Abstract
Pancreatic ductal adenocarcinoma (PDAC) is a deadly cancer in which NF-κB pathways promote biological aggressiveness. In this issue of the JCI, Lesina et al. investigated the role of RelA, the p65 partner of p50 that together form the most common NF-κB complex, in the early stages of pancreatic malignant transformation and in established PDAC. By deleting Rela in the context of an oncogenic Kras-driven autochthonous model of PDAC, the authors demonstrated that RelA is a mediator of oncogene-induced senescence (OIS) and the senescence-associated secretory phenotype (SASP) that attenuates acinar-to-ductal metaplasia, pancreatic intraepithelial neoplasia (PanIN) formation, and PanIN progression to PDAC. Loss of the tumor-suppressor function of RelA in the early stages of Kras-driven pancreatic neoplastic transformation was associated with decreased OIS and SASP and a protumorigenic tumor microenvironment that harbored more M2 macrophages and myeloid-derived suppressor cells. The beneficial effects of RelA were mediated by increased expression of CXCL1 and its activation of CXCR2. By contrast, in advanced stages of Kras-driven murine PDAC, loss of p53 or p16 was associated with senescence bypass, and RelA deficiency in this context attenuated cancer cell proliferation and prolonged mouse survival, indicating that RelA enhances tumor progression in established PDAC.
Pancreatic ductal adenocarcinoma: combined factors promote aggressiveness
Pancreatic ductal adenocarcinoma (PDAC) is a treatment-recalcitrant cancer with an overall 5-year survival rate of 7% (1). It is the fourth leading cause of cancer death in adults in the United States but is predicted to become second only to lung cancer by 2030, due to improved survival among patients with colon and breast cancer (2). This anticipated increase in PDAC incidence is also a consequence of the rising prevalence of risk factors, such as advanced age, type 2 diabetes, and obesity (3, 4). There are multiple reasons for the biological aggressiveness of PDAC. These include a high frequency of major driver mutations, such as KRAS (~95%), CDKN2A (~90%), p53 (~70%), and SMAD4 (~50%), and many low-frequency driver mutations (5). Recent, deep whole-genome sequencing and copy number variation analysis have revealed additional alterations, including mutations in genes such as ARID2, ROBO2, and ATM, small regions of hypermutation (kataegis), chromosomal deletions, rearrangements and amplifications, and epigenetic changes (6–8). For example, when not mutated in PDAC, CDKN2A is epigenetically silenced, allowing for aberrant entry into the cell cycle. PDAC is also associated with suppression of cancer-directed immune mechanisms, a characteristically dense stroma that interferes with intratumor drug delivery, loss of negative growth constraints, and constitutive activation of prosurvival pathways such as STAT3 and NF-κB, all of which combine to confer a survival advantage to pancreatic cancer cells and to render them resistant to chemotherapy and radiation therapy (9).
NF-κB is a transcription factor that was first described by Sen and Baltimore in 1986, when they identified a nuclear factor that bound to the enhancer element of the Ig κ light-chain in B cells (10). Subsequently, it was established that there are five members of this family of transcription factors: RelA (also known as p65), RelB, c-Rel, p50, which derives from a p105 precursor, and p52, which derives from a p100 precursor (11). All five factors possess a Rel homology domain (RHD) and can combine to form homodimers or heterodimers (11). The most common combination is a heterodimer of RelA-p50, and this complex is crucial for nuclear translocation of NF-κB. In addition to the N-terminal RHD, which enables DNA binding and dimerization, RelA possesses a C-terminal transactivation domain (TAD) that mediates interactions with transcription complexes. In the basal state, NF-κB is sequestered in the cytoplasm through its association with inhibitors of κB (IκB) proteins. There are several IκB proteins, including IκBα, IκBβ, and BCL-3 (11), all of which contain ankyrin repeats that promote IκB binding to NF-κB proteins. A variety of stimuli, including growth factors, cytokines, and stress, activate IkB kinases (IKKs), most commonly IKKα and IKKβ, that induce IκB phosphorylation, leading to its polyubiquitination and degradation, thereby allowing for NF-κB nuclear translocation. A noncatalytic accessory protein, NF-κB essential modulator (NEMO), which is also known as IKKγ, also plays a role in this process.
Oncogene-induced senescence
In 1997, Serrano and colleagues reported that attempts to induce transformation of human diploid fibroblasts with oncogenic KRAS led to oncogene-induced senescence (OIS) due to enhanced expression of p16 and p53, as evidenced by cell-cycle arrest in G1 and a senescence-like phenotype, and that this proliferation arrest was reversed by inactivating either p16 or p53 (12). In contrast, Lee and Bar-Sagi reported that oncogenic KRAS prevents senescence in primary pancreatic duct epithelial cells (PDECs) by upregulating the expression of Twist, which then acts to suppress p16 (13). Moreover, in vivo, oncogenic Kras is the major driver of PDAC initiation (9), which is dependent on both upstream factors, such as the EGF receptor (14), and downstream factors, such as IKKβ (15). Thus, Lin and colleagues demonstrated that oncogenic KRAS upregulates the AP-1 transcription factor, which subsequently enhances IL-1α expression, thereby inducing lysine 63 (K63) polyubiquitination of TNF receptor-associated factor 6 (TRAF6), which binds to NEMO (15). Consequently, the IKK complex was activated, leading to the nuclear translocation of NF-κB and the upregulation of p62, a protein that regulates the turnover of K63-polyubiquitinated proteins. The net result was the demonstration of a vicious circle that enabled oncogenic KRAS to sustain deleterious NF-κB activity and enhance pancreatic cancer cell proliferation and survival.
As reviewed relatively recently (16), senescence is designed to eliminate stressed and damaged cells and is associated with the secretion of various cytokines, growth factors, and proteases, a phenomenon that has been described as the senescence-associated secretory phenotype (SASP). Senescent cells are also characterized by an abundance of senescence-associated β-gal (SA-β-gal), often express p16, and exhibit senescence-associated heterochromatin foci, as well as other markers (16). In this issue, a study by Lesina et al. provides a plethora of novel findings regarding OIS in the context of PDAC (17). First, the authors showed that RelA was important for maintaining OIS and SASP (Figure 1). Second, these results have implicated a key cytokine, CXCL1, and its receptor, CXCR2, in this process. Third, they demonstrated that deletion of Rela from the pancreas of mice expressing oncogenic Kras (Kras RelA mice) accelerated the formation of acinar-to-ductal metaplasia (ADM) lesions, promoted a more rapid conversion to pancreatic intraepithelial neoplasia (PanIN), and enhanced the progression of PanIN to PDAC. Fourth, Lesina and colleagues observed an increased tumor frequency in Kras RelA mice, further underscoring a previously unrecognized tumor-suppressive role of RelA (17).
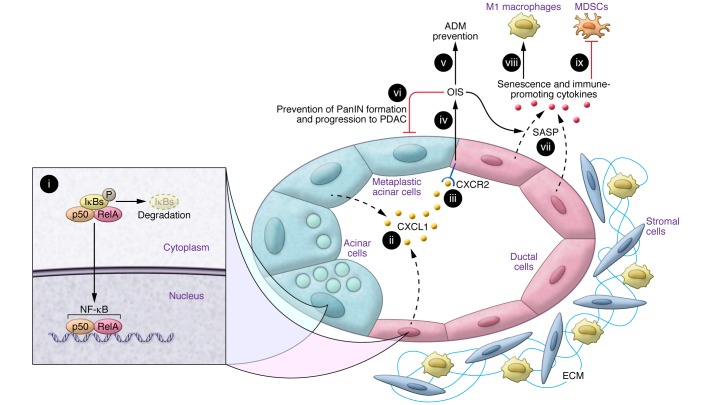
Figure 1. RelA exerts tumor-suppressive actions during the early steps of PDAC initiation.
An early pancreatic lesion, in which acinar cells are undergoing ADM and are actively forming a PanIN lesion. (i) Activated RelA translocates to the nucleus with its p50 partner, and the NF-κB complex enhances the production of CXCL1 (ii). After binding to CXCR2 (iii), CXCL1 enhances OIS (iii), which attenuates the formation of ADM lesions (v), as well as PanIN formation and progression to PDAC (vi). As a result of the induction of senescence, the ductal cells in PanIN lesions secrete multiple additional cytokines (vii) through a mechanism termed SASP. These RelA-induced changes are associated with paracrine immune–promoting actions on the tumor microenvironment, as evidenced by an increase in the number of M1 macrophages (viii) and a decrease in the number of myeloid-derived suppressor cells (MDSCs) (ix). Minimal stroma is shown for these early lesions. By contrast, in established PDAC, loss of p53 or p16/Rb pathways leads to senescence bypass, which converts RelA into a tumor promoter. ECM, extracellular matrix.
Importantly, Lesina et al. (17) went on to show that cell proliferation, both in mouse models and in isolated PDECs, was attenuated in RelA-deficient cells. By contrast, RelA-proficient pancreata harboring oncogenic Kras (Kras mice) had a gene expression profile that was consistent with enhanced proliferation, chemokine signaling, and cytokine-cytokine receptor interactions. Moreover, pancreata from Kras RelA mice exhibited cell differentiation– and cell stress–related signatures, which were confirmed by immunostaining. In support of these findings, a greater proportion of acinar cells from pancreas explants of oncogenic Kras RelA pancreata underwent transdifferentiation into ductal cells in response to TGFα, indicating that RelA functions, in part, to suppress ADM formation. Loss of RelA was also associated with a decrease in multiple OIS-associated markers, the disappearance of the SASP signature seen in RelA-proficient pancreata bearing oncogenic Kras, and increased numbers of M2 macrophages and myeloid-derived suppressor cells in the stroma. Thus, in addition to its cell-autonomous actions, RelA functions to prevent a protumorigenic tumor microenvironment.
Five approaches were used to further confirm that RelA is required for OIS and SASP and that these effects are mediated by a CXCL1/CXCR2 axis. First, Lesina et al. examined the consequences of pharmacological inhibition of NF-κB with JSH-23 and demonstrated that JSH-23 treatment led to inhibition of RelA transcriptional activity and attenuated expression of inflammatory cytokines. Notably, CXCL1-induced SA-β-gal activity was restored in vitro by the addition of exogenous CXCL1. Second, mice carrying oncogenic Kras but deficient for RelA were bred with mice expressing the human ortholog of murine CXCL1 (IL-8), which resulted in increased SA-β-gal activity in low-grade PanIN and marked suppression of PanIN progression. Third, PDECs were incubated in the absence or presence of CXCL1 and the CXCR2 inhibitor SB225002. CXCL1 increased SA-β-gal activity, and this effect was reversed by SB225002. The inhibitor also increased SA-β-gal activity in low-grade PanIN in vivo. Fourth, global loss of CXCR2 in oncogenic Kras–expressing mice resulted in rapid PanIN progression and early PDAC formation. Fifth, similar results were also observed in mice in which Cxcr2 deletion was limited to the pancreas. Taken together, these findings provide definitive evidence that OIS is dependent on CXCR2 activation by CXCL1 downstream of RelA with respect to the early events that occur during pancreatic neoplastic transformation.
Conclusions
Later stages of pancreatic tumorigenesis are associated with mutations or deletions of the genes encoding p53 and p16 (5), and in spite of the paucity of RB1 mutations in PDAC, Rb is frequently functionally inactivated in human PDAC (18). Since loss of these powerful tumor suppressors has been associated with senescence bypass, Lesina et al. next examined the role of RelA in Kras mice that lacked either p53 or p16 in the pancreas. In such mice, deletion of Rela resulted in decreased pancreatic cancer cell proliferation and prolonged survival, indicating that RelA and NF-κB enhance tumor progression once PDAC is established. Thus, RelA and CXCR2 can be double-edged swords that both suppress and promote the carcinogenic process, depending on the PDAC stage. The dual modalities of these factors clearly complicate the development of therapeutic options designed to target these pathways. Additionally, the findings of Lesina and colleagues also raise the possibility that targeting the RelA/CXCL1/CXCR2 pathway could be of special benefit to patients who are at a high risk for developing PDAC by attenuating PanIN formation and progression and providing the proverbial “stitch in time.”
Acknowledgments
MK is supported by the National Cancer Institute (NCI) grant CA-75059.
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Reference information:J Clin Invest. 2016;126(8):2799–2801. doi:10.1172/JCI89156.
See the related article beginning on page 2919.
References
- 1.Miller KD, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. doi: 10.3322/ca. [published online ahead of print June 2, 2016]. doi: 10.3322/caac.21349. [DOI] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Tang H, Dong X, Hassan M, Abbruzzese JL, Li D. Body mass index and obesity- and diabetes-associated genotypes and risk for pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(5):779–792. doi: 10.1158/1055-9965.EPI-10-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 5.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20(10):1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 6.Biankin AV, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491(7424):399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waddell N, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexandrov LB, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleeff J, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2: doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 10.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- 11.Hayden MS, Ghosh S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26(3):203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88(5):593–602. doi: 10.1016/S0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 13.Lee KE, Bar-Sagi D. Oncogenic KRas suppresses inflammation-associated senescence of pancreatic ductal cells. Cancer Cell. 2010;18(5):448–458. doi: 10.1016/j.ccr.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ardito CM, et al. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell. 2012;22(3):304–317. doi: 10.1016/j.ccr.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ling J, et al. KrasG12D-induced IKK2/β/NF-κB activation by IL-1α and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(1):105–120. doi: 10.1016/j.ccr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192(4):547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lesina M, et al. RelA regulates CXCL1/CXCR2-dependent oncogene-induced senescence in murine Kras-driven pancreatic carcinogenesis. J Clin Invest. 2016;126(8):2919–2932. doi: 10.1172/JCI86477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gore AJ, Deitz SL, Palam LR, Craven KR, Korc M. Pancreatic cancer-associated retinoblastoma 1 dysfunction enables TGF-β to promote proliferation. J Clin Invest. 2014;124(1):338–352. doi: 10.1172/JCI71526. [DOI] [PMC free article] [PubMed] [Google Scholar]