonazepam: 7%; diazepam: 6%), and 59% patients received concomitant medications other than benzodiazepines (zopiclone and zolpidem: 10% each). During the DB phase, 238 (24%) of the 995 mITT (DB) patients received benzodiazepines (lorazepam: 13%; clonazepam: 6%; diazepam: 4%), and 631 patients (63%) received concomitant medications other than benzodiazepines (risperidone [10%], zolpidem, biperiden, and trihexyphenidyl [6% each]). Concomitant antipsychotics were prohibited during the study (both OL and DB phases); risperidone (10%) and paliperidone (4%) were started mainly on the disposition/relapse date during the DB phase. During the DB phase, more patients in the PP3M group received concomitant medications other than benzodiazepines (zolpidem, biperiden, trihexiphenidyl [6% each], paracetamol, zopiclone, herbal formulation [5% each]) compared with the PP1M group (66% vs 61%), while similar percentages of patients in either group received benzodiazepines (24% in each group) as well as beta blockers (propranolol: 3% vs 2%). In total, 154 (11%) patients received antidepressant therapy during the OL phase; 53 (11%) patients in the PP3M group and 61 (12%) in the PP1M group received antidepressants during the DB phase. During the OL phase, 211 patients (15%) received anti-EPS therapy; biperiden and trihexyphenidyl were the most common (5% and 7%) anti-EPS medications used. More patients in the PP3M group received anti-EPS treatment than in the PP1M group (16% vs 13%) during the DB phase.
Drug Exposure
A total of 318 (22%) of 1423 patients underwent oral tolerability testing during the screening period; none experienced tolerability issues. A majority of the patients (79%) received 5 injections of PP1M during the OL phase with 448 patients (40%) stabilized on the 100-mg eq. dose and 551 (49%) patients on the 150-mg eq. dose. The mean (SD) duration of exposure to PP1M in the OL phase was 106.1 (31.53) days and the mean (SD) dose of PP1M was 123.50 (16.742) mg eq. The majority of patients (80%) in the PP3M group received 4 active injections, while 73% patients in the PP1M group received 12 active injections during the DB phase; the mean (SD) duration of exposure was 295.1 (88.12) days in the PP3M group and 286.7 (95.92) days in the PP1M group with a mean (SD) PP3M dose of 414.75 (106.062) mg eq. and a PP1M dose of 119.11 (30.193) mg eq.
Pharmacokinetics
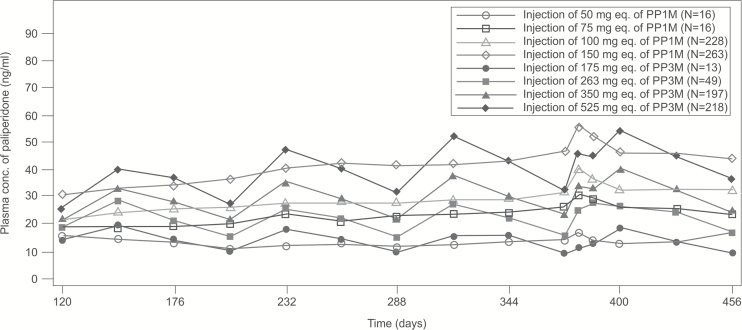
The paliperidone plasma concentration-time profiles for the corresponding PP1M and PP3M doses (50mg eq. vs 175mg eq., 75mg eq. vs 263mg eq., 100mg eq. vs 350mg eq., and 150mg eq. vs 525mg eq.) completely overlapped from day 120 until day 456.
No clinically relevant differences were observed with regard to exposure between PP3M and PP1M across all dose groups. Dose-normalized Cmax and AUCτ were generally comparable and dose proportional in both PP3M and PP1M groups (Figure 2). However, the predose plasma concentrations following PP3M administration (doses: 175, 263, 350, and 525mg eq.) were 21% lower than the concentrations observed following PP1M administration (doses: 50, 75, 100, or 150mg eq.). Similar results were seen while comparing the corresponding dose groups for PP1M and PP3M separately for individual stratification (injection site, race, BMI, gender, age, and creatinine clearance category). Mean peak-to-trough ratios were independent of dose; they were higher following PP3M administration (range: 1.86–2.54) than PP1M administration (range: 1.30–1.63).
Figure 2.
Linear median plasma concentration-time profiles of paliperidone following paliperidone palmitate 1-month formulation (PP1M)a and 3-month formulation (PP3M)b administration during the double-blind (DB) phase. aPP1M doses: 50, 75, 100, or 150mg eq. (78, 117, 156, or 234mg); all patients were to receive the first PP1M injection of 150mg eq. (234mg) on day 1 and the second injection of 100mg eq. (156mg) on day 8, both in the deltoid muscle. bPP3M doses: 175, 263, 350, or 525mg eq., ie, 273, 410, 546, or 819mg.
Efficacy
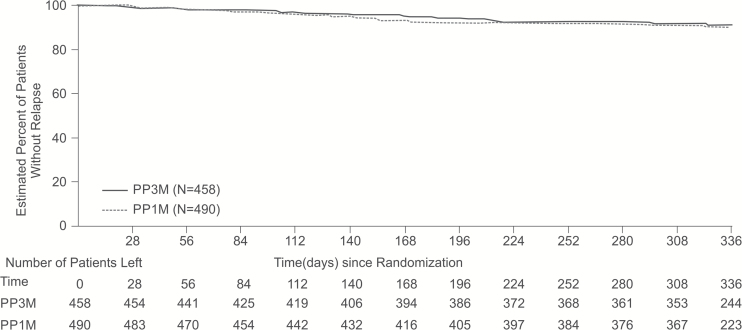
A similar percentage of patients in both groups (PP3M: n=37 [8%]; PP1M: n=45 [9%]) experienced a relapse event during the DB phase (PP analysis set). Based on the Kaplan-Meier estimates, the lower bound of the 95% CI (-2.7%, 5.1%) between the treatment groups (PP3M-PP1M) in the percentages of patients who remained relapse free was larger than the prespecified noninferiority margin of -15%. Further, the estimate based on the mITT (DB) analysis set was consistent with the PP analysis set. Therefore, PP3M can be declared noninferior to PP1M. The median time-to-relapse (median survival time refers to the time at which the cumulative survival function equals 0.5 [or 50%]) was not estimable for either the PP3M or PP1M groups (Figure 3; Table 2) due to the low number of relapses. The most common reasons for relapse were an increase of ≥25% in the total PANSS score (PP3M: n=23 [5%]; PP1M: n=24 [5%]) and psychiatric hospitalizations (PP3M: n=16 [3%]; PP1M: n=22 [4%]). The ratio (95% CI) of the instantaneous risk (hazard) of relapse for a patient switching from PP1M to PP3M during the DB phase vs the risk for a patient remaining on PP1M in the DB phase using Cox Proportional Hazards Model was 0.87 (95% CI: 0.56, 1.34).
Figure 3.
Kaplan-Meier plot of time-to-relapse during the double-blind (DB) phase (PP analysis set).
Table 2.
Time-to-Relapse during the Double-Blind Phase and Number (%) of Patients That Remained Relapse Free (PP Analysis Set)
| PP3M | PP1M | Total | ||
|---|---|---|---|---|
| Number of assessed | 458 | 490 | 948 | |
| Number of censored (%)* | 421(92) | 445(91) | 866(91) | |
| Number of relapsed (%) | 37(8) | 45(9) | 82(9) | |
| Time-to-relapse (days)a | ||||
| 25% Quantile (95% CI) | ( ; ) | ( ; ) | ( ; ) | |
| Median (95% CI) | ( ; ) | ( ; ) | ( ; ) | |
| 75% Quantile (95% CI) | ( ; ) | ( ; ) | ( ; ) | |
| Relapse-freea | ||||
| Week 48 (DB) | ||||
| Percentage relapse-free | 91.2 | 90.0 | ||
| Difference (PP3M-PP1M) | 1.2 | |||
| 95% CI | (-2.7; 5.1) | |||
Abbreviations: DB, double-blind; PP, per protocol; PP1M, paliperidone palmitate 1-month formulation; PP3M, paliperidone palmitate 3-month formulation.
a Based on Kaplan-Meier product limit estimates. Note: 25%, 50%, and 75% quantiles of time-to-relapse are not estimable.
* Censored include patients who completed the DB phase without relapses and patients who withdrew early during the DB phase.
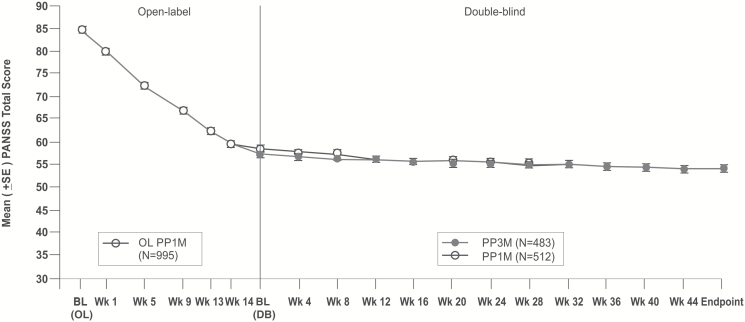
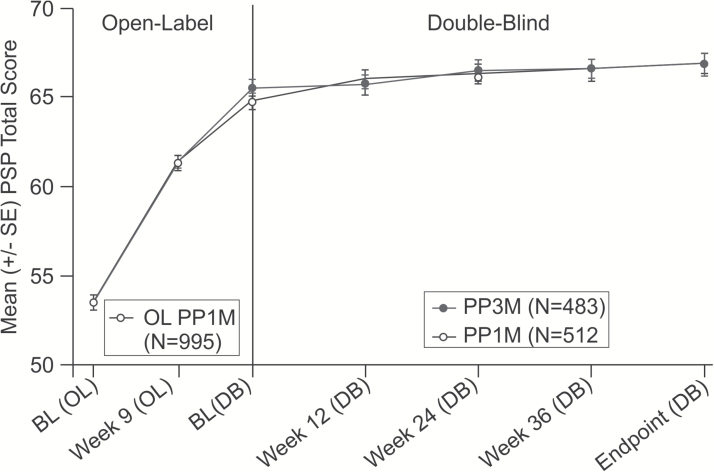
Consistent with the primary efficacy findings, the secondary efficacy results (PANSS total and subscale scores, Marder factor scores, CGI-S and PSP scores) showed similar improvements in both groups from DB baseline to DB endpoint (Figures 4 and 5; Table 3). A similar percentage of patients in both the PP3M and PP1M treatment groups showed an improvement of ≥20%, ≥30%, and ≥40% in the PANSS total score from DB baseline to DB endpoint (Table 3; supplementary Table 2). These improvements in PANSS total score were in addition to the clinically meaningful improvements (PP3M: -27.5; PP1M: -26.9) recorded during the OL phase. More than 50% of patients in both groups showed symptomatic remission for the last 6 months of the DB phase (PP3M: 58%; PP1M: 59%).
Figure 4.
Mean (±SE) in Positive and Negative Syndrome Scale (PANSS) total scores (last observation carried forward) over time during the double-blind (DB) phase (modified intent-to-treat [ITT] DB analysis set).
Figure 5.
Arithmetic mean (±SE) Personal and Social Performance (PSP) total score (last observation carried forward) over time (mITT [DB] analysis set).
Table 3.
Change in Secondary Efficacy Measures during Double-Blind Phase (mITT [DB] Analysis Set)
| PP3M | PP1M |
Between Group Difference-LS Means (SE) (95% CI) |
|
|---|---|---|---|
| PANSS total score,a n | N=481 | N=503 | |
| Baseline, mean (SD) | 57.4 (8.56) | 58.1 (8.88) | 0.9 (0.75) |
| Change from baseline, mean (SD) | -3.5 (12.50) | -4.3 (11.78) | (-0.61; 2.34) |
| PANSS subscales scores,a mean (SD) | N=483 | N=512 | |
| Positive subscale | |||
| Baseline | 11.9 (3.12) | 12.0 (3.19) | 0.2 (0.24) (-0.24; 0.72) |
| Change from baseline | -0.6 (4.31) | -0.9 (3.70) | |
| Negative subscale | |||
| Baseline | 17.3 (4.27) | 17.3 (4.11) | -0.0 (0.22) (-0.43; 0.43) |
| Change from baseline | -1.4 (3.63) | -1.4 (3.67) | |
| General psychopathology subscale | |||
| Baseline | 28.2 (4.55) | 28.8 (4.79) | 0.5 (0.41) (-0.31; 1.29) |
| Change from baseline | -1.4 (6.77) | -2.0 (6.57) | |
| PANSS Marder Standardized Factor Scores,a Mean (SD) | |||
| Positive symptoms | |||
| Baseline | 15.7 (3.66) | 15.8 (3.88) | 0.3 (0.27) (-0.21; 0.84) |
| Change from baseline | -1.1 (4.61) | -1.4 (4.16) | |
| Negative symptoms | |||
| Baseline | 16.2 (4.03) | 16.3 (3.90) | -0.0 (0.22) (-0.48; 0.40) |
| Change from baseline | -1.4 (3.57) | -1.3 (3.80) | |
| Disorganized thoughts | |||
| Baseline | 14.2 (3.20) | 14.3 (3.17) | 0.0 (0.20) (-0.35; 0.43) |
| Change from baseline | -1.2 (3.36) | -1.2 (3.24) | |
| Uncontrolled hostility/excitement | |||
| Baseline | 5.2 (1.64) | 5.4 (1.77) | 0.2 (0.14) (-0.03; 0.50) |
| Change from baseline | 0.2 (2.31) | -0.2 (2.21) | |
| Anxiety/depression | |||
| Baseline | 6.1 (2.02) | 6.3 (2.12) | 0.1 (0.15) (-0.15; 0.44) |
| Change from baseline | -0.0 (2.69) | -0.2 (2.43) | |
| CGI-S score,a n | 481 | 504 | |
| Baseline, Mean (SD) | 2.9 (0.57) | 2.9 (0.66) | 0.0 (0.05) (-0.05; 0.13) |
| Change from baseline, Mean (SD) | -0.1 (0.84) | -0.1 (0.75) | |
| PSP Score,a n | 474 | 495 | |
| Baseline, Mean (SD) | 65.5 (10.40) | 65.0 (11.06) | -0.5 (0.60) (-1.73; 0.64) |
| Change from baseline, Mean (SD) | 1.3 (10.22) | 1.9 (9.21) | |
| Improvement in PANSS total, n (%) | N=481 | N=501 | Relative risk (95% CI of relative risk)c |
| ≥20%, | 241 (50.1) | 237 (47.3) |
1.05
(0.93; 1.19) |
| <20% | 240 (49.9) | 264 (52.7) | |
| ≥30% | 175 (36.4) | 181 (36.1) | 0.98 (0.84; 1.16) |
| <30% | 306 (63.6) | 320 (63.9) | |
| ≥40% | 127 (26.4) | 136 (27.1) | 0.95 (0.78; 1.16) |
| <40% | 354 (73.6) | 365 (72.9) | |
| DB 6-month remission statusb, n (%) | N=483 | N=512 | |
| Yes | 282 (58.4) | 303 (59.2) | 0.98 (0.89; 1.08) |
Abbreviations: CGI-S, Clinical Global Impression-Severity; DB, double-blind; OL, open-label; PANSS, Positive and Negative Symptom Scale; PP1M, paliperidone palmitate 1-month formulation; PP3M, paliperidone palmitate 3-month formulation; PSP, Personal and Social Performance.
a Based on ANCOVA model with treatment and country as factors, and baseline value as a covariate.
b Remission is defined as having a score of ≤3 on all of the following 8 PANSS items: P1, P2, P3, N1, N4, N6, G5, and G9 for the last 6 months of DB treatment, with one excursion allowed.
c Point estimate (95% CI) of relative risk (PP3M vs. PP1M) is based on Cochran-Mantel-Haenszel test controlling for country.
Safety
During the OL phase, 59% of patients experienced TEAEs, 7% of patients experienced ≥1 serious TEAEs, and 4% of patients had TEAEs leading to study drug discontinuation during the OL phase (Table 4). During the DB phase, a similar percentage of patients in the PP3M and PP1M groups experienced TEAEs (68% vs 66%). Overall, 5% patients in the PP3M group and 7% of patients in the PP1M group experienced serious TEAEs; most serious TEAEs were “psychiatric,” usually indicating worsening of the underlying disease. Three percent of patients in each group reported TEAEs leading to discontinuation of study drug in the DB phase, most commonly related to worsening of the psychiatric symptoms (<2% in each group). There were 6 deaths (OL phase: n=2 [1 each due to arteriosclerosis and cardiac arrest]; DB phase: n=4; PP3M: n=1 [hepatocellular carcinoma]; PP1M: n=3 [1 each due to suicide attempt, toxicity to other agents [self-inflicted], and bacterial meningitis). The most common (≥5% patients) TEAEs occurring in either group during the DB phase included increased weight (21% in both groups) followed by nasopharyngitis (PP3M: 7%; PP1M: 6%), anxiety (5% in both groups), and headache (PP3M: 4%; PP1M: 5%) (Table 4; supplementary Table 3).
Table 4.
Summary of Treatment Emergent Adverse Events during the Study (ITT [OL] Analysis Set and Safety Analysis Set)
| ITT (OL) | Safety | ||
|---|---|---|---|
| OL PP1M (N=1429) n (%) | PP3M (N=504) n (%) | PP1M (N=512)n (%) | |
| Patients with adverse events | 846 (59) | 342 (68) | 340 (66) |
| At least 1 possibly related TEAE | 562 (39) | 210 (42) | 209 (41) |
| 1 or more serious TEAE | 101 (7) | 26 (5) | 37 (7) |
| Most common (>2%) serious TEAEs | |||
| Schizophrenia | 31 (2) | 12 (2) | 11 (2) |
| TEAEs leading to drug withdrawala | 60 (4) | 15 (3) | 13 (3) |
| Most common (>0.3%) TEAEs leading to drug withdrawal | |||
| Akathisia | 7 (0.5) | 1 (0.2) | 2 (0.4) |
| Anxiety | 3 (0.2) | 2 (0.4) | 0 |
| Delusion | 1 (0.1) | 2 (0.4) | 0 |
| Galactorrhoea | 1 (0.1) | 1 (0.2) | 2 (0.4) |
| TEAEs leading to death | 2 (0.1) | 1 (0.2) | 3 (1) |
| Most common (≥2%) TEAEs | |||
| Weight increased | 64 (4) | 105 (21) | 109 (21) |
| Nasopharyngitis | 66 (5) | 36 (7) | 33 (6) |
| Anxiety | 83 (6) | 27 (5) | 24 (5) |
| Headache | 46 (3) | 18 (4) | 26 (5) |
| Insomnia | 96 (7) | 16 (3) | 24 (5) |
| Akathisia | 82 (6) | 20 (4) | 14 (3) |
| Schizophrenia | 41 (3) | 18 (4) | 14 (3) |
| Weight decreased | 10 (1) | 14 (3) | 14 (3) |
| Injection site pain | 127 (9) | 12 (2) | 14 (3) |
| Somnolence | 29 (2) | 5 (1) | 5 (1) |
| Hyperglycemia | 3 (0.2) | 4 (1) | 10 (2) |
| Depression | 11 (1) | 11 (2) | 6 (1) |
| Hypertension | 11 (1) | 12 (2) | 7 (1) |
| Diarrhea | 13 (1) | 10 (2) | 6 (1) |
| Fatigue | 17 (1) | 10 (2) | 5 (1) |
| Injection site induration | 40 (3) | 14 (3) | 6 (1) |
| EPS-related TEAEs | 180 (13) | 42 (8) | 38 (7) |
| Akathisia | 82 (6) | 20 (4) | 14 (3) |
| Restlessness | 8 (1) | 2 (<1) | 2 (<1) |
| Restless legs syndrome | 2 (<1) | 0 | 0 |
| Parkinsonism | 17 (1) | 1 (<1) | 5 (1) |
| Hypertonia | 15 (1) | 2 (<1) | 0 |
| Muscle rigidity | 15 (1) | 5 (1) | 0 |
| Musculoskeletal stiffness | 10 (1) | 3 (1) | 9 (2) |
| Extrapyramidal disorder | 4 (<1) | 1 (<1) | 1 (<1) |
| Drooling | 3 (<1) | 0 | 0 |
| Bradykinesia | 2 (<1) | 2 (<1) | 1 (<1) |
| Muscle tightness | 2 (<1) | 2 (<1) | 1 (<1) |
| Akinesia | 1 (<1) | 0 | 0 |
| Cogwheel rigidity | 1 (<1) | 1 (<1) | 0 |
| Hypokinesia | 1 (<1) | 1 (<1) | 1 (<1) |
| Masked facies | 1 (<1) | 0 | 0 |
| Nuchal rigidity | 1 (<1) | 0 | 0 |
| Parkinsonian gait | 0 | 1 (<1) | 0 |
| Tremor | 22 (2) | 9 (2) | 3 (1) |
| Dyskinesia | 11 (1) | 3 (1) | 3 (1) |
| Muscle twitching | 3 (<1) | 0 | 0 |
| Tardive dyskinesia | 2 (<1) | 1 (<1) | 1 (<1) |
| Dystonia | 4 (<1) | 0 | 1 (<1) |
| Muscle spasms | 1 (<1) | 0 | 1 (<1) |
| Myotonia | 1 (<1) | 0 | 0 |
| Trismus | 1 (<1) | 0 | 0 |
| Oculogyric crisis | 0 | 0 | 1 (<1) |
| Torticollis | 0 | 0 | 1 (<1) |
Abbreviations: DB, double-blind; EPS, extrapyramidal syndrome; OL, open-label; PP1M, paliperidone palmitate 1-month formulation; PP3M, paliperidone palmitate 3-month formulation; TEAE, treatment-emergent adverse event.
All percentages are rounded off to nearest whole integer.
a An adverse event that started in the OL phase and resulted in study drug being discontinued in the DB phase was counted as treatment-emergent in the OL phase.
The incidence of TEAEs related to EPS, suicidality, agitation and aggression, somnolence and sedation, tachycardia, orthostatic hypotension, QT prolongation, potentially prolactin-related, and weight gain was comparable between the PP3M and PP1M groups (Table 5, Table 6). Diabetes mellitus and hyperglycemia-related TEAEs were reported at a lower frequency in the PP3M group (2.6%) than in the PP1M group (4.9%). During the study, tardive dyskinesia was reported as a TEAE in 3 patients: 2 in the OL phase and 2 in the DB phase (PP3M: n=1; PP1M: n=1, who also experienced the TEAE in the OL phase earlier). A lower rate of injection site-related TEAEs was reported in the PP1M group (6%) vs the PP3M group (8%). The local injection-site tolerability was good in both groups. Induration, redness, and swelling as evaluated by the investigator were observed in ≤5% of patients in both groups and were mostly mild in nature. The level of induration, redness, and swelling was generally similar between the PP3M and PP1M groups over time (Table 5).
Table 5.
Change from Baseline to Endpoint of OL and DB Phases in Body Weight, EPS Scales, ECG, Injection Site Evaluations and Laboratory Parameters (ITT [OL] Analysis Set and Safety Analysis Set)a
| Parameter | N | OL | N | PP3M | N | PP1M |
|---|---|---|---|---|---|---|
| Body weight, kg | ||||||
| Change from baseline | 495 | 2.19 (6.97) | 495 | 3.07 (6.71) | ||
| Abnormal weight percent change | ||||||
| Decrease ≥7% | 494 | 37 (7) | 493 | 21 (4) | ||
| Increase ≥7% | 494 | 75 (15) | 493 | 81 (16) | ||
| Fasting glucose (mmol/L) | ||||||
| Change from baseline | 473 | -0.004 (1.02) | 476 | 0.086 (0.95) | ||
| Fasting cholesterol (mmol/L) | ||||||
| Change from baseline | 471 | 0.034 (0.74) | 475 | 0.043 (0.72) | ||
| Fasting LDL cholesterol (mmol/L) | ||||||
| Change from baseline | 471 | 0.0533 (0.65) | 475 | 0.0579 (0.63) | ||
| Fasting HDL cholesterol (mmol/L) | ||||||
| Change from baseline | 471 | -0.0396 (0.30) | 475 | -0.0234 (0.24) | ||
| Fasting triglycerides (mmol/L) | ||||||
| Change from baseline | 471 | 0.086 (0.77) | 475 | 0.010 (0.78) | ||
| Insulin (pmol/L) | ||||||
| Change from baseline | 489 | 1.1 (116.40) | 485 | 6.9 (124.10) | ||
| Prolactin (µg/L) | ||||||
| Change from baseline | 495 | -2.29 (24.31) | 493 | 0.56 (20.03) | ||
| Prolactin (µg/L), high relative to OL baseline, n (%) | ||||||
| Men | 256 | 99 (38.7) | 267 | 119 (44.6) | ||
| Women | 239 | 76 (31.8) | 226 | 74 (32.7) | ||
| AIMS total score, Median (range) | ||||||
| Change from baseline | 494 | 0.0 (-5;6) | 495 | 0.0 (-12;4) | ||
| BARS Global Clinical Rating of Akathisia (baseline [DB]), n (%) | ||||||
| Absent | 495 | 460 (92.9) | 495 | 460 (92.9) | ||
| Questionable | 495 | 27 (5.5) | 495 | 26 (5.3) | ||
| Mild akathisia | 495 | 6 (1.2) | 495 | 9 (1.8) | ||
| Moderate akathisia | 495 | 2 (0.4) | 495 | 0 (0.0) | ||
| Marked akathisia | 495 | 0 (0.0) | 495 | 0 (0.0) | ||
| Severe akathisia | 495 | 0 (0.0) | 495 | 0 (0.0) | ||
| BARS Global Clinical Rating of Akathisia (End point [DB]), n (%) | ||||||
| Absent | 495 | 460 (92.9) | 495 | 456 (92.1) | ||
| Questionable | 495 | 25 (5.1) | 495 | 29 (5.9) | ||
| Mild Akathisia | 495 | 8 (1.6) | 495 | 10 (2.0) | ||
| Moderate Akathisia | 495 | 2 (0.4) | 495 | 0 (0.0) | ||
| Marked Akathisia | 495 | 0 (0.0) | 495 | 0 (0.0) | ||
| Severe Akathisia | 495 | 0 (0.0) | 495 | 0 (0.0) | ||
| SAS Global Score, Median (Range) | ||||||
| Change from Baseline | 495 | 0.00 (-0.9;0.5) | 495 | 0.00 (-1.4;1.5) | ||
| QTcF, n (%) | ||||||
| ≤30 (msec) | 1366 | 1308 (96) | 494 | 435 (88) | 494 | 464 (94) |
| >30–60 (msec) | 1366 | 58 (4) | 494 | 58 (12) | 494 | 29 (6) |
| >60 (msec) | 494 | 1 (<1) | 494 | 1 (<1) | ||
| Induration, DB baseline, Absent | 504 | 479 (95) | 512 | 491 (96) | ||
| Mild | 504 | 24 (5) | 512 | 21 (4) | ||
| Moderate | 504 | 1 (<1) | 512 | 0 | ||
| DB endpoint, Absent | 501 | 484 (97) | 503 | 488 (97) | ||
| Mild | 501 | 17 (3) | 503 | 15 (3) | ||
| Redness, DB baseline, Absent | 504 | 487 (97) | 512 | 494 (96) | ||
| Mild | 504 | 17 (3) | 512 | 18 (4) | ||
| DB endpoint, Absent | 501 | 498 (99) | 503 | 498 (99) | ||
| Mild | 501 | 3 (1) | 503 | 5 (1) | ||
| Swelling, DB baseline Absent | 504 | 482 (96) | 512 | 496 (97) | ||
| Mild | 504 | 22 (4) | 512 | 16 (3) | ||
| DB endpoint, Absent | 501 | 500 (>99) | 503 | 502 (>99) | ||
| Mild | 501 | 1 (<1) | 503 | 1 (<1) | ||
Abbreviations: AIMS, abnormal involuntary movement scale; BARS, Barnes Akathisia Rating Scale; DB, double-blind; OL, open-label; QTcF, QTc interval calculated using the Fridericia formula; PP1M, paliperidone palmitate 1-month formulation; PP3M, paliperidone palmitate 3-month formulation; SAS, Simpson-Angus Scale.
a The data presented is change from OL baseline to OL endpoint for OL analysis, and for DB, the data presented is change from DB baseline to DB endpoint.
Table 6.
Treatment-emergent Potentially Prolactin-related Adverse Events during OL and DB phases (ITT [OL] Analysis Set and Safety Analysis Set)
| Sex | OL PP1M (N=1429) n (%) | PP3M (N=504) n (%) | PP1M (N=512) n (%) |
|---|---|---|---|
| Both | 1429 | 504 | 512 |
| Galactorrhoea | 17 (1.2) | 3 (0.6) | 5 (1.0) |
| Sexual dysfunction | 4 (0.3) | 0 | 0 |
| Libido decreased | 3 (0.2) | 0 | 0 |
| Anorgasmia | 1 (0.1) | 0 | 0 |
| Breast enlargement | 1 (0.1) | 0 | 0 |
| Breast pain | 1 (0.1) | 2 (0.4) | 0 |
| Blood prolactin increased | 0 | 1 (0.2) | 0 |
| Breast discharge | 0 | 1 (0.2) | 1 (0.2) |
| Orgasm abnormal | 0 | 1 (0.2) | 0 |
| Orgasmic sensation decreased | 0 | 1 (0.2) | 0 |
| Male | 782 | 258 | 281 |
| Gynaecomastia | 3 (0.4) | 2 (0.8) | 0 |
| Erectile dysfunction | 1 (0.1) | 1 (0.4) | 1 (0.4) |
| Female | 647 | 246 | 231 |
| Amenorrhoea | 17 (2.6) | 8 (3.3) | 4 (1.7) |
| Menstruation irregular | 9 (1.4) | 5 (2.0) | 3 (1.3) |
Abbreviations: DB, double-blind; OL, open-label; PP1M, paliperidone palmitate 1-month formulation; PP3M, paliperidone palmitate 3-month formulation.
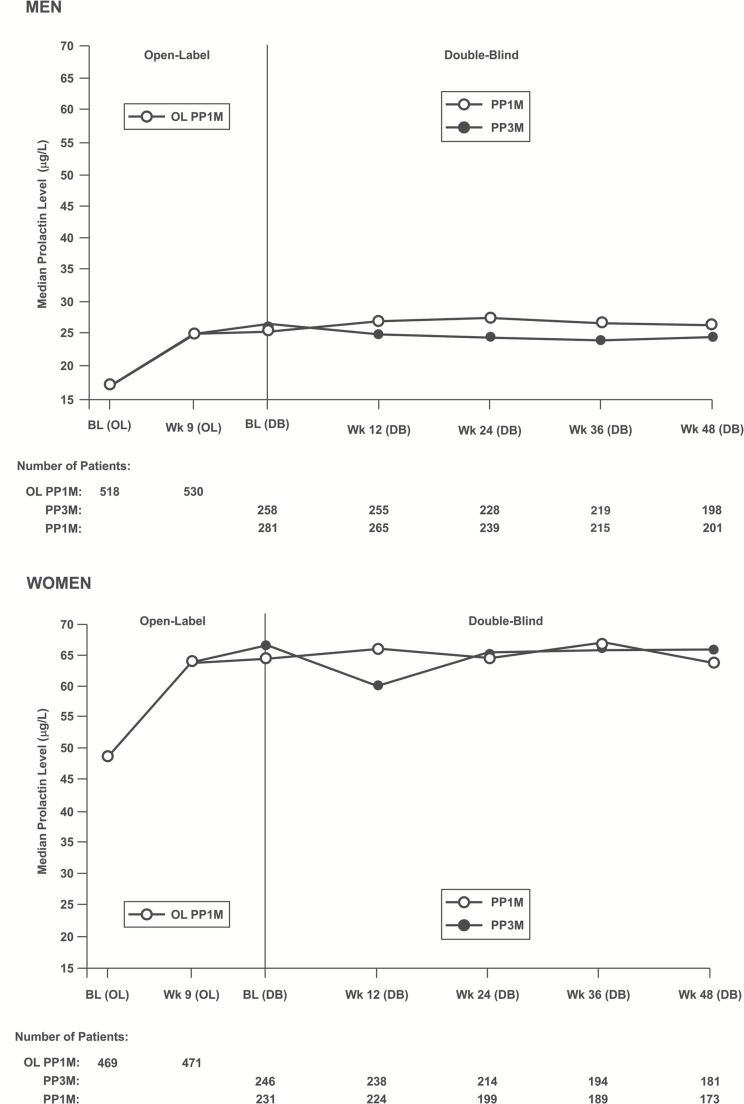
The mean (SD) change from OL baseline to OL endpoint in serum prolactin levels was 7.65 (16.717) µg/L for men and 18.56 (55.208) µg/L for women; mean (SD) change from DB baseline to DB end point was -1.28 (10.351) µg/L for men and -3.37 (33.307) µg/L for women in the PP3M group, while it was 0.45 (8.943) µg/L for men and 0.69 (27.983) µg/L for women in the PP1M group (Table 5). More men in the PP1M group vs the PP3M group had treatment-emergent abnormally high prolactin levels in the DB phase relative to OL baseline (45% vs 39%); a similar percentage of women in both groups had high prolactin levels in the DB phase relative to the OL phase (32% vs 33%) (Figure 6).
Figure 6.
Median prolactin level over time (safety analysis set).
One patient in the PP1M group (and none in the PP3M group) had a shift from a normal average predose value to maximum corrected QT interval value of ≥500 milliseconds based on QTcB, QTcF, QTLc, and QTcLD during the DB phase. Based on QTcLD, 10% of patients in the PP3M group and 6% of patients in the PP1M group had a maximum increase of >30 to 60 milliseconds from the average predose value, and 1 patient each in the PP3M and PP1M groups had a maximum increase of >60 milliseconds from the average predose value. Changes in vital signs, including orthostatic hypotension, were minimal and similar in both the groups.
The mean (SD) increases from OL baseline to DB endpoint (65 weeks) in body weight were 2.19 (6.966) kg in the PP3M group and 3.07 (6.713) kg in the PP1M group. Overall, 136 (27%) patients in the PP3M group and 150 (30%) in the PP1M group had ≥7% increase in their body weight from OL baseline to DB endpoint (Table 5).
Discussion
The results of the current study demonstrate that PP3M was noninferior to PP1M, as measured by the percentage of patients who remained relapse free after 48 weeks based on the Kaplan-Meier estimate. Overall, most patients on both medications completed the 65-week study without a relapse.
The secondary endpoint results corroborated the primary efficacy findings and further suggested that the efficacy of PP3M was similar to PP1M in the maintenance treatment of patients with schizophrenia. The majority of patients in both groups achieved symptomatic remission for the last 6 months of DB treatment. These results are consistent with the efficacy of PP1M in maintaining symptomatic control in comparable relapse prevention studies in patients with schizophrenia (Hough et al., 2009; Gopal et al., 2010; Kramer et al., 2010; Nasrallah et al., 2010; Pandina et al., 2010; Gopal et al., 2011; Coppola et al., 2012) as well as in an earlier long-term maintenance study using PP3M compared with placebo (Berwaerts et al., 2015).
An LAI antipsychotic that requires a less frequent dosing than those currently available introduces several potential advantages to patients, caregivers, and prescribers. An obvious potential advantage of PP3M is that its administration would require injections only 4 times a year (rather than the every 2 weeks or monthly regimens previously available) and would thus be expected in particular to facilitate treatment access among patients with schizophrenia who have only irregular access to treatment or simply cannot coordinate biweekly or once-monthly transportation for injection visits. In addition, an injection with a longer duration of effective plasma levels allows for more leeway with respect to injection intervals and missed appointments, allowing clinicians to have more time to intervene prior to a relapse. Less frequent injections would also allow more time for patients and physicians to address other important treatment objectives such as psychosocial treatment, substance abuse treatment, smoking cessation, health maintenance, vocational rehabilitation, etc.
A limitation of the study is that unlike real-life practice, patients in the PP3M group had only 2 opportunities to adjust their doses. It is also noteworthy that patients in the DB phase were already shown to be responsive to paliperidone and were able to tolerate it, which may explain the high rate of completion and low relapse rate. Differences due to frequency of injections were not tested, since all patients received monthly injections during the DB phase.
The safety and tolerability profiles of PP3M and PP1M were comparable over the 48-week DB phase and consistent with that observed in other trials with PP1M (Hough et al., 2009; Gopal et al., 2010, 2011; Kramer et al., 2010; Nasrallah et al., 2010; Pandina et al., 2010; Coppola et al., 2012). Withdrawal rates due to TEAEs were low and comparable for both treatments. These results are consistent with an earlier PP3M phase-3 study (Berwaerts et al., 2015). Serious TEAEs were mostly of a psychiatric nature and similar between both groups. Weight gain, nasopharyngitis, and anxiety were the most common TEAEs observed in both groups. No new safety signals emerged during this study. Overall, incidence of TEAEs related to EPS, suicidality, agitation and aggression, somnolence and sedation, tachycardia, orthostatic hypotension, QT prolongation, potentially prolactin-related, and weight gain-related TEAEs were similar between the PP3M and PP1M groups. The incidence of QT prolongation of paliperidone was similar in both groups and was without clinical consequences (according to ICH E14 guidelines, increase in QTc interval from baseline of more than 60 milliseconds is considered clinically significant); no patient was excluded from the study because of QT prolongation. Weight gain was consistent with previous studies and somewhat less with PP3M compared with PP1M. Consistent with the known pharmacology of paliperidone, mean prolactin levels increased during OL treatment with PP1M.
The study demonstrated PP3M’s ability to maintain the treatment benefit achieved in patients with schizophrenia who have been adequately treated with PP1M for at least 4 months. These results are consistent with the interim analysis findings of a previous maintenance trial that demonstrated efficacy in 93% of patients previously adequately stabilized with PP1M for at least 4 months and who were subsequently treated with PP3M (Berwaerts et al., 2015). Considered together with the data demonstrating its efficacy and tolerability, PP3M could offer advantages by enhancing to treatment options and outcomes for patients with schizophrenia.
Conclusions
These results confirm the primary study hypothesis that the efficacy (determined by percentage of patients who remained relapse free) of PP3M is noninferior to that of PP1M. The safety and tolerability profiles of PP3M and PP1M were comparable over the 48-week DB phase and consistent with that observed in other trials with PP. No new safety signals emerged during this study. Thereby, PP3M can be seen to complement the treatment options for relapse prevention in schizophrenia.
Interest Statement
Dr Fleischhacker is an employee of the Medical University Innsbruck and received research grants from Otsuka, Janssen Cilag, and Lundbeck; advisory board honoraria from Lundbeck, Roche, Otsuka, Janssen Cilag, Takeda, Amgen, Teva, Boehringer Ingelheim, and Targacept; speaker honoraria from Lundbeck, Janssen Cilag, Otsuka, Roche, and Takeda; and owns stocks of MedAvante. Drs Savitz, Xu, Gopal, Ravenstijn, Nuamah, Janik, Schotte, and Hough are employees of Janssen Research & Development, LLC (a Johnson & Johnson company) and hold stock in Johnson & Johnson.
Supplementary Material
Acknowledgments
Dr. Shruti Shah (SIRO Clinpharm Pvt. Ltd.) provided writing assistance and Dr. Wendy P. Battisti (Janssen Research & Development, LLC) provided additional editorial and writing support for this manuscript. The authors also thank the study participants, without whom this study would never have been accomplished, and all the investigators for their participation in this study.
This work was supported by Janssen Research & Development, LLC, USA
The abstract was presented at the 54th Annual Meeting of the American College of Neuropsychopharmacology (ACNP), December 6–10, 2015; Hollywood, Florida.
Drs Savitz, Gopal, Janik, Schotte, and Hough were involved in study design, data collection, analysis and interpretation. Dr Fleischhacker was the principal investigator for the study, contributed data, and participated in data interpretation as well as in the development of the manuscript. Drs Xu and Nuamah were responsible for the statistical analyses and design/interpretation of study results. Dr Ravenstijn was responsible for the pharmacokinetic analyses. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors meet ICMJE criteria and all those who fulfilled those criteria are listed as authors. All authors provided direction and comments on the manuscript, made the final decision about where to publish these data, and approved submission to this journal.
References
- Alphs L, Bossie CA, Sliwa JK, Fu D-J, Ma Y-W, Hulihan J. (2013) Paliperidone palmitate and risperidone long-acting injectable in subjects with schizophrenia recently treated with oral risperidone or other oral antipsychotics. Neuropsychiatr Dis Treat 9:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Carpenter WT, Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. (2005) Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry 162:441–449. [DOI] [PubMed] [Google Scholar]
- Berwaerts J, Liu Y, Gopal S, Nuamah I, Xu H, Savitz A, Coppola D, Schotte A, Remmerie B, Maruta N, Hough DW. (2015) Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry 72:830–839. [DOI] [PubMed] [Google Scholar]
- Coppola D, Liu Y, Gopal S, Remmerie B, Samtani MN, Hough DW, Nuamah I, Sulaiman A, Pandina G. (2012) A one-year prospective study of the safety, tolerability and pharmacokinetics of the highest available dose of paliperidone palmitate in patients with schizophrenia. BMC Psychiatry 12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Mahmoud R, Brenner R; Risperidone-USA-79 Study Group (2002). A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med 346:16–22. [DOI] [PubMed] [Google Scholar]
- Emsley R, Oosthuizen P, Koen L, Niehaus DJ, Medori R, Rabinowitz J. (2008) Oral versus injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clin Ther 30:2378–2386. [DOI] [PubMed] [Google Scholar]
- Fleischhacker WW, Arango C, Arteel P, Barnes TRE, Carpenter W, Duckworth K, Galderisi S, Halpern L, Knapp M, Marder SR, Moller M, Sartorius N, Woodruff P. (2014) Schizophrenia – time to commit to policy change. Schizophr Bull 40:165–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal S, Hough DW, Xu H, Lull JM, Gassmann-Mayer C, Remmerie BM, Eerdekens MH, Brown DW. (2010) Efficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled, dose-response study. Int Clin Psychopharmacol 25:247–256. [DOI] [PubMed] [Google Scholar]
- Gopal S, Vijapurkar U, Lim P, Morozova M, Eerdekens M, Hough DW. (2011) A 52-week open-label study of the safety and tolerability of paliperidone palmitate in patients with schizophrenia. J Psychopharmacol 25:685–697. [DOI] [PubMed] [Google Scholar]
- Guy W. (1976) ECDEU assessment manual for psychopharmacology: revised (DHEW publication number ADM 76–338). Rockville, MD: US Department of Health, Education and Welfare, Public Health Service, Alcohol, Drug Abuse and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs, pp 534–537. [Google Scholar]
- Heres S, Davis J, Maino K, Jetzinger E, Kissling W, Leucht S. (2006) Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine: an exploratory analysis of head-to-head comparison studies of second-generation antipsychotics. Am J Psychiatry 163:185–194. [DOI] [PubMed] [Google Scholar]
- Higashi K, Medic G, Littlewood KJ, Diez T, Granstrom O, De Hert M. (2013) Medication adherence in schizophrenia: factors influencing adherence and consequences of nonadherence, a systematic literature review. Ther Adv Psychopharmacol 3:200–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough D, Gopal S, Vijapurkar U, Lim P, Morozova M, Eerdekens M. (2010) Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res 116:107–117. [DOI] [PubMed] [Google Scholar]
- Hough D, Lindenmayer JP, Gopal S, Melkote R, Lim P, Herben V, Yuen E, Eerdekens M. (2009) Safety and tolerability of deltoid and gluteal injections of paliperidone palmitate in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 33:1022–1031. [DOI] [PubMed] [Google Scholar]
- Invega Sustenna Prescribing Information (2015) https://www.janssencns.com/shared/product/invegasustenna/prescribing-information.pdf Last updated in June 2015.
- Invega Trinza Prescribing Information (2015) http://www.janssencns.com/shared/product/invegatrinza/prescribing-information.pdf last updated in May 2015.
- Kramer M, Litman R, Hough D, Lane R, Lim P, Liu Y, Eerdekens M. (2010) Paliperidone palmitate, a potential long-acting treatment for patients with schizophrenia. Results of a randomized, double-blind, placebo-controlled efficacy and safety study. Int J Neuropsychopharmacol 13:635–647. [DOI] [PubMed] [Google Scholar]
- Leucht S, Barnes TR, Kissling W, Engel RR, Correll C, Kane JM. (2003) Recurrence prevention in schizophrenia with new-generation antipsychotics: a systematic review and exploratory meta-analysis of randomized, controlled trials. Am J Psychiatry 160:1209–1222. [DOI] [PubMed] [Google Scholar]
- McEvoy JP. (2006) Risks versus benefits of different types of long-acting injectable antipsychotics. J Clin Psychiatry 67:15–18. [PubMed] [Google Scholar]
- Nasrallah HA, Gopal S, Gassmann-Mayer C, Quiroz JA, Lim P, Eerdekens M, Yuen E, Hough D. (2010) A controlled, evidence-based trial of paliperidone palmitate, a long-acting injectable antipsychotic, in schizophrenia. Neuropsychopharmacology 35:2072–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandina GJ, Lindenmayer JP, Lull J, Lim P, Gopal S, Herben V, Kusumakar V, Yuen E, Palumbo J. (2010) A randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophrenia. J Clin Psychopharmacol 30:235–244. [DOI] [PubMed] [Google Scholar]
- Rauch AS, Fleischhacker WW. (2013) Long-acting injectable formulations of new generation antipsychotics: a review from a clinical perspective. CNS Drugs 27:637–652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.