Abstract
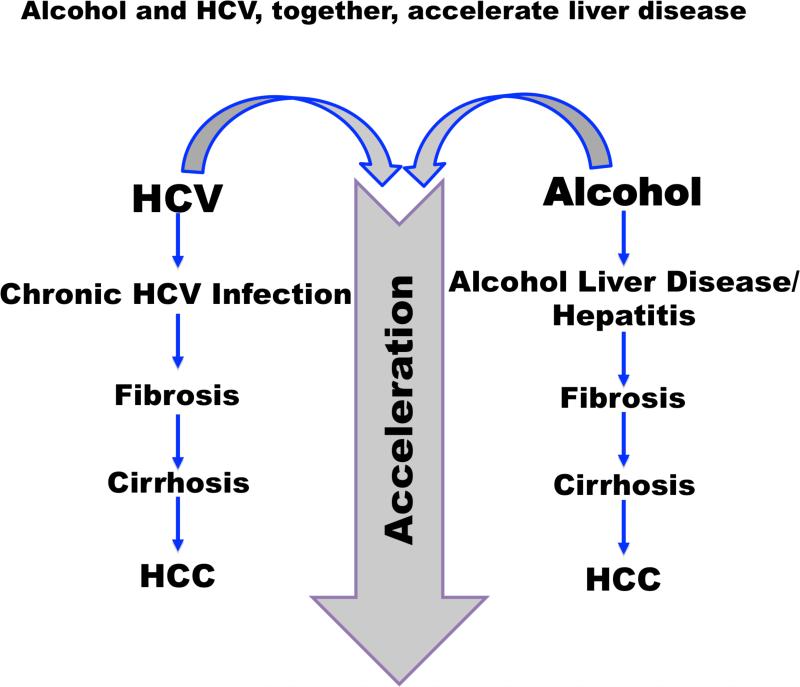
Most HCV infected patients regularly consume alcohol. Alcoholic liver disease (ALD) and chronic hepatitis C virus (HCV) infection together are the most common causes of liver disease worldwide. Although both factors independently cause liver disease, they synergistically promote liver disease progression with devastating outcomes for patients. This review focuses on the prevalence, clinical characteristics and molecular pathophysiologic mechanisms of HCV infection associated with alcohol abuse. Recent findings have centered on the synergistic effect of alcohol and HCV on viral replication, hepatocyte apoptosis, oxidative stress, alcohol-induced ‘leaky gut’, miR-122 and immune dysregulation. Clinical and basic research findings presented here summarize key scientific findings with the aim of highlighting potential areas for new therapies and identifying ways of optimizing current treatments for alcoholics with HCV infection.
Keywords: Acute on chronic liver injury, inflammation, fibrosis, miR-122
Introduction
Alcoholic hepatitis (AH) is an alcohol-induced inflammatory disease of the liver due to excessive intake of alcohol found in about 10-35% of hospitalized alcoholics and has a mortality of up to 50% (1,2). Alcohol abuse which involves recurring use of alcohol despite negative consequences is widespread in patients with chronic hepatitis C (HCV) infection. An estimated 1.6% of the US population has chronic HCV and 14-40% of these patients abuse alcohol (3-5). Not surprisingly, chronic HCV infection is common in patients who are diagnosed with AH. The synergistic effect of alcohol and HCV on the liver can also present as acute on chronic liver damage.
Numerous studies have demonstrated the synergistic effect of alcohol and HCV on the progression of liver disease. Patients with liver disease from both HCV and alcohol abuse have a worse prognosis, compared to patients who present with only one disease. Those who have HCV and abuse alcohol show an increased rate of fibrosis and hepatocellular carcinoma (HCC), (6-9) and have a higher rate of early death, compared to patients with HCV who do not abuse alcohol (10). Patients with alcohol-related admissions who have HCV have been shown to have a 24% longer length of stay and are twice as likely to die during the hospitalization (11). A study by Kim et al also concluded that the risk of death in HCV patients who also abuse alcohol increases by 40% (12). Patients with cirrhosis from both etiologies are more likely to be hospitalized than patients with cirrhosis due to alcohol alone (13). Although there have been numerous studies evaluating the impact of alcohol on HCV, there are few studies on the effect of HCV on patients admitted with AH.
Epidemiology
The prevalence of AH and chronic HCV infection varies. Up to 40% of alcoholics are infected with HCV (14,15), however the percentage of HCV patients admitted with AH varies from 7.7-38% (16-23). The difference in prevalence may reflect HCV detection methods as the first generation anti-HCV antibodies were less sensitive resulting in higher prevalence in later studies (22,23). One study found an increase in the proportion of patients admitted with both AH and HCV between 1998 and 2007, and an increase in discharge diagnosis of HCV in all hospital admissions suggesting an increase in diagnosis rather than in HCV prevalence (16). Inclusion of cirrhosis also increased the prevalence of both diseases from about 6.5-13.2% in studies that excluded cirrhosis to about 19-27% when cirrhosis was included (16,18,20). Up to 50% of patients with AH are cirrhotic (1).
The demographics of patients with both HCV and AH are similar to the groups who have high prevalence of either of the two diseases. Patients with a higher prevalence of both HCV and AH are between ages 40-60 years and Hispanic or African-American (16). Non-Hispanic African-Americans between 40-49 years old have the highest prevalence of HCV at 9.4%, compared to other ethnic and age groups (23). Similarly, non-Hispanic African-Americans have the highest prevalence of AH with 4.4 cases per 100,000 persons followed by Caucasians with 3.1 cases per 100,000, and Hispanics and American Indians with 2.9 per 100,000, respectively (24). In 2007, the mean age of patients admitted with AH was 53.2 years (20). Males in all age and ethnic groups have higher prevalence of HCV and AH than females (20,23-27).
The increased prevalence of HCV in adults admitted with AH is likely multifaceted. The hypothesized etiologies behind the increased rates of HCV and heavy alcohol use may be extended to those with AH and include coexisting intravenous drug use, increased risky behavior requiring blood transfusions, socioeconomic factors, and possible immune suppression from alcohol (3,5,14,28).
Clinical Course
Compared to patients with AH alone, patients with chronic HCV infection who develop AH have a poorer prognosis. Patients with AH or HCV present with similar MELD and discriminant function (DF) scores, which indicates similar severity on admission (17,29). Despite this similarity, patients with AH and HCV have an increased 6 month and possibly in-patient mortality (17). One study found a 29% increased incident of death in patients with both diseases, but another demonstrated no difference in mortality between patients with HCV and AH, compared to those with AH alone (16,20). Patients admitted with AH and HCV are more likely to present with variceal bleeding and hepatic encephalopathy which are associated with in-hospital mortality in patients admitted with AH (17,20,29).
Poorer prognosis in patients with both AH and HCV could be related to multiple factors including increased complications, underlying physiology, and/or treatment. Alcohol use has been identified as an independent risk factor for progression of liver disease in patients with chronic HCV (6-10,12). The underlying mechanism in patients with chronic HCV infection and heavy alcohol use that lead to more rapid fibrosis and HCC development may also cause a worse clinical course in HCV patients with AH (3,5).
Compared to patients with AH alone, those with both liver diseases are less likely to be treated with corticosteroids or Pentoxyfylline, even when they have a DF score ≥ 32 (17). One explanation for the difference in treatment may be that steroids are generally contraindicated in complications such as GI bleed, which is increased in this group (16,17,30). Specific AH treatment may also be delayed or avoided because the primarily etiology of the presentation is unclear. In these cases, liver biopsy may be beneficial (4).
Data on treatment methods and prognostic scores for patients with both diseases is limited. Most of the studies evaluating AH treatment or prognostic scores exclude patients with HCV (31-37). Studies that include patients with HCV do not generally stratify this group (38,39). In addition, some older studies were done before HCV antibodies were readily detected (40). One study examined the outcomes of AH patients treated with Pentoxifylline, which included patients with and without HCV, found similar survival outcomes in the two groups (41). Overall, data is inadequate on treatment and prognostication for patients with both diseases.
Because patients with concomitant HCV infection have poorer prognosis, alternative treatment for AH should be considered. A controversial method of treatment for patients with severe AH in HCV patients is liver transplant (LT). Early LT increases survival of AH patients at 6 months compared to medical treatment (42). Although cirrhosis from alcohol and HCV are leading indications for LT, AH is a relative contraindication. By definition, patients with AH have consumed alcohol within a few weeks of presentation. Most transplant centers in the United States require alcohol sobriety for at least 6 months, primarily because of the concern of relapse post-transplant and organ shortage. European studies have demonstrated that LT improves mortality in patients with AH, and graft survival is similar compared to patients who are transplanted for alcoholic cirrhosis (42-44). One study fo und similar graft and survival in patients with AH and alcoholic cirrhosis. Twenty-six percent of the AH patients were also infected with HCV and, in a subgroup analysis, had similar graft and survival to those transplanted for either AH or alcoholic cirrhosis. None of the patients transplanted for AH had graft failure or died, or had documented alcohol relapse or noncompliance of immunosuppression (43). A retrospective study from Spain found that patients with superimposed AH on cirrhosis based histology had similar survival rates and recidivism compare to patients with alcoholic cirrhosis alone. Graft loss did not occur in the patients who returned to drinking (44). LT for AH will continue to be controversial however may be an option in the future for those with underlying chronic HCV infection in the era of new antivirals.
Mechanisms
Acute Alcoholic Hepatitis
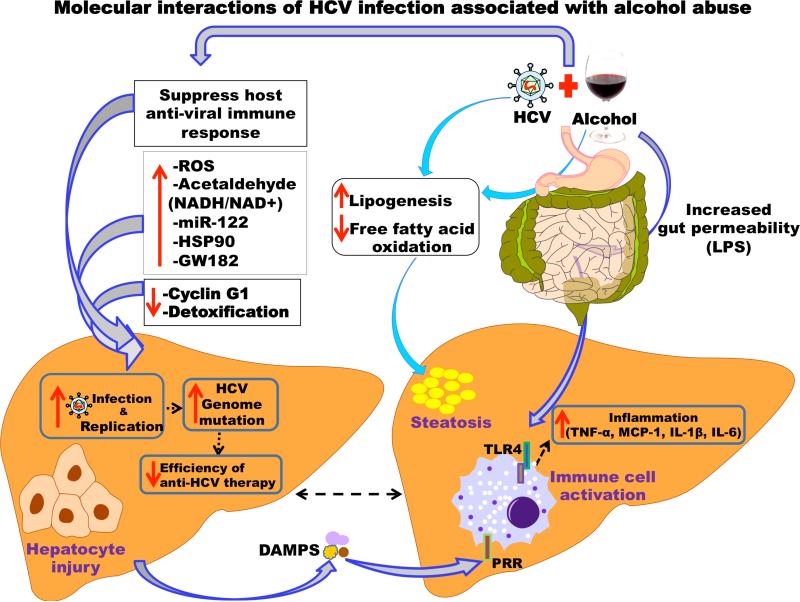
The pathogenesis of AH is based on inflammation and increased oxidative stress (30,45). In AH, elevated levels of endotoxin, a component of the cell wall of Gram negative bacteria, is associated with upregulation of inflammatory cytokines (46-48). Endotoxemia is caused by increased intestinal permeability in the presence of chronic alcohol use, alcoholic cirrhosis, AH, and even after binge drinking (48-50). Intestinal permeability allows intestinal bacteria to migrate to mesenteric lymph nodes and other extraintestinal sites, otherwise known as bacterial translocation (51). A short period of abstinence not only reduces gut permeability, but also the level of endotoxins detected in serum (48,52), and selective intestinal decontamination with antibiotics decreases endotoxin levels (51,53).
In AH and alcoholic cirrhosis, the translocation of the endotoxins from the gut into portal circulation leads to the activation of Toll-like receptor -4 (TLR4) on Kupffer cells that produce TNF-α (50). Other pathways are also activated including the inflammasome that produces IL-1β that amplifies liver inflammation (54). Anti-inflammatory pathways such as the signal transducer and activator transcription factor (STAT) pathway, which leads to production of IL-10 and stimulation of CD4+ and CD8+ T lymphocytes, are suboptimally activated (30,45). Together, this leads to activation of inflammatory cells, production of pro-inflammatory cytokines, and apoptosis (30,45). Hepatic damage in AH is also caused by increased reactive oxygen species (ROS) generated during alcoholic metabolism and depleted antioxidants, such as glutathione (28,45). The increased susceptibility to infection is also related to neutrophil dysfunction with high resting oxidative burst and reduced phagocytic capacity (55,56). Additionally, alcohol use results in impaired antigen presenting cell function affecting dendritic cells and antigen-specific T-cell activation (28,57). Together, these immune alternations contribute to impaired immune clearance of HCV infection (28).
Hepatitis C Infection
HCV damages the liver by modulating hepatocytes and immune responses. Furthermore, HCV, like alcohol, increases reactive oxygen species (ROS) leading to hepatic injury. The HCV core protein binds to the mitochondria and facilitates the uptake of Ca2+ into the endosplasmic reticulum on a cell which also increases ROS production and glutathione depletion (5,28). Therefore both alcohol and HCV worsen the balance between oxidative stress and antioxidants, and impair antiviral immunity.
HCV, Alcohol, and Host Response
Immune modulation by alcohol during HCV infection has been shown to contribute to the higher incidence of hepatitis C virus (HCV) infection (58-60). HCV is a single stranded RNA recognized by host cell pattern recognition receptors (PRRs) and induces Type I IFNs and inflammatory mediators (61,62). Despite this host immune surveillance, HCV can escape host immune recognition systems and establish chronic infection during alcohol abuse (61,63,64). Additionally, alcohol and HCV infection, independently or synergistically, can attenuate the capacity of myeloid dendritic cells to fulfill their antigen presenting and T cell stimulatory function (65-68). Activation of primary human dendritic cells in the presence of alcohol and HCV significantly decreased IL-12 and increased IL-10 production (57,67). Chronic alcohol consumption also increases non-specific inflammatory responses while decreasing Type I IFN production (65, 78) that is important for antiviral immunity.
Alcohol and HCV interactions at the cellular and molecular levels
Alcohol and HCV are independent risk factors that cause liver inflammation, cirrhosis, and HCC with similar and distinct molecular mechanisms (5,25,69). Studies suggest that increased HCV replication, increased hepatic oxidative stress, impaired host immune response, and enhanced hepatocyte cell death contribute to the development of liver disease when alcohol abuse is associated with HCV infection.
HCV infection induces the production of ROS (29) and nitric oxide (NO) in the liver (30). Of the 10 viral proteins, HCV core protein increases oxidative stress in hepatocytes (39). Other HCV proteins, including NS3, or NS5A (42-44), E1 (40), E2 (30,45), and NS4B (40,46), are also involved in inducing oxidative stress. In addition to their other functions, HCV core, NS5A, and NS3 proteins increase calcium uptake by mitochondria and cause oxidation of mitochondrial glutathione leading to increased ROS (13,48,51).
Chronic alcohol enhances inflammation, because HCV-infected patients who consume alcohol exhibit greater liver inflammation than patients who consume no alcohol (70). Upregulated programmed cell death pathways (apoptosis) play a crucial role on HCV-infected hepatocytes in the presence of alcohol (71,72). Damage-associated molecular patterns (DAMPs) from cell death induced by alcohol or HCV can exacerbate immune activation together with increased gut bacteria as a consequence of alcohol abuse (73).
Alcohol can modulate HCV replication by diverse mechanisms including modulation of host proteins, micro-RNAs and suppressing cellular immune signaling. While there are conflicting findings as to direct role of alcohol in modulating HCV replication in-vitro, most studies found that alcohol can increase HCV replication through alcohol metabolites (74), increased GW812 (75), increased HSP90 (75) and decreasing cyclinG1 expression (76). Studies using Huh7.5-CYP2E1 cells or HCV replicon cell lines found that alcohol metabolites can increase HCV replication in-vitro (74,76). Importantly, alcohol exposure can increase HCV replication by increasing cellular host protein expression including HSP90 and GW182 while decreasing Cyclin G1 expression (75).
MiR-122, HCV and alcohol
Previous studies indicated that alcohol use in HCV infected patients can significantly increase serum HCV RNA levels (77,78). In recent reports, we demonstrated that alcohol can increase HCV replication in vitro and identified a critical role of microRNA-122 (miRNA-122) in the process (75,76). While numerous clinical studies have advanced the role of alcohol, in enhancing HCV replication, a meta-analysis showed that alcoholics with HCV infection showed no significant differences in HCV expression (79). miRNAs are non-coding RNAs that modulate gene expression regulate the numerous pathological processes. In hepatocytes, miRNA-122 is highly expressed compared to other miRNAs (80,81). Recently we found that alcohol exposure could significantly increase miRNA-122 levels in Huh7.5 hepatoma cells and through this mechanism, increases HCV replication (76). Additionally, miRNA-122 regulation of HCV is enhanced by the RISC-complex molecules Argonaute 2(Ago2) (80), HSP90, and GW182 which also increased during alcohol exposure in cultured human hepatoma cell line Huh7.5 (75). Independent of HCV, increased serum miRNA-122 appear to positively correlate with the severity of liver damage in alcoholic hepatitis (82-86) and virologic response to pegylated interferon therapy against HCV infection (87). Given that alcohol and HCV infection increase miR-122 and RISC complex proteins that can increa se HCV replication, their synergism in this molecular process might account for the reason that alcoholics show faster progression toward advanced liver disease.
Acute on chronic liver damage
Regardless of etiology, decompensated cirrhotics have a higher prevalence of bacterial translocation, likely related to increased intestinal permeability, compared to compensated cirrhotics and controls (51,88). Endotoxin concentrations, although higher in alcoholic liver disease, are also elevated in chronic HCV (66,67) and HCV cirrhosis, and are associated with increased hepatic venous pressure gradients (89). Mouse models have demonstrated increased cytokines and nitric oxide production in the presence of bacterial translocation and cirrhosis (51).
In patients with HCV cirrhosis, AH precipitates acute decompensation, causes acute on chronic liver injury, or if organ failure develops, acute on chronic liver failure (ACLF). The definition of ACLF is still developing but is primarily based on the presence of acute decompensation (e.g ascites, encephalopathy, GI bleed) and organ failure, from a precipitating factor or superimposed liver insult (55,90). ACLF is also characterized by rapid progression and high mortality, based primarily on the severity of the organ failure (55,90,91). One month mortality is estimated at 30-53% in patients who develop ACLF (90,92). The precipitating factor may be due to liver injury including viral or AH or non-hepatic stress such as sepsis or surgery. One retrospective study on etiologies of ACLF found that 47% of cases were triggered by extra-hepatic causes and AH caused 29% of the hepatic causes (92).
The underlying mechanisms of ACLF injury may be related to the underlying cirrhosis and the triggering event that induces the inflammatory response (55). Elevated inflammatory cytokines including TNF-α, IFN-γ, IL 2, IL2R,IL-6, IL-8 and IL-10, many of which are key factors in hepatic damage induced by alcohol and HCV, are found in cases of ACLF (93,94). Infection may not only precipitate ACLF, but is also associated with increased mortality and morbidity in other triggers of ACLF (55,91). Immune dysfunction in ACLF is multifactorial. Decreased activity by phagocytic cells and the reticuloendothelial system leads to accumulation of microbes including damaging endotoxins (91). Endotoxins and other bacteria that may be present trigger the release of inflammatory cytokines and ultimately a systemic response leading to Systemic Inflammatory Response Syndrome (SIRS). These are characteristics of acute alcoholic hepatitis. ACLF develops from a cycle in which sepsis or SIRS continues the pro-inflammatory response further depressing the immune system and increasing susceptibility to further infections (91,94).
The mechanism and alcoholic hepatitis superimposed on chronic HCV liver disease is a prime example of the pathophysiology of ACLF. The inflammatory response and cytokine release in the chronic liver disease is likely exacerbated by the additional inflammation caused by AH. Increased gut permeability, endotoxemia, cytokine release, and decreased immune response lead to susceptibility to infection which is the most common cause of mortality in both ACLF and AH (91).
Conclusion
The prevalence of AH and chronic HCV is significant but data on epidemiology, prognosis, and treatment is limited. Because up to 38% of patients with AH are also chronically infected with HCV, antibodies to HCV should be obtained on patients admitted with AH. Also, the presence of HCV antibodies should not exclude a diagnosis of AH. Liver biopsy may be useful if etiology at presentation is unclear and may be instrumental in assessing the severity of underlying liver damage and fibrosis.
Studies suggest that mortality may be worse for patients with both AH and HCV compared to AH alone. The worse outcome is multifactorial and related to decreased treatment rates in the former group or related to the mechanisms, which lead to more rapid progression to cirrhosis and HCC in patients with HCV infection who also drink alcohol heavily. Because most studies evaluating treatment and prognosis scoring systems in patients with AH do not analyze those who have concomitant HCV infection, the generalizability to this group is unclear. Further studies examining AH in patients with HCV are necessary.
Acknowledgments
Funding: AA014372 to G. Szabo.
References
- 1.O'Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010 Jan;105(1):14–32. doi: 10.1038/ajg.2009.593. quiz 33. [DOI] [PubMed] [Google Scholar]
- 2.McCullough AJ, O'Shea RS, Dasarathy S. Diagnosis and management of alcoholic liver disease. J Dig Dis. 2011 Aug;12(4):257–262. doi: 10.1111/j.1751-2980.2010.00470.x. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharya R, Shuhart MC. Hepatitis C and alcohol: interactions, outcomes, and implications. J Clin Gastroenterol. 2003 Mar;36(3):242–252. doi: 10.1097/00004836-200303000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Rongey C, Kaplowitz N. Current concepts and controversies in the treatment of alcoholic hepatitis. World J Gastroenterol. 2006 Nov 21;12(43):6909–6921. doi: 10.3748/wjg.v12.i43.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singal AK, Anand BS. Mechanisms of synergy between alcohol and hepatitis C virus. J Clin Gastroenterol. 2007 Sep;41(8):761–772. doi: 10.1097/MCG.0b013e3180381584. [DOI] [PubMed] [Google Scholar]
- 6.Donato F, Tagger A, Gelatti U, Parrinello G, Boffetta P, Albertini A, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002 Feb 15;155(4):323–331. doi: 10.1093/aje/155.4.323. [DOI] [PubMed] [Google Scholar]
- 7.Khan KN, Yatsuhashi H. Effect of alcohol consumption on the progression of hepatitis C virus infection and risk of hepatocellular carcinoma in Japanese patients. Alcohol Alcohol. 2000 May-Jun;35(3):286–295. doi: 10.1093/alcalc/35.3.286. [DOI] [PubMed] [Google Scholar]
- 8.Monto A, Patel K, Bostrom A, Pianko S, Pockros P, McHutchison JG, et al. Risks of a range of alcohol intake on hepatitis C-related fibrosis. Hepatology. 2004 Mar;39(3):826–834. doi: 10.1002/hep.20127. [DOI] [PubMed] [Google Scholar]
- 9.Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004 Nov;127(5 Suppl 1):S87–96. doi: 10.1053/j.gastro.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Chen CM, Yoon YH, Yi HY, Lucas DL. Alcohol and hepatitis C mortality among males and females in the United States: a life table analysis. Alcohol Clin Exp Res. 2007 Feb;31(2):285–292. doi: 10.1111/j.1530-0277.2006.00304.x. [DOI] [PubMed] [Google Scholar]
- 11.Tsui JI, Pletcher MJ, Vittinghoff E, Seal K, Gonzales R. Hepatitis C and hospital outcomes in patients admitted with alcohol-related problems. J Hepatol. 2006 Feb;44(2):262–266. doi: 10.1016/j.jhep.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 12.Kim WR, Gross JB, Jr, Poterucha JJ, Locke GR, 3rd, Dickson ER. Outcome of hospital care of liver disease associated with hepatitis C in the United States. Hepatology. 2001 Jan;33(1):201–206. doi: 10.1053/jhep.2001.20798. [DOI] [PubMed] [Google Scholar]
- 13.Corrao G, Arico S. Independent and combined action of hepatitis C virus infection and alcohol consumption on the risk of symptomatic liver cirrhosis. Hepatology. 1998 Apr;27(4):914–919. doi: 10.1002/hep.510270404. [DOI] [PubMed] [Google Scholar]
- 14.Novo-Veleiro I, Calle Cde L, Dominguez-Quiben S, Pastor I, Marcos M, Laso FJ. Prevalence of hepatitis C virus infection in alcoholic patients: cohort study and systematic review. Alcohol Alcohol. 2013 Sep-Oct;48(5):564–569. doi: 10.1093/alcalc/agt044. [DOI] [PubMed] [Google Scholar]
- 15.Fong TL, Kanel GC, Conrad A, Valinluck B, Charboneau F, Adkins RH. Clinical significance of concomitant hepatitis C infection in patients with alcoholic liver disease. Hepatology. 1994 Mar;19(3):554–557. doi: 10.1002/hep.1840190303. [DOI] [PubMed] [Google Scholar]
- 16.Singal AK, Kuo YF, Anand BS. Hepatitis C virus infection in alcoholic hepatitis: prevalence patterns and impact on in-hospital mortality. Eur J Gastroenterol Hepatol. 2012 Oct;24(10):1178–1184. doi: 10.1097/MEG.0b013e328355cce0. [DOI] [PubMed] [Google Scholar]
- 17.Singal AK, Sagi S, Kuo YF, Weinman S. Impact of hepatitis C virus infection on the course and outcome of patients with acute alcoholic hepatitis. Eur J Gastroenterol Hepatol. 2011 Mar;23(3):204–209. doi: 10.1097/MEG.0b013e328343b085. [DOI] [PubMed] [Google Scholar]
- 18.Mendenhall CL, Seeff L, Diehl AM, Ghosn SJ, French SW, Gartside PS, et al. Antibodies to hepatitis B virus and hepatitis C virus in alcoholic hepatitis and cirrhosis: their prevalence and clinical relevance. The VA Cooperative Study Group (No. 119). Hepatology. 1991 Oct;14(4 Pt 1):581–589. doi: 10.1016/0270-9139(91)90042-t. [DOI] [PubMed] [Google Scholar]
- 19.Nishiguchi S, Kuroki T, Yabusako T, Seki S, Kobayashi K, Monna T, et al. Detection of hepatitis C virus antibodies and hepatitis C virus RNA in patients with alcoholic liver disease. Hepatology. 1991 Dec;14(6):985–989. [PubMed] [Google Scholar]
- 20.Liangpunsakul S. Clinical characteristics and mortality of hospitalized alcoholic hepatitis patients in the United States. J Clin Gastroenterol. 2011 Sep;45(8):714–719. doi: 10.1097/MCG.0b013e3181fdef1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokoyama H, Ishii H, Moriya S, Nagata S, Watanabe T, Kamegaya K, et al. Relationship between hepatitis C virus subtypes and clinical features of liver disease seen in alcoholics. J Hepatol. 1995 Feb;22(2):130–134. doi: 10.1016/0168-8278(95)80419-6. [DOI] [PubMed] [Google Scholar]
- 22.Rosman AS, Paronetto F, Galvin K, Williams RJ, Lieber CS. Hepatitis C virus antibody in alcoholic patients. Association with the presence of portal and/or lobular hepatitis. Arch Intern Med. 1993 Apr 26;153(8):965–969. [PubMed] [Google Scholar]
- 23.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006 May 16;144(10):705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 24.Yang AL, Vadhavkar S, Singh G, Omary MB. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008 Mar 24;168(6):649–656. doi: 10.1001/archinte.168.6.649. [DOI] [PubMed] [Google Scholar]
- 25.Hutchinson SJ, Bird SM, Goldberg DJ. Influence of alcohol on the progression of hepatitis C virus infection: a meta-analysis. Clin Gastroenterol Hepatol. 2005 Nov;3(11):1150–1159. doi: 10.1016/s1542-3565(05)00407-6. [DOI] [PubMed] [Google Scholar]
- 26.Pessione F, Ramond MJ, Peters L, Pham BN, Batel P, Rueff B, et al. Five-year survival predictive factors in patients with excessive alcohol intake and cirrhosis. Effect of alcoholic hepatitis, smoking and abstinence. Liver Int. 2003 Feb;23(1):45–53. doi: 10.1034/j.1600-0676.2003.01804.x. [DOI] [PubMed] [Google Scholar]
- 27.Schiff ER. Hepatitis C and alcohol. Hepatology. 1997 Sep;26(3 Suppl 1):39S–42S. doi: 10.1002/hep.510260707. [DOI] [PubMed] [Google Scholar]
- 28.Szabo G, Aloman C, Polyak SJ, Weinman SA, Wands J, Zakhari S. Hepatitis C infection and alcohol use: A dangerous mix for the liver and antiviral immunity. Alcohol Clin Exp Res. 2006 Apr;30(4):709–719. doi: 10.1111/j.1530-0277.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- 29.Kulkarni K, Tran T, Medrano M, Yoffe B, Goodgame R. The role of the discriminant factor in the assessment and treatment of alcoholic hepatitis. J Clin Gastroenterol. 2004 May-Jun;38(5):453–459. doi: 10.1097/00004836-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009 Jun 25;360(26):2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 31.Mathurin P, O'Grady J, Carithers RL, Phillips M, Louvet A, Mendenhall CL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011 Feb;60(2):255–260. doi: 10.1136/gut.2010.224097. [DOI] [PubMed] [Google Scholar]
- 32.Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KV, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005 Feb;41(2):353–358. doi: 10.1002/hep.20503. [DOI] [PubMed] [Google Scholar]
- 33.Forrest EH, Evans CD, Stewart S, Phillips M, Oo YH, McAvoy NC, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005 Aug;54(8):1174–1179. doi: 10.1136/gut.2004.050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dominguez M, Rincon D, Abraldes JG, Miquel R, Colmenero J, Bellot P, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008 Nov;103(11):2747–2756. doi: 10.1111/j.1572-0241.2008.02104.x. [DOI] [PubMed] [Google Scholar]
- 35.Phillips M, Curtis H, Portmann B, Donaldson N, Bomford A, O'Grady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis--a randomised clinical trial. J Hepatol. 2006 Apr;44(4):784–790. doi: 10.1016/j.jhep.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 36.Srikureja W, Kyulo NL, Runyon BA, Hu KQ. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol. 2005 May;42(5):700–706. doi: 10.1016/j.jhep.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Verma S, Ajudia K, Mendler M, Redeker A. Prevalence of septic events, type 1 hepatorenal syndrome, and mortality in severe alcoholic hepatitis and utility of discriminant function and MELD score in predicting these adverse events. Dig Dis Sci. 2006 Sep;51(9):1637–1643. doi: 10.1007/s10620-006-9099-z. [DOI] [PubMed] [Google Scholar]
- 38.Cabre E, Rodriguez-Iglesias P, Caballeria J, Quer JC, Sanchez-Lombrana JL, Pares A, et al. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology. 2000 Jul;32(1):36–42. doi: 10.1053/jhep.2000.8627. [DOI] [PubMed] [Google Scholar]
- 39.Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007 Jun;45(6):1348–1354. doi: 10.1002/hep.21607. [DOI] [PubMed] [Google Scholar]
- 40.Ramond MJ, Poynard T, Rueff B, Mathurin P, Theodore C, Chaput JC, et al. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med. 1992 Feb 20;326(8):507–512. doi: 10.1056/NEJM199202203260802. [DOI] [PubMed] [Google Scholar]
- 41.Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000 Dec;119(6):1637–1648. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 42.Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011 Nov 10;365(19):1790–1800. doi: 10.1056/NEJMoa1105703. [DOI] [PubMed] [Google Scholar]
- 43.Singal AK, Bashar H, Anand BS, Jampana SC, Singal V, Kuo YF. Outcomes after liver transplantation for alcoholic hepatitis are similar to alcoholic cirrhosis: exploratory analysis from the UNOS database. Hepatology. 2012 May;55(5):1398–1405. doi: 10.1002/hep.25544. [DOI] [PubMed] [Google Scholar]
- 44.Tome S, Martinez-Rey C, Gonzalez-Quintela A, Gude F, Brage A, Otero E, et al. Influence of superimposed alcoholic hepatitis on the outcome of liver transplantation for end-stage alcoholic liver disease. J Hepatol. 2002 Jun;36(6):793–798. doi: 10.1016/s0168-8278(02)00047-8. [DOI] [PubMed] [Google Scholar]
- 45.Jampana SC, Khan R. Pathogenesis of alcoholic hepatitis: Role of inflammatory signaling and oxidative stress. World J Hepatol. 2011 May 27;3(5):114–117. doi: 10.4254/wjh.v3.i5.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanck C, Rossol S, Bocker U, Tokus M, Singer MV. Presence of plasma endotoxin is correlated with tumour necrosis factor receptor levels and disease activity in alcoholic cirrhosis. Alcohol Alcohol. 1998 Nov-Dec;33(6):606–608. doi: 10.1093/alcalc/33.6.606. [DOI] [PubMed] [Google Scholar]
- 47.von Baehr V, Docke WD, Plauth M, Liebenthal C, Kupferling S, Lochs H, et al. Mechanisms of endotoxin tolerance in patients with alcoholic liver cirrhosis: role of interleukin 10, interleukin 1 receptor antagonist, and soluble tumour necrosis factor receptors as well as effector cell desensitisation. Gut. 2000 Aug;47(2):281–287. doi: 10.1136/gut.47.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000 May;32(5):742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 49.Bala S, Marcos M, Gattu A, Catalano D, Szabo G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One. 2014 May 14;9(5):e96864. doi: 10.1371/journal.pone.0096864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szabo G, Bala S, Petrasek J, Gattu A. Gut-liver axis and sensing microbes. Dig Dis. 2010;28(6):737–744. doi: 10.1159/000324281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia-Tsao G, Wiest R. Gut microflora in the pathogenesis of the complications of cirrhosis. Best Pract Res Clin Gastroenterol. 2004 Apr;18(2):353–372. doi: 10.1016/j.bpg.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991 Mar;12(2):162–169. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- 53.Vlachogiannakos J, Saveriadis AS, Viazis N, Theodoropoulos I, Foudoulis K, Manolakopoulos S, et al. Intestinal decontamination improves liver haemodynamics in patients with alcohol-related decompensated cirrhosis. Aliment Pharmacol Ther. 2009 May 1;29(9):992–999. doi: 10.1111/j.1365-2036.2009.03958.x. [DOI] [PubMed] [Google Scholar]
- 54.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012 Oct 1;122(10):3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, et al. Acute-on chronic liver failure. J Hepatol. 2012 Dec;57(6):1336–1348. doi: 10.1016/j.jhep.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 56.Mookerjee RP, Stadlbauer V, Lidder S, Wright GA, Hodges SJ, Davies NA, et al. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology. 2007 Sep;46(3):831–840. doi: 10.1002/hep.21737. [DOI] [PubMed] [Google Scholar]
- 57.Mandrekar P, Catalano D, Dolganiuc A, Kodys K, Szabo G. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J Immunol. 2004 Sep 1;173(5):3398–3407. doi: 10.4049/jimmunol.173.5.3398. [DOI] [PubMed] [Google Scholar]
- 58.Schiff ER, Ozden N. Hepatitis C and alcohol. Alcohol Res Health. 2003;27(3):232–239. [PMC free article] [PubMed] [Google Scholar]
- 59.Oshita M, Hayashi N, Kasahara A, Hagiwara H, Mita E, Naito M, et al. Increased serum hepatitis C virus RNA levels among alcoholic patients with chronic hepatitis C. Hepatology. 1994 Nov;20(5):1115–1120. [PubMed] [Google Scholar]
- 60.Siu L, Foont J, Wands JR. Hepatitis C virus and alcohol. Semin Liver Dis. 2009 May;29(2):188–199. doi: 10.1055/s-0029-1214374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pang M, Bala S, Kodys K, Catalano D, Szabo G. Inhibition of TLR8- and TLR4-induced Type I IFN induction by alcohol is different from its effects on inflammatory cytokine production in monocytes. BMC Immunol. 2011 Sep 30;12:55–2172-12-55. doi: 10.1186/1471-2172-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu BS, Janssen HL, Boonstra A. Type I and III interferons enhance IL-10R expression on human monocytes and macrophages, resulting in IL-10-mediated suppression of TLR-induced IL-12. Eur J Immunol. 2012 Sep;42(9):2431–2440. doi: 10.1002/eji.201142360. [DOI] [PubMed] [Google Scholar]
- 63.Bala S, Tang A, Catalano D, Petrasek J, Taha O, Kodys K, et al. Induction of Bcl-3 by acute binge alcohol results in toll-like receptor 4/LPS tolerance. J Leukoc Biol. 2012 Sep;92(3):611–620. doi: 10.1189/jlb.0112050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Norkina O, Dolganiuc A, Catalano D, Kodys K, Mandrekar P, Syed A, et al. Acute alcohol intake induces SOCS1 and SOCS3 and inhibits cytokine-induced STAT1 and STAT3 signaling in human monocytes. Alcohol Clin Exp Res. 2008 Sep;32(9):1565–1573. doi: 10.1111/j.1530-0277.2008.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Szabo G, Dolganiuc A. Subversion of plasmacytoid and myeloid dendritic cell functions in chronic HCV infection. Immunobiology. 2005;210(2-4):237–247. doi: 10.1016/j.imbio.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 66.Szabo G, Dolganiuc A, Mandrekar P, White B. Inhibition of antigen-presenting cell functions by alcohol: implications for hepatitis C virus infection. Alcohol. 2004 Jul;33(3):241–249. doi: 10.1016/j.alcohol.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 67.Dolganiuc A, Kodys K, Kopasz A, Marshall C, Mandrekar P, Szabo G. Additive inhibition of dendritic cell allostimulatory capacity by alcohol and hepatitis C is not restored by DC maturation and involves abnormal IL-10 and IL-2 induction. Alcohol Clin Exp Res. 2003 Jun;27(6):1023–1031. doi: 10.1097/01.ALC.0000071745.63433.32. [DOI] [PubMed] [Google Scholar]
- 68.Dolganiuc A, Oak S, Kodys K, Golenbock DT, Finberg RW, Kurt-Jones E, et al. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 2004 Nov;127(5):1513–1524. doi: 10.1053/j.gastro.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 69.Wiley TE, McCarthy M, Breidi L, McCarthy M, Layden TJ. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology. 1998 Sep;28(3):805–809. doi: 10.1002/hep.510280330. [DOI] [PubMed] [Google Scholar]
- 70.Cromie SL, Jenkins PJ, Bowden DS, Dudley FJ. Chronic hepatitis C: effect of alcohol on hepatitic activity and viral titre. J Hepatol. 1996 Dec;25(6):821–826. doi: 10.1016/s0168-8278(96)80284-4. [DOI] [PubMed] [Google Scholar]
- 71.Kountouras J, Zavos C, Chatzopoulos D. Apoptosis in hepatitis C. J Viral Hepat. 2003 Sep;10(5):335–342. doi: 10.1046/j.1365-2893.2003.00452.x. [DOI] [PubMed] [Google Scholar]
- 72.Fischer R, Baumert T, Blum HE. Hepatitis C virus infection and apoptosis. World J Gastroenterol. 2007 Sep 28;13(36):4865–4872. doi: 10.3748/wjg.v13.i36.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol Rev. 2010 Jul;90(3):1165–1194. doi: 10.1152/physrev.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCartney EM, Semendric L, Helbig KJ, Hinze S, Jones B, Weinman SA, et al. Alcohol metabolism increases the replication of hepatitis C virus and attenuates the antiviral action of interferon. J Infect Dis. 2008 Dec 15;198(12):1766–1775. doi: 10.1086/593216. [DOI] [PubMed] [Google Scholar]
- 75.Bukong TN, Hou W, Kodys K, Szabo G. Ethanol facilitates hepatitis C virus replication via up-regulation of GW182 and heat shock protein 90 in human hepatoma cells. Hepatology. 2013 Jan;57(1):70–80. doi: 10.1002/hep.26010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hou W, Bukong TN, Kodys K, Szabo G. Alcohol facilitates HCV RNA replication via up-regulation of miR-122 expression and inhibition of cyclin G1 in human hepatoma cells. Alcohol Clin Exp Res. 2013 Apr;37(4):599–608. doi: 10.1111/acer.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pessione F, Degos F, Marcellin P, Duchatelle V, Njapoum C, Martinot-Peignoux M, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998 Jun;27(6):1717–1722. doi: 10.1002/hep.510270635. [DOI] [PubMed] [Google Scholar]
- 78.Safdar K, Schiff ER. Alcohol and hepatitis C. Semin Liver Dis. 2004 Aug;24(3):305–315. doi: 10.1055/s-2004-832942. [DOI] [PubMed] [Google Scholar]
- 79.Anand BS, Thornby J. Alcohol has no effect on hepatitis C virus replication: a meta-analysis. Gut. 2005 Oct;54(10):1468–1472. doi: 10.1136/gut.2004.056697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004 Jul;1(2):106–113. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- 81.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002 Apr 30;12(9):735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 82.Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci U S A. 2009 Mar 17;106(11):4402–4407. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Laterza OF, Scott MG, Garrett-Engele PW, Korenblat KM, Lockwood CM. Circulating miR-122 as a potential biomarker of liver disease. Biomark Med. 2013 Apr;7(2):205–210. doi: 10.2217/bmm.12.107. [DOI] [PubMed] [Google Scholar]
- 84.van der Meer AJ, Farid WR, Sonneveld MJ, de Ruiter PE, Boonstra A, van Vuuren AJ, et al. Sensitive detection of hepatocellular injury in chronic hepatitis C patients with circulating hepatocyte-derived microRNA-122. J Viral Hepat. 2013 Mar;20(3):158–166. doi: 10.1111/jvh.12001. [DOI] [PubMed] [Google Scholar]
- 85.Bala S, Szabo G. MicroRNA Signature in Alcoholic Liver Disease. Int J Hepatol. 2012;2012:498232. doi: 10.1155/2012/498232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Szabo G, Bala S. MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol. 2013 Sep;10(9):542–552. doi: 10.1038/nrgastro.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Su TH, Liu CH, Liu CJ, Chen CL, Ting TT, Tseng TC, et al. Serum microRNA-122 level correlates with virologic responses to pegylated interferon therapy in chronic hepatitis C. Proc Natl Acad Sci U S A. 2013 May 7;110(19):7844–7849. doi: 10.1073/pnas.1306138110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cirera I, Bauer TM, Navasa M, Vila J, Grande L, Taura P, et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001 Jan;34(1):32–37. doi: 10.1016/s0168-8278(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 89.Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, et al. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995 Feb;22(2):165–172. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- 90.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013 Jun;144(7):1426–37. 1437, e1–9. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 91.Katoonizadeh A, Laleman W, Verslype C, Wilmer A, Maleux G, Roskams T, et al. Early features of acute-on-chronic alcoholic liver failure: a prospective cohort study. Gut. 2010 Nov;59(11):1561–1569. doi: 10.1136/gut.2009.189639. [DOI] [PubMed] [Google Scholar]
- 92.Duseja A, Chawla YK, Dhiman RK, Kumar A, Choudhary N, Taneja S. Non-hepatic insults are common acute precipitants in patients with acute on chronic liver failure (ACLF). Dig Dis Sci. 2010 Nov;55(11):3188–3192. doi: 10.1007/s10620-010-1377-0. [DOI] [PubMed] [Google Scholar]
- 93.Sen S, Davies NA, Mookerjee RP, Cheshire LM, Hodges SJ, Williams R, et al. Pathophysiological effects of albumin dialysis in acute-on-chronic liver failure: a randomized controlled study. Liver Transpl. 2004 Sep;10(9):1109–1119. doi: 10.1002/lt.20236. [DOI] [PubMed] [Google Scholar]
- 94.Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghoner A, Vidacek D, Siewert E, et al. Patients with acute on chronic liver failure display “sepsis-like” immune paralysis. J Hepatol. 2005 Feb;42(2):195–201. doi: 10.1016/j.jhep.2004.10.019. [DOI] [PubMed] [Google Scholar]