Abstract
Rupture of atherosclerotic plaques causing thrombosis is the main cause of acute coronary syndrome and ischemic strokes. Inhibition of thrombosis is one of the important tasks developing biomedical materials such as intravascular stents and vascular grafts. Shear stress (SS) influences the formation and development of atherosclerosis. The current review focuses on the vulnerable plaques observed in the high shear stress (HSS) regions, which localizes at the proximal region of the plaque intruding into the lumen. The vascular outward remodelling occurs in the HSS region for vascular compensation and that angiogenesis is a critical factor for HSS which induces atherosclerotic vulnerable plaque formation. These results greatly challenge the established belief that low shear stress is important for expansive remodelling, which provides a new perspective for preventing the transition of stable plaques to high-risk atherosclerotic lesions.
Keywords: high shear stress, angiogenesis, outward remodelling, vulnerable plaque, vascular smooth muscle cells
1. Introduction
The current review focuses on the vulnerable plaques observed in the HSS regions. Evidence is provided to support that ACS and ischemic strokes occur at or near the proximal region of the stenosis. Arterial diseases such as acute coronary syndrome (ACS) and ischemic strokes are the leading causes of death worldwide [1]. ACS and ischemic strokes are frequently caused by rupture of vulnerable plaque leading to thrombus formation and distal cessation of blood flow. The morpho-mechanical characteristics of vulnerable plaques are critical for their tendency to rupture [2, 3]. ACS and ischemic strokes often occur at sites where the stenosis level is lower than 50% [4, 5]. Atherosclerotic plaque rupture or damage of the vascular surface leads to incomplete or complete obstructive thrombus formation and ultimately cause ACS or ischaemic strokes [6–8]. Vulnerable plaques have the following pathological characteristics: (1) A huge lipoprotein core being larger than 40% of the plaque volume; (2) A thin fibrous cap [9]; (3) High content of inflammatory cells (including macrophages, T lymphocytes and mast cells) [10, 11, 13]; (4) Reduced number of vascular smooth muscle cells (VSMCs); and (5) Plenty of new born blood vessels in the plaques [12, 13].
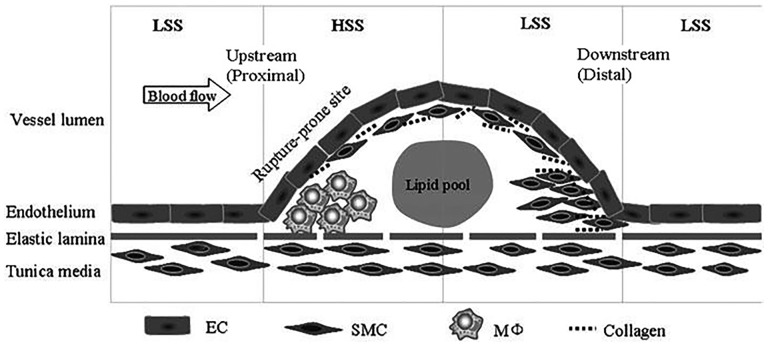
Shear stress participates in the formation of atherosclerosis, vascular remodelling, plaque stability, and restenosis after stent implantation and in intimal hyperplasia after blood vessel grafting [14, 15]. The magnitude and spatial distribution of SS change with the development of the plaque [16–18] (Table 1). When plaques protrude into the lumen, high shear stress (HSS) is formed at the proximal end of the stenosis whereas low shear stress (LSS) is formed at the distal part [16, 19 and 20] (Fig. 1).
Table 1.
The magnitude of HSS and LSS
Figure 1.
When plaques protrude into the lumen, high shear stress (HSS) is formed at the proximal end of the stenosis also whereas low shear stress (LSS) is formed at the distal part [16].
2. Vulnerable Plaque Animal Model for Shear Stress Research
Change of SS is a critical external factor for the plaque characteristics [21]. Therefore, a proper experimental model of atherosclerotic vulnerable plaques is fundamental for our understanding of SS-mediated vulnerable plaque formation [22]. One model used perivascular carotid collar placement, which rapidly induced atherosclerosis in apolipoprotein E-deficient or low-density lipoprotein receptor-deficient mice [23]. Our group has previously demonstrated the efficacy of this model in studying the development of atherosclerotic plaques induced by SS [24, 25, and 28]. The collar develops HSS in the proximal region and LSS in the distal region of the plaque similar to the plaques intruding into the lumen [26]. Cheng and coworkers improved the perivascular SS modifier that induces regions of lowered, increased and lowered/oscillatory shear stresses in mouse carotid arteries [27, 28].
Another model is ligation of the left external and internal carotid branches. In this situation left carotid blood flow is reduced to flow via the occipital artery. In response to partial ligation of the left carotid artery (LCA), blood flow significantly decreased by 90% in the LCA and increased by 70% in the right carotid artery (RCA) [29–31]. The major advantage of the first model is the similarity to well-defined plaques, the accelerated atherosclerosis formation and induction of at least two kinds of SS stimulations simultaneously. HSS occurs inside the stenosis and low and/or oscillatory SS is localized to the distal region of the stenosis. However, the change of SS in the proximal and the distal regions is more serious with the ligation model which can induce very LSS in the ligated LCA. However, the ligation model is not localized, i.e. the SS is changed throughout the vessel.
3. High Shear Stress Induces Vascular Outward Remodelling
Vascular remodelling encompasses chronic changes of the vascular lumen size and morphology, vessel wall structure, and vascular function [32]. SS induced vascular remodelling is a very complex process involving nitric oxide (NO) expression, extracellular matrix (ECM) synthesis and degradation, and VSMCs proliferation and migration.
3.1. High shear stress up-regulates the expression of NO
NO is an important vasodilator participant in vascular remodelling [33]. Endothelial cells (ECs) are the main sensor of the SS and also the critical player in vascular remodelling [34]. In the early stages of atherosclerosis, LSS occurs at the two sides of an arterial bifurcation and on the inside of vascular curvatures whereas HSS occurs at the apex of an arterial bifurcation [35] and on the outside of the vascular curvature [36]. In resistance arteries as well as in large blood vessels, chronic increase in blood flow enhances endothelial nitric oxide synthase (eNOS) expression and NO-dependent vasorelaxation [28, 32–39] whereas LSS decreases endothelial NO synthesis [28]. Furthermore, reduction in blood flow induces inward remodelling and reduced arteriolar contractility [33, 40]. Moreover, NO is essential for arterial outward hypertrophic remodelling after a chronic rise in flow [33, 41]. In addition, NO can induce ECM degradation through increasing the expression of matrix metalloproteinases (MMPs) [33]. This remodelling allows the effect of altered SS on the vascular wall to be normalized [42]. Therefore, HSS induces vascular outward remodelling through increasing NO expression [43, 44].
3.2. High shear stress induces the degradation of ECM
ECM synthesis and degradation plays an important role in vascular wall remodelling [45]. MMPs regulate vascular remodelling by ECM degradation [46]. Hence, the study of SS regulating the expression of MMPs can clarify the understanding of vascular remodelling under SS [47]. HSS induces MMPs expression and vascular outwards remodelling [48]. The likely mechanism involves NO in MMPs expression where HSS induces NO synthesis [28, 36–39] and NO increases the expression of MMP [49, 50]. HSS also induces secretion of plasmin (a strong specific activator protein for MMP-specific precursors secreted by macrophages) [51]. In addition, Pro-MMP-2, activated MMP-2, and proMMP-9 levels were modestly increased by high flow after 7 days [51]. Therefore, HSS may induce high MMPs expression [52]. MMPs promote plaque wall structural changes, severe internal elastic lamila (IEL) degradation. This provides a channel for inflammatory cell and SMC invasion which in turn produces intensive MMPs to degrade collagen and elastic fibres. These processes lead to severe wall and lumen expansion and may be the cause that the HSS region forms a thin fibrous cap in vulnerable plaque on the proximal side of the vascular stenosis [19, 53].
3.3 High shear stress induces the apoptosis of VSMCs
Under physiological conditions, SS does not directly act on VSMCs. However, when medial VSMCs migrate into intima after endothelial injury, they become directly exposed to blood flow [54]. The studies in apoE-deficient mice have revealed that VSMCs in atherosclerotic plaques are derived exclusively from the local vessel wall rather than from circulating progenitor cells [55]. LSS induces VSMCs migration into the intima in the ECs-VSMCs coculture model [56]. Therefore, LSS may be important for VSMCs proliferation and migration and for promoting blood vessel wall thickening which all are factors leading to atherosclerosis stenosis formation [57]. LSS-associated intimal hyperplasia was dependent on platelet endothelial cell adhesion molecule-1 (PECAM-1) [58], suggesting that PECAM-1 is necessary for flow-induced vascular remodelling.
High laminar SS inhibits SMCs proliferation and promotes the apoptosis of VSMCs [59]. This has been demonstrated as a direct factor for vulnerable plaque formation [60]. The finding is consistent with the clinical finding that apoptosis of VSMCs is mainly localized in the HSS region of the stenosis [61–63]. Therefore, vulnerable plaques are mainly found where SS is high because HSS induces apoptosis of VSMCs [48, 51, 64]. In vessel grafts, increasing SS inhibits smooth muscle cell proliferation and reduces intimal hyperplasia [65, 66]. The mechanism could be linked with Bone morphogenetic protein 4 (BMP4) [67] and NO [68] signalling pathways. HSS promotes release of endothelial NO mediating apoptosis of VSMCs [66, 69]. HSS also upregulated the expressions of NF-kappa B phosphorylation and MMP2 and MMP9, facilitating vascular outward remodelling [70]. SS induces vascular NADPH oxidase to comprise p47phox but not gp91phox. Generated Reactive oxygen species (ROS) interact with NO to produce peroxynitrite, which in turn activates MMPs and facilitates vessel remodelling [71]. ECs have an important regulatory role in the biological behaviour of VSMCs [72]. HSS promotes progressive arterial remodelling, which consequently causes blood vessel rupture [35, 73]. In summary, HSS induces adaptive and serious outward vascular remodelling through promoting apoptosis of VSMCs [74].
3.4 The remodelling process under shear stress
Systemic factors such as hyperlipidemia, hyperglycemia, and hypertension and genetics [75] exacerbate the local HSS and inflammatory response and may facilitate the transition of early atherosclerotic plaques into high-risk plaques. Vascular remodelling is governed to maintain the previous (normal) SS. For example the brachial artery remodels to maintain local SS despite the presence of cardiovascular risk factors [76].
After plaque formation and protrusion into the lumen, HSS is mainly apparent in the proximal region of the stenosis whereas LSS is at the distal region [19, 77]. HSS leads to the expansive remodelling [78, 79], which is a compensatory process [30, 80]. Expansive remodelling in response to chronic or repetitive flow increase involves a coordinated sequence of events in the arterial wall as extensively reviewed by others [81–83]. HSS induces aneurysmal remodelling through vascular expansive remodelling for maintaining the local SS [35, 36, 84]. Research showed that HSS increased the vascular diameter by 23%, while LSS reduced the diameter 23% [37, 85]. Outward remodelling is the critical factor for high-risk plaque formation [32, 86, 87]. NO release from ECs exposed to excessive shear is a fundamental step in the remodelling process. NO potentially triggers a cascade of events, including growth factor induction and MMP activation that together contribute to remodelling of the vessel wall [88]. Furthermore, high flow rates not only induce HSS but also increase cyclic strains which are found to induce arterial expansive remodelling [89]. Evaluation of vascular local SS and cyclic strain was used to predict vascular remodelling and plaque development [90].
Although several reports show that LSS promotes vascular expansive remodelling [27, 75, 91–93], both clinical and animal models prove that vascular expansive remodelling mainly localizes in the HSS region [29, 94]. The increased atherosclerotic wall thickness in HSS regions is associated with loss of compensatory remodelling [95]. Vascular remodelling maintains luminal SS stability; hence excessive outward expansion is the direct way to reduce the local HSS.
4. High Shear Stress Induces the Vulnerable Plaque Formation
In vivo colour mapping with intravascular ultrasound and magnetic resonance imaging (MRI) data show that coronary plaque rupture are localized in the arterial regions with elevated SS [64, 97–102] (Table 2). Animal models confirm that vulnerable plaques mainly occur in the HSS region of the stenosis [62, 103].
Table 2.
High shear stress induce rapture-prone plaque formation or rapture in clinical report
| Sample | Proximal | Shear stress | Phenomenon | Device (detected method) | Reference |
|---|---|---|---|---|---|
| Twenty patients | Proximal | High shear stress >25dyn/cm2 | Increase necrosis area | Virtual histology-IVUS and CFD | 100 |
| A 67-year-old woman | Proximal | High shear stress >32dyn/cm2 | Lipid/necrotic core, intraplaque hemorrhage | MRI at 10-month follow up | 97 |
| 20 patients | Proximal to the point of maximum stenosis | Blood wall pressure was 82 ± 18 mm Hg | Coronary plaquerupture | 3-dimensional IVUS | 98 |
| 119 patients | Proximal to the point of maximum stenosis | Higher than the distal | Ulceration | Angiographic ulceration | 103 |
| 42 human carotid atherosclerotic plaques | Proximal to the point of maximum stenosis | Higher than the distal | Apoptosis in the distal | Immunohistochemical (anti-CD31, anti-Ki-67) | 110 |
| 12 patients | Proximal | 38.9 versus 14.4 dyn/cm2 | Ruptured plaques | MRI | 101 |
| 12 patients | Proximal | >25dyn/cm2 | Angiography and IVUS | 102 |
A main difference between stable plaques and high-risk plaque is inflammatory cell accumulation [96, 97, 104, 105]. Inflammatory cell invasion into atherosclerotic plaques is modulated by ECs. The recruitment and infiltration of inflammatory cells into the endothelium are mediated by upregulating adhesion molecules, chemokines and integrins [98, 106, 107]. The viewpoint that LSS induces vulnerable plaque formation is based on the high expression of inflammation-related proteins on ECs [27, 107, 108]. However, LSS induces apoptosis of ECs and endothelial dysfunction [64, 109–112]. Hence, it is inconsistent with the established role of LSS in destabilizing atherosclerotic plaques regarding the expression and activity of MMPs [113]. In addition, LSS induces the VSMCs proliferation, migration and ECMs synthesis [114].
At present, the cross-sectional morphological characteristics of atherosclerotic plaque have been extensively investigated. However, less attention was paid to the axial distribution of plaques in the artery. Clinical pathology research shows that vascular plaque rupture mainly occurs in the proximal region of the stenosis, where macrophages aggregate and thrombosis is found under the endothelium [62, 103]. Connective tissue growth factor is released from platelets exposed to HSS and is differentially expressed in endothelium along atherosclerotic plaques [115]. In vivo MRI 3D FSI studies show that 63.5 dyn/cm2 SS induces high-risk plaque formation [116]. Taken together, these studies demonstrate that there is a high correlation between HSS and vulnerable plaque formation in the axial direction.
5. Angiogenesis May Be the Main Reason That High Shear Stress Induces Atherosclerotic Vulnerable Plaque Formation
A growing body of evidence shows that HSS prevails in the proximal region of atherosclerotic plaques protruding into the lumen [28, 117]. Significant differences in plaque morphology between the proximal and distal parts of plaques indicate a role in arterial flow in the distribution of different cell types [28, 53, 62, 98, 117, 118]. It was shown that 86% of ruptured plaques are located proximal to the stenosis [118]. The reason that atherosclerotic plaque rupture occurs in this region is currently unknown. Oxidized low-density lipoprotein was proposed, because oxLDL activates/induces subsets of smooth muscle cells and macrophages to gelatinase production [62]. However, it is well known that HSS is endothelium-protective and the endothelium may prevent the low-density lipoprotein (LDL) from entering into the vessel wall [19]. Furthermore, some studies showed that oxidized low-density lipoprotein (ox-LDL) is mainly accumulated in the distal region where SS is low [19, 62, 119].
Neovascularization in the vessel wall promotes the formation of atherosclerosis and vulnerable plaque development. The new vasa vasorum (VV) can transport cellular and soluble components such as red blood cells, inflammatory cells and lipid/lipoproteins into the vessel wall [120–122]. A recent report showed that bFGF and VEGFR-2 overexpression in the adventitia induced development of VV and accelerated plaque progression [122, 123]. Furthermore, most microvessels in atherosclerotic arteries were immature with abnormality of intraplaque microvascular ECs with incomplete endothelial junctions and membrane detachment. This may link the association between the microvascular leakage and intraplaque haemorrhage in advanced human coronary atherosclerosis [124, 125].
HSS plays a critical role in the expression of vascular endothelial growth factor (VEGF) [126] and endothelial NO synthesis [28, 34–36, 68]. VEGF induces angiogenesis [127] and also disrupts the vascular barrier function in diseased tissues [128]. NO mediates shear-induced angiogenesis in ECs [129] and increases vascular permeability [130]. Furthermore, the highest concentration of NO is also critical for the loss of VSMCs and ECM [131]. Thus, HSS causes the ECs to form tube-like structures and increases endothelial permeability by increasing the expression of VEGF and NO. The leaky vasculature with high endothelial permeability and without a restrictive basement membrane exhibits no adequate barrier function (Fig. 2).
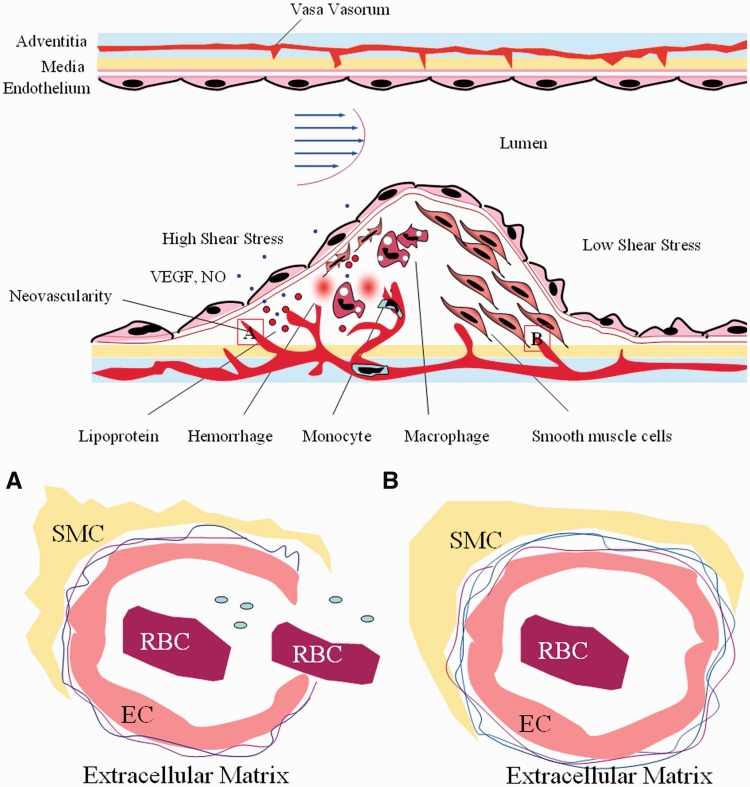
Figure 2.
High shear stress induces atherosclerotic vulnerable plaque formation through angiogenesis. High shear stress promotes the expression of vascular endothelial growth factor (VEGF) and endothelial nitric oxide (NO), resulting in angiogenesis of endothelial cells (EC) that form vasa vasorum and increases the endothelial cell permeability. Furthermore, NO induces smooth muscle cell (SMC) apoptosis and matrix degradation, resulting in loss of mural cells and the basement membrane around newborn microvessels. This results in microvascular leakage. The leaky vasculature becomes entry points for inflammatory cells, red blood cells (RBC) and lipid/lipoproteins. This may result in inflammation, intra-plaque haemorrhage, lipid core accumulation and eventually plaque rupture.
We propose that angiogenesis is the reason that vulnerable plaques are localized in HSS regions. Furthermore, NO induced smooth muscle cell apoptosis and matrix degradation. The result is loss of mural cell and basement membrane around newborn microvessels, causing microvascular leakage. The leaky vasculature becomes entry points for inflammatory cells, red blood cells and lipid/lipoproteins. This may result in inflammation, intra-plaque haemorrhage, lipid core accumulation and eventually plaque rupture.
6. The Mechanical Mechanism Underlying Plaque Rupture
As pointed out above, SS in the proximal region of stenosis is significantly higher than in the distal region. HSS is critical for vulnerable plaque [132]. Intraplaque haemorrhage is associated with higher SS and higher structural stresses in human atherosclerotic plaques as shown by in vivo examining MRI-based 3D fluid-structure interaction [133]. Numerical simulation shows that the SS in the proximal region of stenosis may reach 50-60 dyn/cm2 when the stenosis degree is 50%. The SS in the proximal region does not exceed 20 dyn/cm2 once 70% stenosis is reached. This may precipitate the rupture of vulnerable plaque in the proximal regions when less than 50% stenosis [130, 134]. Although increased SS in the proximal region may lead to plaque fibrous cap rupture, 75% of the plaque rupture is believed not to be due to SS since the wall SS is much smaller than tensile stress during the cardiac cycle [19].
The haemostatic system is a modulator of atherosclerosis [135]. HSS induces intra-thrombus fibrin deposition and platelet adhesion to the arterial wall [136–138]. HSS also promotes platelet aggregation [139]. Hence the haemostatic dysregulation caused by HSS may contribute to our understanding of why ACS and ischemic strokes are located preferentially in the distal region of the stenosis. SS rate is the rate change of the local SS and it is an important factor for vulnerable plaque rupture [136, 140]. Microfluidics is an important tool for blood clotting [141, 142] where platelets preferentially adhere in low-shear zones downstream of the formed thrombus, with stabilization of aggregates dependent on the dynamic restructuring of membrane tethers [143].
Under HSS conditions, blood pressure decreased and uniaxial tensile stress increased at the site of vascular injury. The magnitude of SS is smaller compared with the overall loading of plaques. Hence, pressure may be the main mechanical trigger for plaque rupture and risk stratification [144]. 3D critical plaque wall stress in prior rupture plaques is 100% higher than that for plaques that do not rupture. However, flow SS is 92.94 dyn/cm2 for rupture plaque, which is 76% higher than that for non-rupture plaques (52.70 dyn/cm2) [145]. Rupture sites in human atherosclerotic carotid plaques are associated with high structural stresses [146]. Once the thin fibrous cap is formed, the internal stress increased 200% when the fibrous cap thickness decreased by 50% [147]. These results demonstrate that intravascular haemodynamic factors are responsible for the progression of coronary atherosclerosis and development of vulnerable plaques [148]. Autopsy data have shown that there are obvious difference between circumferential plaque stress and vulnerable plaques. The plaque rupture zone is associated with a high degree of stress concentration [149]. Circumferential stress and Young's modulus are important direct factors for plaque rupture [150, 151]. Furthermore, plaque wall stress and flow SS may produce a significant uniaxial strain [152]. Research results have shown that the small pressure difference in the order of 20 mmHg can generate quite a high uniaxial strain in 75 μm thick plaques. Eccentric plaques would be exposed to a more serious uniaxial strain [153]. Hence HSS and the vessel wall thickness are also responsible for plaque rupture [154–156]. In summary, increased wall SS, circumferential stress and pressure are all important for plaque rupture, especially the pressure of the plaque. However, SS is closely related to plaque formation and progression [157].
7. Research Perspective
The current review focuses on the vulnerable plaques observed in the HSS regions. Evidence is provided to support that ACS and ischemic strokes occur at or near the proximal region of the stenosis. Having reviewed the published results in the literature, we noted that data on the relationship between SS and plaque rupture is contradictory and inconsistent. Previous research mainly focused on the biological function of SS, and less attention was paid to the mechanical properties of extracellular surroundings and the blood vessel itself [158]. The roles of blood vessels, vessel wall thickness and elastic modulus factors have been somewhat ignored considering plaque rupture [159]. From the literature reviews we can conclude that LSS is the main mechanical factor in plaque formation while HSS may be the main cause for the transition of stable plaques into inflamed lesions. Vascular mechanical stress may be the direct trigger for plaque rupture. How and when do those mechanical stresses function to regulate vulnerable plaque formation and destabilization? And what is the association between blood pressure and mechanical stresses? These issues remain uncertain, but it is quite necessary to further illuminate the molecular mechanisms underlying the plaque formation in response to SS [159–164]. SS and chemical stimuli may synergistically regulate vascular remodelling [165].
Currently, numerical analyses have been effectively used to simulate the physical and geometrical parameters characterizing the haemodynamics of various arteries during physiological and pathological conditions [166–168]. Numerical analysis can contribute to reveal the mechanism for development of plaques and predict the tendency for a plaque to rupture [169, 170]. Moreover, clinical imaging techniques such as magnetic resonance or computed tomography (CT) combined with numerical analysis methods have assisted considerably in gaining a detailed patient-specific picture of blood flow and structure dynamics, which could effectively prevent and treat this disease [171, 172].
8. Clinical Implications
SS changes with the degree of stenosis, and the changed stress regulates the development of plaques into high risk plaques [173]. Locally increased SS using a developed flow divider indicates that SS reduces in-stent neointimal formation by 50% [174, 175]. Attempts to increase SS to inhibit intimal hyperplasia are not applicable to atherosclerotic vulnerable plaque treatment [68] because HSS is the critical factor for high-risk coronary plaque formation. After the treatment of stenosis with percutaneous transluminal coronary angioplasty (PTCA) balloon and stent, the SS increases, which promotes vascular outward remodelling. This eventually leads to restenosis or even vulnerable plaque formation [176, 177]. Besides SS, the average wall shear stress (AWSS), average wall shear stress gradient (AWSSG), oscillatory shear index (OSI) and relative residence time (RRT) are important parameters for reducing the number of false positives. AWSS identifies the largest number of plaques, but produces more false positives than OSI and RRT [178]. It is necessary to increase the variety of detection methods, especially to pay attention to the proximal region of the vascular stenosis for detecting the SS [98, 179, 180]. Evaluation of the volume of the plaque is also an indirect method for the SS around the plaque [181]. A 3D fusion of intravascular ultrasound and coronary CT are useful for in-vivo wall SS analysis [182, 183]. It is necessary to combine optical CT tomography and coronary angioplasty in vivo for the evaluation of the connection between the SS and the characteristics of vulnerable plaques [180]. Regarding drug development, the regulatory effects of drugs on the SS should be cautiously considered; otherwise it may lead to more serious vascular disease [184, 185].
Lipid-lowering drugs may change the characteristics of plaques and the thickness of blood vessel wall and elastic modulus [186]. The vascular stiffness affects the sensitivity of ECs to SS and thereby participates in the regulation of vascular remodelling [187]. Changes in vascular cyclic stress can also influence SS-mediated vascular remodelling of VSMCs [188]. MRI assessment of plaque biomechanical properties including wall SS and internal plaque strain provides information on early plaque progression and vessel remodelling [189, 190]. More precise magnetic resonance, intravenous ultrasound (IVUS), CT and angiography were applied to analyse and predict plaque development and stability [191]. Morphological and mechanical features should also be considered in an integrated way for more accurate assessment of plaque vulnerability, allowing for early identification of plaques with inflamed phenotypes [191, 192]. Critical plaque stress/strain conditions are affected considerably by stenosis severity, eccentricity, lipid pool size, shape and position, plaque cap thickness, axial stretch, pressure, and fluid-structure interactions. These variables may be used for plaque rupture predictions [193–195].
If our hypothesis that angiogenesis is the main reason that high SS induces atherosclerotic vulnerable plaque formation is true, it may provide new perspectives for clinically predicting the location of plaques vulnerable to rupture and how to prevent plaque instability. Theoretical models could be developed to predict the relationship between the magnitude of SS and atherosclerosis plaque rupture. It also could be applied to arterial bypass grafting through selection of the most appropriate geometry to adjust the SS for reducing the formation of microvessels. Finally, previous studies have shown that plaque microvessels may serve as an interface for plaque expansion. Therefore, we can narrow the range of treatment strategy since plaque angiogenesis is primarily localized in the proximal plaque region.
In summary, SS has been shown to play a role in plaque formation, progression and rupture. The underlying mechanism of plaque formation seems to differ from plaque rupture. Plaque formation is localized in the LSS region whereas plaque rupture occurs primarily in HSS region. HSS induces up-regulation of NO and VEGF of ECs in the proximal region, which leads to microvessel formation in the plaque from VV. Moreover, the pathological angiogenesis is an entry point for infiltration of inflammatory cells, deposition of lipoproteins and the occurrence of intra-plaque haemorrhage. Decreasing the angiogenesis or the leaky vasculature [196, 197] induced by HSS may establish a more favourable microenvironment, which can impede vulnerable plaque formation.
Acknowledgements
This research program was supported by grants from the National Natural Science Foundation of China (31370949, 11332003, 81400329 and 11372364) and Chongqing Science and Technology Commission (cstc2013kjrc-ljrccj10003) as well as the Public Experiment Center of State Bioindustrial Base (Chongqing), China.
Conflict of interest statement. None declared.
References
- 1.Alan S. Go D, Mozaffarian Véronique L, et al. Heart Disease and Stroke Statistics—2013 Update A Report From the American Heart Association. Circulation 2013;127:e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen WQ, Zhang L, Liu YF, et al. Prediction of atherosclerotic plaque ruptures with high-frequency ultrasound imaging and serum inflammatory markers. Am. J Physiol Heart Circ Physiol 2007;293:H2836–44. [DOI] [PubMed] [Google Scholar]
- 3.Jacob FB, Fumiyuki O, Renu V, et al. Mechanisms of Plaque Formation and Rupture. Circ Res 2014;114:1852–66. [DOI] [PubMed] [Google Scholar]
- 4.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226–35. [DOI] [PubMed] [Google Scholar]
- 5.Glaser R, Selzer F, Faxon DP, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation 2005;111:143–9. [DOI] [PubMed] [Google Scholar]
- 6.Fuster V, Stein B, Ambrose JA, et al. Atherosclerotic plaque rupture and thrombosis. Evolving concepts. Circulation 1990;82:II47–59. [PubMed] [Google Scholar]
- 7.Zaman AG, Helft G, Worthley SG, et al. The role of plaque rupture and thrombosis in coronary artery disease. Atherosclerosis 2000;149:251–66. [DOI] [PubMed] [Google Scholar]
- 8.Ward MR, Pasterkamp G, Yeung AC, et al. Arterial remodeling. Mechanisms and clinical implications. Circulation 2000;102:1186–91. [DOI] [PubMed] [Google Scholar]
- 9.Jean-B M, José L, Martin V, et al. Pathology of human plaque vulnerability: Mechanisms and consequences of intraplaque haemorrhages. Atherosclerosis 2014;234:311–9. [DOI] [PubMed] [Google Scholar]
- 10.Buffon A, Biasucci LM, Liuzzo G, et al. Widespread coronary inflammation in unstable angina. N Engl J Med 2002;347:5–12. [DOI] [PubMed] [Google Scholar]
- 11.Russell CJ, Exley AR, Ritchie AJ. Widespread coronary inflammation in unstable angina. N Engl J Med 2003;348:1931.. [DOI] [PubMed] [Google Scholar]
- 12.Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 2003;349:2316–25. [DOI] [PubMed] [Google Scholar]
- 13.Moulton KS, Vakili K, Zurakowski D, et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci USA 2003;100:4736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest 2005;85:9–23. [DOI] [PubMed] [Google Scholar]
- 15.Anusha S, Maarten H, Paul H, et al. Biomechanical factors and macrophages in plaque stability. Cardiovasc Res 2013;99:284–93. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Yang Q, Wang Z, et al. Shear Stress in Atherosclerotic Plaque Determination. DNA Cell Biol 2014;33:1–9. [DOI] [PubMed] [Google Scholar]
- 17.Wentzel JJ, Chatzizisis YS, Gijsen FJ, et al. Endothelial shear stress in the evolution of coronary atherosclerotic plaque and vascular remodelling: current understanding and remaining questions. Cardiovasc Res 2012;96:234–43. [DOI] [PubMed] [Google Scholar]
- 18.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. Jama 1999;282:2035–42. [DOI] [PubMed] [Google Scholar]
- 19.Slager CJ, Wentzel JJ, Gijsen FJ, et al. The role of shear stress in the generation of rupture-prone vulnerable plaques. Nat Clin Pract Cardiovasc Med 2005;2:401–7. [DOI] [PubMed] [Google Scholar]
- 20.Siauw WL, Ngemail EYK, Mazumdar J. Unsteady stenosis flow prediction: a comparative study of non-Newtonian models with operator splitting scheme. Med. Eng. Phys 2000;22:265–77. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Granillo GA, Serruys PW, Garcia-Garcia HM, et al. Coronary artery remodelling is related to plaque composition. Heart 2006;92:388–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Getz GS, Reardon CA. Animal Models of atherosclerosis. Arterioscler Thromb Vasc Biol 2012;32:1104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von der Thusen JH, van Berkel TJ, Biessen EA. Induction of rapid atherogenesis by perivascular carotid collar placement in apolipoprotein E-deficient and low-density lipoprotein receptor-deficient mice. Circulation 2001;103:1164–70. [DOI] [PubMed] [Google Scholar]
- 24.Qiu JH, Wang GX, Hu JJ, et al. High endothelial shear stress preserves endothelial cell and leads to rupture-prone plaques (abstract). Hypertension 2010;56:1172. [Google Scholar]
- 25.Wei D, Wang G, Tang C, et al. Upregulation of SDF-1 is Associated with Atherosclerosis Lesions Induced by LDL Concentration Polarization. Ann Biomed Eng 2012;40:1018–27. [DOI] [PubMed] [Google Scholar]
- 26.Sadeghi MR, Shirani E, Tafazzoli-Shadpour M, et al. The effects of stenosis severity on the hemodynamic parameters-assessment of the correlation between stress phase angle and wall shear stress. J Biomech 2011;44:2614–26. [DOI] [PubMed] [Google Scholar]
- 27.Cheng C, Tempel D, van Haperen R, et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 2006;113:2744–53. [DOI] [PubMed] [Google Scholar]
- 28.Cheng C, van Haperen R, de Waard M, et al. Shear stress affects the intracellular distribution of eNOS: direct demonstration by a novel in vivo technique. Blood 2005;106:3691–8. [DOI] [PubMed] [Google Scholar]
- 29.Korshunov VA, Berk BC. Strain-dependent vascular remodeling: the “Glagov phenomenon” is genetically determined. Circulation 2004;110:220–6. [DOI] [PubMed] [Google Scholar]
- 30.Korshunov VA, Berk BC. Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arterioscler Thromb Vasc Biol 2003;23:2185–91. [DOI] [PubMed] [Google Scholar]
- 31.Ibrahim J, Berk BC. Flow-mediated vascular remodeling in hypertension: relation to hemodyamics. Stroke 2009;40:582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatzizisis YS, Coskun AU, Jonas M, et al. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol 2007;49:2379–93. [DOI] [PubMed] [Google Scholar]
- 33.Dumont O, Loufrani L, Henrion D. Key role of the NO-pathway and matrix metalloprotease-9 in high blood flow-induced remodeling of rat resistance arteries. Arterioscler Thromb Vasc Biol 2007;27:317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto K, Ando J. New molecular mechanisms for cardiovascular disease:blood flow sensing mechanism in vascular endothelial cells. J Pharmacol Sci 2011;116:323–31. [DOI] [PubMed] [Google Scholar]
- 35.Meng H, Wang Z, Hoi Y, et al. Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke 2007;38:1924–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metaxa E, Meng H, Kaluvala SR, et al. 2008 Nitric oxide-dependent stimulation of endothelial cell proliferation by sustained high flow. Am J Physiol Heart Circ Physiol 2007; 295:H736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freidja ML, Toutain B, Caillon A, et al. Heme oxygenase 1 is differentially involved in blood flow-dependent arterial remodeling: role of inflammation, oxidative stress, and nitric oxide. Hypertension 2011; 58:225–231. [DOI] [PubMed] [Google Scholar]
- 38.Jin ZG, Ueba H, Tanimoto T, et al. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res 2003; 93:354–363. [DOI] [PubMed] [Google Scholar]
- 39.Kemeny SF, Figueroa DS, Andrews AM, et al. Glycated collagen alters endothelial cell actin alignment and nitric oxide release in response to fluid shear stress. J Biomech 2011; 44:1927–1935. [DOI] [PubMed] [Google Scholar]
- 40.Pourageaud F, De Mey JG. Structural properties of rat mesenteric small arteries after 4-wk exposure to elevated or reduced blood flow. Am J Physiol 1997; 273:H1699–1706. [DOI] [PubMed] [Google Scholar]
- 41.Tronc F, Wassef M, Esposito B, et al. Role of NO in flow-induced remodeling of the rabbit common carotid artery. Arterioscler Thromb Vasc Biol 1996; 16: 1256–1262. [DOI] [PubMed] [Google Scholar]
- 42.Skalak TC, Price RJ. The role of mechanical stresses in microvascular remodeling. Microcirculation 1996; 3:143–165. [DOI] [PubMed] [Google Scholar]
- 43.Vita JA, Holbrook M, Palmisano J, et al. Flow-induced arterial remodeling relates to endothelial function in the human forearm. Circulation 2008; 117:3126–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen YS, Lu MJ, Huang HS, et al. Mechanosensitive transient receptor potential vanilloid type 1 channels contribute to vascular remodeling of rat fistula veins. J Vasc Surg 2010; 52:1310–1320. [DOI] [PubMed] [Google Scholar]
- 45.Ferri N, Carragher NO, Raines EW. Role of discoidin domain receptors 1 and 2 in human smooth muscle cell-mediated collagen remodeling: potential implications in atherosclerosis and lymphangioleiomyomatosis. Am J Pathol 2004; 164:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Nie L, Razavian M, et al. Molecular imaging of activated matrix metalloproteinases in vascular remodeling. Circulation 2008; 118:1953–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ota R, Kurihara C, Tsou TL, et al. Roles of matrix metalloproteinases in flow-induced outward vascular remodeling. J. Cereb Blood Flow Metab 2009; 29:1547–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wentzel JJ, Kloet J, Andhyiswara I, et al. Shear-stress and wall-stress regulation of vascular remodeling after balloon angioplasty: effect of matrix metalloproteinase inhibition. Circulation 2001; 104:91–96. [DOI] [PubMed] [Google Scholar]
- 49.Death AK, Nakhla S, McGrath KC, et al. Nitroglycerin upregulates matrix metalloproteinase expression by human macrophages. J Am Coll Cardiol 2002; 39:1943–1950. [DOI] [PubMed] [Google Scholar]
- 50.Wang HH, Hsieh HL, Yang CM. Nitric oxide production by endothelin-1 enhances astrocytic migration via the tyrosine nitration of matrix metalloproteinase-9. J Cell Physiol 2011; 226:2244–2256. [DOI] [PubMed] [Google Scholar]
- 51.Kenagy RD, Fischer JW, Davies MG, et al. Increased plasmin and serine proteinase activity during flow-induced intimal atrophy in baboon PTFE grafts. Arterioscler Thromb Vasc Biol 2002; 22:400–404. [DOI] [PubMed] [Google Scholar]
- 52.Haas TL, Doyle JL, Distasi MR, et al. Involvement of MMPs in the outward remodeling of collateral mesenteric arteries. Am J Physiol Heart Circ Physiol 2007; 293:H2429–2437. [DOI] [PubMed] [Google Scholar]
- 53.Samady H, Eshtehardi P, McDaniel MC, et al. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation 2011; 124:779–788. [DOI] [PubMed] [Google Scholar]
- 54.Ekstrand J, Razuvaev A, Folkersen L, et al. Tissue factor pathway inhibitor-2 is induced by fluid shear stress in vascular smooth muscle cells and affects cell proliferation and survival. J Vasc Surg 2010; 52:167–175. [DOI] [PubMed] [Google Scholar]
- 55.Bentzon JF, Weile C, Sondergaard CS, et al. Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler Thromb Vasc Biol 2006; 26:2696–2702. [DOI] [PubMed] [Google Scholar]
- 56.Sakamoto N, Ohashi T, Sato M. Effect of fluid shear stress on migration of vascular smooth muscle cells in cocultured model. Ann Biomed Eng 2006; 34:408–415. [DOI] [PubMed] [Google Scholar]
- 57.Duivenvoorden R, Vanbavel E, de Groot E, et al. Endothelial shear stress: a critical determinant of arterial remodeling and arterial stiffness in humans–a carotid 3.0-T MRI study. Circ Cardiovasc Imaging 2010; 3: 578–585. [DOI] [PubMed] [Google Scholar]
- 58.Chen Z, Tzima E. PECAM-1 is necessary for flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol 2009; 29:1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fitzgerald TN, Shepherd BR, Asada H, et al. Laminar shear stress stimulates vascular smooth muscle cell apoptosis via the Akt pathway. J Cell Physiol 2008; 216:389–395. [DOI] [PubMed] [Google Scholar]
- 60.Clarke MC, Figg N, Maguire JJ, et al. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med 2006; 12:1075–1080. [DOI] [PubMed] [Google Scholar]
- 61.Dirksen MT, van der Wal AC, van den Berg FM, et al. Distribution of inflammatory cells in atherosclerotic plaques relates to the direction of flow. Circulation 1998; 98:2000–2003. [DOI] [PubMed] [Google Scholar]
- 62.Segers D, Helderman F, Cheng C, et al. Gelatinolytic activity in atherosclerotic plaques is highly localized and is associated with both macrophages and smooth muscle cells in vivo. Circulation 2007; 115:609–616. [DOI] [PubMed] [Google Scholar]
- 63.Fagerberg B, Ryndel M, Kjelldahl J, et al. Differences in lesion severity and cellular composition between in vivo assessed upstream and downstream sides of human symptomatic carotid atherosclerotic plaques. J Vasc Res 2010; 47:221–230. [DOI] [PubMed] [Google Scholar]
- 64.Cicha I, Worner A, Urschel K, et al. Carotid Plaque Vulnerability: A Positive Feedback Between Hemodynamic and Biochemical Mechanisms. Stroke 2011; 42:3502–10. [DOI] [PubMed] [Google Scholar]
- 65.Kohler TR, Kirkman TR, Kraiss LW, et al. Increased blood flow inhibits neointimal hyperplasia in endothelialized vascular grafts. Circ Res 1991; 69:1557–1565. [DOI] [PubMed] [Google Scholar]
- 66.Berceli SA, Davies MG, Kenagy RD, et al. Flow-induced neointimal regression in baboon polytetrafluoroethylene grafts is associated with decreased cell proliferation and increased apoptosis. J Vasc Surg 2002; 36:1248–1255. [DOI] [PubMed] [Google Scholar]
- 67.Hsieh PC, Kenagy RD, Mulvihill ER, et al. Bone morphogenetic protein 4: potential regulator of shear stress-induced graft neointimal atrophy. J Vasc Surg 2006; 43:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y, He X, Chen X, et al. Enhanced external counterpulsation inhibits intimal hyperplasia by modifying shear stress responsive gene expression in hypercholesterolemic pigs. Circulation 2007; 116:526–534. [DOI] [PubMed] [Google Scholar]
- 69.Casey PJ, Dattilo JB, Dai G, et al. The effect of combined arterial hemodynamics on saphenous venous endothelial nitric oxide production. J Vasc Surg 2001; 33:1199–1205. [DOI] [PubMed] [Google Scholar]
- 70.Castier Y, Ramkhelawon B, Riou S, et al. Role of NF-kappaB in flow-induced vascular remodeling. Antioxid Redox Signal 2009; 11:1641–1649. [DOI] [PubMed] [Google Scholar]
- 71.Castier Y, Brandes RP, Leseche G, et al. p47phox-dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ Res 2005; 97:533–540. [DOI] [PubMed] [Google Scholar]
- 72.Kaneda H. Complex roles of endothelial shear stress in vascular remodeling response. J Am Coll Cardiol 2007; 50:2171–2172. [DOI] [PubMed] [Google Scholar]
- 73.Hoi Y, Meng H, Woodward SH, et al. Effects of arterial geometry on aneurysm growth: three-dimensional computational fluid dynamics study. J Neurosurg 2004; 101:676–681. [DOI] [PubMed] [Google Scholar]
- 74.Gao YJ, Yang LF, Stead S, et al. Flow-induced vascular remodeling in the mesenteric artery of spontaneously hypertensive rats. Can J Physiol Pharmacol 2008;86:737–44. [DOI] [PubMed] [Google Scholar]
- 75.Chatzizisis YS, Jonas M, Coskun AU, et al. Prediction of the localization of high-risk coronary atherosclerotic plaques on the basis of low endothelial shear stress: an intravascular ultrasound and histopathology natural history study. Circulation 2008;117:993–1002. [DOI] [PubMed] [Google Scholar]
- 76.Chung WB, Hamburg NM, Holbrook M, et al. The brachial artery remodels to maintain local shear stress despite the presence of cardiovascular risk factors. Arterioscler Thromb Vasc Biol 2009;29:606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hyun S, Kleinstreuer C, Archie JP., Jr., Hemodynamics analyses of arterial expansions with implications to thrombosis and restenosis. Med Eng Phys 2000;22:13–27. [DOI] [PubMed] [Google Scholar]
- 78.Ward MR, Jeremias A, Huegel H, et al. Accentuated remodeling on the upstream side of atherosclerotic lesions. Am J Cardiol 2000;85:523–6. [DOI] [PubMed] [Google Scholar]
- 79.Gijsen FJ, Wentzel JJ, Thury A, et al. A new imaging technique to study 3-D plaque and shear stress distribution in human coronary artery bifurcations in vivo. J Biomech 2007;40:2349–57. [DOI] [PubMed] [Google Scholar]
- 80.Silver AE, Vita JA. Shear-stress-mediated arterial remodeling in atherosclerosis: too much of a good thing? Circulation 2006;113:2787–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heil M, Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis). Circ Res 2004;95:449–58. [DOI] [PubMed] [Google Scholar]
- 82.Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol 2003;23:1143–51. [DOI] [PubMed] [Google Scholar]
- 83.Pasterkamp G, Galis ZS, de Kleijn DP. Expansive arterial remodeling: location, location, location. Arterioscler Thromb Vasc Biol 2004;24:650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Metaxa E, Tremmel M, Natarajan SK, et al. Characterization of critical hemodynamics contributing to aneurysmal remodeling at the basilar terminus in a rabbit model. Stroke 2010;41:1774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Freidja ML, Vessieres E, Clere N, et al. Heme oxygenase-1 induction restores high-blood-flow-dependent remodeling and endothelial function in mesenteric arteries of old rats. J Hypertens 2011;29:102–12. [DOI] [PubMed] [Google Scholar]
- 86.Papafaklis MI, Koskinas KC, Chatzizisis YS, et al. In-vivo assessment of the natural history of coronary atherosclerosis: vascular remodeling and endothelial shear stress determine the complexity of atherosclerotic disease progression. Curr Opin Cardiol 2010;25:627–38. [DOI] [PubMed] [Google Scholar]
- 87.Ivan E, Khatri JJ, Johnson C, et al. Expansive arterial remodeling is associated with increased neointimal macrophage foam cell content: the murine model of macrophage-rich carotid artery lesions. Circulation 2002;105:2686–91. [DOI] [PubMed] [Google Scholar]
- 88.Lehoux S, Tronc F, Tedgui A. Mechanisms of blood flow-induced vascular enlargement. Biorheology 2002;39:319–24. [PubMed] [Google Scholar]
- 89.Ben Driss A, Benessiano J, Poitevin P, et al. Arterial expansive remodeling induced by high flow rates. Am J Physiol 1997;272:H851–8. [DOI] [PubMed] [Google Scholar]
- 90.Wellnhofer E, Bocksch W, Hiemann N, et al. Shear stress and vascular remodeling: study of cardiac allograft coronary artery disease as a model of diffuse atherosclerosis. J Heart Lung Transplant 2002;21:405–16. [DOI] [PubMed] [Google Scholar]
- 91.Koskinas KC, Feldman CL, Chatzizisis YS, et al. Natural history of experimental coronary atherosclerosis and vascular remodeling in relation to endothelial shear stress: a serial, in vivo intravascular ultrasound study. Circulation 2010;121:2092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sun HW, Li CJ, Chen HQ, et al. Involvement of integrins, MAPK, and NF-kappaB in regulation of the shear stress-induced MMP-9 expression in endothelial cells. Biochem Biophys Res Commun 2007;353:152–8. [DOI] [PubMed] [Google Scholar]
- 93.Gambillara V, Montorzi G, Haziza-Pigeon C, et al. Arterial wall response to ex vivo exposure to oscillatory shear stress. J Vasc Res 2005;42:535–44. [DOI] [PubMed] [Google Scholar]
- 94.Stone PH, Coskun AU, Kinlay S, et al. Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and in-stent restenosis in humans: in vivo 6-month follow-up study. Circulation 2003;108:438–44. [DOI] [PubMed] [Google Scholar]
- 95.Wentzel JJ, Janssen E, Vos J, et al. Extension of increased atherosclerotic wall thickness into high shear stress regions is associated with loss of compensatory remodeling. Circulation 2003;108:17–23. [DOI] [PubMed] [Google Scholar]
- 96.Cheng C, de Crom R, van Haperen R, et al. The role of shear stress in atherosclerosis: action through gene expression and inflammation? Cell Biochem Biophys 2004;41:279–94. [DOI] [PubMed] [Google Scholar]
- 97.Groen HC, Gijsen FJ, van der Lugt A, et al. Plaque rupture in the carotid artery is localized at the high shear stress region: a case report. Stroke 2007;38:2379–81. [DOI] [PubMed] [Google Scholar]
- 98.Fukumoto Y, Hiro T, Fujii T, et al. Localized elevation of shear stress is related to coronary plaque rupture: a 3-dimensional intravascular ultrasound study with in-vivo color mapping of shear stress distribution. J Am Coll Cardiol 2008;51:645–50. [DOI] [PubMed] [Google Scholar]
- 99.Fujii K, Kobayashi Y, Mintz GS, et al. Intravascular ultrasound assessment of ulcerated ruptured plaques: a comparison of culprit and nonculprit lesions of patients with acute coronary syndromes and lesions in patients without acute coronary syndromes. Circulation 2003;108:2473–8. [DOI] [PubMed] [Google Scholar]
- 100.Corban MT, Eshtehardi P, Suo J, et al. Combination of plaque burden, wall shear stress, and plaque phenotype has incremental value for prediction of coronary atherosclerotic plaque progression and vulnerability. Atherosclerosis 2014;232:271–6. [DOI] [PubMed] [Google Scholar]
- 101.Tang D, Teng Z, Canton G, et al. Sites of Rupture in Human Atherosclerotic Carotid PlaquesAre Associated With High Structural Stresses-An In Vivo MRI-Based 3D Fluid-Structure Interaction Study. Stroke 2009;40:3258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frank JH, Gijsen JJ, Wentzel AT, et al. Strain distribution over plaques in human coronary arteries relates to shear stress. Am J Physiol Heart Circ Physiol 2008;295:1608–14. [DOI] [PubMed] [Google Scholar]
- 103.Lovett JK, Rothwell PM. Site of carotid plaque ulceration in relation to direction of blood flow: an angiographic and pathological study. Cerebrovasc Dis 2003;16:369–75. [DOI] [PubMed] [Google Scholar]
- 104.Ozaki K, Inoue K, Sato H, et al. Functional variation in LGALS2 confers risk of myocardial infarction and regulates lymphotoxin-alpha secretion in vitro. Nature 2004;429:72–5. [DOI] [PubMed] [Google Scholar]
- 105.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation 2004;109:II2–10. [DOI] [PubMed] [Google Scholar]
- 106.Helderman F, Segers D, de Crom R, et al. Effect of shear stress on vascular inflammation and plaque development. Curr Opin Lipidol 2007;18:527–33. [DOI] [PubMed] [Google Scholar]
- 107.Cheng C, Tempel D, van Haperen R, et al. Shear stress-induced changes in atherosclerotic plaque composition are modulated by chemokines. J Clin Invest 2007;117:616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Burns MP, DePaola N. Flow-conditioned HUVECs support clustered leukocyte adhesion by coexpressing ICAM-1 and E-selectin. Am J Physiol Heart Circ Physiol 2005;288:H194–204. [DOI] [PubMed] [Google Scholar]
- 109.Zeng Y, Qiao Y, Zhang Y, et al. Effects of fluid shear stress on apoptosis of cultured human umbilical vein endothelial cells induced by LPS. Cell Biol Int 2005;29:932–5. [DOI] [PubMed] [Google Scholar]
- 110.Tricot O, Mallat Z, Heymes C, et al. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation 2000;101:2450–3. [DOI] [PubMed] [Google Scholar]
- 111.Dimmeler S, Assmus B, Hermann C, et al. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ Res 1998;83:334–41. [DOI] [PubMed] [Google Scholar]
- 112.Gambillara V, Chambaz C, Montorzi G, et al. Plaque-prone hemodynamics impair endothelial function in pig carotid arteries. Am J Physiol Heart Circ Physiol 2006;290:H2320–8. [DOI] [PubMed] [Google Scholar]
- 113.Chatzizisis YS, Baker AB, Sukhova GK, et al. Augmented expression and activity of extracellular matrix-degrading enzymes in regions of low endothelial shear stress colocalize with coronary atheromata with thin fibrous caps in pigs. Circulation 2011;123:621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Palumbo R, Gaetano C, Antonini A, et al. Different effects of high and low shear stress on platelet-derived growth factor isoform release by endothelial cells: consequences for smooth muscle cell migration. Arterioscler Thromb Vasc Biol 2002;22:405–11. [DOI] [PubMed] [Google Scholar]
- 115.Cicha I, Yilmaz A, Suzuki Y, et al. Connective tissue growth factor is released from platelets under high shear stress and is differentially expressed in endothelium along atherosclerotic plaques. Clin Hemorheol Microcirc 2006;35:203–6. [PubMed] [Google Scholar]
- 116.Yang C, Canton G, Yuan C, et al. Advanced human carotid plaque progression correlates positively with flow shear stress using follow-up scan data: an in vivo MRI multi-patient 3D FSI study. J Biomech 2010;43:2530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fagerberg B, Ryndel M, Kjelldahl J, et al. Differences in lesion severity and cellular composition between in vivo assessed upstream and downstream sides of human symptomatic carotid atherosclerotic plaques. J Vasc Res 2009;4:221–30. [DOI] [PubMed] [Google Scholar]
- 118.Pedrigi RM, de Silva R, Bovens SM, et al. Thin-cap fibroatheroma rupture is associated with a fine interplay of shear and wall stress. Arterioscler Thromb Vasc Biol 2014;34:2224–31. [DOI] [PubMed] [Google Scholar]
- 119.Xie X, Tan J, Wei D, et al. In vitro and in vivo investigations on the effects of low-density lipoprotein concentration polarization and haemodynamics on atherosclerotic localization in rabbit and zebrafish. J R Soc Interface 2013;10:20121053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bot I, de Jager SC, Zernecke A, et al. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation 2007;115:2516–25. [DOI] [PubMed] [Google Scholar]
- 121.Virmani R, Kolodgie FD, Burke AP, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 2005;25:2054–61. [DOI] [PubMed] [Google Scholar]
- 122.Tanaka K, Nagata D, Hirata Y, et al. Augmented angiogenesis in adventitia promotes growth of atherosclerotic plaque in apolipoprotein E-deficient mice. Atherosclerosis 2011;215:366–73. [DOI] [PubMed] [Google Scholar]
- 123.Bhardwaj S, Roy H, Babu M, et al. Adventitial gene transfer of VEGFR-2 specific VEGF-E chimera induces MCP-1 expression in vascular smooth muscle cells and enhances neointimal formation. Atherosclerosis 2011;219:84–91. [DOI] [PubMed] [Google Scholar]
- 124.Sluimer JC, Kolodgie FD, Bijnens AP, et al. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J Am Coll Cardiol 2009;53:1517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Le Dall J, Ho-Tin-Noe B, Louedec L, et al. Immaturity of microvessels in haemorrhagic plaques is associated with proteolytic degradation of angiogenic factors. Cardiovasc Re 2010;85:184–93. [DOI] [PubMed] [Google Scholar]
- 126.Lucerna M, Zernecke A, de Nooijer R, et al. Vascular endothelial growth factor-A induces plaque expansion in ApoE knock-out mice by promoting de novo leukocyte recruitment. Blood 2007;109:122–9. [DOI] [PubMed] [Google Scholar]
- 127.Gee E, Milkiewicz M, Haas TL. p38 MAPK activity is stimulated by vascular endothelial growth factor receptor 2 activation and is essential for shear stress-induced angiogenesis. J Cell Physiol 2010;222:120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature 2005;437:497–504. [DOI] [PubMed] [Google Scholar]
- 129.Kolluru GK, Sinha S, Majumder S, et al. Shear stress promotes nitric oxide production in endothelial cells by sub-cellular delocalization of eNOS: A basis for shear stress mediated angiogenesis. Nitric Oxide 2010;22:304–15. [DOI] [PubMed] [Google Scholar]
- 130.Choi YS, Choi HJ, Min JK, et al. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood 2009;114:3117–26. [DOI] [PubMed] [Google Scholar]
- 131.Mattsson EJ, Kohler TR, Vergel SM, et al. Increased blood flow induces regression of intimal hyperplasia. Arterioscler Thromb Vasc Biol 1997;17:2245–9. [DOI] [PubMed] [Google Scholar]
- 132.Suh DC, Park ST, Oh TS, et al. High Shear Stress at the Surface of Enhancing Plaque in the Systolic Phase is Related to the Symptom Presentation of Severe M1 Stenosis. Korean J Radiol 2011;12:515–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang X, Teng Z, Canton G, et al. Intraplaque hemorrhage is associated with higher structural stresses in human atherosclerotic plaques: an in vivo MRI-based 3D fluid-structure interaction study. Biomed Eng Online 2010;9: 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li MX, Beech-Brandt JJ, John LR, et al. Numerical analysis of pulsatile blood flow and vessel wall mechanics in different degrees of stenoses. J Biomech 2007;40:3715–24. [DOI] [PubMed] [Google Scholar]
- 135.Borissoff JI, Spronk HM, ten Cate H. The hemostatic system as a modulator of atherosclerosis. N. Engl J Med 2011;364:1746–60. [DOI] [PubMed] [Google Scholar]
- 136.Hamada M, Sugimoto M, Matsui H, et al. Antithrombotic properties of pravastatin reducing intra-thrombus fibrin deposition under high shear blood flow conditions. Thromb Haemost 2011;105:313–20. [DOI] [PubMed] [Google Scholar]
- 137.Wohner N, Keresztes Z, Sotonyi P, et al. Neutrophil granulocyte-dependent proteolysis enhances platelet adhesion to the arterial wall under high-shear flow. J Thromb Haemost 2010;8:1624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Haynes LM, Dubief YC, Orfeo T, et al. Dilutional control of prothrombin activation at physiologically relevant shear rates. Biophys J 2011;100:765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stone PH, Coskun AU, Yeghiazarians Y, et al. Prediction of sites of coronary atherosclerosis progression: In vivo profiling of endothelial shear stress, lumen, and outer vessel wall characteristics to predict vascular behavior. Curr Opin Cardiol 2003;18:458–70. [DOI] [PubMed] [Google Scholar]
- 140.Lal BK, Beach KW, Sumner DS. Intracranial collateralization determines hemodynamic forces for carotid plaque disruption. J Vasc Surg 2011;54:1461–71. [DOI] [PubMed] [Google Scholar]
- 141.Runyon MK, Kastrup CJ, Johnson-Kerner BL, et al. Effects of shear rate on propagation of blood clotting determined using microfluidics and numerical simulations. J Am Chem Soc 2008;130:3458–64. [DOI] [PubMed] [Google Scholar]
- 142.Colace TV, Jobson J, Diamond SL. Relipidated Tissue Factor Linked to Collagen Surfaces Potentiates Platelet Adhesion and Fibrin Formation in a Microfluidic Model of Vessel Injury. Bioconjug Chem 2011;22:2104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nesbitt WS, Westein E, Tovar-Lopez FJ, et al. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med 2009;15:665–73. [DOI] [PubMed] [Google Scholar]
- 144.Li ZY, Taviani V, Tang T, et al. The mechanical triggers of plaque rupture: shear stress vs pressure gradient. Br J Radiol 2009;82:S39–45. [DOI] [PubMed] [Google Scholar]
- 145.Teng Z, Canton G, Yuan C, et al. 3D critical plaque wall stress is a better predictor of carotid plaque rupture sites than flow shear stress: An in vivo MRI-based 3D FSI study. J Biomech Eng 2010;132:031007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Andreou I, Antoniadis AP, Shishido K, et al. How do we prevent the vulnerable atherosclerotic plaque from rupturing? Insights from in vivo assessments of plaque, vascular remodeling, and local endothelial shear stress. J Cardiovasc Pharmacol Ther 2015;20:261–75. [DOI] [PubMed] [Google Scholar]
- 147.Li ZY, Gillard JH. Simulation of the interaction between blood flow and atherosclerotic plaque. Conf Proc IEEE Eng Med Biol Soc 2007;1699–702. [DOI] [PubMed] [Google Scholar]
- 148.Feldman CL, Stone PH. Intravascular hemodynamic factors responsible for progression of coronary atherosclerosis and development of vulnerable plaque. Curr Opin Cardiol 2000;15:430–40. [DOI] [PubMed] [Google Scholar]
- 149.Burleigh MC, Briggs AD, Lendon CL, et al. Collagen types I and III, collagen content, GAGs and mechanical strength of human atherosclerotic plaque caps: span-wise variations. Atherosclerosis 1992;96:71–81. [DOI] [PubMed] [Google Scholar]
- 150.Baldewsing RA, Schaar JA, Mastik F, et al. Local elasticity imaging of vulnerable atherosclerotic coronary plaques. Adv Cardiol 2007;44:35–61. [DOI] [PubMed] [Google Scholar]
- 151.Baldewsing RA, Mastik F, Schaar JA, et al. Young's modulus reconstruction of vulnerable atherosclerotic plaque components using deformable curves. Ultrasound Med Biol 2006;32:201–10. [DOI] [PubMed] [Google Scholar]
- 152.Doriot PA. Estimation of the supplementary axial wall stress generated at peak flow by an arterial stenosis. Phys Med Biol 2003;48:127–38. [DOI] [PubMed] [Google Scholar]
- 153.Slager CJ, Wentzel JJ, Gijsen FJ, et al. The role of shear stress in the destabilization of vulnerable plaques and related therapeutic implications. Nat Clin Pract Cardiovasc Med 2005;2:456–64. [DOI] [PubMed] [Google Scholar]
- 154.Wentzel JJ, Corti R, Fayad ZA, et al. Does shear stress modulate both plaque progression and regression in the thoracic aorta? Human study using serial magnetic resonance imaging. J Am Coll Cardiol 2005;45:846–54. [DOI] [PubMed] [Google Scholar]
- 155.Fukuda I, Minakawa M, Fukui K, et al. Breakdown of atheromatous plaque due to shear force from arterial perfusion cannula. Ann Thorac Surg 2007;84:e17–8. [DOI] [PubMed] [Google Scholar]
- 156.Carallo C, Lucca LF, Ciamei M, et al. Wall shear stress is lower in the carotid artery responsible for a unilateral ischemic stroke. Atherosclerosis 2006;185:108–13. [DOI] [PubMed] [Google Scholar]
- 157.Li ZY, Taviani V, Gillard JH. The impact of wall shear stress and pressure drop on the stability of the atherosclerotic plaque. Conf. Proc. IEEE Eng Med.Biol Soc 2008;1373–6. [DOI] [PubMed] [Google Scholar]
- 158.Yu F, Lee JY, Jen N, et al. Elevated electrochemical impedance in the endoluminal regions with high shear stress: Implication for assessing lipid-rich atherosclerotic lesions. Biosens Bioelectron 2013;43:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tang D, Yang C, Mondal S, et al. A negative correlation between human carotid atherosclerotic plaque progression and plaque wall stress: in vivo MRI-based 2D/3D FSI models. J Biomech 2008;41:727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dabek J, Kulach A, Gasior Z. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kappaB): a new potential therapeutic target in atherosclerosis? Pharmacol Rep 2010;62:778–83. [DOI] [PubMed] [Google Scholar]
- 161.Wang G, Qiu J, Hu J, et al. Id1: a novel therapeutic target for patients with atherosclerotic plaque rupture. Med Hypotheses 2011;76:627–8. [DOI] [PubMed] [Google Scholar]
- 162.Qiu J, Wang G, Hu J, et al. Id1-induced inhibition of p53 facilitates endothelial cell migration and tube formation by regulating the expression of beta1-integrin. Mol Cell Biochem 2011;357:125–33. [DOI] [PubMed] [Google Scholar]
- 163.Qiu J, Wang G, Peng Q, et al. Id1 induces tubulogenesis by regulating endothelial cell adhesion and cytoskeletal organization through beta1-integrin and Rho-kinase signalling. Int J Mol Med 2011;28:543–8. [DOI] [PubMed] [Google Scholar]
- 164.Qiu J, Wang G, Zheng Y, et al. Coordination of Id1 and p53 Activation by Oxidized LDL Regulates Endothelial Cell Proliferation and Migration. Ann Biomed Eng 2011;39:2869–78. [DOI] [PubMed] [Google Scholar]
- 165.Korshunov VA, Schwartz SM, Berk BC. Vascular remodeling: hemodynamic and biochemical mechanisms underlying Glagov's phenomenon. Arterioscler Thromb Vasc Biol 2007;27:1722–8. [DOI] [PubMed] [Google Scholar]
- 166.Do H, Owida AA, Yang W, et al. Numerical simulation of the haemodynamics in end-to-side anastomoses. Int J Numer Meth Fluids 2011;67:638–50. [Google Scholar]
- 167.Ng EYK, Siauw WL, Chong CK. Simulation of oscillatory wall shear stress in channels with moving indentations. Int J Numer Meth Engng 2002;54:1477–500. [Google Scholar]
- 168.Ng EYK, Siauw WL. Modelling of fluid-wall interactions for viscous flow in a stenotic elastic artery. Prog Comput Fluid Dy 2002;2:33–44. [Google Scholar]
- 169.Freshwater IJ, Morsi YS, Lai T. The effect of angle on wall shear stresses in a LIMA to LAD anastomosis: numerical modelling of pulsatile flow. Proc Inst Mech Eng H 2006;220:743–57. [DOI] [PubMed] [Google Scholar]
- 170.Ahmed Owida A, Doemail H, Morsi YS. Numerical analysis of coronary artery bypass grafts: an overview. Comput Methods Programs Biomed 2012;108:689–705. [DOI] [PubMed] [Google Scholar]
- 171.Keynton Robert S, Evancho Mary M, Sims RL. et al. Intimal Hyperplasia and Wall Shear in Arterial Bypass Graft Distal Anastomoses: An In Vivo Model Study. J Biomech Eng 2001;123:464–73. [DOI] [PubMed] [Google Scholar]
- 172.Soudah E, Ng EYK, Loong TH, et al. CFD modelling of abdominal aortic neurysm on hemodynamic loads using a realistic geometry with CT. Comput Math Method M 2013;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ge J, Erbel R, Gorge G, et al. High wall shear stress proximal to myocardial bridging and atherosclerosis: intracoronary ultrasound and pressure measurements. Br Heart J 1995;73:462–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wentzel JJ, Gijsen FJ, Stergiopulos N, et al. Shear stress, vascular remodeling and neointimal formation. J Biomech 2003;36:681–8. [DOI] [PubMed] [Google Scholar]
- 175.Takahashi S, Papafaklis MI, Sakamoto S, et al. The effect of statins on high-risk atherosclerotic plaque associated with low endothelial shear stress. Curr Opin Lipidol 2011;22:358–64. [DOI] [PubMed] [Google Scholar]
- 176.Thury A, van Langenhove G, Carlier SG, et al. High shear stress after successful balloon angioplasty is associated with restenosis and target lesion revascularization. Am Heart J 2002;144:136–43. [DOI] [PubMed] [Google Scholar]
- 177.Gaddam S, Rizvi M, Nimmagadda KC, et al. Proximal atherosclerotic lesion as a cause of very late stent thrombosis. Med Hypotheses 2011;76:500–2. [DOI] [PubMed] [Google Scholar]
- 178.Knight J, Olgac U, Saur SC, et al. Choosing the optimal wall shear parameter for the prediction of plaque location-A patient-specific computational study in human right coronary arteries. Atherosclerosis 2010;211:445–50. [DOI] [PubMed] [Google Scholar]
- 179.Wu Z, Yang C, Tang D. In vivo serial MRI-based models and statistical methods to quantify sensitivity and specificity of mechanical predictors for carotid plaque rupture: location and beyond. J Biomech Eng 2011;133:064503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bourantas CV, Papafaklis MI, Naka KK, et al. Fusion of optical coherence tomography and coronary angiography - In vivo assessment of shear stress in plaque rupture. Int J Cardiol 2011;155:e24–6. [DOI] [PubMed] [Google Scholar]
- 181.Gossl M, von Birgelen C, Mintz GS, et al. Volumetric assessment of ulcerated ruptured coronary plaques with three-dimensional intravascular ultrasound in vivo. Am J Cardiol 2003;91:992–6. [DOI] [PubMed] [Google Scholar]
- 182.van der Giessen AG, Schaap M, Gijsen FJ, et al. 3D fusion of intravascular ultrasound and coronary computed tomography for in-vivo wall shear stress analysis: a feasibility study. Int J Cardiovasc Imaging 2010;26:781–96. [DOI] [PubMed] [Google Scholar]
- 183.Gijsen FJ, Schuurbiers JC, van de Giessen AG, et al. 3D reconstruction techniques of human coronary bifurcations for shear stress computations. J Biomech 2014;47:39–43. [DOI] [PubMed] [Google Scholar]
- 184.Kastelein JJ, van Leuven SI, Burgess L, et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med 2007;356:1620–30. [DOI] [PubMed] [Google Scholar]
- 185.Nissen SE, Tardif JC, Nicholls SJ, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med 2007;356:1304–16. [DOI] [PubMed] [Google Scholar]
- 186.Yamagishi T, Kato M, Koiwa Y, et al. Evaluation of plaque stabilization by fluvastatin with carotid intima- medial elasticity measured by a transcutaneous ultrasonic-based tissue characterization system. J Atheroscler Thromb 2009;16:662–73. [DOI] [PubMed] [Google Scholar]
- 187.Song S, Kim M, Shin JH. Upstream mechanotaxis behavior of endothelial cells. Conf. Proc. IEEE Eng Med Biol Soc 2009;2106–10. [DOI] [PubMed] [Google Scholar]
- 188.Thacher T, da Silva RF, Stergiopulos N. Differential effects of reduced cyclic stretch and perturbed shear stress within the arterial wall and on smooth muscle function. Am J Hypertens 2009;22:1250–7. [DOI] [PubMed] [Google Scholar]
- 189.Kerwin WS, Canton G. Advanced techniques for MRI of atherosclerotic plaque. Top Magn Reson Imaging 2009;20:217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zheng J, Abendschein DR, Okamoto RJ, et al. MRI-based biomechanical imaging: initial study on early plaque progression and vessel remodeling. Magn Reson Imaging 2009;27:1309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sadat U, Teng Z, Gillard JH. Biomechanical structural stresses of atherosclerotic plaques. Expert Rev Cardiovasc Ther 2010;8:1469–81 [DOI] [PubMed] [Google Scholar]
- 192.Chaniotis AK, Kaiktsis L, Katritsis D, et al. Computational study of pulsatile blood flow in prototype vessel geometries of coronary segments. Phys Med 2010;26:140–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Keshavarz-Motamed Z, Saijo Y, Majdouline Y, et al. Coronary artery atherectomy reduces plaque shear strain s:An endovascular elastography imaging study. Atherosclerosis 2014;235:140–9. [DOI] [PubMed] [Google Scholar]
- 194.Tang D, Yang C, Kobayashi S, et al. Effect of a lipid pool on stress/strain distributions in stenotic arteries: 3-D fluid-structure interactions (FSI) models. J Biomech Eng 2004;126:363–70. [DOI] [PubMed] [Google Scholar]
- 195.Tousoulis D, Papageorgiou N, Synetos A, et al. Assessing vulnerable plaque: Is shear stress enough? Int J Cardio 2013;172:135–8. [DOI] [PubMed] [Google Scholar]
- 196.Mause SF, Weber C. Intrusion through the fragile back door immature plaque microvessels as entry portals for leukocytes and erythrocytes in atherosclerosis. J Am Coll Cardiol 2009;53:1528–31. [DOI] [PubMed] [Google Scholar]
- 197.Schwabl P, Payer BA, Grahovac J, et al. Pioglitazone decreases portosystemic shunting by modulating inflammation and angiogenesis incirrhotic and non-cirrhotic portal hypertensive rats. J Hepatol 2014;60:1135–42. [DOI] [PubMed] [Google Scholar]