Abstract
BACKGROUND. Administration of conventional antithrombotic treatment (low-dose aspirin plus low–molecular weight heparin [LDA+LMWH]) for obstetric antiphospholipid syndrome (APS) does not prevent life-threatening placenta insufficiency–associated complications such as preeclampsia (PE) and intrauterine growth restriction (IUGR) in 20% of patients. Statins have been linked to improved pregnancy outcomes in mouse models of PE and APS, possibly due to their protective effects on endothelium. Here, we investigated the use of pravastatin in LDA+LMWH-refractory APS in patients at an increased risk of adverse pregnancy outcomes.
METHODS. We studied 21 pregnant women with APS who developed PE and/or IUGR during treatment with LDA+LMWH. A control group of 10 patients received only LDA+LMWH. Eleven patients received pravastatin (20 mg/d) in addition to LDA+LMWH at the onset of PE and/or IUGR. Uteroplacental blood hemodynamics, progression of PE features (hypertension and proteinuria), and fetal/neonatal outcomes were evaluated.
RESULTS. In the control group, all deliveries occurred preterm and only 6 of 11 neonates survived. Of the 6 surviving neonates, 3 showed abnormal development. Patients who received both pravastatin and LDA+LMWH exhibited increased placental blood flow and improvements in PE features. These beneficial effects were observed as early as 10 days after pravastatin treatment onset. Pravastatin treatment combined with LDA+LMWH was also associated with live births that occurred close to full term in all patients.
CONCLUSION. The present study suggests that pravastatin may improve pregnancy outcomes in women with refractory obstetric APS when taken at the onset of PE or IUGR until the end of pregnancy.
Introduction
Antiphospholipid syndrome (APS) is characterized by a variety of clinical and immunological manifestations. The clinical hallmarks of this syndrome are thrombosis and poor obstetric outcomes in the presence of antiphospholipid (aPL) antibodies (1). Pregnancy complications in APS (recurrent unexplained abortions, spontaneous fetal loss, preeclampsia [PE], and premature birth) have been attributed to placental thrombosis and infarcts, and management of these patients is based on attenuating the procoagulant state. However, in many cases, there is no evidence of decidual thrombosis or placental vasculopathy, and instead, inflammatory signs are present (2–4). Treatment with low-dose aspirin (LDA) and heparinoids has become a conventional option for pregnant women with APS. While the use of LDA and heparinoids has improved pregnancy outcome in these women, current treatment fails in a significant number of pregnancies (5, 6), raising the need to explore other treatments to improve obstetrical outcome. In particular, antithrombotic therapy has been shown to be ineffective in preventing PE in women with APS. A recent study has shown that women with APS that received conventional LDA plus low–molecular weight heparin (LDA+LMWH) treatment throughout pregnancy had higher rates of PE than control women, suggesting that antithrombotic therapy is unsuccessful in preventing placental insufficiency and associated maternal and fetal risks (7). In this line, our mouse studies suggest that modulating inflammation might be a more effective approach than antithrombotic therapy (8).
PE and intrauterine growth restriction (IUGR) — associated with impaired placentation — are obstetric complications frequently observed in APS (9–12). Both conditions are major causes of maternal and fetal morbidity worldwide, with uncertain prevention and management (13, 14). Ten million women develop PE each year worldwide; from these instances, approximately 76,000 pregnant women and 500,000 babies die (15–17). The only effective treatment to date is delivery of the fetus and the placenta, and current therapy options are predominantly symptomatic and not directed specifically to the underlying causes. Although delivery is always beneficial for the mother, it may not be optimal for the fetus, as it might be extremely premature.
Efforts to reduce the risks of placenta insufficiency in APS remain critically important, and developing effective pharmacological strategies will be of significant clinical benefit for mothers and fetuses.
Clinical studies suggest that the main cause of placental insufficiency is a disturbance in uteroplacental circulation. Although placenta malperfusion is unique to pregnancy, there are many biological and pathological similarities as well as risk factors in common with cardiovascular disease (CVD). Normal development of maternal hyperlipidemia of pregnancy is exaggerated in PE (18). In addition, acute atherosis affects uteroplacental spiral arteries in 20% to 40% of cases of PE and resembles the early stages of atherosclerosis characterized by lipid deposition and inflammatory cells in the walls of maternal uterine arteries (19, 20). Thus, both conditions, PE and atherosclerotic CVD, are characterized by arterial foam cell deposition, endothelial dysfunction, oxidative stress, and inflammation. Moreover, patients with aPL antibodies have a high rate of atherosclerosis (21) and probably also high rates of early gestation development of uteroplacental acute atherosis (22, 23).
Statins block the biosynthesis of cholesterol, and their favorable effects have been well established for the prevention of atherosclerotic CVD. However, the benefit achieved with statin treatment in patients with hypercholesterolemia cannot be attributed to their cholesterol-lowering effect alone. Indeed, lipid-independent pleiotropic effects, including endothelial protection and regulation of immune, inflammatory, and procoagulant responses, have been described (24).
Studies in animal models support the hypothesis that statins may be an effective means of preventing pregnancy complications related to APS and PE (25–29). Pravastatin has been shown to prevent adverse pregnancy outcomes in mouse models of APS and PE by diminishing inflammation, increasing placental blood flow, and reversing angiogenic and redox imbalances (26, 28, 29). Therefore, animal models and the similarities between PE and CVD provide a strong basis for the use of statins in the prevention of placental insufficiency in APS in humans. We have previously reported the clinical improvement in a preeclamptic APS patient treated with pravastatin (30). Similarly, a recent study has reported the successful use of pravastatin in preventing intrauterine fetal death (IUFD) in a case of massive perivillous fibrin deposition in the placenta (31). Another recent study has shown that pravastatin stabilized clinical and biochemical features of PE in 4 women (32). Statins have been shown not to be teratogenic as demonstrated by several studies (33, 34). A study by Costantine et al. reported no identifiable safety risks associated with pravastatin use in a pilot randomized trial designed to determine pravastatin safety and pharmacokinetic parameters in pregnant women at high risk of PE (33). Finally, a recent landmark study did not find congenital malformations and organ-specific malformations in the offspring of over 800,000 women exposed to statins during the first trimester of pregnancy (34).
The objective of this study was to assess pregnancy outcome in women with APS that developed PE and/or IUGR despite the use of LDA+LMWH, with additional pravastatin given at the time of the onset of placental insufficiency.
Results
Twenty-one women with persistent aPL antibodies participated in this study. Most women met APS criteria before the current pregnancy, and a few of them met criteria during the current pregnancy (Tables 1 and 2). All women had a poor obstetric history, as can be observed in Tables 1 and 2. Regardless of the fact that some women did not fulfill the criteria for APS at the beginning of the current pregnancy, all women were treated with antithrombotic therapy from the time of the first positive pregnancy test (35). The live birth rate prior to this study in the control group was 3.5% and 8% in the group that received pravastatin. All women were treated with LMWH (enoxaparin or tinzaparin, 40 mg subcutaneously, once daily) plus LDA (80 mg orally, once daily). Despite treatment, all of the patients developed PE and/or IUGR (Tables 1 and 3). Most of the patients were diagnosed with early PE (21–23 weeks) and received methyldopa (MDP) to treat hypertension. None of the 21 patients had underlying chronic hypertension, and all presented normal blood pressure (BP) values prior to the onset of PE. Increased impedance to blood flow in the uterine arteries as shown by a pulsatility index (PI) above the 95th percentile — frequently associated with pregnancy complications associated with defective trophoblast invasion — (36, 37) was observed in all women during Doppler assessment of uteroplacental perfusion (Tables 1 and 2, and Figure 1). Presence of diastolic notching (a characteristic waveform indicating decreased early diastolic flow) in the uterine artery reflects low vessel elasticity (37). Bilateral diastolic notching in uterine arteries was observed in 7 patients.
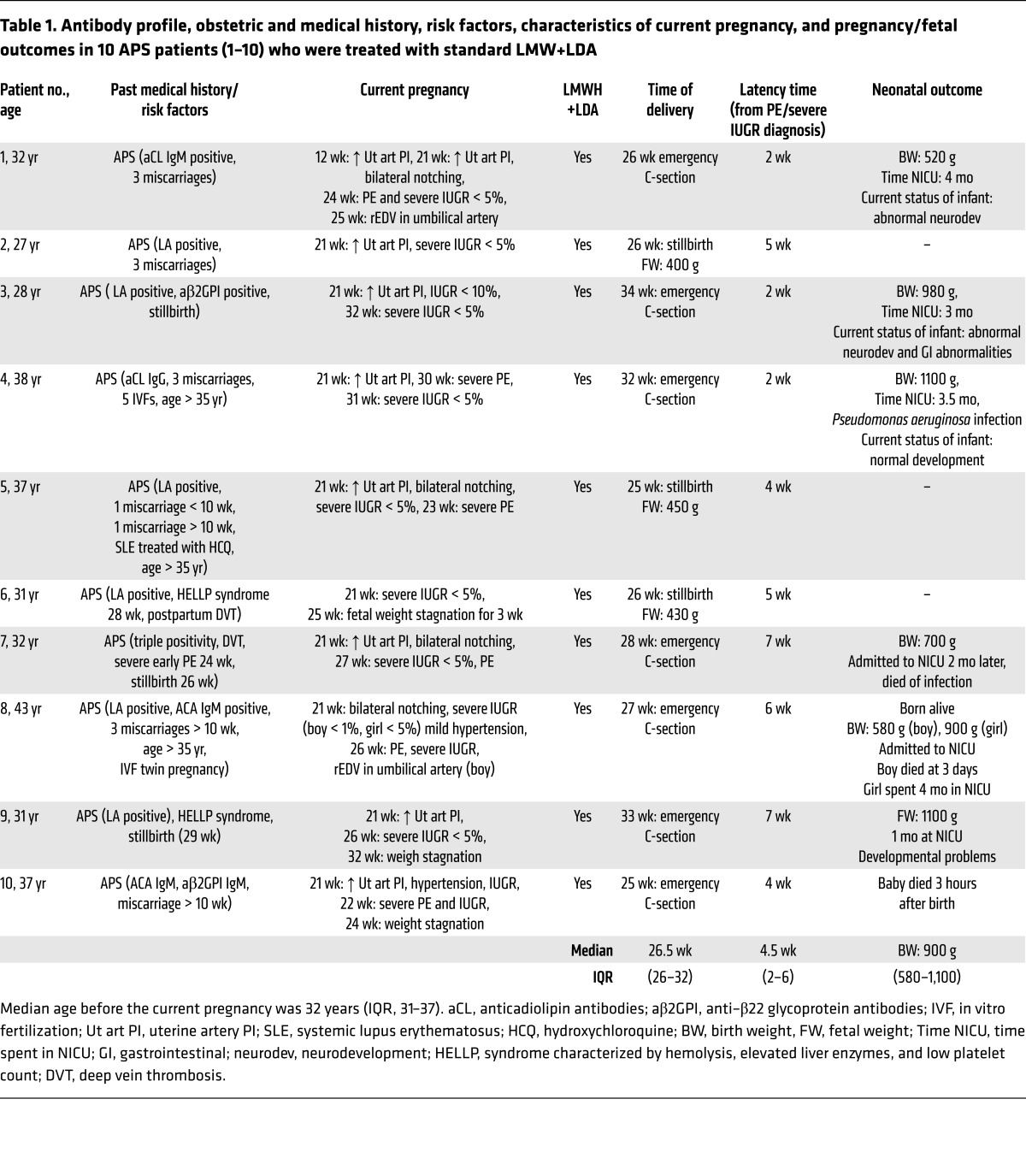
Table 1. Antibody profile, obstetric and medical history, risk factors, characteristics of current pregnancy, and pregnancy/fetal outcomes in 10 APS patients (1–10) who were treated with standard LMW+LDA.
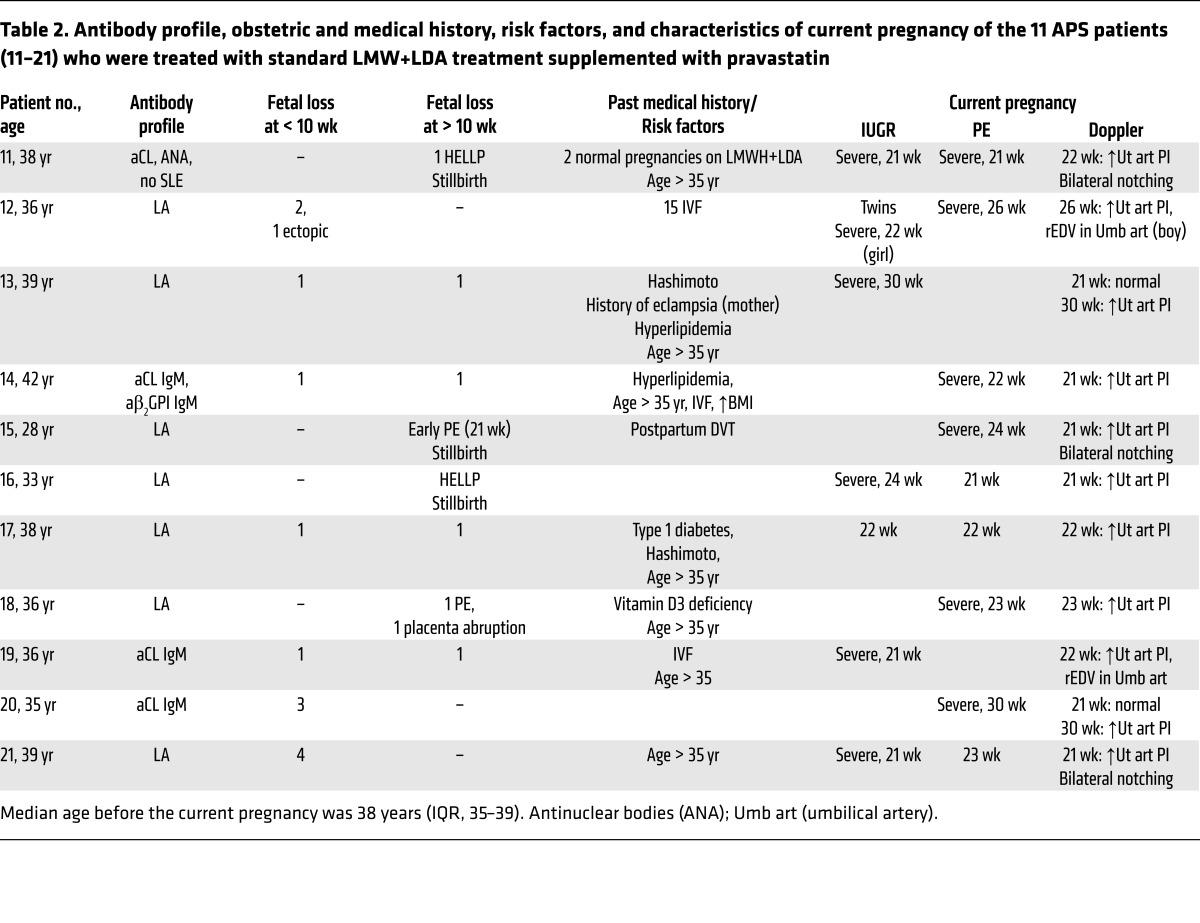
Table 2. Antibody profile, obstetric and medical history, risk factors, and characteristics of current pregnancy of the 11 APS patients (11–21) who were treated with standard LMW+LDA treatment supplemented with pravastatin.
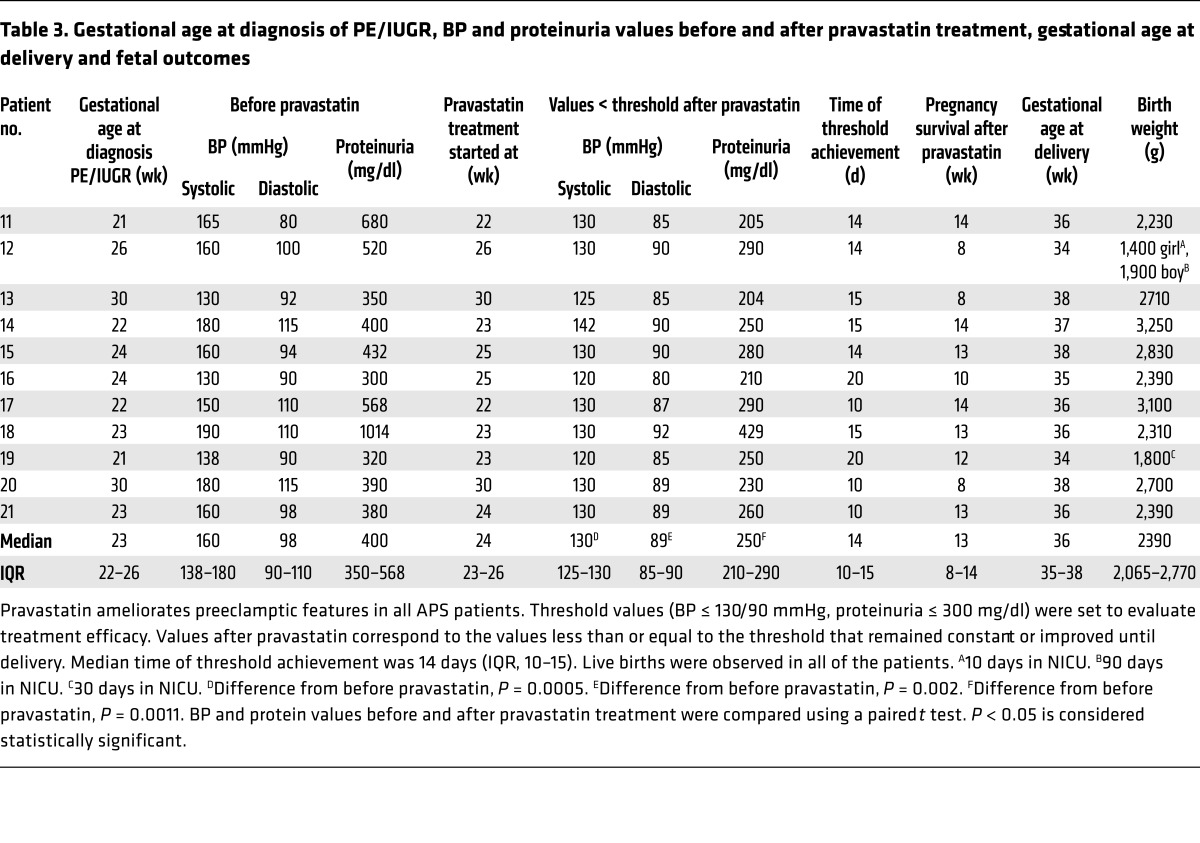
Table 3. Gestational age at diagnosis of PE/IUGR, BP and proteinuria values before and after pravastatin treatment, gestational age at delivery and fetal outcomes.
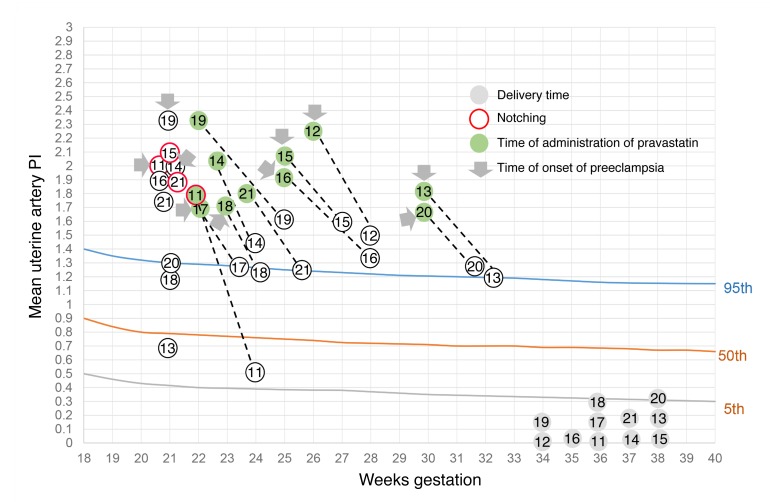
Figure 1. Mean uterine artery PI versus gestational age in the general population (estimated 5th, 50th, and 95th percentiles are shown) and in the 11 patients with APS (11–21) who were treated with LMWH+LDA supplemented with pravastatin.
PI values in women with obstetric APS were over the 95th percentile. After pravastatin treatment (gray arrows), PI values decreased. Time of administration of pravastatin is indicated in green. Red circles indicate patients who presented notching in the uterine arteries.
Patients 1 through 10 continued to be treated with standard LMWH+LDA after PE and/or IUGR diagnosis. Doppler measurements in the uterine arteries remained abnormal with standard LMWH+LDA treatment. A slight decrease in BP was observed after the administration of MDP. After PE and/or IUGR was diagnosed, pregnancies continued for approximately 4 weeks (median, 4.5 weeks; interquartile range [IQR], 2–6). Three pregnancies ended in stillbirth at 25 to 26 weeks. Preterm cesarean delivery (C-section) was performed in 7 women due to fetal distress and or maternal health concerns (median C-section time, 26.3 weeks; IQR, 26–32). One neonate died 3 hours after birth. Because of prematurity (birth weight: median, 900 g; IQR, 580–1,100) all neonates were admitted to the neonatal intensive care unit (NICU). Of the 7 neonates admitted to the NICU, 2 neonates died — one at 3 days and the other at 2 months of age — due to infection. The remaining 5 neonates were discharged from the NICU, and 3 of them currently present neurological and gastrointestinal developmental abnormalities.
In patients 11 to 21, pravastatin (20 mg) was added to the standard of care treatment as soon as signs of PE and/or IUGR were observed until delivery. This group also received MDP to treat hypertension. Following the addition of pravastatin to conventional therapy, uteroplacental blood flow improved (Figure 1) and proteinuria and BP diminished significantly (Table 3). To evaluate improvement of maternal signs of PE after pravastatin treatment, a threshold of BP of 130/90 mmHg or less and proteinuria of 300 mg/dl or less was set. The BP/proteinuria values that reached these thresholds after pravastatin treatment are shown in Table 3. After reaching the threshold values, BP and proteinuria remained within those values or improved until the end of pregnancy. Time (days) of threshold achievement was defined as the shortest time at which BP and proteinuria thresholds were reached (Table 3). BP diminished in patients who developed PE and also in patients 13, 16, and 19, who showed borderline hypertension and severe IUGR. The initial response to treatment was observed at as early as 10 days (median, 14 days; IQR, 10–15) (Figure 1 and Table 3).
Abnormal umbilical artery Doppler (absent end diastolic flow [aEDV] or reverse end diastolic flow [rEDV]) in the presence of IUGR is an ominous finding that requires immediate delivery to prevent IUFD. In the control group that did not receive pravastatin, rEDV was observed in patients 1 and 8. Emergency C-sections were performed in both patients 1 week after the detection of abnormal Dopplers because of fetal distress. One of the neonates died 3 days after birth, and the other spent 4 months in the NICU and currently shows neurological abnormalities.
In the pravastatin-treated group, patients 12 and 19 also showed rEDV and IUGR (Table 2). However, while fetal survival in this situation is very unlikely, fetal weight gain and normal cardiotocography (CTG) were observed with pravastatin treatment, and pregnancies continued for 8 and 12 weeks, respectively. Redistribution of fetal cardiac output in response to abnormal placental perfusion to maintain delivery of oxygen and nutrients to the brain — brain sparing — was observed in all growth-restricted fetuses, but remained stable until delivery in patients treated with pravastatin.
Preterm delivery, spontaneous or iatrogenic, is a complication frequently associated with PE. Infants born preterm are vulnerable to many complications, including respiratory distress syndrome, chronic lung disease, injury to the intestines, compromised immune system, cardiovascular disorders, hearing and vision problems, and neurological insult. Infants born at the lower limit of viability have the highest mortality rates and the highest rates of all complications. All patients in the control group that received standard therapy delivered preterm by emergency C-sections (median, 26.5 weeks; IQR, 26–32) because of fetal and/or maternal health concerns. Pravastatin prolonged pregnancies significantly after the onset of PE (median, 13 weeks; IQR, 10–15) compared with those women who did not receive pravastatin (median, 4.5 weeks; IQR, 2–6; P < 0.01) (Tables 1 and 3). With pravastatin treatment, women delivered close to term (median, 36 weeks; IQR, 35–36), increasing the chances of fetal maturation and survival. In the group that did not receive pravastatin, all premature neonates were admitted to the NICU and 3 perinatal deaths were observed. The obstetric end point that was set for the cohort of patients treated with pravastatin was to deliver them after 37 weeks. Interestingly, 8 (73%) of the patients who received pravastatin delivered at 36 weeks or later, with excellent neonatal outcomes (Figure 1 and Table 3), compared with the control group in which 3 stillbirths occurred and 100% of the remaining deliveries occurred preterm. All of the patients cotreated with pravastatin delivered after 34 weeks, thus diminishing substantially the chances for any prematurity-associated adverse neonatal outcomes. All women received antenatal steroids to improve respiratory neonatal outcome. Three patients (patients 12, 16, and 19) (27%) delivered at 34 to 35 weeks. Patient 12 had a twin pregnancy, and patient 19 showed early signs of severe IUGR. Neonates from patients 12 (twin pregnancy) and 19 (singleton) were admitted to the NICU; the length of stay varied from 10 days to 90 days and was related to gestation and birth weight (Table 3), but the neonates are now healthy and show normal development for their age. Only one patient in the control group delivered at 34 weeks (patient 3). The neonate showed a significant growth restriction (980 g), spent 3 months in the NICU, and currently shows neurological and gastrointestinal abnormalities (Table 1).
There were no congenital abnormalities or late fetal deaths and no evidence of maternal morbidity because of the use of pravastatin, adding to the growing evidence that statins are safe in pregnancy.
Discussion
The currently accepted first-line treatment for obstetric APS is thromboprophylactic treatment with LDA and heparinoids. However, in approximately 20% of obstetric APS cases, good pregnancy outcomes cannot be achieved with this conventional treatment (38). In fact, women with APS that received LDA+LMWH treatment throughout pregnancy had higher rates of PE than control women without APS (7). That the 21 patients described in this study developed PE and/or IUGR despite the use of antithrombotic therapy suggests that averting thrombosis is not always effective in preventing placental insufficiency–associated pregnancy complications in APS (5, 6).
The risk of neonatal death in a preeclamptic pregnancy is considerable, especially the risk associated with prematurity (15–17). Early onset PE is associated with the highest neonatal mortality. Moreover, the risk of prematurity and perinatal death is exacerbated in APS pregnancies. IUGR (26.3% of the total live births) and prematurity (48.2%) are the most frequent fetal morbidities in APS (39). Following pravastatin addition to conventional standard of care therapy, placental blood flow and maternal signs of PE improved significantly in this cohort, leading to live births in 100% of the patients. The beneficial effects of pravastatin were observed within a short time period and allowed pregnancies to survive. On the other hand, only 45% of pregnancies resulted in live births in the patients who received conventional antithrombotic therapy, and 40% of the surviving neonates showed developmental abnormalities.
Considering the adverse outcomes in previous pregnancies and in patients who received LMWH+LDA without pravastatin supplementation, the present study suggests that women with refractory obstetric APS may have improved pregnancy outcomes with pravastatin taken at the time of onset of PE or severe IUGR.
Limitations of the present study include the small number studied.
MDP remains one of the most widely used drugs for the treatment of PE. The use of antihypertensive therapy might also contribute to the improvement of maternal signs of PE in this cohort of women. However, treatment of preeclamptic women remote from term with antihypertensive drugs showed limited benefits in prolonging gestation and improving perinatal outcomes (40). In fact, in 5 out of the 10 women in the control group who did not receive pravastatin, MDP did not decrease BP significantly and pregnancies were prolonged for a short time (median, 4 weeks; IQR, 2–5). Some women in this study were put on bed rest. While some studies suggest this might help control BP, there are no data to support the benefit of bed rest in prolonging gestation (41).
The rapid response to pravastatin observed in uterine artery hemodynamic parameters suggests that pravastatin might be targeting placental vasculopathy in APS patients who develop PE. It is tempting to speculate that pravastatin might increase placental blood flow by its antiatherogenic effects and by stimulating the release of vasoactive substances from the endothelium, such as nitric oxide or carbon monoxide (42, 43). Statins have also been shown to downregulate tissue factor, a crucial molecule in the crosstalk between inflammation and thrombosis in mice and humans with APS (29, 44). These protective effects of pravastatin on the endothelium together with its effect restoring angiogenic balance (24) might also explain the amelioration of placental and maternal preeclamptic signs.
Overall, the results obtained in this study in which pregnant women with APS who did not respond to conventional antithrombotic therapy responded satisfactorily to pravastatin appear encouraging in a patient population with frequent adverse pregnancy outcomes and warrant further investigation. Randomized clinical trials should be organized to confirm these observations.
Methods
Between 2013 and 2015, at the Hippokration General Hospital of Thessaloniki, twenty-one women with APS had been treated with LMWH (enoxaparin or tinzaparin, 40 mg subcutaneously, once daily) plus LDA (80 mg orally, once daily) from the first positive pregnancy test. APS was defined by the presence of clinical criteria and laboratory criteria as follows. Clinical criteria included 1 or more clinical episodes of thrombosis and pregnancy morbidity. Pregnancy morbidity was defined as 1 or more unexplained fetal deaths at or beyond the 10th week of gestation or 1 or more premature births before the 34th week of gestation because of placental insufficiency such as PE or 3 or more unexplained consecutive spontaneous abortions before the 10th week of gestation. Lupus anticoagulant (LA) and/or anticardiolipin (aCL) and/or β2 glycoprotein-I IgG or IgM antibody present in plasma or serum on 2 or more occasions, at least 12 weeks apart, are included in the laboratory criteria for APS (45). While some of the women met the criteria for obstetric APS before this study and others had persistent aPL antibodies and met criteria during the current pregnancy, all women received conventional LDA+LMWH treatment (35).
Despite the aforementioned antithrombotic treatment, all of the patients developed placental insufficiency–related complications such as PE and IUGR. Most of the patients were diagnosed with early PE (21–23 weeks).
PE was defined according to the report of the American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy (46). PE was defined as BP elevation after 20 weeks of gestation with proteinuria or any of the following features: thrombocytopenia, impaired liver function, new development of renal insufficiency, pulmonary edema, or new onset of cerebral or visual disturbances. IUGR was defined as fetal weight below the 10th percentile, and severe IUGR was defined as fetal weight below the 5th percentile for gestational age and abdominal circumference below the 2.5th percentile (47).
The patients’ past obstetric and medical history, risk factors, and characteristics of current pregnancy, including Doppler studies, are presented in Table 1 (patients who were treated with LMWH+LDA) and Table 2 (patients who were supplemented with pravastatin). All women had a poor obstetric history with a low rate of live births. Eleven patients consented and were started on pravastatin (20 mg/d) from the time they were diagnosed with PE or IUGR, and 10 received only standard LMWH+LDA treatment.
Median age for the patients who were supplemented with pravastatin was 38 years (IQR, 35–39), and 32 years (IQR, 31–37) was the median age for the group that received only standard therapy. Women with PE were given MDP (1 g/d in 2 divided doses) to treat hypertension. All patients were admitted to the hospital, most of them until delivery. Because these were inpatients in the high-risk pregnancy unit, close maternal and fetal monitoring was undertaken. Maternal monitoring included measurement of BP 3 times a day, measurement of proteinuria, and blood tests to assess liver and kidney function and number of platelets twice weekly. Ultrasound assessment of fetal growth was performed every week, amniotic fluid volume was assessed twice weekly, and umbilical, middle cerebral artery, ductus venosus, and uterine artery Doppler velocimetry were performed twice weekly to monitor fetal status. Daily monitoring of the fetus with CTG was also performed. Maternal and fetal progress were reviewed regularly. Patients with PE were discharged to the community when BP was less than 130/90 mmHg and blood results were stable or improving and, in the case of IUGR fetuses, when acceleration of fetal weight gain was observed along with stable or improved fetal and maternal Doppler measurements. After discharge, women were advised to check their BP twice daily and to visit the antenatal day assessment unit 3 times a week in order to have a CTG and to review their measurements. Blood tests were also performed during these visits, and the quantity of urinary protein was checked once a week. Doppler assessment after discharge was performed twice weekly. Despite improvement after pravastatin treatment, some patients chose to remain as inpatients in the high-risk pregnancy unit until delivery for close monitoring, mainly because of maternal anxiety.
Statistics.
Statistical analysis to compare BP and proteinuria values before and after pravastatin treatment and time from diagnosis to delivery between pravastatin-treated women and control was conducted using a paired t test. P < 0.05 was considered statistically significant. Medians and IQRs are reported for all variables measured. All analysis was conducted with GraphPad Prism statistical software (GraphPad Software Inc.).
Study approval.
This study was approved by the Ethical Review Committee at Hippokration General Hospital of Thessaloniki, and informed consent was obtained from all pregnant patients.
Author contributions
GG and EL conceived and designed the study. GG and EL analyzed and interpreted data. GG created graphs and wrote the manuscript. TD, CV, and AM collected data. DR was responsible for the overall supervision of the patients.
Supplementary Material
Acknowledgments
This work was funded by local sources from the Hippokration General Hospital of Thessaloniki.
Footnotes
Role of funding source: Hippokration General Hospital of Thessaloniki provided the patients and consumables used for the clinical care and routine exams of the patients. The study was designed, conducted, analyzed, and reported entirely by the authors.
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2016;126(8):2933–2940. doi:10.1172/JCI86957.
See the related Commentary beginning on page 2792.
Contributor Information
Eleftheria Lefkou, Email: elefkou@gmail.com.
Apostolos Mamopoulos, Email: amamop@otenet.gr.
Themistoklis Dagklis, Email: tdagklis@yahoo.gr.
Christos Vosnakis, Email: vosnakisc@gmail.com.
Guillermina Girardi, Email: guillermina.girardi@kcl.ac.uk.
References
- 1.Danza A, Ruiz-Irastorza G, Khamashta M. Antiphospohlipid syndrome in obstetrics. Best Pract Res Clin Obstet Gynaecol. 2012;26(1):65–76. doi: 10.1016/j.bpobgyn.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Stone S, et al. The placental bed in pregnancies complicated by primary antiphospholipid syndrome. Placenta. 2006;27(4–5):457–467. doi: 10.1016/j.placenta.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Girardi G, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003;112(11):1644–1654. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meroni PL, Gerosa M, Raschi E, Scurati S, Grossi C, Borghi MO. Updating on the pathogenic mechanisms 5 of the antiphospholipid antibodies-associated pregnancy loss. Clin Rev Allergy Immunol. 2008;34(3):332–337. doi: 10.1007/s12016-007-8055-9. [DOI] [PubMed] [Google Scholar]
- 5.Laskin CA, et al. Low molecular weight heparin and aspirin for recurrent pregnancy loss: results from the randomized, controlled HepASA Trial. J Rheumatol. 2009;36(2):279–287. doi: 10.3899/jrheum.080763. [DOI] [PubMed] [Google Scholar]
- 6.Branch DW, Silver RM, Blackwell JL, Reading JC, Scott JR. Outcome of treated pregnancies in women with antiphospholipid syndrome: an update of the Utah experience. Obstet Gynecol. 1992;80(4):614–620. [PubMed] [Google Scholar]
- 7.Bouvier S, et al. Comparative incidence of pregnancy outcomes in treated obstetric antiphospholipid syndrome: the NOH-APS observational study. Blood. 2014;123(3):404–413. doi: 10.1182/blood-2013-08-522623. [DOI] [PubMed] [Google Scholar]
- 8.Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med. 2004;10(11):1222–1226. doi: 10.1038/nm1121. [DOI] [PubMed] [Google Scholar]
- 9. Lefkou E, Hunt B. Pre-eclampsia. In: Pavord S, Hunt B, eds. The Obstetric Hematology Manual. Cambridge, United Kingdom: Cambridge University Press; 2010:203–217. [Google Scholar]
- 10.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking:systematic review of controlled studies. BMJ. 2005;330(7491): doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nodler J, Moolamalla SR, Ledger EM, Nuwayhid BS, Mulla ZD. Elevated antiphospholipid antibody titers and adverse pregnancy outcomes: analysis of a population-based hospital dataset. BMC Pregnancy Childbirth. 2009;9: doi: 10.1186/1471-2393-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heilmann L, Schorsch M, Hahn T, Fareed J. Antiphospholipid syndrome and pre-eclampsia. Semin Thromb Hemost. 2011;37(2):141–145. doi: 10.1055/s-0030-1270341. [DOI] [PubMed] [Google Scholar]
- 13.Adams T Yeh C, Bennett-Kunzier N, Kinzler WL. Long-term maternal morbidity and mortality associated with ischemic placental disease. Semin Perinatol. 2014;38(3):146–150. doi: 10.1053/j.semperi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Dekker GA. Management of preeclampsia. Pregnancy Hypertens. 2014;4(3):246–247. doi: 10.1016/j.preghy.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Maternal mortality. [November 2015]; [June 27, 2016];World Health Organization. http://www.who.int/mediacentre/factsheets/fs348/en/
- 16.Cousens S, et al. National, regional, and worldwide estimates of stillbirth rates in 2009 with trends since 1995: a systematic analysis. Lancet. 2011;377(9774):1319–1330. doi: 10.1016/S0140-6736(10)62310-0. [DOI] [PubMed] [Google Scholar]
- 17.Vogel JP, et al. Maternal complications and perinatal mortality: findings of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121(suppl 1):76–88. doi: 10.1111/1471-0528.12633. [DOI] [PubMed] [Google Scholar]
- 18.Wetzka B, Winkler K, Kinner M, Friedrich I, März W, Zahradnik HP. Altered lipid metabolism in preeclampsia and HELLP syndrome: links to enhanced platelet reactivity and fetal growth. Semin Thromb Hemost. 1999;25(5):455–462. doi: 10.1055/s-2007-994950. [DOI] [PubMed] [Google Scholar]
- 19.Staff AC, Dechend R, Pijnenborg R. Learning from the placenta: acute atherosis and vascular remodelling in preeclampsia-novel aspects for atherosclerosis and future cardiovascular health. Hypertension. 2010;56(6):1026–1034. doi: 10.1161/HYPERTENSIONAHA.110.157743. [DOI] [PubMed] [Google Scholar]
- 20.Staff AC, Johnsen GM, Dechend R, Redman CW. Preeclampsia and uteroplacental acute atherosis: immune and inflammatory factors. J Reprod Immunol. 2014;101–102:120–126. doi: 10.1016/j.jri.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Ames PR, Margarita A, Alves JD. Antipohospholipid antibodies and atherosclerosis: insights from systemic lupus erythematosus and primary antiphospholipid syndrome. Clin Rev Allergy Immunol. 2009;37(1):29–35. doi: 10.1007/s12016-008-8099-5. [DOI] [PubMed] [Google Scholar]
- 22.Levy RA, Avvad E, Oliveira J, Porto LC. Placental pathology in antiphospholipid syndrome. Lupus. 1998;7(suppl 2):S81–S85. doi: 10.1177/096120339800700218. [DOI] [PubMed] [Google Scholar]
- 23.Nayar R, Lage JM. Placental changes in a first trimester missed abortion in maternal systemic lupus erythematosus with antiphospholipid syndrome; a case report and review of the literature. Hum Pathol. 1996;27(2):201–206. doi: 10.1016/s0046-8177(96)90377-9. [DOI] [PubMed] [Google Scholar]
- 24.Girardi G. Can statins prevent pregnancy complications? J Reprod Immunol. 2014;101–102:161–167. doi: 10.1016/j.jri.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed A, Singh J, Khan Y, Seshan SV, Girardi G. A new mouse model to explore therapies for preeclampsia. PLoS One. 2010;5(10): doi: 10.1371/journal.pone.0013663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costantine MM, et al. Using pravastatin to improve the vascular reactivity in a mouse model of soluble fms-like tyrosine kinase-1-induced preeclampsia. Obstet Gynecol. 2010;116(1):114–120. doi: 10.1097/AOG.0b013e3181e10ebd. [DOI] [PubMed] [Google Scholar]
- 27.Kumasawa K, et al. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci U S A. 2011;108(4):1451–1455. doi: 10.1073/pnas.1011293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh J, Ahmed A, Girardi G. Role of complement component C1q in the onset of preeclampsia in mice. Hypertension. 2011;58(4):716–724. doi: 10.1161/HYPERTENSIONAHA.111.175919. [DOI] [PubMed] [Google Scholar]
- 29.Redecha P, Franzke CW, Ruf W, Mackman N, Girardi G. Neutrophil activation by the tissue factor/Factor VIIa/PAR2 axis mediates fetal death in a mouse model of antiphospholipid syndrome. J Clin Invest. 2008;118(10):3453–3461. doi: 10.1172/JCI36089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lefkou E, et al. Clinical improvement and successful pregnancy in a preeclamptic patient with antiphospholipid syndrome treated with pravastatin. Hypertension. 2014;63(5):e118–e119. doi: 10.1161/HYPERTENSIONAHA.114.03115. [DOI] [PubMed] [Google Scholar]
- 31.Chaiworapongsa T, et al. Pravastatin to prevent recurrent fetal death in massive perivillous fibrin deposition of the placenta (MPFD) J Matern Fetal Neonatal Med. 2016;29(6):855–862. doi: 10.3109/14767058.2015.1022864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brownfoot FC, et al. Effects of Pravastatin on human placenta, endothelium, and women with severe preeclampsia. Hypertension. 2015;66(3):687–697. doi: 10.1161/HYPERTENSIONAHA.115.05445. [DOI] [PubMed] [Google Scholar]
- 33.Costantine MM, et al. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol. 2015;214(6):720.e1–720.e17. doi: 10.1016/j.ajog.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bateman BT, et al. Statins and congenital malformations: cohort study. BMJ. 2015;350: doi: 10.1136/bmj.h1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arachchillage DR, Machin SJ, Mackie IJ, Cohen H. Diagnosis and management of non-criteria obstetric antiphospholipid syndrome. Thromb Haemost. 2015;113(1):13–19. doi: 10.1160/TH14-05-0416. [DOI] [PubMed] [Google Scholar]
- 36.Campbell S, et al. New doppler technique for assessing uteroplacental blood flow. Lancet. 1983;1(8326 pt 1):675–677. doi: 10.1016/s0140-6736(83)91970-0. [DOI] [PubMed] [Google Scholar]
- 37.Zhong Y, Tuuli M, Odibo AO. First-trimester assessment of placenta function and the prediction of preeclampsia and intrauterine growth restriction. Prenat Diagn. 2010;30(4):293–308. doi: 10.1002/pd.2475. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Pedrera C, et al. Immunotherapy in antiphospholipid syndrome. Int Immunopharmacol. 2015;27(2):200–208. doi: 10.1016/j.intimp.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Cervera R, et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis. 2015;74(6):1011–1018. doi: 10.1136/annrheumdis-2013-204838. [DOI] [PubMed] [Google Scholar]
- 40.Mutch LM, Moar VA, Ounsted MK, Redman CW. Hypertension during pregnancy, with and without specific hypotensive treatment. Perinatal factors and neonatal morbidity. Early Human Develop. 1977;1(1):45–57. doi: 10.1016/0378-3782(77)90029-9. [DOI] [PubMed] [Google Scholar]
- 41.Bigelow C, Stone J. Bed rest in pregnancy. Mt Sinai J Med. 2011;78(2):291–302. doi: 10.1002/msj.20243. [DOI] [PubMed] [Google Scholar]
- 42.Kaesemeyer WH, Caldwell RB, Huang J, Caldwell RW. Pravastatin sodium activates endothelial nitric oxide synthase independent of its cholesterol-lowering actions. J Am Coll Cardiol. 1999;33(1):234–241. doi: 10.1016/S0735-1097(98)00514-2. [DOI] [PubMed] [Google Scholar]
- 43.Ramma W, Ahmed A. Therapeutic potential of statins and the induction of heme oxygenase-1 in preeclampsia. J Reprod Immunol. 2014;101–102:153–160. doi: 10.1016/j.jri.2013.12.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erkan D, et al. A prospective open-label pilot study of fluvastatin on proinflammatory and prothrombotic biomarkers in antiphospholipid antibody positive patients. Ann Rheum Dis. 2014;73(6):1176–1180. doi: 10.1136/annrheumdis-2013-203622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyakis S, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 46.American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 47. Bernstein I, Gabbe SG. Intrauterine Growth Restriction. 3rd ed. New York, New York, USA: Churchill Livingstone, 1996:863–886. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.