Abstract
Background:
Our pilot study suggested that the angiotensin-converting enzyme inhibitor perindopril might reduce some subjective effects produced by i.v. methamphetamine. We characterized the impact of a wider range of perindopril doses on methamphetamine-induced effects in a larger group of non-treatment-seeking, methamphetamine-using volunteers.
Methods:
Before treatment, participants received 30mg methamphetamine. After 5 to 7 days of perindopril treatment (0, 4, 8, or 16mg/d), participants received 15 and 30mg of methamphetamine on alternate days. Before and after treatment, participants rated subjective effects and cardiovascular measures were collected.
Results:
Prior to treatment with perindopril, there were no significant differences between treatment groups on maximum or peak subjective ratings or on peak cardiovascular effects. Following perindopril treatment, there were significant main effects of treatment on peak subjective ratings of “anxious” and “stimulated”; compared to placebo treatment, treatment with 8mg perindopril significantly reduced peak ratings of both anxious (P=.0009) and stimulated (P=.0070). There were no significant posttreatment differences between groups on peak cardiovascular effects.
Conclusions:
Moderate doses of perindopril (8mg) significantly reduced peak subjective ratings of anxious and stimulated as well as attenuated many other subjective effects produced by methamphetamine, likely by inhibiting angiotensin II synthesis. Angiotensin II is known to facilitate the effects of norepinephrine, which contributes to methamphetamine’s subjective effects. The lack of a classic dose-response function likely results from either nonspecific effects of perindopril or from between-group differences that were not accounted for in the current study (i.e., genetic variations and/or caffeine use). The current findings suggest that while angiotensin-converting enzyme inhibitors can reduce some effects produced by methamphetamine, more consistent treatment effects might be achieved by targeting components of the renin-angiotensin system that are downstream of angiotensin-converting enzyme.
Keywords: methamphetamine, renin-angiotensin system, human laboratory study
Introduction
Under conditions of reduced fluid volume and in response to stress, the kidneys release renin, which cleaves angiotensinogen to angiotensin I (Ang I) (Porter, 1990). Ang I is subsequently converted to Ang II by the peptidase angiotensin-converting enzyme (ACE). Ang II, the major mediator of the renin-angiotensin system (RAS), stimulates type-1 Ang II (AT1) receptors. Stimulation of AT1 receptors in the hypothalamic-pituitary-adrenal (HPA) axis regulates the stress response by increasing circulating levels of glucocorticoids and catecholamines, which in turn act in a negative feedback cycle to dampen HPA axis activation.
In addition to the HPA axis, AT1 receptors are also expressed in the striatum (Chai et al., 1990, 1991; Jenkins et al., 1996), a key part of the mesocorticolimbic system implicated in the development of substance use disorders (Verrico et al., 2013). Indeed, both stimulation of AT1 receptors (Cavadas et al., 2003) and drugs of abuse (Weinshenker and Schroeder, 2007; Haile et al., 2012; Goertz et al., 2014) potently increase the release of catecholamines (i.e., dopamine [DA] and norepinephrine [NE]) in the nucleus accumbens and mesocorticolimbic system. For example, cocaine (and probably other drugs of abuse) significantly increases ACE activity and mRNA expression in frontal cortex and striatum (Visniauskas et al., 2012). Moreover, Ang II potentiates stimulant-induced increases of NE (Mota and Guimaraes, 2003), and this is attenuated by ACE inhibition (Storgaard and Nedergaard, 1997). Similarly, genetic mouse models show that overexpression of angiotensinogen significantly increases voluntary consumption of alcohol, whereas genetically ablating angiotensinogen has the opposite effect (Maul et al., 2001). Consistent with the latter, ACE inhibitors reduce alcohol consumption (Lingham et al., 1990) and morphine self-administration (Hosseini et al., 2009) in rodent models. Collectively, biochemical and behavioral evidence strongly implicate central RAS involvement in neural substrates and neurotransmitter systems known to mediate the actions of abused drugs (Lijffijt et al., 2014). In addition to substance use disorders, the central RAS is also implicated in other neuropsychiatric/neurological disorders, including schizophrenia (Kucukali et al., 2010), Alzheimer’s disease (Duron et al., 2009), and Parkinson’s disease (Mertens et al., 2010).
Although the effects of methamphetamine (METH) are mediated chiefly by direct facilitation of DA and NE signaling, METH also alters function of the HPA axis, which likely contributes to the overall effects produced by METH. Because AT1 receptors regulate the HPA axis, blocking the effects of METH on the central RAS could potentially attenuate the effects produced by METH. With this in mind, we previously conducted a double-blind, placebo-controlled, inpatient human laboratory-based study to determine the impact of perindopril treatment (0, 2, 4, or 8mg/d) on the subjective effects produced by acute i.v. METH (30mg) in participants (n=30) with METH use disorder (Newton et al., 2010). Consistent with the idea that blocking the effects of METH on the central RAS might attenuate the effects produced by METH, our previous study found a significant treatment by METH interaction on the subjective ratings of “any drug effect”; however, posthoc tests did not reveal significant differences between groups, most likely due to small group sizes. Thus, the present study was undertaken to replicate and extend these findings to determine whether similar (4 or 8mg/d) or a higher (16mg/d) dose of perindopril would attenuate the subjective effects produced by i.v. METH in volunteers with METH use disorder.
Methods
This double-blind, placebo-controlled study was sponsored by the National Institute on Drug Abuse and approved by the Baylor College of Medicine and Michael E. DeBakey Veteran Affairs Medical Center (MEDVAMC) Institutional Review Boards. Also, this material is the result of work supported with resources from, and the use of facilities at, the MEDVAMC, Houston, TX. All volunteers provided written informed consent after being apprised of potential risks of study participation.
Subjects
All participants were non-treatment-seeking, English-speaking volunteers recruited through advertisements, paid for their participation, and met the DSM-IV-TR criteria for METH dependence. DSM-IV-TR diagnosis criteria for substance dependence has been shown to correlate with DSM5 diagnostic criteria for use disorders. Therefore, we use DSM5 nomenclature throughout this manuscript (Compton et al., 2013). All participants were between 18 and 55 years old with a history of using METH by the smoked or i.v. route. Participants were required to provide a urine sample that was positive for METH but negative for other illicit drugs, except marijuana. In addition, participants were required to provide a medical history and undergo a brief physical examination to ensure they did not have clinically significant contraindications before study participation. Thus, potential participants were excluded if they had a history of seizure disorder, head trauma, dependence on other drugs aside from nicotine, prior adverse reactions to METH, or the presence of any other axis I psychiatric disorder. Serious medical conditions such as heart disease, symptomatic HIV-related disease, asthma, and other serious medical conditions were also exclusionary. Concomitant use of psychotropic medications was not allowed aside from low-dose benzodiazepine (lorazepam, 2mg) for sleep.
General Procedures
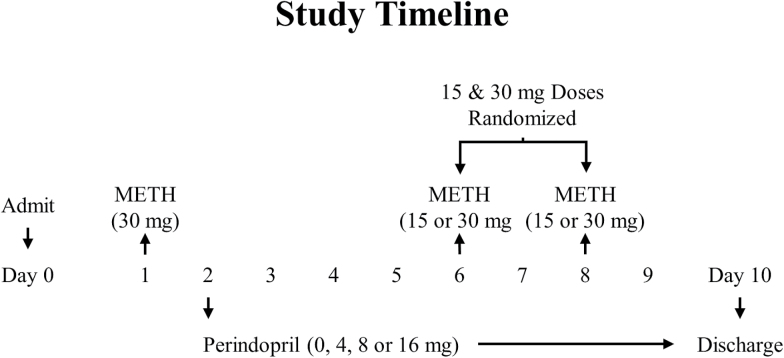
A timeline of study events, including METH and perindopril administrations, are provided in Figure 1. Briefly, following admission on day 0, participants resided at the Research Commons of the MEDVAMC for the duration of this 10-day double-blind study. Before (day 1) and after (day 6 or 8) treatment, cardiovascular and subjective effects in response to 30mg METH were determined for each participant. On day 10, medication was discontinued, and participants were discharged from the study.
Figure 1.
Timeline of study events, including methamphetamine (METH) and perindopril dosing.
METH
A NIDA contractor (RTI International, Research Triangle Park, NC) provided sterile METH solution for human use, and a saline solution of equal volume and appearance served as the control. Single bolus infusions of 15 and 30mg METH were used as in our previous studies, since they consistently produce significant increases in positive subjective effects (Newton et al., 2010; De La Garza et al., 2012; Verrico et al., 2014). METH was infused over 2 minutes by a study physician on days 1, 6, and 8. Within each day, METH infusions were randomized to either 10:00 am or 1:00 pm by the VA pharmacy; at the alternate times (i.e., 10 AM or 1 PM), saline (0mg METH) was infused. The first 30-mg dose of METH was infused on day 1 before treatment randomization. The second (and final) infusion of 30mg METH was randomized to either day 6 or 8 by the VA pharmacy; on the alternate day (i.e., day 6 or 8), the 15-mg dose of METH was infused to maintain the blind. Thus, if a participant received the second, 30-mg dose of METH on day 6, that same participant received the 15-mg dose of METH on day 8 or vice versa.
Subjective Ratings and Cardiovascular Effects of METH
Participants completed visual analog scale forms to rate the subjective effects produced by METH 15 minutes before and 5, 10, 15, 20, 30, 45, 60, 75, 90, 105, and 120 minutes after every infusion. Visual analog scale subjective ratings, which were measured on a continuous scale digitized between 0 (not at all) to 100 (strongest ever), included ratings of anxious, any drug effect, bad effects, depressed, desire (for METH), drug liking, good effects, high, likely to use (METH if accessible), and stimulated. Cardiovascular (i.e., heart rate and blood pressure [BP]) measures were collected at the same time points subjective ratings were collected.
Perindopril
Placebo and commercially available perindopril tablets were overencapsulated in gelatin capsules by the MEDVAMC research pharmacy. Two perindopril or matched placebo capsules were administered orally at 7:00 am beginning on day 2 and continuing through day 10 of the protocol.
Data Analysis
Demographic and baseline drug use characteristics were compared using chi-square tests and ANOVA for categorical and continuous variables, respectively. To simplify analyses of subjective ratings and cardiovascular measures, 2-factor [METH (dose): 0 and 30mg, or 0, 15, and 30mg; and treatment (dose): 0, 4, 8, and 16 mg] ANOVAs were calculated on maximum or peak ratings/measures to determine: (1) whether or not participants responded differentially to METH (0 and 30mg) prior to perindopril maintenance; and (2) treatment effects after perindopril maintenance. Significant main effects were interpreted with Bonferroni-corrected posthoc tests. Data are presented as means ± SD, except as displayed in illustrations where data are presented as means ± SEs.
Results
Participants
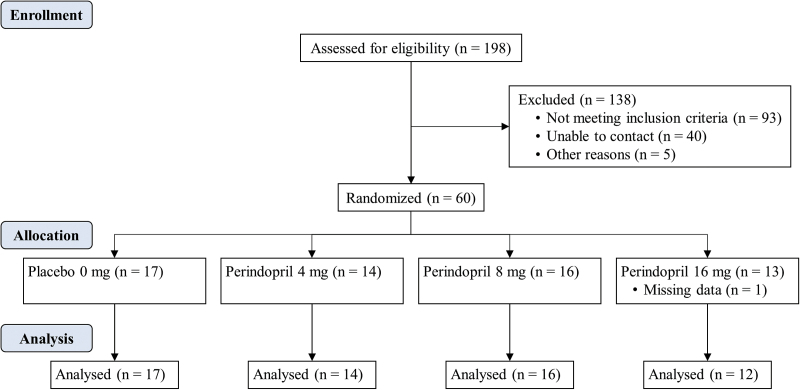
Sixty participants were randomized to medication conditions (placebo [n=17], 4mg perindopril [n=14], 8mg perindopril [n=16], and 16mg perindopril [n=13]). Posttreatment data were missing for 1 participant because of a technicality; thus, analyses were conducted on the 59 participants for whom there was a complete set of data. The flow of participants is shown in Figure 2.
Figure 2.
Participant flow, including the number of participants assessed for eligibility, randomized to a treatment group, and analyzed for the placebo (0mg; n=17) and 4-mg (n=14), 8-mg (n=16) and 16-mg (n=12) perindopril treatment groups.
Demographics
On average, the 59 participants for whom we had complete data were 36 (±1.2) years old, Caucasian (76%), and male (78%) with 12.4 (±0.3) years of education. The majority of participants usually used METH via multiple routes, although all participants reported previously having used METH by the i.v. or smoked routes. On average, participants reported using METH at 0.9 (±0.1) g/d and reported using it on 16 (±1.2) of the last 30 days. Demographic and drug use means for each group are provided in Table 1.
Table 1.
Demographic and Drug Use Characteristics
| Treatment Groups | Analyses | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 mg | 4 mg | 8 mg | 16 mg | χ2 or F (df) | P | |||
| Demographics | Sex | Male | 13 | 9 | 13 | 11 | (3) = 2.96 | .398 |
| Female | 4 | 5 | 3 | 1 | ||||
| Race | African American | 1 | 1 | 2 | 0 | (12) = 11.28 | .506 | |
| Asian | 2 | 0 | 1 | 0 | ||||
| Caucasian | 13 | 10 | 12 | 10 | ||||
| Hispanic | 0 | 3 | 1 | 2 | ||||
| Middle Eastern | 1 | 0 | 0 | 0 | ||||
| Other | Age | 39.4±9.1 | 33.3±8.9 | 36.0±8.8 | 34.3±9.1 | (3,55) = 1.40 | .253 | |
| Education (y>) | 12.2±2.6 | 12.4±1.7 | 12.0±1.3 | 13.0±2.7 | (3,55) = 0.60 | .617 | ||
| Methamphetamine | Use | Years | 15.4±9.7 | 11.1±6.6 | 11.5±10.3 | 13.8±9.0 | (3,55) = 0.76 | .520 |
| Past 30 days | 15.5±10.1 | 15.7±11.2 | 16.9±7.0 | 16.0±9.6 | (3,55) = 0.07 | .974 | ||
| g/d | 1.2±0.8 | 0.8±0.5 | 0.9±0.5 | 0.8±0.2 | (3,49) = 1.55 | .215 | ||
| Routes Used | IV | 12 | 7 | 10 | 8 | (9) = 12.25 | .200 | |
| Smoke | 14 | 10 | 12 | 9 | (3) = 0.55 | .907 | ||
| Oral | 4 | 4 | 8 | 4 | (6) = 6.97 | .323 | ||
| Nasal | 1 | 0 | 4 | 2 | (3) = 5.37 | .147 | ||
| Nicotine | Current users | 15 | 11 | 14 | 11 | (3) = 1.08 | .782 | |
| Years* | 20.3±11.3 | 16.5±8.1 | 19.5±11.1 | 17.2±9.8 | (3,47) = 0.38 | .767 | ||
| Cigarettes per day* | 14.2±7.7 | 15.1±5.9 | 15.3±5.1 | 13.8±8.0 | (3,47) = 0.15 | .931 | ||
| Alcohol | Current users | 7 | 6 | 12 | 4 | (3) = 6.13 | .105 | |
| Years* | 20.4±13.1 | 13.5±9.9 | 18.8±9.6 | 17.3±13.9 | (3,25) = 0.46 | .710 | ||
| Days used of past 30* | 8.9±7.1 | 4.3±5.3 | 6.0±8.1 | 8.0±7.8 | (3,25) = 0.50 | .687 | ||
| Drinks per day* | 3.6±3.4 | 2.0±1.1 | 2.0±1.3 | 1.4±0.8 | (3,25) = 1.40 | .266 | ||
| Cannabis | Current users | 11 | 6 | 9 | 7 | (3) = 1.53 | .675 | |
| Years* | 14.0±9.4 | 16.5±10.4 | 23.3±44.8 | 12.6±12.2 | (3,29) = 1.67 | .194 | ||
| Days used of past 30* | 12.1±11.8 | 7.0±11.5 | 13.1±12.7 | 9.2±10.5 | (3,29) = 0.41 | .745 | ||
| Times per day* | 3.7±3.3 | 2.4±1.7 | 1.8±1.1 | 2.1±1.4 | (3,29) = 1.48 | .242 | ||
*Data reflect current users only. Data are presented as means ± SDs.
Tolerability
Participants generally tolerated perindopril treatment well. There were no significant (F(3, 55)=.33; P=0.806) differences across groups for total number (n=88) of reported complaints or abnormal laboratory findings (adverse events). The most common complaints included headache (n=31; P=.988) and gastrointestinal distress (n=21; P=0.175), which are not common side effects of perindopril. There were no significant (F(3, 55)= 0.63; P=.6002) differences across groups for total number (n=26) of lorazepam doses administered, which were prescribed to 35% (n=6), 57% (n=8), 38% (n=6), and 50% (n=6) of participants in the placebo and 4-, 8-, and 16-mg treatment groups.
Pretreatment
Subjective
Importantly, prior to perindopril maintenance there were nonsignificant (P≥.2383) treatment by METH interactions and nonsignificant (P≥.0977) main effects of treatment on all subjective ratings. Analyses also revealed nonsignificant (P≥.0751) main effects of METH on ratings of anxious, depressed, desire, and likely to use. In contrast, there were significant main effects of METH on ratings of any drug effect (F(1, 110)=49.45; P<.0001), bad effects (F(1, 110)=6.40; P=.0128), drug liking (F(1, 110)=25.10; P<.0001), good effects (F(1, 110)=35.78; P<.0001), high (F(1, 110) =48.38; P<.0001), and stimulated (F(1, 110)=44.03; P<.0001).
Cardiovascular
Similar to subjective ratings, there were nonsignificant (P≥.5491) treatment by METH interactions as well as nonsignificant (P≥.1000) main effects of treatment on all cardiovascular measures. In contrast, there were significant main effects of METH on heart rate (F(1, 110)=21.19; P<.0001), systolic BP (F(1, 110)=43.62; P<.0001), and diastolic BP (F(1, 110)=12.46; P=.0006).
Posttreatment
Subjective
Following 5 to 7 days of perindopril maintenance, analyses revealed nonsignificant (P≥.2343) treatment by METH interactions on all subjective ratings. There were also nonsignificant main effects of both treatment (P≥.0851) and METH (P≥.2665) on ratings of depressed, desire, and likely to use. There were significant main effects of METH (P≤.0114) on all remaining ratings. Bonferroni-corrected posthoc tests revealed that compared with placebo METH ratings of bad effects, ratings were significantly (P=.0085) higher for the 30-mg METH dose only. For all other ratings, Bonferroni-corrected posthoc tests revealed that compared with placebo METH, ratings were significantly higher for the 15- (P≤.0161) and 30- (P≤.0014) mg METH doses.
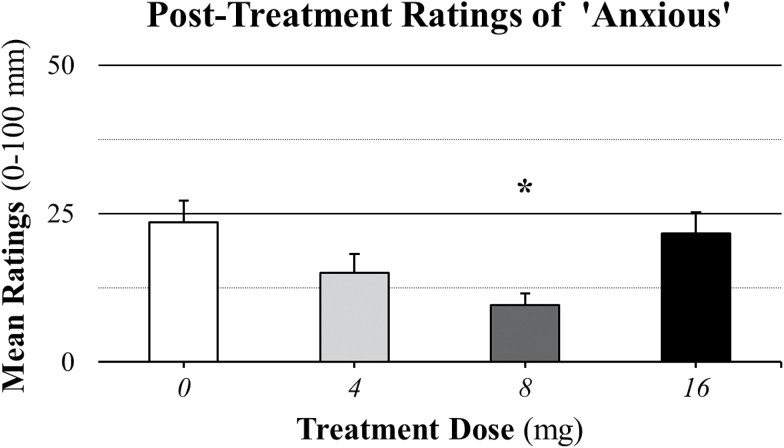
In contrast to pretreatment analyses, posttreatment analyses revealed a significant main effect of treatment (F(3, 224)=5.13; P=.0019) on anxious ratings. As illustrated in Figure 3, Bonferroni-corrected posthoc tests revealed that compared with placebo treatment ratings (23.53±30.16), ratings were significantly (P=.0009) lower for the 8-mg treatment dose (9.53±16.18) and nonsignificantly (P≥.0490) lower for the 4- (15.00±24.12) and 16- (21.67±24.35) mg treatment doses.
Figure 3.
Posttreatment mean ratings of anxious across methamphetamine (METH) doses (0, 15, and 30mg). Comparisons across treatment doses revealed a significant (P=.0019) main effect of treatment dose. The asterisk (*) indicates that anxious ratings were significantly (P=.0009) lower in the 8-mg treatment group compared with the placebo treatment group. Data are presented as means ± SEs for the 0-mg (n=17), 4-mg (n=14), 8-mg (n=16), and 16-mg (n=12) perindopril groups.
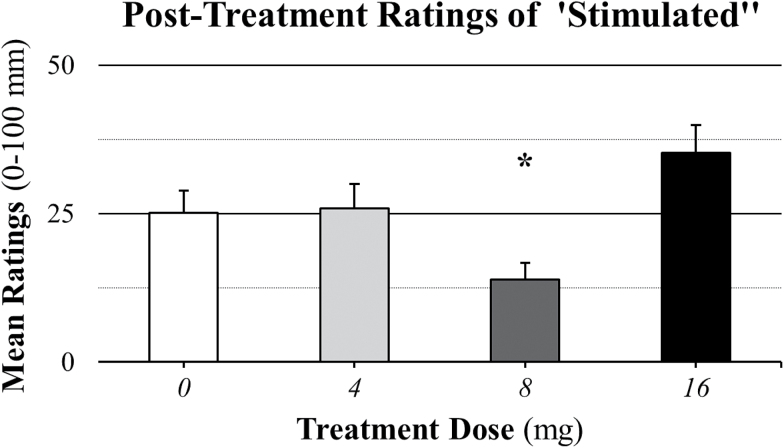
There was a significant main effect of treatment (F(3, 224)=8.63; P<.0001) on stimulated ratings. As illustrated in Figure 4, posthoc tests revealed that compared with placebo treatment ratings (25.15±30.59), ratings were significantly (P=.0070) lower for the 8-mg treatment dose (13.91±22.30) and nonsignificantly (P≥.0254) higher for the 4- (25.89±30.32) and 16- (35.21±32.62) mg treatment doses.
Figure 4.
Posttreatment mean ratings of stimulated across methamphetamine (METH) doses (0, 15, and 30mg). Comparisons across treatment doses revealed a significant (P<.0001) main effect of treatment dose. The asterisk (*) indicates that stimulated ratings were significantly (P=.0070) lower in the 8-mg treatment group compared with the placebo treatment group. Data are presented as means ± SEs for the 0-mg (n=17), 4-mg (n=14), 8-mg (n=16), and 16-mg (n=12) perindopril groups.
There were significant main effects of treatment on ratings of any drug effect (P=.0026), bad effects (P=.0003), drug liking (P=.0489), good effects (P=.0026), and high (P=.0026); Bonferroni-corrected posthoc tests revealed, however, that there were nonsignificant rating differences for the 4- (P≥.0352), 8- (P≥.0240), and 16- (P≥.0250) mg treatment doses compared with placebo treatment.
Cardiovascular
Similar to pretreatment cardiovascular effects, there were nonsignificant (P≥.5015) treatment by METH interactions, as well as nonsignificant (P≥.1899) main effects of treatment, on all cardiovascular measures. In contrast, there were significant (P<.0001) main effects of METH on all cardiovascular measures. Bonferroni-corrected posthoc tests revealed that compared with placebo METH, cardiovascular effects were significantly greater for the 15- (P<.0043) and 30- (P<.0001) mg METH doses. For systolic BP, there was also a significant (P=.0008) difference between the 15- and 30-mg METH doses.
Discussion
This double-blind, placebo-controlled human laboratory study assessed the impact of perindopril treatment on cardiovascular and subjective effects produced by i.v. METH administration in non-treatment-seeking volunteers with METH use disorder. As expected, METH (15 and 30mg) alone increased cardiovascular measures and positive subjective ratings. The most consistent treatment effects on subjective ratings were observed in the 8-mg perindopril group. Specifically, compared to treatment with placebo, treatment with 8mg perindopril significantly reduced METH-induced increases on ratings of anxious and stimulated, and nonsignificantly reduced ratings of any drug effect, good effects, high, and drug liking. Interestingly, as the dose of perindopril increased, however, the response decreased. Thus, while treatment with 8mg perindopril decreased positive subjective ratings produced by METH, treatment with 16mg perindopril tended to increase these same subjective ratings.
The current results are somewhat consistent with our previous report (Newton et al., 2010), in which the effect of perindopril on METH-induced subjective effects did not follow a classic dose-response function. On the other hand and in contrast to the current findings, Newton et al. (2010) previously found that the effects produced by METH were nonsignificantly higher in the 8- vs 4-mg perindopril dose. Nonetheless, there are notable differences between the 2 studies. First, while the current analyses were conducted on peak subjective ratings across 2-hour intervals, our previous report analyzed subjective ratings at only the 30-minute time point. The previous report also analyzed the mean change in ratings; that is, ratings at baseline or the 0-minute time point were subtracted from ratings at the 30-minute time point prior to analyses. The current study also had nearly twice the number of participants in each group. In addition, there could be other factors that underlie, at least in part, the discrepant findings between these studies. For example, given the nonlinear effects of perindopril observed in both studies, it could be that the weight-based dose (i.e., mg/kg) of perindopril between the 2 studies differed, which would produce a shift in the dose-response curve. On the other hand, genetic variations (Wang et al., 2015) and diuretics (e.g., caffeine) are also known to affect the RAS; consequently, between-group differences in either of these factors could also have affected therapeutic responses.
The biological mechanism(s) underlying the nonlinear effects of perindopril on METH-induced subjective effects are likely complex, because ACE inhibitors modulate a variety of peptides. For example, in addition to reducing Ang II synthesis, ACE inhibitors also slow the metabolism of substance P (Hanson and Lovenberg, 1980; Yokosawa et al., 1983; Skidgel, 1985; Yokosawa et al., 1985) and increase striatal pre-proenkephalin mRNA levels (Jenkins et al., 1997b). Substance P is a neurokinin-1 receptor agonist, and they prevent stimulant-induced increases in striatal DA (Loonam et al., 2003). Enkephalins tonically modulate DA release, and inhibiting their degradation increases striatal DA release (Dourmap et al., 1990), which is consistent with the ability of ACE inhibitors to increase DA release in rat striatum (Jenkins et al., 1997; Mendelsohn et al., 1993). These results are compatible with perindopril increasing striatal DA function. Increased striatal DA responsiveness would be expected to enhance the positive subjective effects produced by METH, at least in principle, because it would be the equivalent of administering higher doses. Nonetheless, those significant effects were most consistently observed with moderate doses of perindopril, which is consistent with the efficacy of 2mg/kg/d perindopril, but not 1 or 4mg/kg/d, to increase antioxidant levels in rodent hippocampus (Mashhoody et al., 2014) as well as with 4 and 8mg/d perindopril, but not 16mg/d, to decrease circulating levels of ANG II in normotensive men (Waeber et al., 1989). It could be that larger doses of perindopril progressively inhibit ACE in more brain regions in humans, as has been shown in rodents (Sakaguchi et al., 1988), thereby altering the subjective effects of METH.
The effects of ACE inhibitors in the brain are even more complex when the role of Ang II is considered. In addition to stimulating AT1 receptors, Ang II also stimulates AT2 receptors. While the function of AT2 receptors in the brain is not well defined, AT1 receptor expression in the nucleus accumbens (Jenkins et al., 1997a) and locus coeruleus (Lenkei et al., 1997), as well as modulatory effects of AT1 receptors on NE (Gelband et al., 1998), serotonin (Nahmod et al., 1978), glutamate, and GABA (Barnes et al., 2003; Oz et al., 2005) neurotransmission, have all been described. Considering that AT1 receptor blockade attenuates the release of stress hormones (Armando et al., 2007) and measures of anxiety (Saavedra et al., 2006) suggests at least some effects of ACE inhibitors are mediated via the AT1 receptor. Consistent with this, AT1 receptor blockade attenuates the development of amphetamine-induced behavioral sensitization (Paz et al., 2011) and amphetamine induces persistent alterations of AT1 receptor expression in the nucleus accumbens and caudate putamen of rodents (Paz et al., 2014). These studies suggest that selectively blocking the AT1 receptor in humans may more effectively reduce the acute subjective effects produced by METH compared with the ability of perindopril in the current study. In fact, our preliminary (C. N. Haile, C. D. Verrico, and T. F. Newton, unpublished observations) data indicate that the AT1 receptor antagonist candesartan attenuates METH- and cocaine-induced cravings, supporting this idea.
Regardless of the underlying mechanism(s) by which perindopril modulates the subjective effects of METH, it is also noteworthy that ACE inhibitors decrease depression in hypertensive patients (Germain and Chouinard, 1989) and the release of stress hormones in healthy controls (Zacharieva et al., 1991), which could play a role in reducing stress-related relapse to METH use. This idea is consistent with the current findings; all active perindopril doses reduced anxious ratings (Figure 3). ACE inhibitors have also been reported to decrease the incidence of cognitive impairment in humans (Yagi et al., 2013) and protect against dopaminergic neurotoxicity in animal models of Parkinson’s disease (Sonsalla et al., 2013).
Collectively, the anxiolytic-, antidepressant-, and cognitive-enhancing properties of ACE inhibitors, coupled with their cardio- and neuro-protective qualities, suggest that ACE inhibitors may have some clinical utility for individuals who use METH. On the other hand, numerous factors affect therapeutic responses to perindopril. AT1 receptor blockers confer many of the same benefits as ACE inhibitors, but they do not directly inhibit ACE activity or inhibit the breakdown of bradykinin. Thus, while perindopril might not be an ideal treatment for METH use disorder, the current findings suggest that downstream components of the RAS might provide a more consistent and effective therapeutic target for reducing the effects produced by METH.
Statement of Interest
None.
Acknowledgments
We acknowledge the Baylor College of Medicine General Clinical Research Center nursing staff for their expert assistance. In addition, this material is the result of work supported with resources from, and the use of facilities at, the Michael E. DeBakey VA Medical Center, Houston, TX.
This work was supported by the National Institute on Drug Abuse at the National Institutes of Health (DA023468 to T.F.N.).
References
- Armando I, Volpi S, Aguilera G, Saavedra JM. (2007) Angiotensin II AT1 receptor blockade prevents the hypothalamic corticotropin-releasing factor response to isolation stress. Brain Res 1142:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes KL, DeWeese DM, Andresen MC. (2003) Angiotensin potentiates excitatory sensory synaptic transmission to medial solitary tract nucleus neurons. Am J Physiol Regul Integr Comp Physiol 284:R1340–1353. [DOI] [PubMed] [Google Scholar]
- Cavadas C, Grand D, Mosimann F, Cotrim MD, Fontes Ribeiro CA, Brunner HR, Grouzmann E. (2003) Angiotensin II mediates catecholamine and neuropeptide Y secretion in human adrenal chromaffin cells through the AT1 receptor. Regul Pept 111:61–65. [DOI] [PubMed] [Google Scholar]
- Chai SY, McKenzie JS, McKinley MJ, Mendelsohn FA. (1990) Angiotensin converting enzyme in the human basal forebrain and midbrain visualized by in vitro autoradiography. J Comp Neurol 291:179–194. [DOI] [PubMed] [Google Scholar]
- Chai SY, McKinley MJ, Paxinos G, Mendelsohn FA. (1991) Angiotensin converting enzyme in the monkey (Macaca fascicularis) brain visualized by in vitro autoradiography. Neuroscience 42:483–495. [DOI] [PubMed] [Google Scholar]
- Compton WM, Dawson DA, Goldstein RB, Grant BF. (2013) Crosswalk between DSM-IV dependence and DSM-5 substance use disorders for opioids, cannabis, cocaine and alcohol. Drug Alcohol Depend 132:387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R, II, Newton TF, Haile CN, Yoon JH, Nerumalla CS, Mahoney JJ, III, Aziziyeh A. (2012) Rivastigmine reduces “likely to use methamphetamine” in methamphetamine-dependent volunteers. Prog Neuropsychopharmacol Biol Psychiatry 37:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourmap N, Michael-Titus A, Costentin J. (1990) Local enkephalins tonically modulate dopamine release in the striatum: a microdialysis study. Brain Res 524:153–155. [DOI] [PubMed] [Google Scholar]
- Duron E, Rigaud AS, Dubail D, Mehrabian S, Latour F, Seux ML, Hanon O. (2009) Effects of antihypertensive therapy on cognitive decline in Alzheimer’s disease. Am J Hypertens 22:1020–1024. [DOI] [PubMed] [Google Scholar]
- Gelband CH, Sumners C, Lu D, Raizada MK. (1998) Angiotensin receptors and norepinephrine neuromodulation: implications of functional coupling. Regul Pept 73:141–147. [DOI] [PubMed] [Google Scholar]
- Germain L, Chouinard G. (1989) Captopril treatment of major depression with serial measurements of blood cortisol concentrations. Biol Psychiatry 25:489–493. [DOI] [PubMed] [Google Scholar]
- Goertz RB, Wanat MJ, Gomez JA, Brown ZJ, Phillips PE, Paladini CA. (2014) Cocaine increases dopaminergic neuron and motor activity via midbrain alpha1 adrenergic signaling. Neuropsychopharmacology 40:1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile C, Hao Y, O’Malley P, Newton T, Kosten T. (2012) The α1 antagonist doxazosin alters the behavioral effects of cocaine in rats. Brain Sci 2:619–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson GR, Lovenberg W. (1980) Elevation of substance P-like immunoreactivity in rat central nervous system by protease inhibitors. J Neurochem 35:1370–1374. [DOI] [PubMed] [Google Scholar]
- Hosseini M, Alaei HA, Havakhah S, Neemati Karimooy HA, Gholamnezhad Z. (2009) Effects of microinjection of angiotensin II and captopril to VTA on morphine self-administration in rats. Acta Biol Hung 60:241–252. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Allen AM, Chai SY, MacGregor DP, Paxinos G, Mendelsohn FA. (1996) Interactions of angiotensin II with central dopamine. Adv Exp Med Biol 396:93–103. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Chai SY, Mendelsohn FA. (1997. a) Upregulation of angiotensin II AT1 receptors in the mouse nucleus accumbens by chronic haloperidol treatment. Brain Res 748:137–142. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Mendelsohn FA, Chai SY. (1997. b) Angiotensin-converting enzyme modulates dopamine turnover in the striatum. J Neurochem 68:1304–1311. [DOI] [PubMed] [Google Scholar]
- Kucukali CI, Aydin M, Ozkok E, Bilge E, Zengin A, Cakir U, Kara I. (2010) Angiotensin-converting enzyme polymorphism in schizophrenia, bipolar disorders, and their first-degree relatives. Psychiatr Genet 20:14–19. [DOI] [PubMed] [Google Scholar]
- Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. (1997) Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol 18:383–439. [DOI] [PubMed] [Google Scholar]
- Leone A. (2011) Does smoking act as a friend or enemy of blood pressure? Let release pandora’s box. Cardiol Res Pract 2011:264894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M, Hu K, Swann AC. (2014) Stress modulates illness-course of substance use disorders: a translational review. Front Psychiatry 5:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingham T, Perlanski E, Grupp LA. (1990) Angiotensin converting enzyme inhibitors reduce alcohol consumption: some possible mechanisms and important conditions for its therapeutic use. Alcohol Clin Exp Res 14:92–99. [DOI] [PubMed] [Google Scholar]
- Loonam TM, Noailles PA, Yu J, Zhu JP, Angulo JA. (2003) Substance P and cholecystokinin regulate neurochemical responses to cocaine and methamphetamine in the striatum. Life Sci 73:727–739. [DOI] [PubMed] [Google Scholar]
- Mashhoody T, Rastegar K, Zal F. (2014) Perindopril may improve the hippocampal reduced glutathione content in rats. Adv Pharm Bull 4:155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul B, Siems WE, Hoehe MR, Grecksch G, Bader M, Walther T. (2001) Alcohol consumption is controlled by angiotensin II. FASEB J 15:1640–1642. [DOI] [PubMed] [Google Scholar]
- Mertens B, Vanderheyden P, Michotte Y, Sarre S. (2010) The role of the central renin-angiotensin system in Parkinson’s disease. J Renin Angiotensin Aldosterone Syst 11:49–56. [DOI] [PubMed] [Google Scholar]
- Mota A, Guimaraes S. (2003) Influence of alpha2-autoreceptor stimulation on the facilitation by angiotensin II and bradykinin of noradrenaline release. Naunyn Schmiedebergs Arch Pharmacol 368:443–447. [DOI] [PubMed] [Google Scholar]
- Nahmod VE, Finkielman S, Benarroch EE, Pirola CJ. (1978) Angiotensin regulates release and synthesis of serotonin in brain. Science 202:1091–1093. [DOI] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, II, Grasing K. (2010) The angiotensin-converting enzyme inhibitor perindopril treatment alters cardiovascular and subjective effects of methamphetamine in humans. Psychiatry Res 179:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz M, Yang KH, O’Donovan M J, Renaud LP. (2005) Presynaptic angiotensin II AT1 receptors enhance inhibitory and excitatory synaptic neurotransmission to motoneurons and other ventral horn neurons in neonatal rat spinal cord. J Neurophysiol 94:1405–1412. [DOI] [PubMed] [Google Scholar]
- Paz MC, Assis MA, Cabrera RJ, Cancela LM, Bregonzio C. (2011) The AT angiotensin II receptor blockade attenuates the development of amphetamine-induced behavioral sensitization in a two-injection protocol. Synapse 65:505–512. [DOI] [PubMed] [Google Scholar]
- Paz MC, Marchese NA, Stroppa MM, Gerez de Burgos NM, Imboden H, Baiardi G, Cancela LM, Bregonzio C. (2014) Involvement of the brain renin-angiotensin system (RAS) in the neuroadaptive responses induced by amphetamine in a two-injection protocol. Behav Brain Res 272:314–323. [DOI] [PubMed] [Google Scholar]
- Porter JP. (1990) Effect of stress on the control of renin release in spontaneously hypertensive rats. Hypertension 15:310–317. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Armando I, Bregonzio C, Juorio A, Macova M, Pavel J, Sanchez-Lemus E. (2006) A centrally acting, anxiolytic angiotensin II AT1 receptor antagonist prevents the isolation stress-induced decrease in cortical CRF1 receptor and benzodiazepine binding. Neuropsychopharmacology 31:1123–1134. [DOI] [PubMed] [Google Scholar]
- Sakaguchi K, Chai SY, Jackson B, Johnston CI, Mendelsohn FA. (1988) Differential angiotensin-converting enzyme inhibition in brain after oral administration of perindopril demonstrated by quantitative in vitro autoradiography. Neuroendocrinology 48:223–228. [DOI] [PubMed] [Google Scholar]
- Skidgel RA. (1985) Characterization of the metabolism of substance P and neurotensin by human angiotensin I converting enzyme and “enkephalinase”. Prog Clin Biol Res 192:371–378. [PubMed] [Google Scholar]
- Sonsalla PK, Coleman C, Wong LY, Harris SL, Richardson JR, Gadad BS, Li W, German DC. (2013) The angiotensin converting enzyme inhibitor captopril protects nigrostriatal dopamine neurons in animal models of parkinsonism. Exp Neurol 250:376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storgaard T, Nedergaard OA. (1997) Prejunctional modulation by angiotensins of noradrenaline release from sympathetic neurons in isolated rabbit aorta. Naunyn Schmiedebergs Arch Pharmacol 356:706–711. [DOI] [PubMed] [Google Scholar]
- Verrico CD, Haile CN, Newton TF, Kosten TR, De La Garza R. (2013) Pharmacotherapeutics for substance-use disorders: a focus on dopaminergic medications. Expert Opin Investig Drugs 22:1549–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrico CD, Mahoney JJ, III, Thompson-Lake DG, Bennett RS, Newton TF, De La Garza R., II (2014) Safety and efficacy of varenicline to reduce positive subjective effects produced by methamphetamine in methamphetamine-dependent volunteers. Int J Neuropsychopharmacol 17:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visniauskas B, Perry JC, Oliveira V, Dalio FM, Andersen ML, Tufik S, Chagas JR. (2012) Cocaine administration increases angiotensin I-converting enzyme (ACE) expression and activity in the rat striatum and frontal cortex. Neurosci Lett 506:84–88. [DOI] [PubMed] [Google Scholar]
- Waeber B, Nussberger J, Perret L, Santoni JP, Brunner HR. (1989) Experience with perindopril in normal volunteers. Arch Mal Coeur Vaiss 82 Spec No 1:35–41. [PubMed] [Google Scholar]
- Wang X, Wang G, Shi J, Aa J, Comas R, Liang Y, Zhu HJ. (2015) CES1 genetic variation affects the activation of angiotensin-converting enzyme inhibitors. Pharmacogenomics J. Advance online publication. doi: 10.1038/tpj.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. (2007) There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology 32:1433–1451. [DOI] [PubMed] [Google Scholar]
- Yagi S, Akaike M, Ise T, Ueda Y, Iwase T, Sata M. (2013) Renin-angiotensin-aldosterone system has a pivotal role in cognitive impairment. Hypertens Res 36:753–758. [DOI] [PubMed] [Google Scholar]
- Yokosawa H, Endo S, Ogura Y, Ishii S. (1983) A new feature of angiotensin-converting enzyme in the brain: hydrolysis of substance P. Biochem Biophys Res Comm 116:735–742. [DOI] [PubMed] [Google Scholar]
- Yokosawa H, Endo S, Ohgaki Y, Maeyama J, Ishii S. (1985) Hydrolysis of substance P and its analogs by angiotensin-converting enzyme from rat lung. Characterization of endopeptidase activity of the enzyme. J Biochem 98:1293–1299. [DOI] [PubMed] [Google Scholar]
- Zacharieva S, Matrozov P, Stoeva I, Andonova K. (1991) The effect of angiotensin-converting enzyme inhibition on ACTH response to corticotropin-releasing hormone (CRH) in normal men. Horm Metab Res 23:245–246. [DOI] [PubMed] [Google Scholar]