Abstract
G-rich oligonucleotides have attracted considerable interest as therapeutic agents. In this report, we tested two G-rich aptamers selected against EGFR-transfected A549 cells, and their G-rich domains (S13 and S50) were identified to account for the binding of parental aptamers. CD spectra showed that S13 and S50 bind to their targets by forming parallel quadruplexes. Their binding, internalization and antiproliferation activity in cancer and noncancer cells were investigated by flow cytometry and MTS assay, and compared with those of nucleolin-binding AS1411 and thrombin-binding aptamer. The two truncated aptamers (S13 and S50) have good binding and internalization in cancer cells and noncancer cells; however, only S50, like AS1411, shows potent antiproliferation against cancer cells. Our data suggest that tumor-selective antiproliferation of G-rich oligonucleotides does not directly depend on the binding of the G-rich aptamer to cells.
Keywords: G-rich aptamer, G-quadruplex, antiproliferation, internalization
Graphical Abstract
The secondary structure, binding ability, internalization and antiproliferation activity of two truncated G-rich aptamer S13 and S50 were investigated in cancer and noncancer cells, and compared with those of nucleolin-binding AS1411 and thrombin-binding aptamer. Our data suggest that tumor-selective antiproliferation of G-rich oligonucleotides may not directly depend on the binding of the G-rich aptamers to cells.
Introduction
DNA containing contiguous guanine (G) bases can self-assemble into intramolecular or intermolecular quadruplex structures stabilized by G-quartets. Extensive interest has been shown in exploring the biological roles of G-quadruplex structures in vivo. G-rich sequences have been identified in the single-stranded DNA overhang (telomeric DNA) at the end of human chromosomes, as well as in the regions of some oncogene promoters, e.g., c-myc and c-kit oncogenes.[1–3] Studies have shown that G-quadruplex DNA plays important roles in vivo. For example, a telomeric DNA folding into a quadruplex structure can inhibit telomerase, which is overexpressed in about 85% of cancer cells and related to their immortalization.[4] In addition, there has been increasing evidence in the last 10 years that G-quadruplex sequences are involved in gene transcription.[5]
It has also been reported that some synthesized G-rich oligonucleotides have a non-antisense antiproliferative effect.[6–9] In these cases, the antiproliferation activity of G-rich sequences is associated with specific binding to cellular protein(s) by forming quadruplexes, rather than by inhibiting protein expression. For example, a 26-mer G-rich oligonucleotide, AS1411, reported by Bates et al., has shown potent tumor-selective antiproliferative effects and could inhibit the growth of many kinds of cancer cells, including leukemia, lung cancer, breast cancer and prostate cancer.[10–15] In addition, other synthetic G-rich oligonucleotides have also been reported to exhibit antiproliferative activity against tumor cell lines.[16, 17] However, the molecular basis of the antitumor activity of these sequences remains unclear.
Because of the diversity of the initial polynucleotide library and the complexity of cell surfaces, live cell-based Systematic Evolution of Ligands by EXponential enrichment (cell-SELEX),[18–23] offers the possibility of isolating cell-binding G-rich aptamers with diversified functions. Studies of these G-rich aptamers may be helpful in elucidating the antitumor mechanism of G-rich DNA. In this report, two DNA aptamers containing G-rich domains selected against EGFR-transfected A549 cells[24] are further investigated, and their binding ability, internalization and antiproliferation abilities are tested and compared with those of AS1411 and thrombin-binding aptamer in malignant cells and normal cells. Our study may provide new insight for cell recognition by G-rich DNA and help in the investigation of the properties of G-rich oligonucleotides and their applications.
Results and Discussion
Truncation of G-rich aptamer
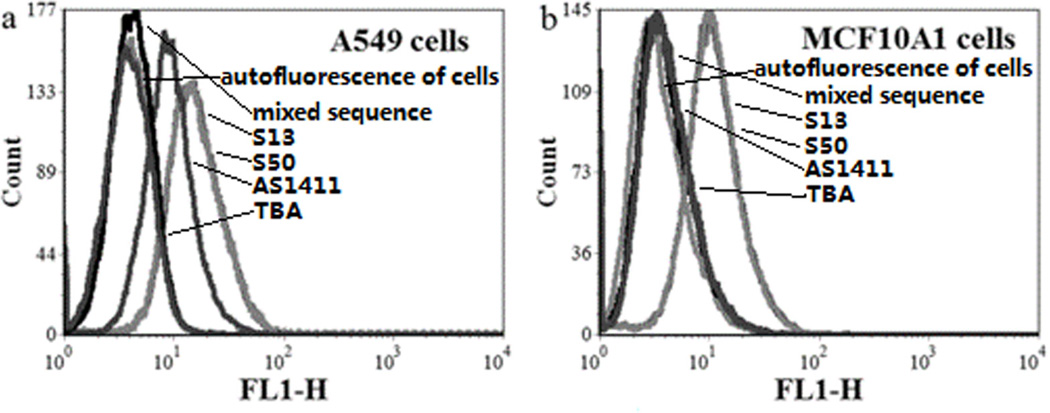
In our previous study, a panel of aptamers was was selected by cell-SELEX against EGFR-transfected A549 cell line, and their targets were suggested to be nucleolin.[24] Herein, two G-rich aptamer (R13 and R50) containing runs of contiguous guanine bases were chosen for further investigation. Due to the fact that G-rich DNA oligonucleotides could bind to cells[17], it was deduced that the G-rich domain in R13 and R50 also accounted for their binding ability to cells. To test this assumption, the G-rich domains (S13 and S50) of the parental aptamers (R13 and R50 in Table 1) were synthesized and evaluated for their binding ability to A549 cells with thrombin-binding aptamer (TBA) as comparison. As shown in Figure 1a and 1b, the two truncated G-rich aptamers, but not TBA, could bind to A549 cells at a concentration of 250 nM. However, the resulting fluorescence signal could be inhibited by 20-fold of their parental sequence, but not 20-fold of the mixed sequence (Table 1). When the G-rich domains were replaced with random bases, the two random parental sequences (Table 1) showed little binding to A549 cells (Figure 1). Thus, S13 and S50 were suggested as the core domains for the binding ability of the parental aptamers, respectively.
Table 1.
DNA sequences used in this study.
| Name | DNA sequences (5’→3’) |
|---|---|
| parental R13 | ACGCTCGGATGCCACTACAGTCTCTAGTTAT TGAGTTTTCTTTTATGGGTGGGTGGGGGGTT TTTCTCATGGACGTGCTGGTGAC |
| partental R50 | ACGCTCGGATGCCACTACAGTAAAGGGCGG GGGGTGGGGTGGTTGGTAGTTGTTTTTTCTG TTTTCTCACTCATGGACGTGCTGGTGAC |
| random R13 | ACGCTCGGATGCCACTACAGTCTCTAGTTAT TGAGTTTTCTTTTANNNNNNNNNNNNNNNTTT TTCTCATGGACGTGC TGGTGAC |
| random R50 | ACGCTCGGATGCCACTACAGTAAANNNNNNN NNNNNNNNNNNNNNNTAGTTGTTTTTTCTGTT TTCTCACTCATGGACGTGCTGGTGAC |
| S13 | TGG GTG GGT GGG GGG |
| S50 | GGG CGG GGG GTG GGG TGG TTG G |
| AS1411 | GGT GGT GGT GGT TGT GGT GGT GGT GG |
| Mixed sequence (Mixed) | GAC TGT ACC GAG CAA GTA CTC TAT |
| Thrombin-binding aptamer (TBA) | GGT TGG TGT GGT TGG |
Figure 1.
Flow cytometric analysis of the binding of the FAM-labeled G-rich oligonucleotides S13 (a) and S50 (b) to A549 cells in the presence of corresponding random sequence or parental aptamer. In the competition assay, FAM-labeled DNA sequences were 250 nM, and DNA sequences without modification were 5 µM. (c) Flow cytometric analysis of the binding of thrombin-binding aptamer (TBA) to A549 cells.
To investigate whether the truncated aptamers S13 and S50 could bind to other cell lines, HBE 135-E6E7 (normal human bronchial epithelial cell line), MCF10A1 (normal immortalized breast epithelial cell line) and MCF10CA1d (malignant breast cell line)[25] were used for the assay. AS1411 and TBA were also tested. The results are shown in Figure 2. Compared with the mixed sequence, TBA showed little binding to the three cell lines at the concentration of 250 nM; however, two aptamers (S13 and S50) and AS1411 at 250 nM could bind to malignant breast MCF10CA1d cells and the two noncancer cell lines. From these data, it can be concluded that the targets of S13, S50 and AS1411 are expressed not only on cancer cell surfaces, but also on normal cell surfaces.
Figure 2.
Flow cytometric analysis of the binding of FAM-labeled oligonucleotides to MCF10CA1d cells (a), MCF10A1 cells (b) and HBE 135–E6E7 cells (c). FAM-labeled DNA sequences were 250 nM.
Competition between truncated aptamers and AS1411 on A549 cells
Due to the fact that the parental aptamers of S13 and S50 could bind to the 323-710 AA domain of nucleolin, which was suggested as the target of AS1411, competition assays between the truncated aptamers (S13 and S50) and AS1411 were carried out to help identify their binding sites. It can be seen from Figure 3 that 20-fold of truncated aptamers S13 and S50, but not 20-fold of the mixed sequence, completely blocked the binding of FAM-labeled AS1411 to A549. On the other hand, AS1411, but not the mixed sequence, blocked the binding of the truncated aptamers to A549 cells. The competition between truncated aptamers and AS1411 could also be seen on the K562 cell line (data not shown). The results showed that the truncated aptamers and AS1411 may share the same binding target site, and that nucleolin may be a potential target for the truncated aptamers.
Figure 3.
Competition assays between the truncated aptamers (S13 and S50) and AS1411 on A549 cells. In the competition assay, FAM-labeled DNA sequences were 250 nM, and DNA sequences without modification were 5 µM.
Circular dichroism (CD) spectroscopy of truncated aptamers
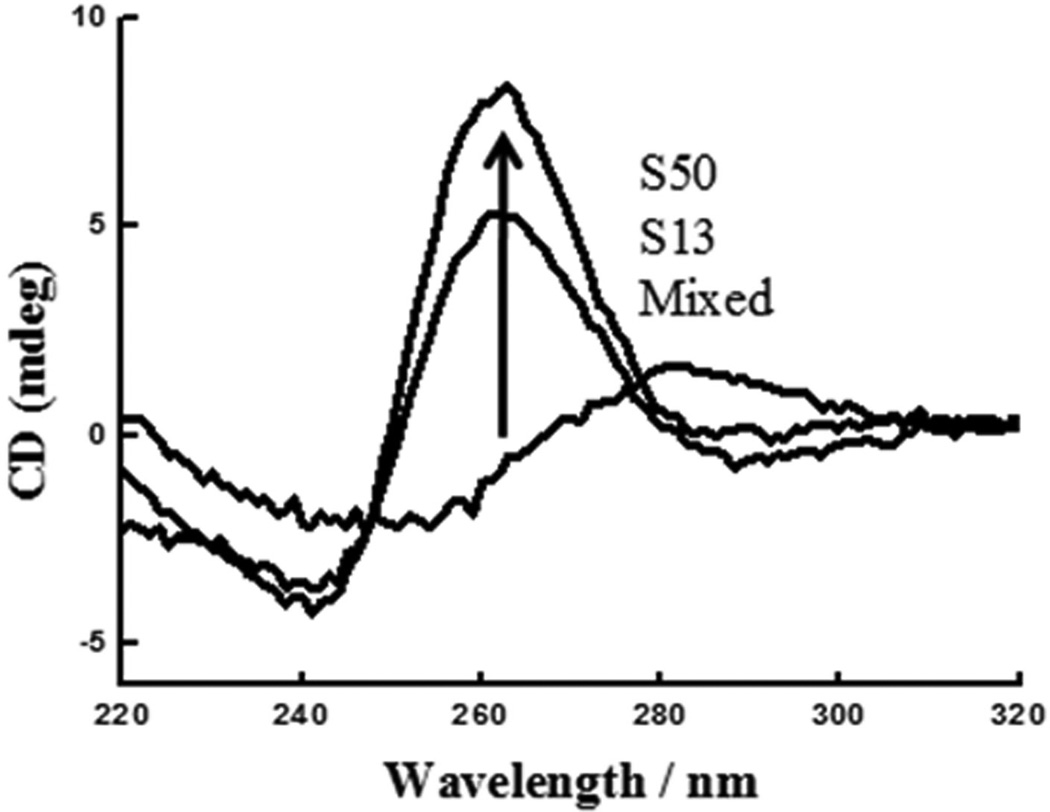
Circular dichroism (CD), originating from the difference of absorption of right- and left-handed circularly polarized light by a chiral substance, provides important information about the conformational characteristics of DNA. It is widely accepted that CD spectra of antiparallel quadruplexes are characterized by a positive ellipticity maximum at 295 nm and a negative minimum at 265 nm, while CD spectra of parallel quadruplexes have a positive maximum at 265 nm and negative minimum at 240 nm. According to a previous report,[25] TBA and AS1411 form antiparallel and antiparallel/parallel quadruplexes, respectively. The two truncated G-rich aptamers, S13 and S50, exhibited typical spectra of parallel quadruplexes with a positive peak at 264 nm and a negative peak at 245 nm in physiological buffer (D-PBS) (Figure 4). However, the mixed sequences showed a spectrum with a positive peak at ~280 nm and a negative peak at ~250 nm (Figure 4), which is typical of single-stranded or double-stranded DNA. It can be inferred from these CD spectra that the two G-rich aptamers bound to the cell surface in physiological buffer by forming parallel quadruplexes.[26]
Figure 4.
CD spectroscopy studies of 5 µM of G-rich oligonucleotides in D-PBS at 25°C with a path length of 1 mm. The curved lines at 260 nm from the bottom to top represent the CD spectroscopy of mixed sequence, S13 and S50, respectively.
Internalization studies of G-rich aptamers
For this study, the truncated aptamers labeled with FAM at the 3’-end with a spacer of 10 thymidine bases to avoid fluorophore interference were synthesized, and their uptake ability in A549 cells and MCF10A1 cells at 250 nM was evaluated by using FAM-labeled mixed sequence as control. It can be seen in Figure 5 that S13 and S50 could be internalized in A549 cells and MCF10A1 cells, and TBA could not enter the two cell lines at 250 nM. However, it was noted that AS1411 could enter A549 cells, but not MCF10A1 cells, even though it could bind to both of the cell lines. Confocal microscope imaging demonstrated that S13, S50 and AS1411 could be swiftly internalized into A549 cells in 20 min (data not shown). Moreover, flow cytometric analysis indicated that the cellular uptakes of S50 and AS1411 were not enhanced with increasing incubation time from 1 to 4 h (Figure 6); however, the cellular uptake of S13 reached a maximum at about 2 hours and then decreased with increasing incubation time from 2 to 4 hours. The time-independent internalization process is different from the receptor-mediated internalization process of atpamer Sgc8 into RRCF-CEM cells, in which the internalization increased with incubation time.[27]
Figure 5.
Flow cytometric analysis of the internalization of G-rich oligonucleotides in A549 cells (a) and MCF10A1 cells (b) at the concentration of 250 nM.
Figure 6.
Internalization dynamic analysis of S13, S50 and AS1411 in A549 cells.
A colocalization experiment was also performed to study the internalization pathway of the truncated aptamers in A549 cells. After incubation with FAM-labeled truncated aptamers and either Alexa Fluor 633 conjugated to transferrin from human serum or LysoTracker Red DND-99, either one of which is typically used as an indicator of endosomes and lysosomes, respectively, The A549 cells were analyzed by confocal microscopy. As seen in Figure 7, the truncated aptamer was clearly in the cytoplasm, but little intracellular colocalization of aptamer and respective indicator could be observed. These results show that neither endosome nor lysosome is in the internalization pathway of S13 and S50, in agreement with previous reports on the internalization pathway of AS1411.[28, 29]
Figure 7.
Studies of the internalization pathway of G-rich sequences (S13 and S50) in A549 cells by colocalization with endosome indicator Alexa Fluor 633 conjugated with transferrin from human serum or lysosome indicator Red DND-99. Bars indicated 10 µm.
Antiproliferation studies of truncated aptamers
To determine whether the truncated G-rich aptamers could inhibit the growth of cancer cells, the S13 and S50, as well as TBA and AS1411, were tested without any modification, and their antiproliferative activity was assessed by MTS assay. The effect of incubation time on the antiproliferative activity of truncated aptamers at 10 µM was first investigated. During the first 4-day incubation, A549 cells treated with S50 or the positive control AS1411 showed late proliferation, compared with the cells treated with PBS, the mixed sequences or S13 aptamer. However, the cells treated with the two G-rich sequences (S50 and AS1411) also showed rapid growth on the fifth day (Figure 8a). Therefore, the cells treated for 96 h with G-rich sequence were used to test the antiproliferation effect of G-rich sequences.
Figure 8.
MTS assays of the antiproliferation activity of G-rich sequences. (a) Effect of incubation time of G-rich sequences on the growth of A549 cells; (b) Dose effect of G-rich sequences on the growth of A549 cells after 96-h incubation (△ mixed, ▲ S13, ○ S50, ●AS1411); (c) The antiproliferation of TBA on A549 cells at 0 µM, 5 µM, 10 µM, 20 µM; Antiproliferation activity of 20 µM of 5 kinds of DNA sequences on malignant breast MCF10CA1d cells (d) and normal immortalized breast epithelial MCF10A1 cells (e).
The concentration effect of G-rich sequences on antiproliferation was also tested (Figure 8b). After treatment for 96 h with different concentrations of G-rich sequences, the number of viable A549 cells was measured. It can be seen that the antiproliferative effects of S50 and AS1411 are concentration-dependent. The antiproliferation effect increases swiftly with increasing concentration of DNA from 1 µM to 4 µM, and then it reaches a plateau at about 5 µM. However, S13 has little inhibitory effect on the growth of A549 cells even at 16 µM, even if it showed similar internalization in A549 cells with S50 (Figure S1a). It was noted that TBA had potent inhibitory effect on the growth of A549 cells (Figure 8c), even if it could not bind to A549 cells at 250 nM and also showed weak internalization at 5 µM (Figure S1a).
Malignant breast MCF10CA1d cell line and normal immortalized breast epithelial MCF10A1 cell line were also used for the antiproliferation assay. It can be seen from Figures 8d and 8e that aptamer S50 and AS1411, but not aptamer S13 and TBA, could inhibit the growth of MCF10CA1d cells; in addition, all the tested G-rich sequences have little antiproliferative effect on the normal MCF10A1 cell line at the concentration of 20 µM, even though S13, S50 and AS1411 could bind to and be internalized into MCF10A1 cells at 5 µM (Figure S1b).
Cancer-selective antiproliferative activity is a general property of G-rich oligonucleotides.[16, 17] Nucleolin was reported to be involved in antiproliferation as a potential target and cellular surface receptor of AS1411 and some G-rich oligonucleotides.[11, 15] In this report, competition between truncated aptamers (S13 and S50) and AS1411 indicated that nucleolin may also be the target of truncated aptamers. It was found that S13, S50 and AS1411 can bind not only to A549 and MCF10CA1d cancer cells, but also to normal HBE 135-E6E7 and MCF10A1 cells. However, distinct differences in antiproliferation were observed for these tested G-rich sequences. G-rich sequences S50 and AS1411 showed potent antiproliferative effect on A549 cells and MCF10CA1d cells, but not MCF10A1 cells. Aptamer S13 showed little antiproliferation effect on the cell lines tested. Thrombin-binding aptamer had potent antiproliferation on A549 cells, but not on the other two cell lines. These lines of evidence suggest that the cancer-selective antiproliferation of G-rich oliogonucleotides may be independent of their cellular binding.
Conclusion
In summary, we have studied two G-rich aptamers selected against EGFR-transfected A549 cells. Their G-rich domains are identified as the core of the parental aptamers. The binding, internalization and antiproliferation of the two truncated aptamers were studied in cancer cells amd noncancer cells and compared with nucleolin-binding AS1411 and thrombin-binding aptamer. Our observations suggest that cancer-selective antiproliferation of G-rich oligonucleotides may be independent of their cellular binding. These results provide new clues or tools for investigation of the functions of G-rich oligonucleotides.
Experimental Section
Cell culture
Lung adenocarcinoma A549 cell line (a gift from Prof. Richard W. Moyer of the Department of Molecular Genetics and Microbiology at the University of Florida) was cultured in MEM cell medium (Invitrogen) with 10% fetal bovine serum (FBS, Invitrogen) and 1% penicillin-streptomycin (Invitrogen). Normal human bronchial epithelial cell line (HBE 135-E6E7) was purchased from ATCC and cultured in cell medium recommended by ATCC. Malignant breast cells (MCF10CA1d cell line) and normal immortalized breast epithelial cells (MCF10A1 cell line) were kindly provided by Dr. Goodison Steven (Orlando Health). The two cell lines were cultured in D-MEM/F12 cell medium (Invitrogen) with 5% horse serum (Invitrogen) and 1% penicillin-streptomycin. MCF10A1 growth medium was supplemented with 20 ng/mL epidermal growth factor (EGF, Invitrogen), 10 ug/mL insulin (Sigma), 10 ng/mL cholera toxin (Sigma) and 500 ng/mL hydrocortisone (Sigma). All of the cell lines were incubated at 37°C in humid atmosphere with 5% CO2.
Synthesis, purification and preparation of DNA
All oligonucleotides (Table 1) and fluorophore-labeled DNA were synthesized by AB 3400 DNA synthesizer (Applied Biosystems, Foster City, CA, USA) and then purified by reversed-phase HPLC (Prostar, Varian, Walnut Creek, CA, USA) on a C-18 column after deprotection. The extinction coefficient of each oligonucleotide was obtained by mfold software (www.idtdna.com), and the concentration of DNA was determined with a Cary 100 Bio UV-vis spectrophotometer (Varian). For oligonucleotides without any modification, the oligonucleotides were dissolved in Dulbecco’s PBS (D-PBS, Invitrogen), sterilized by passing through a 0.2-µm filter (Corning Co.), and diluted with sterile D-PBS to 500 µM for use. For the truncated oligonucleotides with modification, Fluorescein (FAM) was labeled at the 3’-end of oligonucleotides. An additional 10 thymidine bases were incorporated between FAM and the G-rich oligonucleotides.
Flow cytometric analysis
Preparation of samples for the binding and the competition assays: After overnight incubation, the adherent cells were washed twice with D-PBS, detached with non-enzyme dissociation solution (MP Biomedicals), washed twice with D-PBS and then filtered by cell strainer with a 40-µm nylon mesh (BD). Fifty pmol of FAM-labeled ssDNA was incubated with 300,000 cells in 200 µL of binding buffer for 1 h at 4°C. Finally, the cells were washed twice with 500 µL binding buffer. The fluorescence intensity of cells was determined with a FACScan cytometer (BD Biosciences) by counting 20, 000 events. For the competitive binding assay, all the steps were the same as described above, except that the cells were incubated with 50 pmol of FAM-labeled aptamer and 1 nmol of unlabeled aptamers in 200 µL of binding buffer at 4°C.
Preparation of samples for the internalization assays: 2 × 105 tested cells in fresh complete medium were seeded in a 6-well plate for 24 hours. Then the medium was replaced with preheated cell medium with 10% FBS. FAM-labeled DNA sequences were added to the fresh cell medium to give a final concentration of 250 nM or 5 µM and incubated for at 37°C. After washing twice with preheated D-PBS at predesigned times, the cells were treated with trypsin-EDTA (Invitrogen) for 5 min. The detached cells were centrifuged and washed another two times with D-PBS with 1 mg/mL BSA (Sigma). Finally, the cell pellets were resuspended in D-PBS for flow cytometric analysis, as described above. All of the flow cytometric data were analyzed with FCS Express V3 software. Each experiment was replicated at least three times.
Fluorescence microscopy assays
The samples were prepared as described below: A549 cells were seeded in confocal dishes (Corning Co.) and incubated for 24 h. After two washes with preheated D-PBS, the cells were incubated with 250 nM FITC-labeled aptamers in 1 mL of fresh cell medium and 10% FBS for 30 min at 37°C, after which Alexa Fluor 633 conjugated with transferrin from human serum (5 µg/mL, Invitrogen), or LysoTracker Red DND-99 (50 nM, Invitrogen) was added and co-incubated for another 30 min at 37°C. After washing three times with D-PBS, the fluorescence images of internalization were collected on a FV500-IX81 confocal microscope (Olympus America Inc., Melville, NY) with 20× and 100× objectives (Olympus, Melville, NY). Excitation wavelength and emission filters were as follows: FAM, 488 nm laser line excitation, emission BP520_12 nm filter; TAMRA, 543 nm laser line excitation, emission BP580_20 nm filter; Alexa 633, 633 nm laser line excitation, emission LP650 filter.
Antiproliferation assay of truncated aptamers
Each cell line was seeded in a 96-well plate (Coring Co.) at about 500 cells per well. After incubation overnight, the cell medium was removed, and 200 µL of preheated fresh medium was added to each well. Then DNA sequences were directly added to the cell medium at designated concentrations. The culture medium was not changed until cell proliferation analysis. Cell proliferation was determined by using MTS assay (CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay, Promega). Briefly, after incubation, the cell medium containing oligonucleotides was replaced with 100 µL of preheated fresh cell medium, and then 20 µL of MTS solution was added to each well. Following 2 h of incubation, cell viability was determined by measuring the absorbance at 490 nm, using a 550 BioRad plate-reader (Bio-Rad, Hertfordshire, UK). Experiments were repeated in triplicate, and the error bars represent the standard derivations.
Circular dichroism spectroscopy
All G-rich DNA sequences were diluted to a concentration of 5 µM with D-PBS. Samples were denatured at 95°C for 10 min and cooled on ice until analysis. Circular dichroism (CD) spectra were recorded from 340 to 220 nm at 25°C using an Aviv 202 Circular Dichroism spectrometer, 2 s response time, 5 nm bandwidth and 1-cm path length. The spectra were normalized to zero ellipticity at 320 nm.
Supplementary Material
Acknowledgments
We thank Prof. Steve Goodison who has provided us insights and also samples for this study. This work is supported by the National Key Scientific Program of China (2011CB911000), NSFC grants (NSFC 21405041, NSFC 21221003, NSFC 21327009), and China National Instrumentation Program 2011YQ03012412. The authors wish to thank Prof. Stephen Hagen of the Department of Physics, University of Florida, for assistance with CD data.
References
- 1.Henderson E, Hardin CC, Walk SK, Tinoco I, Jr, Blackburn EH. Cell. 1987;51:899–908. doi: 10.1016/0092-8674(87)90577-0. [DOI] [PubMed] [Google Scholar]
- 2.Siddiqui-Jain A, Grand CL, Bearss DJ, Hurley LH. Proc. Natl. Acad. Sci. USA. 2002;99:11593–11598. doi: 10.1073/pnas.182256799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rangan A, Fedoroff OY, Hurley LH. J. Biol. Chem. 2002;276:4640–4646. doi: 10.1074/jbc.M005962200. [DOI] [PubMed] [Google Scholar]
- 4.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 5.Balasubramanian S, Neidle S. Curr. Opin. Chem. Biol. 2009;13:345–353. doi: 10.1016/j.cbpa.2009.04.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anselmet A, Mayat E, Wietek S, Layer PG, Payrastre B, Massoulié J. FEBS Letters. 2002;510:175–180. doi: 10.1016/s0014-5793(01)03248-3. [DOI] [PubMed] [Google Scholar]
- 7.Qi H, Lin CP, Fu X, Wood LM, Liu AA, Tsai YC, Chen Y, Barbieri CM, Pilch DS, Liu LF. Cancer Res. 2006;66:11808–11816. doi: 10.1158/0008-5472.CAN-06-1225. [DOI] [PubMed] [Google Scholar]
- 8.Bates PJ, Kahlon JB, Thomas SD, Trent JO, Miller DM. J. Biol. Chem. 1999;274:26369–26377. doi: 10.1074/jbc.274.37.26369. [DOI] [PubMed] [Google Scholar]
- 9.Goodchild A, King A, Gozar MM, Passioura T, Tucker C, Rivory L. Nucleic Acids Res. 2007;35:4562–4572. doi: 10.1093/nar/gkm465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bates PJ, Laber DA, Miller DM, Thomas SD, Trent JO. Exp. Mol. Pathol. 2009;86:151–164. doi: 10.1016/j.yexmp.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soundararajan S, Wang L, Sridharan V, Chen W, Courtenay-Luck N, Jones D, Spicer EK, Fernandes DJ. Mol. Pharmacol. 2009;76:984–991. doi: 10.1124/mol.109.055947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girvan AC, Teng Y, Casson LK, Thomas SD, Jüliger S, Ball MW, Klein JB, Pierce WM, Jr, Barve SS, Bates PJ. Mol. Cancer Ther. 2006;5:1790–1799. doi: 10.1158/1535-7163.MCT-05-0361. [DOI] [PubMed] [Google Scholar]
- 13.Xu X, Hamhouyia F, Thomas SD, Burke TJ, Girvan AC, McGregor WG, Trent JO, Miller DM, Bates PJ. J. Biol. Chem. 2001;276:43221–43230. doi: 10.1074/jbc.M104446200. [DOI] [PubMed] [Google Scholar]
- 14.Teng Y, Girvan AC, Casson LK, Pierce WM, Jr, Qian M, Thomas SD, Bates PJ. Cancer Res. 2007;67:10491–10500. doi: 10.1158/0008-5472.CAN-06-4206. [DOI] [PubMed] [Google Scholar]
- 15.Soundararajan S, Chen W, Spicer EK, Courtenay-Luck N N, Fernandes DJ. Cancer Res. 2008;168:2358–2365. doi: 10.1158/0008-5472.CAN-07-5723. [DOI] [PubMed] [Google Scholar]
- 16.Choi EW, Nayak LV, Bates PJ. Nucleic Acids Res. 2010;38:1623–1635. doi: 10.1093/nar/gkp1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dapić V, Abdomerović V, Marrington R, Peberdy J, Rodger A, Trent JO, Bates PJ. Nucleic Acids Res. 2003;31:2097–2107. doi: 10.1093/nar/gkg316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuerk C, Gold L. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 19.Ellington AD, Szostak JW. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 20.Blank M, Weinschenk T, Priemer M, Schluesener H. J. Biol. Chem. 2001;276:16464–16468. doi: 10.1074/jbc.M100347200. [DOI] [PubMed] [Google Scholar]
- 21.Daniels DA, Chen H, Hicke BJ, Swiderek KM, Gold L. Proc. Natl. Acad. Sci. USA. 2003;100:15416–15421. doi: 10.1073/pnas.2136683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerchia L, Ducongé F, Pestourie C, Boulay J, Aissouni Y, Gombert K, Tavitian B, de Franciscis V, Libri D. Plos, Biol. 2005;3:697–704. doi: 10.1371/journal.pbio.0030123. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Shangguan DH, Li Y, Tang ZW, Cao ZH, Chen HL, Mallikaratchy P, Sefah K, Yang CY, Tan WH. Proc. Natl. Acad. Sci. USA. 2006;103:11838–11843. doi: 10.1073/pnas.0602615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu L, Zhang Z, Zhao ZL, Liu QL, Tan WH, Fang XH. Am. J. Biomed. Sci. 2012;5:47–58. [Google Scholar]
- 25.Santner SJ, Dawson PJ, Tait L, Soule HD, Eliason J, Mohamed AN, Wolman SR, Heppner GH, Miller FR. Breast Cancer Res. Treat. 2001;65:101–110. doi: 10.1023/a:1006461422273. [DOI] [PubMed] [Google Scholar]
- 26.Chang T, Qi C, Meng J, Zhang N, Bing T, Yang X, Cao ZH, Shangguan DH. PLoS One. 2013;8:e62348. doi: 10.1371/journal.pone.0062348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao ZY, Shangguan DH, Cao ZH, Fang XH, Tan W WH. Chem. A. Eur. J. 2008;14:1769–1775. doi: 10.1002/chem.200701330. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Kube DM, Cooper MJ, Davis PB. Mol. Ther. 2008;16:333–342. doi: 10.1038/sj.mt.6300365. [DOI] [PubMed] [Google Scholar]
- 29.Reyes-Reyes EM, Teng Y, Bates PJ. Cancer Res. 2010;70:8617–8629. doi: 10.1158/0008-5472.CAN-10-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.